Abstract
High diversity, complex topography and geological factors make the Neotropics an interesting region for the study of avian diversification. Phylogeography, in conjunction with ecological niche modeling, offers an interesting complementary approach to identify the tempo and mode of diversification in different species. In this work, we analyzed the genetic structure of Habia fuscicauda based on multilocus analyses using mtDNA (ND2 and ND4) and nuDNA (ACOI-I9 and ODC) sequences. Additionally, we transferred the optimal conditions for current distribution to the Mid-Holocene, Last Glacial Maximum and the Last Inter-Glacial in order to evaluate the shift in species distribution and compare with the genetic data. Our results indicate that H. fuscicauda comprises two clades: one with genetic correspondence to south-east Mexico to the Nicaragua Depression and the other from the south of the Nicaragua Depression to Central Panama. Within these clades, we identified genetic differentiation between populations across the Isthmus of Tehuantepec, Costa Rica—associated with the Talamanca Cordillera—and Central Panama. The two principal clades showed contrasting demographic histories, with the northern clade showing demographic changes and the southern clade demographic stasis. The ecological niche models identified areas as refugia for the northern clade fin Central America and for the southern clade in the Talamanca Cordillera and Central Panama, supporting the hypothesis that these clades were isolated from each other during the climate shifts of the Pleistocene.
Zusammenfassung
Die Phylogeographie des Rotkehlkardinals (Habia fuscicauda, Cardenalidae) zeigt Populationen-Isolierung, genetische Divergenz und demographische Veränderungen während der Klimaveränderungen im mittelamerikanischen Regenwald des Quartärs. Eine hohe Diversität, komplexe Topographie sowie geologische Faktoren machen die Neotropen zu einer interessanten Region für die Untersuchung der Artenbildung bei Vögeln. Die Phylogeographie bietet in Verbindung mit der ökologischen Nischenmodellierung einen interessanten ergänzenden Ansatz, um die Geschwindigkeit und die Art und Weise der Ausbildung verschiedener Arten zu untersuchen. In dieser Arbeit haben wir die genetische Struktur des Rotkehlkardinals (Habia fuscicauda) anhand von Multi-Locus-Sequenzanalysen unter Verwendung von mtDNA- (ND2 und ND4) und nuDNA- (ACOI-I9 und ODC) Sequenzen analysiert. Zusätzlich dazu übertrugen wir die optimalen Bedingungen für die derzeitige Verbreitung auf das Mittelholozän, das Letzteiszeitliche Maximum und die letzte Zwischeneiszeit, um die Verschiebung der Artenverteilung zu bewerten und mit den genetischen Daten zu vergleichen. Unsere Ergebnisse deuten darauf hin, dass H. fuscicauda zwei Kladen umfasst: eine mit genetischer Entsprechung im Südosten Mexikos bis zum Nicaragua-Graben und die zweite vom Süden des Nicaragua-Grabens bis nach Zentralpanama. Innerhalb dieser Kladen konnten wir genetische Unterschiede zwischen Populationen am Isthmus von Tehuantepec, in Costa Rica,—in Verbindung mit der Talamanca-Gebirgskette—und in Zentralpanama feststellen. Die beiden Hauptkladen zeigten gegensätzliche demographische Verläufe, wobei die nördliche Klade demographische Veränderungen und die südliche Klade einen demographischen Stillstand aufwies. Die ökologischen Nischenmodelle identifizierten bestimmte Gebiete als Rückzugsorte für die nördliche Klade in Mittelamerika und für die südliche Klade in der Talamanca-Gebirgskette und Zentralpanama, was die Hypothese unterstützt, dass diese Kladen während der Klimaverschiebungen im Pleistozän isoliert voneinander waren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remarkable diversity, complex topography and intricate array of highly contrasting habitats make the Neotropics an interesting region for the study of the evolutionary processes that have promoted avian diversification (Lavinia et al. 2015). Currently, Neotropical avian biodiversity represents a mixture of species of different ages, with no single time or mode of diversification (Lovette 2005; Rull 2008; Zink et al. 2004). However, the way in which glacial–interglacial cycles during the Pleistocene (sensu Haffer 1969) shaped biological diversity at interspecific and intraspecific levels remains a point of discussion (Rull 2008). Allopatric speciation is the main mode of species diversification in the Pleistocene refugia model, in which isolated rainforest refuges limited the genetic exchange between populations, promoting their divergence (Haffer 1969; Hewitt 1996, 2000; Pennington et al. 2000; Prado and Gibbs 1993). In southern Mexico and Central America, Pleistocene climatic fluctuations seem to have played a major role in avian diversification isolating populations, due to the expansion and contraction of humid and dry habitats (Soubiès et al. 1991; Prado and Gibbs 1993; Pennington et al. 2000, 2004; Smith et al. 2013; Castillo-Chora et al. 2021). However, the effects of isolation in populations seem not to have been homogeneous among different species, so different degrees of genetic differentiation are expected depending on the duration and strength of the isolation (Hewitt 1996, 2000). Therefore, more phylogeographic studies are needed, as they provide information about the different processes that have led to the high diversity of Neotropical avifauna.
The genus Habia comprises five species: H. fuscicauda (Red-throated Ant Tanager), H. gutturalis (Sooty Ant Tanager), H. cristata (Crested Ant Tanager), H. atrimaxillaris (Black-cheeked Ant Tanager) and H. rubica (Red-crowned Ant Tanager), but the phylogenetic relationships among them are unclear (Dwight and Griscom 1924; Hilty 2011; Klicka et al. 2007; Oberholser 1922). The genus Habia was long classified as part of the Thraupidae family, but phylogenetics analyses based on mtDNA indicated that Habia rather belonged to Cardinalidae (Burns et al. 2003; Klicka et al. 2007). Later, molecular studies using multilocus data placed the Habia genus within the well-supported “cardinal-tanager” clade and found the genus Habia to be paraphyletic, with H. rubica more closely related to the genus Chlorothraupis (Barker et al. 2015; Ramírez-Barrera et al. 2018).
The Red-throated Ant Tanager H. fuscicauda is distributed in lowland forests from eastern Mexico to northern Colombia, mainly in the undergrowth of humid to semiarid, evergreen to semideciduous forest and edge habitats. This species is also common in second growth forest, woodlands and thickets. The Red-throated Ant Tanager occurs at low elevations, from sea level to 1200 m (Hilty 2011). Up to six geographically delimited subspecies of H. fuscicauda have been proposed based on differences in morphology and plumage coloration (Hilty 2011). However, subspecies delimitation based on phenotypic characters does not necessarily reflect lineages’ evolutionary history (Ball and Avise 1992; Cheviron et al. 2005); therefore, a phylogeographic approach using molecular data could reveal different geographic patterns and demographic processes about the evolutionary history of the species (Avise et al. 1987; Hewitt 2004).
