Abstract
A new chromatography support was synthesized from Sepharose CL-6B containing a benzothiazolium bromide salt as a ligand. The original goal of this study was the evaluation of the azolium salt individual contribution as a constituent part of thiacarbocyanine dyes in an affinity or multi-modal relationship with standard proteins. This dye was previously post-grafted onto beaded cellulose by a curing method and successfully used as an affinity ligand for the interaction and isolation of different proteins. Following this idea, the immobilization of an azolium bromide salt onto Sepharose CL-6B in different concentrations was performed by Steglich esterification, using dicyclohexylcarbodiimide as a coupling reagent and 4-dimethylaminopyridine as basic catalyzer. The obtained crafted beaded Sepharose supports were qualitatively and quantitatively characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy and elemental analysis. The interactions between the chromatographic supports obtained and several standard proteins were finally evaluated. The best results were achieved using a chromatographic support with a ligand density of 0.18 mmol of benzothiazolium salt/g of chromatography support, enabling the separation of ribonuclease, α-chymotrypsin and bovine serum albumin from an artificial mixture using a decreasing ammonium sulfate stepwise gradient. In conclusion, this study demonstrates the potential applicability of small multi-modal cationic ligands such as benzothiazolium salts, compared to these congener cyanine dyes previously studied, for the separation and purification of proteins by chromatography, revealing a distinct and useful chromatographic behavior by itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Affinity chromatography (AC) is potentially the most selective method for protein purification and is one of the most powerful techniques employed in the selective isolation of target molecules in bio systems [1, 2]. The technique is based on the complementary relationship between structure, charge and hydrophobicity between a given protein and a stationary phase [3]. The use of AC offers several advantages when compared to other protein purification techniques: it has the power to eliminate purification steps, increases the yield of purified product and thereby improves economic processes [2]. These advantages make this technique ideal for large scale use and stabilization of bound protein through the use of biologically based conditions to purify specific proteins. In this technique, the gradient established promotes the interaction of biomolecules with the ligand enabling its purification, through biorecognition, maintaining the stability and activity of the protein.
To obtain high yields and purity it is necessary to consider mainly the type of ligand, the matrix to which it is attached and the purification procedure [4].
Besides the low cost, an ideal matrix will be rigid, highly porous, will allow high ligand substitution, and will not interact with the sample [5]. Common matrices are materials such as agarose, cellulose, dextran, silica, polyacrylamide, among others [6]. However, the beaded agarose derivatives such as Sepharose matrixes are even more desirable because of their very loose network [7].
Another important and probably the most crucial parameter to take in mind is the nature of the ligand. Most existing affinity ligands have origin in natural sources such as heparin, gelatin, monoclonal antibodies, binding or receptor proteins, which offer defined selectivity and specificity. However, biological ligands present some limitations, since this requires purification in their own right, may be contaminated with host DNA and viruses, show lot-to-lot variation, tend to be fragile, costly to produce and are not readily amenable to scale up [8, 9]. On the other hand, the existence of synthetic ligands, based on chemically synthesized small molecules offers several advantages. They eliminate biological contamination from infective agents (such as prions), inflammatory agents (such as endotoxins), or other biological contaminants such as DNA and RNA normally associated with the biological ligand source. Moreover, synthetic ligands usually present high stability and can be prepared from fairly inexpensive chemicals [10–12].
Cyanine dyes came to the limelight during the 19th century [13]. Our research group had synthesized several carboxyalkylthiacarbocyanines [14, 15] and investigated their new applicability as ligands for AC [16].
Following these studies, the evaluation of the individual chromatographic contribution of a benzothiazolium salt as the core constituent part of a thiacarbocyanine dye used as ligand in a successful affinity or multi-modal methodology [16, 17] seems to be crucial to understand the role of each molecular interaction occurring between this complementary ligand-proteins pair. For the best of our knowledge, this is the first time that an ionic liquid [18] was used as ligand in AC.
