Abstract
Sex ratio is a fundamental concept in evolutional biology, and theory predicts that parents should invest in sons and daughters according to the fitness returns they expect from them. The fitness returns may depend on the timing of breeding and on parental conditions leading to sex ratios that depend on breeding date and/or parental quality. Here, we investigate the offspring sex ratio in a small shorebird, the Kentish Plover Charadrius alexandrinus, in a large breeding population in Eastern China, and test whether the parents adjust their offspring’s sex in response to hatch date, brood age and their own body condition. Using 1264 chicks from 676 broods that were molecularly sexed, we show that hatchling sex ratio was not significantly different from unity. Hatchling sex ratios were not related to hatch date or to the body condition of parents. In addition, we sexed 138 eggs that were confiscated from illegal egg collectors and found that the mortality of female and male embryos was not significantly different. The latter result is important by suggesting that neither primary sex ratio (i.e., at conception) nor secondary sex ratio (i.e., at hatching) is biased. Taken together, the even offspring sex ratio in Chinese Kentish Plovers is consistent with recent analyses of six plover populations that found even sex ratios at hatching. Future works should investigate whether the even sex ratio persists into adulthood, or it may shift toward more males (or females) due to sex-biased mortalities of juveniles and/or adults.
Zusammenfassung
Das Geschlechterverhältnis des Nachwuchses einer Küstenlimikole ist unabhängig von den Eigenschaften der Eltern und dem Brutzeitbeginn
Das Geschlechterverhältnis ist ein fundamentales Konzept der Evolutionsbiologie, und die Theorie sagt voraus, dass Eltern in Söhne und Töchter proportional zu dem von ihnen erwarteten Fitnessertrag investieren sollten. Der Fitnessertrag hängt möglicherweise vom Brutzeitbeginn und der elterlichen Qualität ab, was dazu führen kann, dass das Geschlechterverhältnis selbst vom Beginn der Brutzeit und den Eigenschaften der Eltern abhängt. Hier untersuchen wir das Geschlechterverhältnis des Nachwuchses einer kleinen Küstenvogelart, dem Seeregenpfeifer Charadrius alexandrinus, in einer großen Brutpopulation in Ostchina und testen, ob die Eltern das Geschlechterverhältnis ihres Nachwuchses dem Schlüpfzeitpunkt, Brutalter und den eigenen körperlichen Bedingungen anpassen. Mittels 1264 Küken aus 676 Bruten, die alle molekular geschlechtsbestimmt waren, zeigen wir, dass das Geschlechterverhältnis nicht signifikant von gleichförmig abwich. Das Geschlechterverhältnis beim Schlupf war weder vom Schlüpfzeitpunkt noch von der Körperkondition der Eltern abhängig. Zusätzlich bestimmten wird das Geschlecht von 138 Eiern, die von illegalen Eisammlern beschlagnahmt wurden, und fanden, dass die Sterblichkeit von weiblichen und männlichen Embryos nicht signifikant verschieden war. Dies zeigt, dass weder das primäre Geschlechterverhältnis (bei der Zeugung) noch das sekundäre Geschlechterverhältnis (beim Schlupf) unausgeglichen sind. Das ausgeglichene Geschlechterverhältnis in den chinesischen Seeregenpfeifern stimmt mit jüngsten Ergebnissen von sechs Regenpfeiferpopulationen überein, die auch ausgeglichene Geschlechterverhältnisse beim Schlupf fanden. Zukünftige Arbeiten sollten untersuchen, ob das ausgeglichene Geschlechterverhältnis bis in das Erwachsenenalter fortbesteht, oder ob es sich zu mehr Männchen (oder Weibchen) verschiebt aufgrund von geschlechtsabhängigen Sterberaten von Jung- und/oder Altvögeln.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ever since the work of Darwin (1871), evolutionary biologists have focused on sex ratios as they are a key aspect of the natural history of sexually reproducing organisms and are particularly important for an understanding of social behaviour, breeding strategies and population dynamics (Hardy 2002; West 2009). Therefore, understanding how natural and sexual selection act on sex ratios is a significant component in studies on the evolutionary basis of animal behaviour. The core idea of frequency-dependent sex allocation was developed by Fisher (1930), who argued that the ratio of males to females at birth should not deviate from parity as long as the costs of producing males and females are identical. Numerous theoretical models have been developed following Fisher’s seminal argument that predicted skewed offspring sex ratios under certain environmental and genetic conditions (Hamilton 1967; Trivers and Willard 1973; Daan et al. 1996; reviewed by Trivers 1985; Hardy 2002; West 2009).
A shared prediction of several of these models is that the offspring sex ratio should deviate from unity as a result of temporal variation in costs and/or benefits of reproduction. For instance, bias is expected if the environment has sex-specific effects on offspring fitness (Trivers and Willard 1973; Bell et al. 2014; Bowers et al. 2015). Parents in poor environments might produce more of the sex that costs less (Myers 1978; Gomendio et al. 1990) or survives better (Heinsohn et al. 2011). As environmental factors often change seasonally and/or annually, offspring sex ratios are expected to be influenced by time of breeding (Pen et al. 1999; Bordier et al. 2014; Minias 2016).