The current distribution of the Red-throated Ant Tanager includes geographic regions that are recognized for their geological and biogeographic complexity. In Mexico, it is distributed across the region of the Isthmus of Tehuantepec, which is recognized as a biogeographic barrier for many taxa (e.g. Barber and Klicka 2010; Mulcahy et al. 2006; Ornelas et al. 2013; Rocha-Méndez et al. 2018). The Isthmus of Tehuantepec, together with the Nicaragua Depression, delimits a geographic zone that is considered the oldest geologic North Central America, whose main orogenic feature is the highlands of Chiapas and Guatemala (Marshall and Liebherr 2000; Villar-Anleu 1994). South of the Nicaragua Depression are the lowlands of Central America, comprising the territories of Costa Rica and Panama. This zone is positioned at the intersection of four tectonic plates, whose boundaries create a rugged landscape, which contributes to local processes such as extinction and genetic isolation of populations (Miguez-Gutiérrez et al. 2013). The complexity of this area is due to its role as a transition zone between the biota of North and South America, in addition to its varied physiography, geomorphology and geological history (Bagley and Johnson 2014; Jackson et al. 1996).
We consider the Red-throated Ant Tanager, H. fuscicauda complex, an ideal model to investigate evolutionary mechanisms that promote genetic differentiation in Mesoamerican rainforests for the following reasons: (1) it is a complex of several subspecies with morphological differences; (2) the species’ distribution is separated by important geographic barriers including the Isthmus of Tehuantepec, the highlands of Chiapas and Central America, the Nicaragua Depression and the Talamanca Cordillera, which could promote genetic divergence and (3) the complex topography in conjunction with the Pleistocene climatic shifts could have had demographic effects and isolated the populations. We used extensive sampling of H. fuscicauda using both mtDNA and nuDNA genetic markers to address the following questions: (1) What type of phylogeographic pattern of genetic structure is recovered within this widespread taxon across its range? (2) What are the mechanisms by which physical or ecological barriers promoted the divergence within this complex? And (3) have past climatic conditions during the Quaternary and shifts in habitat suitability promoted distributional changes in H. fuscicauda that help explain genetic variation? Low levels of gene flow followed by high genetic differentiation are expected among disjunct populations of H. fuscicauda in the presence of geographic barriers, limiting genetic exchange.
Materials and methods
Taxon sampling
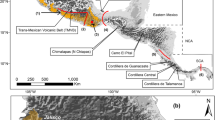
Tissue samples of H. fuscicauda were obtained from collections of the Museo de Zoología de la Facultad de Ciencias (MZFC) and the Burke Museum of Natural History and Culture (BMNHC) in Mexico and the USA, respectively. We delimitated nine groups based on their geographic distribution and the genetic results (Fig. 1). The genetic data spanned most of the H. fuscicauda distribution and included all subspecies recognized except for the H. f. erythrolaema, restricted to Colombia (Fig. 1). In total, we used 126 individuals of H. fuscicauda, including samples from Mexico, Belize, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica and Panama (Table SM1, see Online Resources).
Geographic delimitation of H. fuscicauda populations. Color of ovals indicates the corresponding populations according to the geographic groups, and red solid circles indicate localities from collected individuals. Populations from San Luis Potosí, Isthmus, Chiapas and East Central America correspond to the subspecies H. f. salvini; Yucatan Peninsula to H. f. insularis; Nicaragua to H. f. discolor; Costa Rica-North Panama to H. f. fuscicauda; Central Panama to H. f. willisi and Colombia to H. f. erythrolaema
For 56 tissue samples from the MZFC, we extracted genomic DNA using the Qiagen DNAeasy kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. We amplified the mitochondrial marker Nicotinamine Dehydrogenase Subunit 2 (ND2) using the primers L5219 and H6313 (Sorenson et al. 1999). We also used 60 ND2 sequences provided by the BMNHC, under the charge of the curator John Klicka, and ten sequences downloaded from GeneBank (NCBI; Table SM1, see Online Resources). Then, three additional molecular markers were amplified for a subset of individuals (n = 28–34) of the main clades of the ND2 Bayesian tree: the mitochondrial marker Nicotinamine Dehydrogenase Subunit 4 (ND4) was amplified using the primers LEU–ND4 (Arévalo et al. 1994), the nuclear locus Z-linked intron Acointase I (ACOI-I9) was amplified using the primers ACOI-I9F and ACOI-I9R (Kimball et al. 2009), and the autosomal locus Ornithine Decarboxylase gene (ODC) was amplified using the primers ODCF and ODCR (Primmer et al. 2002). We used annealing temperatures of 54 °C for ND2 and ND4, and 60–65 °C for ACOI and ODC. We visualized all PCR products on agarose gels stained with BrEt, including a negative control to monitor contamination. DNA sequencing was performed in the High Throughput Genomic Unit Service of the University of Washington. Sequences were edited and aligned by eye using Sequencher v4.8 (GeneCodes Corporation, Ann Arbor, MI, USA). We also included two ND2 sequences of the congeneric species H. gutturalis and H. atrimaxillaris from GenBank (Klicka et al. 2007; Barker et al. 2015), and as an outgroup we used 30 ND2 sequences from Habia rubica deposited in FigShare (https://figshare.com/s/fe9f9f6fed1686782f62; Ramírez-Barrera et al. 2018) and nine ND2 sequences from Chlorothraupis stolzmanni, C. olivacea, C. carmioli and Piranga flava, downloaded from GenBank (Lavinia et al. 2015; Online Resource 1). Finally, sequences were aligned using Clustal X 2.1 (Thompson et al. 1997). All new sequences were deposited in GenBank under accession numbers MW884324–MW884530.
Phylogenetic analyses and divergence times
We reconstructed the phylogenies using the following two different matrices: (1) a matrix from mitochondrial marker ND2 that included 122 sequences from H. fuscicauda and 41 from related taxa, and (2) a matrix that included sequences from mtDNA markers ND2 and ND4 (n = 34), plus two nuclear markers ACOI-9 (n = 28) and ODC for 32 individuals of H. fuscicauda, and three congeneric species. The model parameters of nucleotide evolution for each DNA region were obtained with jModeltest v3.8 (Posada 2008) using the Akaike Information Criterion (Akaike 1973). For phylogenetic reconstructions, we used the Bayesian Inference method implemented in MrBayes v3.2 (Ronquist et al. 2012), and the Maximum Likelihood (ML) approach with raxmlGUI 2.0 (Edler et al. 2020). The parametrization of the analyses for Bayesian analysis consisted of 1 × 107 generations, retaining a tree every 1000 generations and discarding the initial 20% as burn-in. Two independent runs were performed to verify the convergence of the chains and the verification of adequate effective sample size for each parameter (ESS > 200) was assessed in Tracer v1.6.0 (Rambaut and Drummond 2013). The sampled trees were used to construct a majority rule consensus tree with a posterior probability distribution in TreeAnnotator v1.8.4. (Drummond et al. 2013). For ML, we used 1000 bootstrap replicates to assess the support of the clades. All trees were visualized and edited in FigTree v1.2.3 (http://tree.bio.ed.ac.uk/software/figtree/). Some of these analyses were run via the CIPRES Science Gateway web application (Miller et al. 2010), following the parameters described above.