The toxicity obtained for 91 different benzothiazolium salts related to the salt herein used against Euglena gracilis, with an observed enhance of the activity found in salts with substituents on the benzene ring were already described [19, 20]. From this data and from other previous studies [21] it can be concluded that benzothiazolium salts, as the ligand herein used not substituted in the benzene ring, but possessing a methyl group in the position 2, presents values of log ED50 between 2.755 and 3.214 which generally corresponds to an absence of a toxicity activity below 50 μg mL−1. Other biological activity studied on benzothiazolium salts and related ones, revealed inhibitory activity against transmissible gastroenteritis virus [22], antibacterial, antifungal and antimicrobial activities [23–28] and anthelmintic activity [29]. Besides, benzothiazolium salts have been also described as germination inhibitors and activity stimulators on cucumber and corn seedlings depending on the range of concentration used [30] and both in the production of chlorophyll in Chlorella vulgaris [31], the already described effect of plants growth and chlorophyll synthesis on Euglena gracillis [23, 24], increase sugar and chlorophyll contents in plants [32], and growth-regulating activity in plants [25–27].
Attempts to immobilize the benzothiazolium salt by the previously developed curing method [33] using higher temperatures used for the congener’s benzothiazolium carbocyanine dyes [16], induced the undesirable decomposition of the ligand. Moreover, the use of lower temperatures revealed to be unsuccessful since the immobilization of the ligand onto the chromatographic support has not been observed. Within different esterification methods alternatively used, namely thionyl chloride, N,N-dimethylformamide (DMF) in the presence of sulfuric acid or tosylic acid, the so called Steglich esterification showed to be effective and easy to perform with this cationic ligand.
The Steglich esterification is generally performed at room temperature without the use of extreme acid or basic conditions, allowing the reaction to proceed smoothly in mild conditions. This esterification method uses N,N′-dicyclohexylcarbodiimide (DCC) as a coupling reagent and 4-dimethylaminopyridine (DMAP) as a basic catalyst. Under these conditions, DCC and the carboxylic acid are able to form an O-acylisourea intermediate, which possesses a similar reactivity with the corresponding carboxylic acid anhydride. Finally, the alcohol easily reacts with this activated carboxylic acid to form the desired ester. Accordingly, the carboxylic group moiety of the benzothiazolium salt makes this compound reactive and potentially able to bind onto hydroxylic macromolecules as agarose (Fig. 1).
The interactions between the prepared chromatographic supports with different amounts of ligands and several standard proteins were finally evaluated. The best results were achieved using a chromatographic support with a ligand density of 0.18 mmol of benzothiazolium salt/g of chromatographic support enabling to separate ribonuclease (RNase), α-chymotrypsin and bovine serum albumin (BSA) from an artificial mixture using a decreasing stepwise gradient of ammonium sulfate.
Materials and Methods
Materials and Reagents
All reagents were purchased from Sigma–Aldrich Company (St Louis, MO) and used as received. Solvents were of analytical grade and were dried over 4 Å molecular sieves prior to use. 3-(10-carboxydecyl)-2-methylbenzothiazol-3-ium bromide (herein designed as benzothiazolium salt) was synthesized according to the literature procedure [13]. All reactions were monitored by thin-layer chromatography using 0.20 mm Al-backed silica-gel plates (Macherey–Nagel 60 F254). Sepharose CL-6B was obtained from GE Healthcare Life Sciences (Uppsala, Sweden). Polypropylene econo-pac chromatographic columns were acquired from Bio-Rad (Hercules, CA). RNase, α-chymotrypsin and BSA were purchased from Sigma–Aldrich Company (St Louis, MO). Vivaspin concentrators were acquired from Sartorius Stedim Biotech (Goettingen, Germany). NZYTech color protein marker II was bought from NZYTech (Lisbon, Portugal).
Instrumentation
Sepharose CL-6B was dried in a Büchi TO-51 Glass Drying Oven, under vacuum, over phosphorus pentoxide. Elemental analysis (EA) was performed on a Carbo-Erba, CHNS EA-1108 Elemental Analyzer.
Scanning electron microscopy (SEM) images were acquired on a Hitachi S-2700, with a UHV Dewar detector (Rontec EDX). The sulfur mapping was made by an energy dispersive X-ray spectroscopy (EDS).
The absorbance measurements were recorded on a Thermo Scientific Evolution 160 UV–Vis spectrophotometer.
To provide constant voltage for electrophoresis applications, the PowerPac™ HC power supply from Bio-Rad was used.
Preparation of the Chromatographic Matrices
Sepharose CL-6B was washed in a sintered glass funnel with several portions of ethanol and diethyl ether and suction filtered to near dryness. Prior to use, the gel was dried for at least 24 h at 80 °C, under vacuum, over phosphorous pentoxide.