Parental quality may also influence offspring sex ratios. The parental condition hypothesis predicts that parents in good condition should bias their allocation towards the sex that produces the greater increase in reproductive value for a given level of investment (Trivers and Willard 1973). The results from numerous studies are consistent with this hypothesis (Whittingham and Dunn 2000; Lu et al. 2013; Bowers et al. 2017), although the patterns are certainly not at all general (Leimar 1996; Hewison and Gaillard 1999). According to the attractive mate hypothesis (Burley 1981), if females get higher relative fitness returns from sons than daughters by mating with an attractive male, they bias the sex ratio of their offspring towards sons (Ellegren et al. 1996). For many sexually dimorphic species, variation in the ornamentation or body size of males influences offspring sex ratios (Sheldon et al. 1999; Romano et al. 2015; Booksmythe et al. 2017).
Sex ratio variation may emerge during early development. For instance, in sexually dimorphic species in which the males are larger, male offspring have a higher mortality rate than female offspring (Kalmbach and Benito 2007), presumably due to their higher sensitivity to starvation (Kalmbach et al. 2005). The reverse mortality pattern, i.e. higher female than male chick mortality, has also been reported for raptors, ducks, swifts and passerines (Arroyo 2002; Hipkiss et al. 2002; Bize et al. 2005; Råberg et al. 2005; Lehikoinen et al. 2008). Although the causes of these sex-dependent mortalities are rarely identified, the sex bias in juvenile mortalities may have knock-on effects on population viability with major conservation implications, especially for endangered birds (Donald 2007; Eberhart-Phillips et al. 2017; Ramula et al. 2018).
Here, we investigate sex allocation in a small shorebird, the Kentish Plover, Charadrius alexandrinus, which has a body mass of about 32–56 g (Wiersma et al. 2018). The Kentish Plover provides a good model system for understanding the evolution of mating systems, and has been used for ecological and evolutionary studies for the following main reasons. First, they have an unusually wide geographic distribution that includes Europe, North Africa and Asia (Wiersma et al. 2018), and they inhabit different types of habitat that include hot deserts, temperate coast and dunes, inland salt marshes and cold subalpine lakes. This diversity offers unique opportunities to test behavioural, ecological and physiological adaptation to different environments. Second, the Kentish Plover exhibits diverse mating systems and parental care strategies, and has been used as a model system of mating system evolution as either the male or the female parent may desert the family shortly and then re-mate, and, therefore, create a variable degree of polygamy in different populations (Lessells 1984; Székely and Lessells 1993; Fraga and Amat 1996; Székely 2014). Third, recent work showed that closely related plover populations exhibit different sex ratios, and that these sex ratio biases are related to the extent of parental cooperation (Eberhart-Phillips et al. 2018). These observations raise the possibility that different plover populations have divergent sex allocation patterns that lead to biased sex ratio that have knock-on effects on mating system and parental care. For example, a previous study of Kentish Plover in Turkey found that hatch date was associated with the hatchling sex ratio, as the hatchling sex ratio became progressively female biased over the breeding season (Székely et al. 2004), and consistent with theoretical expectations (Kokko and Jennions 2008; Székely et al. 2014b), the male parent looks after the young (Eberhart-Phillips et al. 2018).
Here, we focus on a population of Kentish Plover that breeds in Eastern China and investigate whether parental quality and/or hatch date predict the offspring sex ratio. In Eastern China, both parents incubate the eggs, although after chicks hatch, many females desert their brood during the first week, so that the males look after the chicks until they fledge or die (P. Que, unpublished data). As a male-skewed sex ratio could lead to brood desertion in female plovers (Székely et al. 1999), the offspring of the Chinese population may have a biased sex ratio. The specific aims of our study were to test: (1) whether hatchling sex ratios are biased; (2) whether sex ratios vary in relation to hatching date, brood age and breeding year; and (3) whether parental quality predicts hatchling sex ratio. In addition, by incubating eggs that were confiscated from illegal egg-collectors (see below), we also test (4) whether sex ratio shift may occur between laying and hatching of the eggs.
Methods
Study sites
We conducted fieldwork during April-July 2009 and 2012 at Nanpu Wetland (27,000 ha, 39°09′N, 118°09′E to 39°02′N, 118°19′E; Fig. 1S), and during April–July 2013–2016 at Daqinghe Wetland (9700 ha, 39°08′N, 118°46′E to 39°14′N, 118°52′E; Fig. 1S). Both field sites are important stopover and breeding sites for migratory waterbirds in the East Asian-Australasian Flyway and are located in Bohai Bay, Eastern China. Over 200,000 waterbirds stop or breed in these areas each year (Yang et al. 2011). These two sites are composed of large artificial salt pans used for salt processing and prawn aquaculture. Approximately 1800 pairs of Kentish Plover breed at Nanpu and 600 pairs at Daqinghe (Lei 2017). We treated Nanpu and Daqinghe as one study area in this study as they are less than 60 km apart and have similar breeding habitats.