Divergence times were estimated by calculating a maximum clade credibility tree (MCCT) using a Yule speciation process (Gernhard 2008; Yule 1925), under a strict clock model with a mtDNA substitution rate of 0.0125 subs/site/lineage/My (Ramírez-Barrera et al. 2018; Smith and Klicka 2010), using mitochondrial DNA, in Beast v1.8.4 (Drummond et al. 2013). To test whether our dataset fits a strict clock model or a relaxed clock model, we performed selection tests using the stepping-stone method (Xie et al. 2011) as implemented in MrBayes 3.2 (Ronquist et al. 2012). The mean marginal likelihood under a strict clock model (− Ln − 9783.22) performed better than the relaxed clock model (− Ln − 9891.26). Calibrations for nodes were based on the estimates of Lavinia et al. (2015), and Ramírez-Barrera et al. (2018), see Table SM2. Therefore, chains were run for 5 × 107 generations, discarding the first 20% as burn-in. Stationarity was analyzed with TRACER v1.6 (Rambaut and Drummond 2013). Mean heights and 95% credibility interval values for node estimates were generated in TreeAnnotator v1.8.4 (Drummond et al. 2013), with a posterior probability limit of 0.5. Final tree was visualized and edited in FigTree v1.4.3.
Genetic diversity
We used DnaSP v5.0 (Librado and Rozas 2009) to estimate genetic diversity from delimited populations (using mitochondrial DNA). We estimated the number of segregating sites (S), number of haplotypes (H) and haplotype and nucleotide diversity (Hd, π). To test whether there was evidence of genetic structure between the two main clades identified in phylogenetic analyses (see “Results”), we performed a hierarchical analysis of molecular variance (AMOVA) using pairwise differences. The genetic divergence between groups was also measured using the FST fixation index. All tests were performed in ARLEQUIN 3.1 (Excoffier et al. 2005), and their significance was assessed with 1000 permutations. In addition, to visualize the relationships between haplotypes, we constructed haplotype networks from our different data sets with the Median-Joining algorithm available in Network v4.6 (Bandelt et al. 1999), and in Population Analysis with Reticulate Trees PopART v1.7 (Leigh and Briant 2015).
Demographic history
We tested for evidence of recent demographic changes in the two lineages identified in phylogenetic analyses (see “Results”). Specifically, we described historic demographic dynamic of both lineages based on mismatch distributions, which test the null hypothesis of population growth (Rogers and Harpending 1992). Unimodal and positively skewed mismatch distributions are frequently observed in populations that have undergone a recent range expansion, while multimodal distributions are observed in populations that have undergone a diminishing or structured population size (Harpending 1994). We obtained the sum of squared deviations (SSD; Excoffier and Schneider 1999), in which significant values are interpreted as demographic stability (Excoffier and Schneider 1999). We also estimated the raggedness index (RI) on mismatch distributions (Harpending 1994). RI values greater than 0.05 are expected for multimodal distributions, while RI values less than 0.05 are obtained for unimodal distributions, typically observed in populations that have experienced sudden demographic expansion (Harpending 1994; Morando et al. 2008). Additionally, we calculated the statistics of neutrality tests, corresponding to Fu and Li's F (Fu and Li 1993) and Tajima's D (Tajima 1989a). Significance for these summary statistics was inferred from 1000 replicates of the coalescent algorithm using ARLEQUIN 3.1. Departures from neutrality in populations could suggest not only selection, but also demographic changes (Tajima 1989b). Evidence of historical signatures of fluctuations in population size was also examined using a Bayesian Skyline Plot model (Drummond et al. 2005), implemented in BEAST v2.6. (Bouckaert et al. 2019), using a coalescent-based estimation of population size changes over time. We ran the analysis for 2 × 107 generations, sampling every 1000 generations, discarding the first 20% as burn-in. We used the appropriate substitution model obtained from jModeltest, with empirical frequencies of bases. The verification of adequate effective sample size for each parameter (ESS > 200) and the Bayesian Skyline reconstruction was performed in Tracer v1.6.0 (Rambaut and Drummond 2013).
Range shift estimations with Ecological Niche Modelling
We used an Ecological Niche Modelling (hereafter ENM) approach to test the hypothesis that demographic changes occurred in response to climate shifts during the Quaternary, promoting expansion and contraction of population ranges. We used the database of geographical occurrences for H. fuscicauda available in the Global Biodiversity Information Facility (GBIF.org, Occurrence Download https://doi.org/10.15468/dl.gnk2ue). First, we cleaned the input presence points by eliminating ambiguities, misidentifications and duplicated records within a vicinity of 5 km. In total we used 418 (see Table SM3). We used the 19 bioclimatic variables (2.5 arc-minutes resolution) from the WorldClim data project (available at: http://www.worldclim.org; Hijmans et al. 2005), and also the past climatic variables available for the Mid-Holocene (Mid-Hol; 6,000 YA), Last Glacial Maximum (LGM; 21,000 YA) and Last Inter-Glacial (LIG; ~ 140,000–120,000 YA) periods.
We delimited the calibration area”M” (the historically accessible area for the species; Soberón and Peterson 2005) by matching occurrence records with terrestrial ecoregions (Dinerstein et al. 2017). This method avoids artificial projections of extreme values of the ecological variables (Elith et al. 2011; Owens et al. 2013; Stohlgren et al. 2001). In order to reduce over-fitting and collinearity, models were performed using only uncorrelated or weakly correlated (r ≤ 0.7) environmental variables, selected by Pearson’s correlation coefficient analyses performed in R v3.4.2 (R Core Team 2017). We prioritized bioclimatic layers that have been used in other ENM studies and which best represent the climatic conditions of the Neotropical rainforest (see Sobral-Souza et al. 2015). We depicted the cluster of bioclimatic variables in a dendrogram (Fig. S1, see Online Resources) with 1000 bootstrap replicates and with approximately unbiased (AU) probability values (p-values). Clusters with AU ≥ 95% are considered strongly supported by data (Suzuki and Shimodaira 2006). Additionally, the correlation matrix was plotted in a correlogram (Fig. S1, see Online Resources). This process resulted in the inclusion of the following bioclimatic variables: BIO3 (isothermality), BIO5 (max temperature of warmest month), BIO7 (temperature annual range), BIO11 (mean temperature of coldest quarter), BIO13 (precipitation of wettest month), BIO14 (precipitation of driest month), BIO15 (precipitation seasonality) and BIO18 (precipitation of warmest quarter).
Tuning of the best parameters was performed using the libraries “ENMeval” (Muscarella et al. 2014), selecting the best parameter combination of Regularization Multiplayer (RM) and feature types based on the AIC values (Fig. SM1, see Online Resources). ENM hypotheses were obtained and evaluated in Maxent 3.4.1 (Phillips and Dudik 2008), whose algorithms have shown good performance in transferring present niche space conditions onto past scenarios. Models were generated using cross-validation, with no extrapolation and no clamping. We evaluated model performance using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve (Elith et al. 2006, 2011) and the ROC-partial test (Lobo et al. 2008; Peterson et al. 2008). We used the threshold values of suitability from “10 percentile training presence” to reduce commission errors in our final maps, recovering more conservative species distribution ranges (Phillips and Dudik 2008). Processing and visualization of ENMs was performed in ArcMap 10.2.2 (ESRI 2010).