The dried Sepharose (1.0 g) was placed in a round-bottom flask with the benzothiazolium salt (0.125–1.00 g, 0.30–2.41 mmol). Two molar equivalents of DCC and 0.2 molar equivalents of DMAP were added together 3 times each 2 h. The mixture thus obtained was swirled in DMF (30 mL) at room temperature for 96 h. To evaluate the chromatographic performance of the spacer arm and to assess its contribution to the proteins’ retention, the 11-bromoundecanoic acid (0.125–1.00 g, 0.47–3.77 mmol) was also immobilized onto dried Sepharose using the same methodology.
The derivative gels thus obtained were sequentially washed off with large volumes of DMF and methanol to remove the excess of ligand and then subjected to Soxhlet extraction with the same solvents separately for 24 h each. The gels were stored at 25 °C in deionized water. The Sepharose beads samples were submitted under the same conditions in the absence of ligand as a control experiment, for each assay.
Chromatographic supports based on Sepharose beads derivative thus obtained were qualitatively and quantitatively characterized using SEM, SEM–EDS and EA.
Chromatographic Method
All chromatographic assays were executed using a workbench column (econo-pac column) at room temperature. The derivative Sepharose CL-6B (1 mL) was packed in a 1.5 × 12 cm econo-pac column and equilibrated with the buffer used for the binding step (ammonium sulfate [(NH4)2SO4] or sodium chloride [NaCl] in 10 mM Tris–HCl (pH 8.0)).
Standard protein solutions of RNase, α-chymotrypsin and BSA were used for AC studies with the concentration of 25, 12.5 and 25 mg mL−1, respectively, prepared in the same buffer used for the binding step. An artificial mixture of standard proteins was also prepared with equal amounts of the three protein solutions.
The protein or protein mixture solution (150 μL) was loaded onto the column and the elution performed with different concentrations of (NH4)2SO4 (0.1–1.5 M) or NaCl (0.1–1 M), depending on the conditions under study (hydrophobic or ionic conditions, respectively).
After several experiments, the best chromatographic conditions for the binding and elution were established. Briefly, after elution of the not retained species with 10 mL of 1.5 M (NH4)2SO4 in 10 mM Tris–HCl (pH 8.0), the ionic strength of the buffer was decreased to 0.6 M (NH4)2SO4 in 10 mM Tris–HCl (pH 8.0). Finally, the column was loaded with 10 mL of 10 mM Tris–HCl (pH 8.0) buffer to promote the desorption of the most strongly bound protein. Fractions of 1 mL of eluate were collected and the absorbance was measured at 280 nm.
The fractions were concentrated and desalted using Vivaspin concentrators and checked for purity by acrylamide gel electrophoresis (SDS-PAGE).
SDS-PAGE
The protein fractions recovered from the chromatographic experiments were analyzed by 15 % SDS-PAGE using a Bio-Rad system.
Briefly, protein samples were denatured by the addition of loading dye (62.5 mM Tris-HCI, pH 6.8, 2 % SDS, 25 % glycerol, 0.01 % bromophenol blue, 3 % DL-Dithiothreitol), followed by incubation at 100 °C for 8 min. Initially, an electrical potential of 80 V was applied until the bromophenol blue dye reached the running gel. Then, the electrical potential was increased to 150 V for 1 h with Tris/glycine/SDS buffer (25 mM Tris, 192 mM glycine, 0.1 % SDS, pH 8.3) and the gel was stained by Coomassie Brilliant Blue R-250.
Results and Discussion
Benzothiazolium Salt Synthesis and Sepharose CL-6B Derivatization
The required benzothiazolium salt was obtained by condensation of 2-methylbenzothiazole with 11-bromoundecanoic acid in the absence of a solvent, as previously described [14]. The obtained compound showed spectral data fully consistent with the assigned structure.
This salt presents two suitable properties expected for an AC ligand. In one hand, the alkyl chain constitutes a convenient spacer arm between the aromatic moiety of the salt and the matrix. The use of a spacer arm will ensure that a ligand is placed at a suitable distance from the surface of the support, with ample space for complete attachment to the immobilized ligand, which potentially increases the affinity or multi-modal interaction between the protein and the derivatized Sepharose CL-6B [6]. On the other hand, the presence of a reactive carboxylic group in the benzothiazolium salt enables the opportunity of a successful fixation onto Sepharose by esterification.