Field methods
On finding a nest, we floated the eggs to estimate the stage of incubation, initiation date and hatch date (Liebezeit et al. 2007; Que et al. 2015). Nests were checked at least daily near the time of hatching (approximately the 22nd day after the start of incubation) to capture freshly hatched chicks in the nest scrape (Székely et al. 2008). As the Kentish Plover is not very sensitive to cars, we could hide inside a car to search through a spotting scope for chicks and brooded plovers that left the nest scrape (Székely et al. 2008). After successful capture, body mass, tarsus length and bill length were measured for each chick. Body mass was measured to the closest 0.01 g with an electronic scale (G&G MS501), while bill and tarsus lengths were measured to the closest 0.01 mm using Vernier callipers (Mitutoyo 500-762-10) following Redfern and Clark (2001). A blood sample (< 20 μl) was also taken from the left leg vein and stored below − 20 °C in a freezer.
Parents were caught using funnel traps or spring traps during incubation or brood care (Hall and Cavitt 2012). Adults were sexed according to the colour of the head and breast (Hayman et al. 1986), and for a subset, morphological sexing was confirmed by molecular sexing (see below). Body mass, tarsus length and bill lengths were measured following the above protocol, and flattened wing chord length was measured to the closest 1 mm with a wing ruler (Redfern and Clark 2001). Plovers were marked with a numbered aluminium ring from the National Bird Banding Centre of China, and a unique combination of one to three coloured plastic leg bands for visual identification. All handling methods were approved by the Hebei Province Forestry Bureau.
On 5 May 2015, two people illegally collected 138 Kentish Plover eggs for human consumption at Hangu, Tianjin (Fig. 1S). Local policemen and conservation volunteers apprehended these individuals and brought the eggs to a wildlife rescue centre based at Daqinghe Saltworks. The eggs were immediately put into an incubator (type Yikebeite RK-60); we estimated that less than 6 h elapsed from when the eggs were collected until they were put in the incubator. During these 6 h, the eggs were exposed to the ambient temperature of approximately 30 °C. We measured and floated these eggs to estimate the number of incubated days and hatching status using the protocol of Székely et al. (2008), and placed all of the eggs into an incubator at 37.6 °C with 80–85% humidity (Page et al. 1989). We considered an egg to have failed when it remained unhatched after 7 days of estimated hatch date. Out of 138 eggs, 104 eggs hatched and 34 failed to hatch. For the hatched eggs, we took blood samples from the chicks for molecular sexing. Developed embryos were found in all unhatched eggs; we took tissue samples from these embryos. As we did not know from how many clutches the eggs had been taken, we considered each egg as an individual datum. After the measurements and blood samples were taken, the chicks were reared and released back into the wild by the Daqinghe Wildlife Rescue Centre when they fledged and had gained adult weight.
Molecular sexing
DNA was extracted from the blood and tissue samples using TIANamp Genomic DNA Kits (Tiangen Biotech, Beijing) and primers 2550F/2718R (Fridolfsson and Ellegren 1999). Polymerase chain reaction (PCR) amplification was carried out in a total volume of 20 μl. The final reaction conditions were as follows: 0.5 μl of each primer, 4 μl of target DNA, 10 μl of 2 × EasyTaq PCR SuperMix and 5 μl of deionized water. Thermal cycling was carried out in a Biometra UnoII: initial denaturation for 5 min at 94 °C was followed by 35 cycles of 30 s at 94 °C, 45 s at 48 °C, 45 s at 72 °C, with a final extension for 7 min at 72 °C. As molecular sex determination is known to be prone to errors (Robertson and Gemmell 2006), we randomly chose 300 samples, included 40 of 104 hatched confiscated eggs and 20 of 34 unhatched confiscated eggs, and used another set of primers, 2602F/2669R (van der Velde et al. 2017), to test the result of primers 2550F/2718R. PCR amplification was carried out in a total volume of 20 μl: with 1 μl of each primer, 2 μl of target DNA, 10 μl of 2× EasyTaq PCR SuperMix and 6 μl of deionized water. The PCR program was as follows: initial denaturation for 1 min at 94 °C, 35 cycles of 30 s at 94 °C, 60 s at 61 °C, 60 s at 72 °C, and a final extension for 2 min at 72 °C. Electrophoresis of the PCR products was carried out on 2.5% agarose gel stained at 120 V. The products were then stained with SYBR and photographed under ultraviolet (UV) light. The male samples had one band, while the female samples had double bands.
The results of the sex determination experiment with two different primer pairs were identical, and the bands on the UV-transillumination were clearer and brighter than before. In addition, 24 chicks were tested blind with regards to the first set of results, and the second sexing provided identical results to the first one. Finally, we molecularly sexed 48 adults (19 males and 29 females) that had been sexed in the field using plumage characteristics. The results of molecular sexing matched the field-based sexing for all adults.