Results
Phylogeny, divergence times and genetic diversity
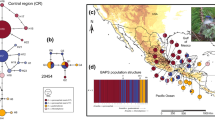
We obtained ~ 900–1041 base pairs (bp) for the mtDNA ND2 and 925 bp for ND4, 851 bp for the nDNA COI9 and 635 bp for the ODC gene. The length of the final multilocus matrix was 3454 bp. The best-fit models for each molecular marker were GTR + I + G for ND2, HKY + I for ND4 and TrN + I for ACOI-I9 and ODC. The Bayesian and ML phylogenies constructed using the mitochondrial and multilocus datasets revealed well-supported sister clades, as shown in Figs. 2 and 3, respectively. The following clades: North Central America (hereafter NCA) and Low Central America (hereafter LCA), which were reciprocally monophyletic, with only one putative migrant from Nicaragua (Nic_02), nested in the Costa Rica–North Panama clade (Fig. 2). The clade NCA comprises individuals from the geographic groups of San Luis Potosí (SLP), Isthmus, Chiapas, Yucatan Peninsula, East Central America and Nicaragua, distributed from eastern Mexico to Nicaragua. The LCA clade was composed of two populations: one from the Costa Rica-North Panama region, and another from Central Panama. None of the five subspecies included were monophyletic according to the loci analyzed. Phylogenetic trees for concatenated mtDNA matrix showed the NCA–LCA split with high support (pp = 1, bootstrap = 97), while for the nDNA matrix, no geographic structure was recovered and none of these trees support subspecies differentiation (see Fig. SM2 in Online Resources).
Bayesian phylogenetic tree from the mtDNA marker ND2. Color tips indicate populations according to geographic delimitations. Polygons or symbols indicate the subspecies corresponding to each individual. The values at the nodes indicate the support for posterior probability/bootstrap. The mean divergence time estimated in Beast in Million years for the mtDNA marker ND2 and 95% High Posterior Density range in brackets
Molecular dating of the divergence between the NCA and LCA groups yielded a time estimate during the Late Pliocene—Early Pleistocene around 2.50 Million years ago (Mya) [HPD95 2.96–2.08 Mya], whereas the estimate for the differentiation between the two populations of the LCA clade occurred around 0.54 Mya [HDP95 0.74–0.36 Mya], during the Middle and Upper Pleistocene (Fig. 2).
Overall, genetic diversity values for the geographic groups delimitated showed low nucleotide diversity (π < 0.009), but high haplotype diversity (Hd > 0.67; Table 1), the different groups defined in the Fig. 1 are the same in the Table 1.
The mtDNA-based haplotype network (Fig. 4) showed two main haplogroups separated by 20 mutational steps: one corresponding to the North Central America (NCA) haplogroup (San Luis Potosi, Isthmus, Chiapas, Yucatan Peninsula, East Central America and Nicaragua), and the other to the Low Central America (LCA) haplogroup (Costa Rica—North Panama and Central Panama, with one individual from Nicaragua group). In the NCA haplogroup, haplotype 1 had the highest frequency (n = 28), which was widely distributed, and included samples from five populations. The LCA group had a unique high frequency haplotype (Haplotype 3, n = 14), which includes individuals from three different populations. The ND4 haplotype network indicates the divergence between NCA and LCA with 17 mutational steps, while the ACOI-I9 network showed no divergence between NCA and LCA clades and ODC recovered no haplotype variation between individuals (see Fig. SM3 in Online Resources).
Median-joined haplotype network from the mtDNA ND2. Colors indicate populations according to geographic delimitations. Numbers inside the circles indicate the name of the haplotype (see the text for detailed information). Black dots depict the mutational steps between haplotypes for more than one step. Gray circles indicate the number of individuals within each haplotype
The AMOVA between geographic clades indicated that genetic variation was mainly explained by differences between the North Central America and Low Central America clades (83.18%), with variation among populations within groups roughly homogeneous (5.33%), and 11.5% of the variation explained by differences within populations (Table SM4, Online Resources). Comparisons among pairs of geographic groups using FST values (Table 2) revealed moderate genetic differentiation within NCA clade (0.011 < FST < 0.435). In contrast, groups within the LCA clade showed high and significant values of geographic structure (0.67 < FST < 0.75).
Historical demography
The historical demography of H. fuscicauda inferred through mismatch distributions (Fig. 5) showed an observed distribution of pairwise differences similar to that expected by a null model of demographic growth for the North Central America (NCA) group. The RI index value showed not significant p-value for population expansion (RI = 0.03, P-value = 0.12), unlike the SSD value wich rejected the nule hypothesis of demographic stability (SSD = 0.02, P-value = 0.17) for the NCA group. However, both neutrality test Tajima's D (− 1.52, P-value < 0.01) and Fu and Li's F (− 7.54, P-value < 0.01) showed negative and significant values for the NCA group; therefore, demographic fluctuations are suggested based on these values. Both mismatch distribution and the RI (0.05, P-value = 0.02) and SSD values (0.04, P-value = 0.02) do not rejected the hypothesis of demographic stability, therefore suggesting no demographic changes for the Low Central America group. Likewise, the D and F neutrality indices showed no significant values, suggesting demographic stability for the LCA group. The historical demography estimated through a Bayesian skyline plot (Fig. 5) confirmed a pattern of change in population size for NCA throughout the late Pleistocene, approximately 250,000 years ago, suggesting a population bottleneck followed by a rapid population expansion to the present. In contrast, the skyline plot for LCA group suggests demographic stability at least during the past 400,000 years.
Analyses of effective population size changes for North Central America (NCA) and Low Central America (LCA) groups using the mtDNA ND2 marker. a Mismatch plot for the NCA group. b Mismatch plot for the LCA group. For the mismatch plots the dotted lines show expected values through a growth demographic model, and solid lines show the observed values. c Bayesian skyline plot for the NCA group. d Bayesian skyline plot for the LCA group. Mean estimate is shown as a thick solid line, and the 95% HDP limits are shown in solid blue color area surrounding the mean estimate
Ecological Niche Modelling and past range shifts
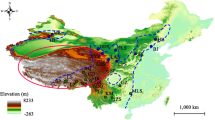
The parameters selected by AICc used in the Ecological Niche Modelling (ENM) were RM = 0.5 (regularization multiplier), and feature type: Quadratic, Product and Hinge “QPH” (Fig. SM1, see Online Resources). The ENM performed well according to the AUC (0.71) and partial-ROC (1.504; P < 0.05). From this, we infer that models performed better than a random model and could be considered statistically descriptive of the climatic conditions that define the ecological niche for the species. These high suitability conditions are shown geographically for the present and for the past climatic scenarios in Fig. 6. Overall, transferring the present high suitability conditions to the three past climatic scenarios indicated a dramatic reduction in distribution range in North Central America clade during the Last Glacial Maximum and Last Inter-Glacial, and re-expansion to the Mid-Holocene and present. For the Low Central America group, there were no drastic changes in habitat suitability during the Last Glacial Maximum and Last Inter-Glacial, but for the Mid-Holocene a reduction in habitat suitability is inferred. Finally, we identified four main putative areas of climate stability (ACS) or areas of high suitability for the presence of the species in all periods modeled (Carnaval et al. 2009): (1) a portion of the highlands of Chiapas (Mexico) and Guatemala, (2) southeastern Nicaragua and northern Costa Rica, (3) both slopes of the Talamanca Cordillera in Costa Rica and Panama and (4) Central Panama (Fig. 6).