The attempts, to use the curing method previously used to immobilize a thiacarbocyanine dye, [16] were not effective in our case, due to the decomposition of the benzothiazolium salt observed at the high temperatures used. Some variations were attempted without success, namely lowering temperatures and using other catalysts such as thionyl chloride, sulfuric acid and tosylic acid. Within different esterification methods alternatively used, the so called Steglich esterification showed to be effective and easy to be used with this cationic ligand and therefore it was herein used for the purpose of this work.
Some of our initial studies, both in curing and Steglich esterification conditions were performed in cellulose beads following the previous literature [16]. However, we have found that Sepharose beads were easier and more successful to derivatize with the benzothiazolium salt under Steglich esterification. Additionally, the selection of Sepharose matrix in this study was supported by its suitable properties to be used in ligands immobilization, namely the ability to form a loose porous network [5] and the high mechanical and chemical stability after coupling.
Accordingly, benzothiazolium salt AC support was synthesized by derivatization of the Sepharose CL-6B with benzothiazolium salt performed by Steglich esterification (Fig. 1). Following the same procedure, a support derivatized with the spacer arm 11-bromoundecanoic acid was prepared to evaluate the contribution of the alkyl chain in ligand–protein interaction. The bromine atom at the end of the alkyl chain allows us to determine the ligand concentration by EA.
To remove all unbound ligand, the derivatized Sepharose CL-6B supports were sequentially subject to 24 h Soxhlet extraction with DMF and methanol.
The amount of benzothiazolium salt and 11-bromoundecanoic acid immobilized onto Sepharose CL-6B was, respectively, calculated from the percentage of nitrogen (Table 1), and the bromine present was determined by elemental analysis.
A control sample obtained in the same Steglich esterification conditions in the absence of any type of ligands was performed for each immobilization experiment, revealing an amount of nitrogen below the detection limit (0.07 g of nitrogen per g of chromatographic support).
The characterization of the Sepharose beads morphology by SEM was also performed (Fig. 2). Sepharose beads submitted to the same reaction conditions in the absence of benzothiazolium salt (Fig. 2b) and after benzothiazolium salt immobilization (Fig. 2c), showed no significant differences capable of changing the chromatographic performance of the final supports.
The sulfur analysis map by SEM–EDS and the respective SEM micrograph (Fig. 3) show a uniform benzothiazolium salt distribution over the Sepharose beads.
Chromatographic Behavior of the New Supports
Pursuing the final goal of this work, namely the purification of proteins using benzothiazolium salts as ligands for AC, several experiments were carried out to study the interactions established between the matrices previously prepared and standard proteins. In other words, protein binding is affected by several studied parameters, such as: ligand density, salt type (sodium chloride or ammonium sulfate), salt concentration in the eluent and pH.
RNase, α-chymotrypsin and BSA were the standard proteins that presented a more distinct and selective binding to the chromatographic supports obtained and therefore herein used. Moreover, since the structure and functions of the studied proteins are well known and data are available, they were chosen as suitable models for this study.
Initially, the chromatographic behavior of the matrices bearing benzothiazolium salt as ligand was compared with the control matrices and with the matrices bearing 11-bromoundecanoic acid. Different stepwise gradients of NaCl (0.1–1.0 M) and (NH4)2SO4 (0.1–1.5 M) were considered and the results obtained with these elution strategies revealed some differences. None of the three proteins interact with the control experiment, what clearly shows that the ligand is responsible for the interactions observed with the three proteins. In fact, when using the NaCl gradient, different retention patterns were found for the RNase loaded onto the matrices with different ligands (benzothiazolium salt or 11-bromoundecanoic acid), which indicates that the aromatic moiety and the cationic charge of the benzothiazolium salt are responsible for some interactions, namely related to the ionic character of the ligand (Fig. 4). On the other hand, when using the (NH4)2SO4 gradient, the same chromatographic behavior was observed for both matrices. These results suggest that the alkyl chain in the matrices is mainly responsible for the hydrophobic interactions. Considering these results, the evaluation of the binding/elution behavior of the proteins was undertaken using the (NH4)2SO4 elution strategy.
The effect of ligand density in the interaction with proteins was evaluated to ascertain the most suitable ligand density. The ligand concentrations used in the immobilization procedure were 2.41, 1.21, 0.60 and 0.30 mmol of benzothiazolium salt per g of dry Sepharose affording 0.38, 0.36, 0.24 and 0.18 mmol of benzothiazolium salt bounded per g of chromatographic support, respectively.