Statistical analyses
One male and two females produced two broods either within a year or in subsequent years, although only the first complete brood was included in the analysis for each adult. These complete broods were included in the statistical analyses. We created the following sets of data: (1) broods in which all the hatched chicks were captured in the nest, which we termed the offspring sex ratio at hatching, were used to analyse the relationship between offspring sex ratio and hatching date; (2) all broods that were either captured in the nest or off the nest, which were used to analyse the relationship between the offspring sex ratio and the chick age. The equation given by Székely and Cuthill (1999) was used to estimate the age of chicks which were captured off the nest. (3) Broods in which all the hatched chicks were captured in the nest, and of which both parents were captured during incubation, were used to analyse the relationship between the offspring sex ratio at hatching and parental body size and condition.
Binomial tests were used to determine whether offspring sex ratio bias occurred at hatching. χ2-tests were used to ascertain whether variations in the sex ratio occurred between years.
We calculated a scaled mass index (SMI) to quantify the body condition of the parents from the body mass and a linear body measurement (Peig and Green 2009). SMI is a good indicator of body condition as it accounts for the changing relationship between mass and length as body size changes (Peig and Green 2010), and it has been used to indicate the energy reserves and body components in birds (Bell et al. 2014; Cooper et al. 2015), including the Red-capped Plover (Charadrius ruficapillus) (Tan et al. 2015). As wing length was the structural measure most strongly correlated with body mass (Table S1), we used wing length as a measure of structural size. At first, we created a bivariate plot of mass and wing length to excluded any outliers that may distort the expected relationship. Then standardised major axis regression was carried out to calculate the slope. Thus, the SMI of body condition can be computed for each individual following the equation described by Peig and Green (2009).
To assess the effect of parental condition and time of breeding on the offspring sex ratio, we used generalized linear mixed models (GLMMs) with binomial errors and a logit link. We fitted the brood sex ratio as the dependent variable, the brood size as weights, the brood identifier and year as random factors, and body size (mass and tarsus length) and the body condition (SMI) of both parents, hatching date, and brood age as independent variables (fixed effects). We created candidate models of all possible combinations including all considered variables for each data set. As a previous study suggested that hatching date has a non-linear relationship with brood sex ratio in the Snowy Plover (Charadrius nivosus) (Saalfeld et al. 2013), the quadratic effects of hatching date, and the interaction between year and hatching date were also considered.
Statistical analyses were carried out in R version 3.2.2 (R Development Core Team 2015) using the lme4 package (Bates et al. 2015) for fitting GLMMs and the MuMIn package (Barton 2015) for selected models. Akaike’s information criterion corrected for small sample size (AICc) was used to assess candidate models. The best model is the model with the lowest AICc score, and the models with a change in AICc of less than 2 are considered candidates (Burnham and Anderson 2002). We conducted model averaging to obtain robust estimates of the model parameters if there was more than one model with an AICc less than 2 (Burnham and Anderson 2002). Parameter estimates are represented as the mean ± SE, and we calculated two-tailed probabilities. Sex ratio is expressed as the proportion of males.
Results
Brood sex ratio
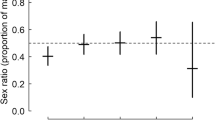
Over the 6 years of the study, blood samples from 1322 chicks were collected from 676 broods, for which the sex was determined in 1264 chicks (95.61%; Table 1). The sex ratio was not significantly biased at hatching (0.49 ± 0.023, χ2 = 0.33, p = 0.57, n = 440 chicks from 146 broods; Fig. 1).
Brood sex ratio and timing of breeding
The sex ratios at hatching did not differ significantly among years (χ2 = 9.48, df = 5, p = 0.09, n = 146 broods; Fig. S2). Year, hatching date, and the interaction between year and hatching date were not included in the candidate model for brood sex ratio at hatching (Table S2). This suggested that breeding year and hatching date did not significantly vary with brood sex ratio at hatching. We have no evidence that the proportion of males changed significantly with brood age [β = 0.19 (− 0.08, 0.45) (± 95% CI), p = 0.16, n = 1252 chicks from 664 broods; Table 2].
Brood sex ratio and parental body condition
The brood sex ratio at hatching was not significantly related to the body size of the female parent [body mass, β = − 0.21 (− 0.73, 0.30) (± 95% CI), p = 0.41, n = 249 chicks from 83; Table 3] or the male parent (body mass, β = 0.19 (− 0.33, 0.70) [± 95% CI], p = 0.47, n = 249 chicks from 83; Table 3); none of the candidate models included male or female tarsus length (n = 249 chicks from 83 broods; Table 3).
Brood sex ratio at hatching was not significantly related to parental body condition (SMI) [female parent, β = − 0.17 (− 0.20, 0.71) [± 95% CI], p = 0.52; male parent, β = 0.17 (− 0.26, 0.59) (± 95% CI), p = 0.52, n = 249 chicks from 83; Table 4].