Maxent ecological niche model for H. fuscicauda species projected into present and past scenarios. Scale bar brown to Dark blue depicts higher suitability values. The legends refer to the following: Mid-Hol: Mid-Holocene 6000 YA. LGM: Last Glacial Maximum 21,000 YA. LIG: Last Inter-glacial 120,000–140,000 YA. ACS: Areas of Climate Stability
Discussion
Our results show that the H. fuscicauda complex is composed of two main clades with correspondence in geography throughout its distribution range. The principal biogeographic barrier promoting this genetic differentiation is the Nicaragua Depression. Population dynamics within these two main groups are contrasting, as the northern lineage showed signs of demographic expansion, while the southern lineage showed population demographic stasis. At the population level, geographic structure is subtle and indicates a low influence of orogeny in the current pattern of the species (northern lineage), while Pleistocene climate shifts promoted important range and demographic changes, in addition to isolation reflected in the overall evolutionary pattern for this species. Time estimated for genetic divergence between the two major lineages was 2.5 Mya.
Phylogeny, population structure and demographic history
The mitochondrial DNA tree (Fig. 2) indicates the presence of two highly differentiated genetic groups with geographic correspondence to the following: (1) northeastern Mexico, from San Luis Potosí (Mexico) and Central America to the Nicaraguan Depression that correspond to the North Central America (NCA) clade, and (2) from south of the Nicaragua Depression, along the Caribbean slope of the Talamanca Cordillera to Central Panama that corresponds to the Low Central America (LCA) clade. In the NCA clade, the most differentiated region corresponds to the Tuxtlas (Veracruz, Mexico), where all individuals from this biogeographic area are nested in one clade. The populations from San Luis Potosí, the Yucatan Peninsula and those from Chiapas and East Central America showed no genetic differentiation in the phylogenetic tree or the haplotype network. Three individuals from Nicaragua corresponding to subspecies H. f. discolor were included, of which two were grouped within the NCA clade and one within the LCA clade. Retained ancestral polymorphisms or dynamics of gene flow between the two main clades could explain this pattern, but additional sampling in this region is necessary to test these hypotheses (Moore 1995; Ottenburghs et al. 2017; Templeton et al. 2000). Within the LCA clade, only two groups were highly genetic differentiated: one with distribution across the Caribbean slope of the Talamanca Cordillera corresponding with H. f. fuscicauda and the other population restricted to Central Panama belonging to H. f. willisi.
The haplotype network (Fig. 4), as well shows two main groups (North Central America and Low Central America). However, both groups are not geographically limited, and both have at least one widely distributed haplotype, which indicates low or not geographic structure within them. For example, the North Central America (NCA) group showed a star-shaped haplotype network with a unique highly frequency haplotype (Haplotype 1), which suggest recent demographic expansion in the group. A star-like pattern indicates an excess of low-frequency genetic variants, which is a common pattern in populations with recent demographic expansion (Excoffier et al. 2009; Slatkin and Hudson 1991). Haplotype 1 is proposed as the haplotype from which the NCA clade had a demographic growth (e.g. Cheviron et al. 2005), because of its high frequency, in addition to being widely distributed (present in 16 localities) throughout all the NCA clade, except in the Tuxtlas region. We also observed that individuals from the Tuxtlas region, belonging to the Isthmus group, are the most differentiated within the NCA and can be found in haplotype 2 along with six related haplotypes (Fig. 4). Meanwhile in the Low Central America, we found highlight haplotype 3, which is distributed in both the Costa Rica-Northern Panama and Central Panama populations and in Nicaragua, with no clear genetic structure.
From the AMOVA results (Table SM4), we inferred significant differentiation between the two main geographic groups. The FST values (Table 2) also showed that populations from Costa Rica–North Panama, Central Panama and the Isthmus of Tehuantepec are significantly different from all other populations. These results suggest a scenario in which these populations have diverged in isolation, with limited gene flow between surrounded regions, in contrast to individuals from Yucatan Peninsula and Central America, with low values of population differentiation FST.
The North Central America clade shows signs of demographic changes, as indicated by the neutrality and demographic statistics. Likewise, skyline plots and mismatch distribution both suggest demographic growth to the present. Moreover, low nucleotide diversity and high haplotype diversity in mtDNA (Table 1) are consistent with a pattern of populations passing through genetic bottlenecks, followed by rapid population growth (Burney 2009; Grant and Bowen 1998; Li and Sadler 1991; Liu et al. 2006). High haplotype diversity can indicate population growth because many genetic variants are conserved (Harpending 1994), or that insufficient time has passed since range expansion, so there is not yet equilibrium between migration events and drift (Slatkin 1993). In contrast, for the Low Central America clade, the neutrality test (D = 0.92, P-value = 0.03 and F = 5.08, P-value = 0.60) and demographic, as well as mismatch and skyline plots, suggest a pattern of demographic stasis.
Geographical barriers and past scenarios that modulated gene flow
The Nicaragua Depression appears to be the main geographic barrier limiting gene flow between North Central America and Low Central America clades. Several studies confirm the importance of the Nicaragua Depression as a vicariant barrier in birds (D’Horta et al. 2013; Puebla-Olivares et al. 2007; Rocha-Méndez et al. 2018) and other taxa (Bagley and Johnson 2014; Marshall and Liebherr 2000). In general, evolutionary patterns of differentiation are consistent with a major influence of lowlands (depressions) in species inhabiting highlands and viceversa, when talking about gene flow dynamics (e.g. Arbeláez-Cortés et al. 2010). In the case of H. fuscicauda (a lowland species), the Nicaragua Depression is acting more like an ecological barrier, where individuals remain isolated because of the particular conditions found at both sides of it, more than a physical barrier that prevents actual interchange of individuals. In contrast, the effects of the Isthmus of Tehuantepec, recognized as one of the most important barriers that limits gene flow (e.g. Barber and Klicka 2010; González et al. 2011; Maldonado-Sánchez et al. 2016; Mulcahy et al. 2006; Rocha-Méndez et al. 2018), is a permeable region for this species.
Within the northern phylogroup, the individuals from Los Tuxtlas grouped in a clade embedded with the rest. This biogeographic region has a particular evolutionary history, and the isolation through this area is a recurrent pattern in the Mexican avifauna (Arbeláez-Cortés 2012), and some other groups (Flores-Villela and Martínez-Salazar 2009; Ornelas et al. 2013). Another interesting result found in the northern group is the lack of differentiation over the Yucatan Peninsula, which has a unique topography and vegetation, and where processes of differentiation have occurred in some other birds: Psilorhinus morio (dos Anjos et al. 2009), Campylopterus curvipennis (González et al. 2011), Doricha eliza (Licona-Vera and Ornelas 2014), Amazona sp. (Silva et al. 2017) and might have been expected here.
In the Low Central America clade, the Talamanca Cordillera may be influencing the shifts in genetic variation along the Caribbean slope, added to the geographic separation of the Costa Rica-Northern Panama and Central Panama groups by the Panama Canal basin. Some studies have concluded that the Talamanca Cordillera has played an important role in gene flow dynamics and isolation across this region (Mendoza et al. 2019; Ramírez-Barrera et al. 2018).
Moreover, geographic barriers are not the only factor responsible for present genetic patterns, as our results suggest. There have been processes, such as demographic changes, range expansions and isolation promoted by Pleistocene climate shifts that affected the current genetic structure of populations. According to the niche models projected into past scenarios, different suitability areas were obtained for each main clade (North Central America and Low Central America), and given the estimated divergence times, it is inferred that both clades remained isolated from each other during the Quaternary. This implies that the divergence between these two groups (~ 2.50 Mya) arose during Pleistocene, keeping these phylogroups in isolation. For example, and according to the Pleistocene refugia hypothesis (Haffer 1969), climatic shifts isolated multiple populations from diverse taxonomic groups, currently recognized as conspecifics, and that consequently promoted high intraspecific diversification (Hewitt 2000, 2004; Ibrahim et al. 1996; Lovette 2005).