When a 1.5 M (NH4)2SO4 in 10 mM Tris–HCl (pH 8.0) buffer solution was used as binding condition, a strong interaction mainly of α-chymotrypsin and BSA proteins with all the matrices prepared with the highest ligand concentrations was observed, preventing the desirable recovery of these retained proteins. On the other hand, the chromatographic support obtained using the lower ligand concentration (0.18 mmol of benzothiazolium salt bounded per g of chromatographic support) showed a desirable and differential interaction with all three proteins, when a decreasing stepwise gradient of (NH4)2SO4 was used. This result allowed us to elect this support as the best from all herein obtained. A close ligand concentration of 0.20 mmol of 11-bromoundecanoic acid per g chromatographic support was obtained using an initial amount of 11-bromoundecanoic acid of 1.88 mmol per g of dry Sepharose in the immobilization protocol.
The better results verified for the ligand lower concentrations could be easily understood since low ligand density can be advantageous in most of the cases, since the access of an isolated immobilized ligand to the buried binding sites of the protein is not hindered [34].
Figure 5 shows the chromatographic profile obtained by injecting an artificial mixture of the three studied proteins, after selecting the most appropriated matrix and the best chromatographic conditions, which allowed the binding and elution of each protein. For instance, RNase did not interact with the support when 1.5 M (NH4)2SO4 in 10 mM Tris–HCl (pH 8.0) was used, whereas both α-chymotrypsin and BSA were totally retained with the same condition. When the ionic strength of the buffer was successively decreased to 0.6 M (NH4)2SO4 in 10 mM Tris–HCl (pH 8.0), α-chymotrypsin was completely unbound from the support. BSA showed the strongest interaction with the ligand and eluted at the final gradient step.
Chromatographic profile obtained for the support prepared with benzothiazolium salt (grey dashed line) loaded with a mixture of RNase, α-chymotrypsin and BSA, using 1.5 M (NH4)2SO4 in the initial washing step, followed by a stepwise gradient of (NH4)2SO4 from 0.6 to 0 M (represented by the black dashed line). Reproducibility and reusability assay: chromatographic profile after fifty chromatographic runs (dashed line)
This result shows that the three proteins under study interact differently with the ligand. Due to the properties of α-Chymotrypsin and BSA, these proteins tend to bind to the ligand mainly by hydrophobic interactions, what was confirmed by the retention of the proteins with high ionic strength and elution with the decrease of the ammonium sulfate concentration. On the other hand, RNase is not able to interact with the ligand at high salt concentration and it is immediately recovered in the flowthrough. These results indicated that the hydrophobic interactions are not the predominant forces established between RNase and the ligands, suggesting that in this case the electrostatic interactions may play a more relevant role. At the pH used in this study (pH 8.0) the RNase (pI 9.6) is positively charged, not interacting with the cationic ligand. This fact, confirms the importance of ionic interactions to mediate the interaction of RNase with the support.
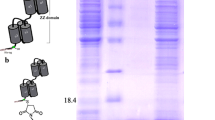
The purity of the recovered protein fractions corresponding to the three eluting peaks was monitored by SDS-PAGE, as shown in Fig. 6. Lane M represents the artificial mixture of the three proteins that was loaded onto the prepared column. By comparing with the control samples represented in lanes A, B and C, it is possible to identify in lane 1, the protein eluted in the first peak of the chromatogram, the RNase. In lane 2, the protein eluted in the second peak of the chromatogram, corresponds to α-chymotrypsin and finally the protein recovered from the third chromatographic peak is BSA (lane 3).
SDS-PAGE analysis of the fractions recovered from each chromatographic peak [lane M, mixture of the three proteins; lane 1 RNase (first peak eluted); lane 2 α-chymotrypsin (second peak eluted); lane 3 BSA (third peak eluted); lane A RNase (control); lane B α-chymotrypsin (control); lane C BSA (control)]
The presence of more than one band in the SDS-PAGE of α-chymotrypsin may not be related to an isoform separation phenomenon during the purification process, but is probably associated with the SDS-PAGE procedure. Moreover, α-chymotrypsin is a serine protease of the peptidase S1 family consisting of 245 amino acid residues. This biomolecule has three peptidic chains held together by disulphide bonds which can be broken by addition of reducing agents (dl-Dithiothreitol or β-mercaptoethanol). These reducing agents are usually included in the SDS-PAGE sample buffer to cleave disulphide bonds, allowing molecules to adopt an extended monomeric form. On the other hand, self digestion may occur when temperatures above 37 °C are used, which is used to denature proteins in the SDS-PAGE procedure. Consequently, the number of visible bands in this sample can also be originated by the fragments arising from the cleavage of intermolecular disulphide bonds or from the autolysis process [35–38].