Sex ratio of the confiscated eggs
Of 104 hatched eggs, 49 were male (binomial test, p = 0.62), whereas of 34 unhatched eggs, nine were male (binomial test, p = 0.01). The sex ratio bias in unhatched eggs is significantly different from that in hatched eggs (9:25 cf. 49:55; Fisher exact test, p = 0.045). The overall sex ratio of 138 eggs was not significantly different from even (binomial test, p = 0.07). The sex ratio of confiscated eggs did not vary significantly during incubation [GLMMs, β = 0.04 (− 0.01, 0.09) (± 95% CI), p = 0.10, n = 117 eggs; Table 4].
Discussion
Our study produced four main findings. First, the chick sex ratio did not differ significantly from even at laying or at hatching, which is consistent with previous studies that found unbiased sex ratios in plovers (Székely et al. 2004; Saalfeld et al. 2013; Riordan et al. 2015; Eberhart-Phillips et al. 2017). However, the sample size was limited for inferring sex ratio at egg-laying, these data were from only 1 year, and the eggs were pooled for the analyses rather than accounting for clutch identity or parental identity. Nevertheless, these results suggest that the hatchling sex ratio is not strongly biased in Charadrius plovers, which is consistent with results for most birds that have been studied to date (Donald 2007; Székely et al. 2014a).
Our finding that there was no significant shift in sex ratio during egg development underpins that primary and secondary sex ratios are not different, and suggests that there is no sex-specific offspring mortality before hatching. Such data are quite rare for wild bird populations for ethical reasons, although we were lucky to have a dedicated conservationist in Bohai Bay willing to incubate confiscated eggs for us. We wish to note two findings here. First, the sex ratio was significantly biased towards males in unhatched eggs, suggesting that female embryos may be more sensitive to disturbance and/or fluctuation in incubation temperatures than males. Although female eggs might have been treated more harshly than male eggs for unknown reasons (e.g. female eggs may, for unknown reasons, have spent more time in the collector’s bag than male eggs), there is a possibility that male and female eggs have genuinely different sensitivity to perturbations. Second, to detect shifts in pre-birth offspring, large sample sizes are needed, and such samples are rarely available for ornithologists. For instance, Orzack et al. (2015) reported significant shifts in foetal abortion in humans, although this study used over 90,000 individuals.
Second, unlike previous studies in Kentish and Snowy Plovers (Székely et al. 2004; Saalfeld et al. 2013), the hatchling sex ratio did not show significant seasonal variation in Bohai Bay. This suggests that different plover populations may exhibit different seasonal trends in offspring sex ratios: in Turkey the proportion of males decreased over the breeding season (Székely et al. 2004), in Texas the seasonal pattern was quadratic as the proportion of males was high both earlier and later than in the middle of the breeding season (Saalfeld et al. 2013), whereas we found no significant trend in Eastern China. A seasonal trend in offspring sex ratio may vary among species (Smallwood and Smallwood 1998; McIntosh et al. 2003; Goławski et al. 2016; Minias 2016), and even among different populations of a species (e.g. Fiala 1981 vs. Weatherhead 1983; Howe 1977 vs. Maddox and Weatherhead 2009).
Third, in previous studies of Kentish and Piping Plovers (Charadrius melodus) the proportion of males increased with brood age (Székely et al. 2004; Saunders and Cuthbert 2015), which appears to result from female-biased chick mortality (Székely et al. 2004; Saunders and Cuthbert 2015). The lack of a significant association between brood sex ratio and brood age in Bohai Bay is consistent with our previous finding that mortality did not differ significantly between male and female chicks (Que 2015).
Fourth, we found no evidence for offspring sex ratio adjustment relative to parental body size. These results did not support the parental condition hypothesis or the attractiveness hypothesis. The underlying assumption of the parental condition hypothesis, the function indicating that investment to fitness returns differ for male and female offspring, is difficult to measure in most study systems. An in-depth investigation of the underlying assumptions is necessary for each study testing the parental condition hypothesis. Similarly, choosing appropriate traits that indeed relate to the fitness of male offspring and are selected by females is a major challenge for sex-allocation studies.
Recent theoretical and empirical studies are adding a new layer of interest to sex allocation by arguing that an important, yet neglected, aspect of mating system evolution is adult sex ratio (Kokko and Jennions 2008; Székely et al. 2014b; Schacht et al. 2017). Frequency-dependent selection of these social behaviours are expected, as the rarer sex in the population has more opportunities to choose a mate and/or abandon the partner than the more common sex. Phylogenetic analyses of birds including shorebirds are consistent with these propositions (Liker et al. 2013, 2014). Consistent with these phylogenetic comparative results, a recent demographic analysis of six closely related populations showed significantly different adult sex ratios in Charadrius plovers [Eberhardt-Phillips et al. (2018); note that the latter study did not include data from China]. Importantly, Eberhart-Phillips et al. (2018) also showed that a major factor creating biased adult sex ratios is sex-dependent juvenile survival. Whilst we found no significant difference in juvenile survival in Bohai Bay, our brood observations concentrated on small chicks shortly after hatching, so we do not know whether sex-specific mortality may occur in subsequent chick development. Therefore, studies are needed to investigate the transitions in sex ratios from conception till adulthood.