The signals of range expansion of the North Central America Clade, visualized in past scenario projections (from the Last Inter-Glacial 140,000–120,000 years to the present), corroborate the patterns of demographic changes in concordance with genetic data results. Range contraction and expansion may promote demographic signs of bottlenecks, as the results indicated for North Central America populations (e.g. Carnaval and Moritz 2008; Harvey and Brumfield 2015; He et al. 2010; Jezkova et al. 2015).
In the case of the Low Central America populations, Ecological Niche Models showed that the Caribbean slope of Costa Rica-Panama and Central Panama are areas in which suitable conditions were present during all time periods (LIG, LGM, Mid-Hol), with no significant variation in demography. This historical stability in population dynamics have favored the isolation with limited gene flow between groups within this clade, from the past 540,000 years, time estimated for the split between them. In addition, the Ecological Niche Models show that both sides of the Talamanca Cordillera are suitable for the presence of H. fuscicauda, in spite of differences in environmental conditions: wetter climate on the Caribbean slope than the Pacific (Bagley and Johnson 2014). These past projections also show that the Colombian subspecies (H. f. erythrolaema) has been isolated during all of time periods modelled, from which we suspect that this population may be genetically different from the other groups.
In conclusion, H. fuscicauda is a species complex with at least two phylogroups isolated by the Nicaragua Depression. The genetic variation within the northern lineage, from Mexico to Nicaragua (North Central America), has had subtle influences of orogeny in geographic structure (referring specifically to the most differentiated individuals from the Tuxtlas region in Mexico), and climate oscillations during the Quaternary may have led to dynamics in which high levels of gene flow occurred during demographic expansions. Within the southern group, that contains individuals from Costa Rica and Panama (Low Central America), the orogeny (geographic barriers) and demographic stasis (revealed with demographic analyses and projections of niche models into the past), have influenced a process of isolation and divergence between populations across the Talamanca Cordillera (Costa Rica-North Panama) and populations from Central Panama separated by the Panama Canal Basin.
Availability of data and materials
The sequences generated during the current study are available in GenBank (accession numbers: MW884324–MW884530).
Code availability
Not applicable.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (Eds) Proceedings of the 2nd international symposium on information theory, Budapest, Akademiai Kiado, pp 267–281.
Arbeláez-Cortés E (2012) Comparative phylogeography: concepts, methods and general patterns in neotropical birds. Acta Biol Colomb 17:19–38
Arbeláez-Cortés E, Nyári ÁS, Navarro-Sigüenza AG (2010) The differential effect of lowlands on the phylogeographic pattern of a Mesoamerican montane species (Lepidocolaptes affinis, Aves: Furnariidae). Mol Phylogenet Evol 57:658–668
Arévalo E, Davis SK, Sites JW (1994) Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in central Mexico. Syst Biol 43:387–418
Avise JC, Arnold J, Ball RM, Bermingham E, Lamb T, Neigel JE, Reeb CA, Saunders NC (1987) Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annu Rev Ecol Evol Syst 18:489–522
Bagley JC, Johnson JB (2014) Phylogeography and biogeography of the lower central american neotropics: diversification between two continents and between two seas. Biol Rev 89:767–790
Ball RM, Avise JC (1992) Mitochondrial DNA phylogeographic differentiation among avian populations and the evolutionary significance of subspecies. Auk 109:626–636
Bandelt H, Forster P, Rohl A (1999) Median joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Barber BR, Klicka J (2010) Two pulses of diversification across the Isthmus of Tehuantepec in a montane Mexican bird fauna. Proc R Soc B 277:2675–2681
Barker FK, Burns KJ, Klicka J, Lanyon SM, Lovette IJ (2015) New insights into New World biogeography: an integrated view from the phylogeny of black- birds, cardinals, sparrows, tanagers, warblers, and allies. Auk 132:333–348
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, du Plessis L, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu C-H, Xie D, Zhang C, Stadler T, Drummond JA (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15:e1006650
Burney CW (2009) Comparative phylogeography of Neotropical birds. LSU Doctoral Dissertations. 2682.
Burns KJ, Hackett SJ, Klein NK (2003) Phylogenetic relationships of Neotropical honeycreepers and the evolution of feeding morphology. J Avian Biol 34:360–370
Carnaval AC, Moritz C (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J Biogeogr 35:1187–1201
Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C (2009) Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 323:785–789
Castillo-Chora VdeJ, Sánchez-González LA, Mastretta-Yanes A, Prieto-Torres DA, Navarro-Sigüenza AG (2021) Insights into the importance of areas of climatic stability in the evolution and maintenance of avian diversity in the Mesoamerican dry forests. Biol J Linn Soc Lond 132:741–758
Cheviron Z, Hackett SJ, Capparella AP (2005) Complex evolutionary history of a neotropical lowland forest bird (Lepidothrix Coronata) and its implications for historical hypotheses of the origin of neotropical avian diversity. Mol Phylogenet Evol 36:338–357
D’Horta FM, Cuervo AM, Ribas CC, Brumfield RT, Miyaki CY (2013) Phylogeny and comparative phylogeography of Sclerurus (Aves: Furnariidae) reveal constant and cryptic diversification in an old radiation of rain forest understorey specialists. J Biogeogr 40:37–49
Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E, Hahn N, Palminteri S, Hedao P, Noss R, Hansen M, Locke H, Ellis EC, Jones B, Barber CV, Hayes R, Kormos C, Martin V, Crist E, Sechrest W, Price L, Baillie JEM, Weeden D, Suckling K, Davis C, Sizer N, Moore R, Thau D, Birch T, Potapov P, Turubanova S, Tyukavina A, de Souza N, Pintea L, Brito JC, Llewellyn OA, Miller AG, Patzelt A, Ghazanfar SA, Timberlake J, Klöser H, Shennan-Farpón Y, Kindt R, Barnekow Lillesø J-P, van Breugel P, Graudal L, Voge M, Al-Shammari KF, Saleem M (2017) An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67:534–545
dos Anjos L, Debus SJS, Madge SC, Marzluff JM (2009) Family Corvidae (Crows). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world: bush-shrikes to old world sparrows, vol 14. Lynx Edicions, Barcelona, pp 678–588
Drummond AJ, Rambaut A, Shapiro B, Pybus OG (2005) Bayesian coalescent inference of past population dynamics from molecualr sequences. Mol Biol Evol 22:1185–1192
Drummond AJ, Suchard MA, Xie D, Rambaut A (2013) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Dwight J, Griscom L (1924) Descriptions of new birds from Costa Rica. Am Mus Novit 142:1–5
Edler D, Klein J, Antonelli A, Silvestro D (2020) raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol 00:1–5
Elith J, Graham CH, Anderson RP, Dudık M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McC J, Overton M, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurence data. Ecography 29:129–151
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57
ESRI (2010) ArcGIS desktop. Environmental Systems Research Institute, Redlands