Several additional assays were performed to check for leakage of the ligand, reproducibility and reusability of this support. The derivative support was washed meticulously several times and the color never faded. It was also left in water and ethanol at 4 °C for almost 6 months and the liquid did not present any measured ligand molecules. After more than one hundred injections the color of the support remained the same.
Good reproducibility of the elution pattern was demonstrated for fifty chromatographic runs (Fig. 5) and the stability of the column was additionally confirmed by half year of consecutive runs (approximately one hundred), which did not influence the quality of the chromatographic performance.
These results indicate the possibility of application of the proposed derivative support in the purification of specific proteins from different crude extracts. The chromatographic results herein described can arise from a judicious combination of various possible molecular interactions established within the ligand and the target protein in a specific spatial configuration, namely aromatic (π–π), electrostatic (ionic), and hydrophobic (including van der Waals) interactions due to the benzothiazole, azolium and N-decyl chain moieties of this benzothiazolium salt, respectively.
In addition, and regarding the toxicity data already described for benzothiazolium salts against Euglena gracilis [19–21] it can be concluded that the ligand herein, should be absent of toxicity below 50 μg mL−1. It should be stressed that such high leakage never occurs in liquid chromatography, where the highest leakage found in high molecular weights as dyes in extreme conditions is around 1–2 μg mL−1 [39] pointing to a minimum risk of toxicity when using the chromatographic support herein described.
Therefore, it can be finally concluded that the benzothiazolium salt herein used represents a specialized ligand which combines structural and stereochemical features that are able to offer a pseudospecificity to a large number of proteins, when appropriate binding and elution conditions are used in a multi-modal chromatographic process, with a good reproducibility and a minimum risk of toxicity.
Conclusions
A new multi-modal chromatographic support was synthesized from Sepharose CL-6B and a benzothiazolium salt. The studied support had a ligand density of 0.18 mmol/g support. This ligand exhibited the ability to separate RNase, α-chymotrypsin and BSA from an artificial mixture using a decreasing stepwise gradient of ammonium sulfate. Nevertheless, we believe that a multi-modal interaction is responsible for the specific binding observed between the immobilized benzothiazolium salt and the standard proteins tested, it cannot be disregarded as the primordial hydrophobic interaction role was also observed in the congener chromatographic support derivatized with the spacer arm. Hence, shorter alkyl spacer arms should be used in future studies to achieve a better balance favoring the azolium ionic and π–π interactions at the expenses of the spacer arm hydrophobic interaction. Furthermore, it can also be concluded that the use of a concentration nearby 0.20 mmol per g of immobilized ligand per g of support is fundamental to achieve a good AC performance.
Since it was the first time that azolium salts had been used for protein purification by AC, a promising unexplored field is herein opened. Namely, the inexpensive cost of the ligand, the mild Steglich esterification conditions used in the ligand immobilization, high stability, reproducibility and minimum risk of toxicity of the chromatographic support and finally the capability to recognize in a specific way different proteins opened a new multi-modal chromatography opportunity.
