Abstract
In birds and mammals with sexual size dimorphism (SSD), the larger sex is typically more sensitive to adverse environmental conditions, such as food shortage, during ontogeny. However, some recent studies of altricial birds have found that the larger sex is less sensitive, apparently because large size renders an advantage in sibling competition. Still, this effect is not an inevitable outcome of sibling competition, because several studies of other species of altricial birds have found the traditional pattern. We investigated if the sexes differ in environmental sensitivity during ontogeny in the blue tit, a small altricial bird with c. 6% SSD in body mass (males larger than females). We performed a cross-fostering and brood size manipulation experiment during 2 years to investigate if the sexes were differently affected as regards body size (body mass, tarsus and wing length on day 14 after hatching) and pre-fledging survival. We also investigated if the relationship between body size and post-fledging survival differed between the sexes. Pre-fledging mortality was higher in enlarged than in reduced broods, representing poor and good environments, respectively, but the brood size manipulation did not affect the mortality rate of males and females differently. In both years, both males and females were smaller on day 14 after hatching in enlarged as compared to reduced broods. In one of the years, we also found significant Sex × Experiment interactions for body size, such that females were more affected by poor environmental conditions than that of males. Body size was positively correlated with post-fledging survival, but we found no interactive effects of sex and morphological traits on survival. We conclude that in the blue tit, females (the smaller sex) are more sensitive to adverse environmental conditions which, in our study, was manifest in terms of fledgling size. A review of published studies of sex differences in environmental sensitivity in sexually size-dimorphic altricial birds suggests that the smaller sex is more sensitive than the larger sex in species with large brood size and vice versa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of studies have documented a sex difference in the effect of environmental conditions (e.g. food availability) during ontogeny on growth and survival (Clutton-Brock et al. 1985). Such a difference in environmental sensitivity is interesting from an ecological and evolutionary perspective for at least three reasons: (1) it can affect the extent of sexual size dimorphism (SSD) in adults, (2) it can affect the population sex ratio, and (3) it can select for the ability to manipulate offspring sex in relation to current environmental conditions (Trivers and Willard 1973).
The general pattern in both birds and mammals is that the larger sex, usually males, is more sensitive to environmental conditions (Clutton-Brock et al. 1985). This is usually considered to be simply an effect of the larger sex having greater nutritional requirements [but see e.g. Sheldon et al. (1998) for other explanations for greater male sensitivity]. However, in situations with strong sibling competition, large size may be advantageous in competition for food delivered by the parents. Indeed, some recent studies of altricial birds have demonstrated that the smaller sex suffers relatively more under poor conditions (Oddie 2000; Hipkiss et al. 2002; see also Dhondt 1970; Smith et al. 1989). Nonetheless, a number of studies of other species of altricial birds have found the traditional pattern of greater sensitivity of the larger sex, even though there is potential for sibling competition also in these species (Howe 1977; Blank and Nolan 1983; Slagsvold et al. 1986; Torres and Drummond 1997; Teather and Weatherhead 1989; Nager et al. 2000). A variety of species of altricial birds are used as model organisms in the studies of SSD and sex allocation, and a sex difference in environmental sensitivity is often an important factor in such studies (Bensch 1999; Badyaev 2002; Blondel et al. 2002). It would therefore be of interest to understand why the relative sensitivity of the sexes differs between different species of altricial birds.
We here report the results of a study investigating if the sexes differ in sensitivity to environmental conditions during the nestling phase in blue tits (Parus caeruleus). We used two approaches: First, we conducted a brood size manipulation experiment to test if the sexes were differently affected as regards growth and pre-fledging survival. Second, we tested if the relationship between morphology (body mass, tarsus length and wing length) at fledging and post-fledging survival differed between the sexes. We also review the literature on sex differences in environmental sensitivity in altricial birds with SSD in an attempt to elucidate what factors determine as to whether the larger or smaller sex is most sensitive.
Methods
The blue tit is a small (c. 11 g), hole-nesting songbird, which mainly inhabits deciduous woodland. We studied a nest box breeding population at Revingehed, 20 km east of Lund, southern Sweden. The area is 64 km2 and consists of permanent pastures and agricultural fields interrupted by groves and small forests. The blue tit exhibits slight SSD; in our population, tarsus length, wing length and body mass in winter is 3–6% larger in males than in females.
The present paper is based on data from two breeding seasons, 2000 and 2001. In both years, we conducted a cross-fostering and brood size manipulation experiment (cf. e.g. Merilä 1996), thereby creating good and poor environments. Nest boxes were visited weekly during the beginning of the breeding season to determine laying date and clutch size, and daily around expected time of hatching to determine hatching date. Nest boxes were matched pair-wise with respect to clutch size (±1 egg) and hatching date. Two days after hatching, nestlings were exchanged reciprocally between boxes, so that each box contained both own and cross-fostered nestlings in equal proportions and the brood size was increased or reduced by c. 30% (cf. Merilä 1996). Before moving nestlings, we weighed them (with a Pesola spring balance to the nearest 0.1 g) and painted their claws with nail polish in different colour combinations, which enabled us to identify each individual nestling. Nestlings were ringed with uniquely numbered aluminium rings on day 7 (day of hatching = day 0). On day 14, we measured body mass, tarsus length (with digital callipers to the nearest 0.1 mm; between observer repeatability: R=0.96, F 19,40=67.1, P<0.001) and wing length (to the nearest 0.5 mm). We also collected blood for sex identification (see below). Nest boxes were visited after fledging to determine fledging success.
To determine post-fledging survival, we made one or two nightly visits to all nest boxes during the winters 2000–2001 to 2002–2003 to catch birds sleeping in the boxes. We also visited all nest boxes and caught breeding birds during the breeding seasons 2001–2003. A bird was scored as survivor if it survived at least to its first winter.