In conclusion, using 440 chicks from a well-studied shorebird species, the Kentish Plover, we found no sex bias at hatching in China. Therefore, it seems unlikely that the male-biased adult sex ratios reported for Kentish and Snowy Plover populations (Kosztolányi et al. 2011; Stenzel et al. 2011; Carmona-Isunza et al. 2017; Eberhart-Phillips et al. 2018) are due to biased secondary (hatching) sex ratios, consistent with birds in general (Székely et al. 2014a). We call for further studies to understand the causes of adult sex ratio variation by testing sex differences in maturation and/or in mortalities of chicks and adults. Such studies are needed not only to clarify one of the key areas of avian evolutionary ecology, i.e. breeding system evolution, but may also have conservation implications for the management of endangered bird populations with biased sex ratios (Donald 2007; Eberhart-Phillips et al. 2017).
References
Arroyo B (2002) Sex-biased nestling mortality in the Montagu’s Harrier Circus pygargus. J Avian Biol 33:455–460
Barton K (2015) MuMIn: multi-model inference. http://cran.r-project.org/web/packages/MuMIn/index.html
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bell SC, Owens IP, Lord AM (2014) Quality of breeding territory mediates the influence of paternal quality on sex ratio bias in a free-living bird population. Behav Ecol 25:352–358
Bize P, Roulin A, Tella JL, Richner H (2005) Female-biased mortality in experimentally parasitized Alpine Swift Apus melba nestlings. Funct Ecol 19:405–413
Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD (2017) Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol Rev 92:108–134
Bordier C, Saraux C, Viblanc VA, Gachot-Neveu H, Beaugey M, Le Maho Y, Le Bohec C (2014) Inter-annual variability of fledgling sex ratio in King Penguins. PLoS One 9:e114052
Bowers EK, Thompson CF, Sakaluk SK (2015) Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol 84:473–486
Bowers EK, Thompson CF, Sakaluk SK (2017) Maternal natal environment and breeding territory predict the condition and sex ratio of offspring. Evol Biol 44:11–20
Burley N (1981) Sex ratio manipulation and selection for attractiveness. Science 211:721–722
Burnham KP, Anderson DR (2002) Model selection and interference: a practical information-theoretic approach, 2nd edn. Springer, New York
Carmona-Isunza MC, Ancona S, Székely T, Ramallo-González AP, Cruz-López M, Serrano-Meneses MA, Küpper C (2017) Adult sex ratio and operational sex ratio exhibit different temporal dynamics in the wild. Behav Ecol 28:523–532
Cooper NW, Sherry TW, Marra PP (2015) Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 96:1933–1942
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–430
Darwin C (1871) The descent of man and selection in relation to sex. Murray, London
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Eberhart-Phillips LJ, Küpper C, Miller TE, Cruz-López M, Maher KH, Dos Remedios N, Stoffel MA, Hoffman JI, Krüger O, Székely T (2017) Sex-specific early survival drives adult sex ratio bias in Snowy Plovers and impacts mating system and population growth. Proc Natl Acad Sci USA 117:E5474–E5481
Eberhart-Phillips LJ, Küpper C, Carmona-Isunza MC, Vincze O, Zefania S, Cruz-López M, Kosztolányi A, Miller TE, Barta Z, Cuthill IC, Burke T (2018) Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat Commun 9:1651
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to parental attractiveness in a wild bird population. Proc Natl Acad Sci USA 93:11723–11728
Fiala KL (1981) Sex ratio constancy in the Red-winged Blackbird. Evolution 35:898–910
Fisher RA (1930) The genetical theory of natural selection: a complete variorum edition. Oxford University Press, Oxford
Fraga RM, Amat JA (1996) Breeding biology of a Kentish Plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola 43:69–85
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 1:116–121
Goławski A, Kasprzykowski Z, Ledwoń M, Mróz E, Morelli F (2016) Brood sex ratio in expansive and non-expansive tern species in east-central Poland. Bird Study 63:31–36
Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ (1990) Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343:261–263
Hall LK, Cavitt JF (2012) Comparative study of trapping methods for ground-nesting shorebirds. Waterbirds 35:342–346
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Hardy ICW (2002) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Hayman P, Marchant J, Prater T (1986) Shorebirds. An identification guide to the waders of the world. Helm, London
Heinsohn R, Langmore NE, Cockburn A, Kokko H (2011) Adaptive secondary sex ratio adjustments via sex-specific infanticide in a bird. Curr Biol 21:1744–1777