References
Chang T, Liu X, Chenga X, Qi C, Mei H, Shangguan D (2012) J Chromatogr A 1246:62–68
Labrou NE (2003) J Chromatogr B 790:67–78
Görner T, Gissinger P, Léonard M, Dellacherie E (1997) J Chromatogr B 694:39–48
Ayyar BV, Arora S, Murphy C, O’Kennedy R (2012) Methods 56:116–129
Subramanian S (1984) Crit Rev Biochem Mol Biol 16:169–205
Zou H, Luo Q, Zhou D (2001) J Biochem Biophys Methods 49:199–240
Atasever A, Ozdemir H, Gulcin I, Kufrevioglu OI (2013) Food Chem 136:864–870
Lowe RC, Lowe AR, Gupta G (2001) J Biochem Biophys Methods 49:561–574
Sproule K, Morrill P, Pearson JC, Burton SJ, Hejnæs KR, Valore H, Ludvigsen S, Lowe CR (2000) J Chromatogr B 740:17–33
Behizad M, Curling JM (2000) Biopharm 13:42–46
Clonis YD, Labrou NE, Kotsira VPh, Mazitsos C, Melissis S, Gogolas G (2000) J Chromatogr A 891:33–44
Feng H, Jia L, Li H, Wang X (2006) Biomed Chromatogr 20:1109–1115
Panigrahi M, Dash S, Patel S, Mishra BK (2012) Tetrahedron 68:781–805
Boto REF, El-Shishtawy RM, Santos PF, Reis LV, Almeida P (2007) Dyes Pigments 73:195–205
Boto REF, Santos PF, Reis LV, Almeida P (2007) Dyes Pigments 75:298–305
Boto REF, Almeida P, Queiroz JA (2008) Biomed Chromatogr 22:278–288
Boto REF, Oliveira AS, Vieira Ferreira LF, Almeida P (2001) Dyes Pigments 48:71–84
Munawar MA, Nadeem S (2011) In: Handy ST (ed) Thiazolium and benzothiazolium ionic liquids. Croatia, InTech
Zahradnik P, Foltinova P, Halgas J (1996) Environ Res 5:51–56
Hatrík S, Zahradník P (1996) J Chem Inf Comput Sci 36:992–995
Foltínová P, Ebringer L, Sutoris V, Zahradník P, Halga J (1986) Folia Microbiol 31:319–328
Yang CH, Yang YN, Liang PH, Chen CM, Chen WL, Chang HY, Chao YS, Lee SJ (2007) Antimicrob Agents Chemother 51:3924–3931
Macdolen P, Zahradník P, Foltínová P (2000) Pharm 55:803–810
Chabreč P, Sutoris V, Foltínová P, Sekerka V, Gáplovsk A (1986) Chem Pap 41:655–669
Sutoris V, Halgaš J, Sekerka V, Foltínová P, Gáplovský A (1983) Chem Zvesti 5:653–662
Sutoris V, Gáplovský A, Sohlerova R, Sekerka V (1984) Chem Pap 39:491–501
Halgaŝ J, Sutoris V, Foltínová P, Sekerka V (1983) Chem Zvesti 6:799–808
Pianka M, Hall JC (1959) J Sci Food Agric 10:385–388
Garmaise DL, Parys GY, Komlossy J, Chambers CH (1968) J Med Chem 12:30–36
Henselová M, Gašparová R, Lácová M (2008) In: Nova Biotechnologica: revue Fakulty prírodných vied, vol 8 (1)—Trnava: Univerzita sv. Cyrila a Metoda, Fakulta prírodných vied, pp 79–86
Králová K, Mitterhauszerová L, Halgaš J (1994) Biol Plant 36:477–479
Sutoris V, Bajči P, Sekerka V, Halgaš J (1988) Chem Pap 42:249–261
Silva A, Boto REF, El-Shishtawy RM, Almeida P (2006) Eur Polym J 42:2270–2282
Murza A, Fernández-Lafuente R, Guisán JM (2000) J Chromatogr B 740:211–218
Buck FF, Vithayathil AJ, Bier M, Nord FF (1962) Arch Biochem Biophys 97:417–424
Marshall T (1984) Clin Chem 30:475–479
Sustrova B, Novotna L, Kucerova Z, Ticha M (2009) J Mol Catal B Enzym 60:22–28
Silva MS, Graça VC, Reis LV, Santos PF, Almeida P, Queiroz JA, Sousa F (2013) Biomed Chromatogr 27:1671–1679
Santambien P, Sdiqui S, Hubert E, Girot P, Roche AC, Monsigny M, Boschetti E (1995) J Chromatogr B 664:241–246
Acknowledgments
The authors would like to thank the Portuguese Foundation for Science and Technology, Programa Operacional “Ciência, Tecnologia, Inovação”—POCTI and Fundo Europeu de Desenvolvimento Regional—FEDER, for the funding of the Project “PTDC/QUI–QUI/100896/2008” and PEst-C/SAU/UI0709/2011 COMPETE (Programa Operacional Factores de Competitividade). L. P. Alves acknowledges a fellowship (BI-1-PTDC/QUI–QUI/100896/2008) in the ambit of this project. We are also grateful to the Optical Centre of University of Beira Interior for the acquisition of the SEM and SEM–EDS images.
Conflict of interest
The authors declare no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, L.P., Ramos, S.S., Sousa, F. et al. A Benzothiazolium Salt as Chromatography Ligand for Protein Purification. Chromatographia 77, 1597–1605 (2014). https://doi.org/10.1007/s10337-014-2774-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2774-x