Excoffier L, Schneider S (1999) Why hunter-gatherer populations do not show signs of Pleistocene demographic expansions. PNAS 96:10597–10602
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Excoffier L, Foll M, Petit RJ (2009) Genetic consequences of range expansions. Annu Rev Ecol Evol Syst 40:481–501
Flores-Villela O, Martínez-Salazar EA (2009) Historical explanation of the origin of the herpetofauna of Mexico. Rev Mex Biodivers 80:817–833
Fu YX, Li WH (1993) Statistical tests of neutrality of mutations. Genetics 133:693–709
Gernhard T (2008) The conditioned reconstructed process. J Theor Biol 253:769–778
González C, Ornelas JF, Gutiérrez-Rodríguez C (2011) Selection and geographic isolation influence hummingbird speciation: genetic, acoustic and morphological divergence in the wedge-tailed sabrewing (Campylopterus curvipennis). BMC Evol Biol 11:38
Grant WS, Bowen BW (1998) Shallow population histories in deep evolutionary lineages of marine fishes: insights from sardines and anchovies and lessons for conservation. J Hered 89:415–426
Haffer J (1969) Speciation in Amazonian forest birds. Science 165:131–137
Harpending RC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harvey MG, Brumfield RT (2015) Genomic variation in a widespread neotropical bird (Xenops Minutus) reveals divergence, population expansion, and gene flow. Mol Phylogenet Evol 83:305–316
He L, Zhang A, Weese D, Zhu C, Jiang C, Qiao Z (2010) Late pleistocene population expansion of Scylla Paramamosain along the Coast of China: a population dynamic response to the last interglacial sea level highstand. J Exp Mar Biol Ecol 385:20–28
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hewitt GM (2000) The genetic legacy of the quaternary ice ages. Nature 405:907–913
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci 359:183–195
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hilty S (2011) Red-throated Ant-tanager (Habia fuscicauda). In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona
Ibrahim KM, Nichols RA, Hewitt GM (1996) Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity 77:282–291
Jackson JCB, Budd AF, Coates AG (1996) Evolution and environment in tropical America. University of Chicago Press, Chicago
Jezkova T, Riddle BR, Card DC, Schield DR, Eckstut ME, Castoe TA (2015) Genetic consequences of postglacial range expansion in two codistributed rodents (genus Dipodomys) depend on ecology and genetic locus. Mol Ecol 24:83–97
Kimball RT, Braun EL, Barker FK, Bowie RCK, Braunm MJ, Chojnowski JL, Hackett SJ, Han KL, Harshman J, Heimer-Torres V, Holznagel W, Huddlestone CJ, Marks BD, Miglia KJ, Moore WS, Reddy S, Sheldon FH, Smith JV, Witt CC, Yuri T (2009) A well-tested set of primers to amplify regions spread across the avian genome. Mol Phylogenet Evol 50:654–660
Klicka J, Burns K, Spellman GM (2007) Defining a monophyletic Cardinalini: a molecular perspective. Mol Phylogenet Evol 45:1014–1032
Lavinia PD, Escalante P, García NC, Barreira AS, Trujillo-Arias N, Tubaro PL, Naoki K, Miyaki CY, Santos FR, Lijtmaer DA (2015) Continental-scale analysis reveals Deep diversification within the polytypic Red-crowned Ant Tanager (Habia rubica, Cardinalidae). Mol Phylogenet Evol 89:182–193
Leigh JW, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116
Li WH, Sadler LA (1991) Low nucleotide diversity in man. Genetics 129:513–523
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Licona-Vera Y, Ornelas JF (2014) Genetic, ecological and morphological divergence between populations of the endangered Mexican Sheartail hummingbird (Doricha eliza). PLoS ONE 9:e101870
Liu J-X, Gao T-X, Yokogawa K, Zhang Y-P (2006) Differential population structuring and demographic history of two closely related fish species, Japanese Sea Bass (Lateolabrax japonicus) and spotted Sea Bass (Lateolabrax maculatus) in Northwestern Pacific. Mol Phylogenet Evol 39:799–811
Lobo JM, Jiménez-Valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr 17:145–151
Lovette IJ (2005) Glacial cycles and the tempo of avian speciation. Trends Ecol Evol 20:57–59
Maldonado-Sánchez D, Gutiérrez-Rodríguez C, Ornelas JF (2016) Genetic divergence in the common bush-tanager Chlorospingus ophthalmicus (Aves: Emberizidae) throughout Mexican cloud forests: the role of geography, ecology and Pleistocene climatic fluctuations. Mol Phylogenet Evol 99:76–88
Marshall C, Liebherr J (2000) Cladistic biogeography of the Mexican Transition Zone. J Biogeogr 27:203–216
Mendoza AM, Bolívar-García W, Vázquez-Domínguez E, Ibáñez R, Parra Olea G (2019) The role of Central American barriers in shaping the evolutionary history of the northernmost glassfrog, Hyalinobatrachium fleischmanni (Anura: Centrolenidae). PeerJ 7:e6115
Miguez-Gutiérrez A, Castillo J, Márquez J, Goyenechea I (2013) Biogeografía de la Zona de Transición Mexicana con base en un análisis de árboles reconciliados. Rev Mex Biodivers 84:215–224
Miller MA, Pfeifeer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing envirnonments workshop (GCE), New Orleans, LA: 1–8.
Moore WS (1995) Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees. Evolution 49:718–726
Morando M, Avila LJ, Turner C, Sites JW (2008) Phylogeography between valleys and mountains: the history of populations of Liolaemus koslowskyi (Squamata, Liolaemini). Zool Scr 37:603–618
Mulcahy D, Benson G, Morrill H, Mendelson JR (2006) Historical biogeography of Lowland Species of Toads (Bufo) across the Trans-Mexican Neovolcanic Belt and the Isthmus of Tehuantepec. J Biogeogr 33:1889–1904
Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP (2014) ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol 5:1198–1205
Oberholser HC (1922) Phoenicothraupis Cabanis becomes Habia Blyth. P Biol Soc Wash 35:79–80
Ornelas JF, Sosa V, Soltis DE, Daza JM, González C, Soltis PS, Gutiérrez-Rodríguez C, Espinosa de los Monteros A, Castoe TA, Bell C, Ruiz-Sanchez E (2013) Comparative phylogeographic analyses illustrate the complex evolutionary history of threatened cloud forests of Northern Mesoamerica. PLoS ONE 8:2
Ottenburghs J, Kraus RHS, van Hooft P, van Wieren SE, Ydenberg RC, Prins HHT (2017) Avian introgression in the genomic era. Avian Res 8:30
Owens HL, Campbell L, Dornak L, Saupe E, Barve N, Soberón J, Ingenloff K, Lira-Noriega A, Hensz CM, Myers CE, Peterson AT (2013) Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Model 263:10–18
Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. J Biogeogr 27:261–273
Pennington RT, Lavin M, Prado DE, Pendry CA, Pell SK, Butterworth CA (2004) Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Philos Trans R Soc Lond B Biol Sci 359:515–538
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Prado DE, Gibbs PE (1993) Patterns of species distributions in the dry seasonal forests of South America. Ann Mo Bot Gard 80:902–927
Primmer CR, Borge T, Lindell J, Saetre GP (2002) Single-nucleotide polymorphism characterization in species with limited available sequence information: high nucleotide diversity revealed in the avian genome. Mol Ecol 11:603–612
Puebla-Olivares F, Bonaccorso E, Espinosa de los Monteros A, Omland KE, Llorente-Bousquets JE, Peterson AT, Navarro-Sigüenza AG (2007) Speciation in the Emerald Toucanet (Aulacorhynchus prasinus) complex. Auk 125:39–50
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Rambaut A, Drummond AJ (2013) Tracer v1.6.0. http://beast.bio.ed.ac.uk/Tracer.