Hewison AJM, Gaillard JM (1999) Successful sons or advantaged daughters? The Trivers-Willard model and sex-biased maternal investment in ungulates. Trends Ecol Evol 14:229–234
Hipkiss T, Hornfeldt B, Eklund U, Berlin S (2002) Year-dependent sex-biased mortality in supplementary-fed Tengmalm’s Owl nestlings. J Anim Ecol 71:693–699
Howe HF (1977) Sex-ratio adjustment in the Common Grackle. Science 198:744–746
Kalmbach E, Benito MM (2007) Sexual size dimorphism and offspring vulnerability in birds. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 133–142
Kalmbach E, Furness RW, Griffiths R (2005) Sex-biased environmental sensitivity: natural and experimental evidence from a bird species with larger females. Behav Ecol 16:442–449
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Kölliker M, Heeb P, Werner I, Mateman AC, Lessells CM, Richner H (1999) Offspring sex ratio is related to male body size in the Great Tit (Parus major). Behav Ecol 10:68–72
Kosztolányi A, Barta Z, Küpper C, Székely T (2011) Persistence of an extreme male-biased adult sex ratio in a natural population of polyandrous bird. J Evol Biol 24:1842–1846
Küpper C, Augustin J, Kosztolányi A, Burke T, Flguerola J, Székely T (2009) Kentish versus Snowy Plover: phenotypic and genetic analyses of Charadrius alexandrinus reveal divergence of Eurasian and American subspecies. Auk 126:839–852
Lehikoinen A, Öst M, Hollmén T, Kilpi M (2008) Does sex-specific duckling mortality contribute to male bias in adult common eiders? Condor 110:574–578
Lei W (2017) Studies on the waterbirds used saltpans in north of Bohai Bay. Dissertation, Beijing Normal University
Leimar O (1996) Life-history analysis of the Trivers and Willard sex-ratio problem. Behav Ecol 7:316–325
Lessells CM (1984) The mating system of Kentish Plovers Charadrius alexandrinus. Ibis 126:474–483
Lessells CM, Mateman AC, Visser J (1996) Great Tit hatchling sex ratios. J Avian Biol 1:135–142
Liebezeit JR, Smith PA, Lanctot RB, Schekkerman H, Tulp I, Kendall SJ, Tracy DM, Rodrigues RJ, Meltofte H, Robinson JA, Gratto-Trevor C (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47
Liker A, Freckleton RP, Székely T (2013) The evolution of sex roles in birds is related to adult sex ratio. Nat Commun 4:1587
Liker A, Freckleton RP, Székely T (2014) Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr Biol 24:880–884
Lu X, Zeng X, Du B (2013) Body attributes of both parents jointly affect offspring sex allocation in a socially monogamous, size-monomorphic passerine. Curr Zool 59:271–277
Maddox JD, Weatherhead PJ (2009) Seasonal sex allocation by Common Grackles? Revisiting a foundational study. Ecology 90:3190–3196
McIntosh RR, Kats R, Berg M, Komdeur J, Elgar MA (2003) Breeding ecology and bias in offspring sex ratio in Little Grassbirds (Megalurus gramineus). Aust J Zool 51:505–514
Minias P (2016) Seasonal trends in brood sex ratio reflect changes in early-life physiological condition of chicks in the Whiskered Tern. Ethol Ecol Evol 28:385–393
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring. Am Nat 112:381–388
Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munné S, Scholl T, Steinsaltz D, Zuckerman JE (2015) The human sex ratio from conception to birth. Proc Natl Acad Sci USA 112:E2102–E2111
Page GW, Quinn PL, Warriner JC (1989) Comparison of the breeding of hand-and wild-reared Snowy Plovers. Conserv Biol 3:198–201
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332
Pen I, Weissing FJ, Daan S (1999) Seasonal sex ratio trend in the European kestrel: an evolutionarily stable strategy analysis. Am Nat 153:384–397
Que P (2015) Breeding success and population genetic structure of Kentish Plover Charadrius alexandrinus in China. Dissertation, Beijing Normal University
Que P, Chang Y, Eberhart-Phillips L, Liu Y, Székely T, Zhang Z (2015) Low nest survival of a breeding shorebird in Bohai Bay, China. J Ornithol 156:297–307
Råberg L, Stjernman M, Nilsson JÅ (2005) Sex and environmental sensitivity in Blue Tit nestlings. Oecologia 145:496–503
Ramula S, Öst M, Lindén A, Karell P, Kilpi M (2018) Increased male bias in eider ducks can be explained by sex-specific survival of prime-age breeders. PLoS ONE 13:e0195415
Redfern CPF, Clark JA (2001) Ringers’ manual. British Trust for Ornithology, Thetford
Riordan MM, Lukacs PM, Huyvaert KP, Dreitz VJ (2015) Sex ratios of Mountain Plovers from egg production to fledging. Avian Conserv Ecol 10:3
Robertson BC, Gemmell NJ (2006) PCR-based sexing in conservation biology: wrong answers from an accurate methodology? Conserv Genet 7:267–271
Romano A, Romano M, Caprioli M, Costanzo A, Parolini M, Rubolini D, Saino N (2015) Sex allocation according to multiple sexually dimorphic traits of both parents in the Barn Swallow (Hirundo rustica). J Evol Biol 28:1234–1247