Ramírez-Barrera SM, Hernández-Baños BE, Jaramillo-Correa JP, Klicka J (2018) Deep divergence of Red-crowned Ant Tanager (Habia rubica: Cardinalidae), a multilocus phylogenetic analysis with emphasis in Mesoamerica. PeerJ 6:e5496
Rocha-Méndez A, Sánchez-González LA, Arbeláez-Cortés E, Navarro-Sigüenza AG (2018) Phylogeography indicates incomplete genetic divergence among phenotypically differentiated montane forest populations of Atlapetes albinucha (Aves, Passerellidae). Zookeys 809:125–148
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian inference and model choice across a large model space. Syst Biol 61:539–542
Rull V (2008) Speciation timing and neotropical biodiversity: the tertiary–quaternary debate in the light of molecular phylogenetic evidence. Mol Ecol 17:2722–2729
Silva T, Guzmán A, Urantówka AD, Mackiewicz P (2017) A new parrot taxon from the Yucatán Peninsula, Mexico—its position within genus Amazona based on morphology and molecular phylogeny. PeerJ 5:e3475
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Smith BT, Klicka J (2010) The profound influence of the Late Pliocene Panamanian uplift on the exchange, diversification, and distribution of New World birds. Ecography 33:333–342
Smith BT, Ribas CC, Whitney BM, Hernández-Baños BE, Klicka J (2013) Identifying biases at different spatial and temporal scales of diversification: a case study in the Neotropical parrotlet genus Forpus. Mol Ecol 22:483–494
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inf 2:1–10
Sobral-Souza T, Lima-Ribeiro MS, Solferini VN (2015) Biogeography of Neotropical Rainforests: past connections between Amazon and Atlantic Forest detected by ecological niche modeling. Evol Ecol 29:643–655
Sorenson MD, Ast CJ, Dimcheff DE, Yuri T, Mindell DP (1999) Primers for PCR-based approach to mitocondrial genome sequencing in birds and other vertebrates. Mol Phylogenet Evol 12:105–114
Soubiès F, Suguio K, Martin L (1991) The Quaternary lacustrine deposits of the Serra dos Carajás (State of Pará, Brazil): ages and other preliminary results. Bol IG-USP 8:223–243
Stohlgren TJ, Otsuki Y, Villa CA, Lee M, Belnap J (2001) Patterns of plant invasions: a case example in native species hotspots and rare habitats. Biol Invasions 3:37–50
Suzuki R, Shimodaira H (2006) Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22:1540–1542
Tajima F (1989a) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tajima F (1989b) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Templeton AR, Maskas SD, Cruzan MB (2000) Gene trees: a powerful tool for exploring the evolutionary biology of species and speciation. Plant Species Biol 15:211–222
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Villar-Anleu L (1994) Informe de País Guatemala: Perfil General. In Vega A (Ed). Corredores Conservacionistas en la Región Centroamericana: Memorias de una Conferencia Regional auspiciada por el Proyecto Paseo Pantera. Florida: Tropical Research and Development, Inc.
Xie WG, Lewis PO, Fan Y, Kuo L, Chen MH (2011) Improving marginal likelihood estimation for bayesian phylogenetic model selection. Syst Biol 60:150–160
Yule GU (1925) A Mathematical theory of evolution based on the conclusions of Dr. J C Willis. Philos Trans R Soc B 213:21–87
Zink RM, Klicka J, Barber BR (2004) The tempo of avian diversification during the Quaternary. Philos Trans R Soc B 359:215–220
Acknowledgements
VJC-C thanks the Posgrado en Ciencias Biológicas from the Universidad Nacional Autónoma de México (UNAM) and CONACYT for the Doctoral scholarship received to perform this research, as well as the support of the scholarship “Ayudante de Investigador SNI III” (EXP. AYTE. 16804).
Funding
The research was supported by PAPIIT/DGAPA, Universidad Nacional Autónoma de México (UNAM) through a grant to Blanca E. Hernández-Baños (IN220620).
Author information
Authors and Affiliations
Contributions
BEHB and VJC-C designed the study. BEHB secured financial support. VJC-C carried out the laboratory work. BEHB, LEZB, and VJC-C analyzed the data. BEHB, VJC-C, LEZB and CP contributed to the writing and improvement of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
The authors declare their consent to participate.
Consent for publication
The authors declare their consent for publication of the manuscript.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki.
Additional information
Communicated by J. T. Lifjeld.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10336_2021_1904_MOESM1_ESM.docx
Supplementary file 1. (DOCX 69974 KB). Table SM1. GenBank accession numbers for the analyzed data and downloaded sequences used as external groups. Table SM2. Prior distribution for calibration of the main nodes used to estimate divergence times for Habia fuscicauda in Beast. Table SM3. Clean occurrence dataset for the Ecological Niche Model analysis. Table SM4. Analyses of molecular variance (AMOVA) between geographical groups NCA and LCA. Figure SM1. Model selection of bioclimatic variables and tuning of the best parameter combination for the Ecological Niche Model. a) Clustering bios in a dendrogram with 1,000 bootstrap replicates with approximately unbiased (AU) probability values (p-values). b) correlation matrix heatmap: Positive correlations are displayed in blue and negative correlations in red color. Color intensity and the size of the circle are proportional to the correlation coefficients. c) Tuning of the best parameter combination of regularization multiplier and feature types through AICc model selection. Bios: bio1 = Annual Mean Temperature; bio2 = Mean Diurnal Range; bio3 = Isothermality; bio4 = Temperature Seasonality; bio5 = Max Temperature of Warmest Month; bio6 = Min Temperature of Coldest Month; bio7 = Temperature Annual Range; bio8 = Mean Temperature of Wettest Quarter; Mean Temperature of Driest Quarter; bio10 = Mean Temperature of Warmest Quarter; bio11 = Mean Temperature of Coldest Quarter; bio12 = Annual Precipitation; bio13 = Precipitation of Wettest Month; bio14 = Precipitation of Driest Month; bio15 = Precipitation Seasonality; bio16 = Precipitation of Wettest Quarter; bio17 = Precipitation of Driest Quarter; bio18 = Precipitation of Warmest Quarter; bio19 = Precipitation of Coldest Quarter. Figure SM2. mtDNA and nDNA phylogenetic trees. Color tips indicate populations according to geographic delimitations. Polygons or symbols indicate the subspecies corresponded to each individual. Support values indicate posterior probability for Bayesian approach and bootstrap support for Maximum Likelihood. Figure SM3. Haplotype networks for the individual genes a) ND4 (mtDNA), b) ACOI-I9 (nDNA), and c) ODC (nDNA). Colors used for geographic delimitation of populations are the same as in the main text.
Rights and permissions
About this article
Cite this article
Castillo-Chora, V.d., Zamudio-Beltrán, L.E., Pozo, C. et al. Phylogeography of Habia fuscicauda (Cardinalidae) indicates population isolation, genetic divergence and demographic changes during the Quaternary climate shifts in the Mesoamerican rainforest. J Ornithol 162, 961–976 (2021). https://doi.org/10.1007/s10336-021-01904-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01904-x