Saalfeld ST, Conway WC, Haukos DA, Johnson WP (2013) Seasonal variation in offspring sex ratio in the Snowy Plover. West N Am Nat 73:60–71
Saunders SP, Cuthbert FJ (2015) Chick mortality leads to male-biased sex ratios in endangered Great Lakes Piping Plovers. J Field Ornithol 86:103–114
Schacht R, Kramer KL, Székely T, Kappeler PM (2017) Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies. Philos Trans R Soc B 372:1729
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences Blue Tit sex ratios. Nature 402:874–877
Smallwood PD, Smallwood JA (1998) Seasonal shifts in sex ratios of fledgling American Kestrels (Falco sparverius paulus): the early bird hypothesis. Evol Ecol 12:839–853
Stenzel LE, Page GW, Warriner JC, Warriner JS, Neuman KK, George DE, Eyster CR, Bidstrup FC (2011) Male-skewed adult sex ratio, survival, mating opportunity and annual productivity in the Snowy Plover Charadrius alexandrinus. Ibis 153:312–322
Székely T (2014) Sexual conflict between parents: offspring desertion and asymmetrical parental care. In: Rice WR, Gavrilets S (eds) The genetics and biology of sexual conflict. Cold Spring Harbor Laboratory Press, New York, pp 245–263
Székely T, Cuthill IC (1999) Brood desertion in the Kentish Plover: the value of parental care. Behav Ecol 10:191–197
Székely T, Lessells CM (1993) Mate change by Kentish Plovers Charadrius alexandrinus. Ornis Scand 24:317–322
Székely T, Cuthill IC, Kis J (1999) Brood desertion in Kentish Plover: sex differences in remating opportunities. Behav Ecol 10:185–190
Székely T, Cuthill IC, Yezerinac S, Griffiths R, Kis J (2004) Brood sex ratio in the Kentish Plover. Behav Ecol 15:58–62
Székely T, Kosztolányi A, Küpper C (2008) Practical guide for investigating breeding ecology of Kentish Plover Charadrius alexandrinus. University of Bath. http://www.bath.ac.uk/bio-sci/biodiversity-lab/pdfs/KP_Field_Guide_v3.pdf. Accessed 2 Feb 2015
Székely T, Liker A, Freckleton RP, Fichtel C, Kappeler PM (2014a) Sex-biased survival predicts adult sex ratio variation in wild birds. Proc R Soc Lond B 281:20140342
Székely T, Weissing FJ, Komdeur J (2014b) Adult sex ratio variation: implications for breeding system evolution. J Evol Biol 27:1500–1512
Tan LXL, Buchanan KL, Maguire GS, Weston MA (2015) Cover, not caging, influences chronic physiological stress in a ground-nesting bird. J Avian Biol 46:482–488
Trivers RL (1985) Social evolution. Benjamin/Cummings, Menlo Park
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
van der Velde M, Haddrath O, Verkuil YI, Baker AJ, Piersma T (2017) New primers for molecular sex identification of waders. Wader Study 124:147–151
Weatherhead PJ (1983) Secondary sex ratio adjustment in Red-winged Blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 12:57–61
West S (2009) Sex allocation. Princeton University Press, Princeton
Whittingham LA, Dunn PO (2000) Offspring sex ratios in Tree Swallows: females in better condition produce more sons. Mol Ecol 9:1123–1129
Wiersma P, Kirwan GM, Boesman P (2018) Kentish Plover (Charadrius alexandrinus). In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (ed) Handbook of the birds of the world alive. Lynx, Barcelona. http://www.hbw.com/node/53835. Accessed 22 Jan 2018
Yang H, Chen B, Barter M, Piersma T, Zhou C, Li F, Zhang Z (2011) Impacts of tidal land reclamation in Bohai Bay, China: ongoing losses of critical Yellow Sea waterbird staging and wintering sites. Bird Conserv Int 21:241–259
Acknowledgements
This study was supported by the National Natural Science Foundation of China (nos. 31600297 and 31572288). We especially thank Zhiwei Tian and Jianli Song, who provided much help in the fieldwork. We are grateful to Yajing Chang, Bingrun Zhu, Jin Liu, Jia Zheng, Boshi Liang, Siyuan Huang, Karen Kim, Siyao Zhong, Zhuoxue Chen, Guang Yang, Pei Luo, Christopher Dudley, Carrie Wendt, Rebecca Gouge, Robert Weber, Kai Chen, Xiaoyan Long, Nan Zhang, and Xuecong Zhang for field assistance. We also thank Xunqiang Mo and Jianmin Wang for intercepting the illegal egg collectors and bringing the confiscated eggs to us. We thank Dr. Benjamin Werner for translating the abstract to German. Tamás Székely was a fellow of the Advanced Institute of Berlin at the time of writing the manuscript, and his work was funded by a Royal Society Wolfson Merit Award (WM170050), and by the Hungarian scientific funding agency, NKFIH (ÉLVONAL KKP-126949, K-116310). All experiments described in this study comply with current Chinese laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Que, P., Székely, T., Wang, P. et al. Offspring sex ratio is unrelated to parental quality and time of breeding in a multiple-breeding shorebird. J Ornithol 160, 443–452 (2019). https://doi.org/10.1007/s10336-018-1620-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-018-1620-6