Data set and statistical analyses
We manipulated 34 pairs of broods in 2000 and 33 pairs in 2001. Broods that were deserted or predated (fully or partly) were excluded from analyses concerning the effect of the experiment. These restrictions reduced the data set to 25 pairs in 2000 and 27 pairs in 2001.
We tested for interactive effects of sex and experimental treatment on morphological traits with mixed linear models with Year, Sex and Experiment as fixed and nest box pair (henceforth Pair; nested within Year) and box of origin (henceforth Origin; nested within Pair and Year) as random effects. This is the same model as usually used in quantitative genetic analyses of cross-fostering experiments (e.g. Merilä 1996), but with the addition of the Sex factor. Analyses were performed with PROC MIXED in SAS 8.2. We used the Satterthwaite approximation for the denominator degrees of freedom of fixed effects (SAS Institute 1999). We first fitted the full model and then eliminated non-significant interactions at P>0.10 in a stepwise manner. Removal of non-significant interactions did not affect the significance of the fixed effects or their interactions, but simplifies the presentation of the results. We report least squares means ± 1SE obtained from the final models.
There was no difference in body mass on day 2 between nestlings allotted to enlarged and reduced broods [mixed model with Experiment as fixed and Pair and Origin (nested within Pair) as random effects, 2000: enlarged 2.08±0.05 g, reduced 2.03±0.06 g, Experiment F 1,462=1.41, P=0.24, Pair Z=2.00, P=0.023, Origin Z=2.22, P=0.013; 2001: enlarged 2.10±0.06 g, reduced 2.12±0.07 g, Experiment F 1,511=0.08, P=0.78, Pair Z=2.27, P=0.012, Origin Z=2.26, P=0.012].
We tested for effects of experimental treatment (enlarged vs. reduced brood) and year on fledging rate and fledgling sex ratio with generalized linear models. The dependent variables were number of fledglings/brood size and number of males/fledglings (i.e. the dependent variable was entered as a ratio). Analyses were performed with PROC GENMOD in SAS 8.2 with binomial error distribution, logit link function and type 3 option (SAS Institute 1999). We used the DSCALE option to correct for overdispersion. Nest boxes that showed signs of predation between day 14 and fledging were excluded from these analyses (three in 2000).
To test if morphology on day 14 after hatching affected post-fledging survival, and especially if the strength of such an effect differed between the sexes, we calculated selection differentials and gradients (Brodie III et al. 1995) for males and females separately and combined. The standardized linear selection differential (i) measures the total change in mean of a trait during the selection episode under consideration, and is estimated from the model w = a + i x, where w is relative fitness, and x is the standardized (mean=0, SD=1) trait value. Such univariate analysis cannot distinguish between direct and indirect selection on a trait. Because body mass, tarsus and wing length are correlated, it would clearly be interesting to disentangle direct and indirect selection on these traits. We therefore also performed multivariate analyses and calculated selection gradients (β), which measure the partial change in mean of a trait, that is the change in mean when statistically controlling for the effect of selection on other traits. β is estimated from the model w = a + β1 x 1 + β2 x 2. Selection coefficients were obtained from general linear mixed models (PROC MIXED in SAS 8.2) with Year and Sex as fixed effects and box of rearing (nested within year) as random effect. Because the dependent variable (survival) in these analyses is binary, the P values obtained from general linear models are not reliable. To assess the statistical significance of the selection coefficients, and test for interactions between Sex and morphological traits, we therefore used generalized linear mixed models (binomial error structure, logit link function, and Satterthwaite approximation) with Year and Sex as fixed effects, the morphological trait(s) under consideration as covariates, and box of rearing (nested within year) as random effect. Analyses were performed with the GLIMMIX macro in SAS 8.2 (Littell et al. 1996). The data set for these analyses included, besides nestlings from the brood size manipulation experiment, also nestlings from un-manipulated broods. Nestlings from nest boxes that showed signs of predation between day 14 and fledging were excluded from the selection analyses (three in 2000). In 2000, the data set consisted of 316 nestlings from enlarged broods, 168 nestlings from reduced broods, and 231 nestlings from control broods, and in 2001 of 298 nestlings from enlarged broods, 170 nestlings from reduced broods, and 21 nestlings from control broods.
Sex identification
Blood was collected in SET buffer and samples were stored at −20°C until analysis. DNA was extracted by the proteinase-K/phenol chloroform method following standard procedures (Sambrook et al. 1989). Sex was determined by PCR amplification of the CHD1-W and CHD1-Z genes using the primers P2 and P8 (Griffiths et al. 1998). The PCR products were separated by electrophoresis through 2% agarose gels containing ethidium bromide. Gels were scanned with a FluorImager (Molecular Dynamics, Sunnyvale, CA, USA).
For those birds that were recruited into the breeding population, there was a perfect match between sex as determined by molecular methods and sex determined when catching the bird during the breeding season (when sex can be determined unequivocally by the presence/absence of a brood patch).
Results
Morphology
Analyses with data from both years pooled showed that tarsus length differed between years (2000: 19.39±0.08 mm, 2001: 19.07±0.08 mm, F 1,134=9.03, P=0.0032). Body mass tended to differ in the same way (2000: 11.23±0.13 g, 2001: 10.91±0.13 g, F 1,49.7=3.01, P=0.089), while there was no difference in wing length between years (2000: 42.09±0.46 mm, 2001: 41.48±0.44 mm, F 1,49.2=0.94, P=0.34). There were also interactions between Year, Experiment and/or Sex for all traits (tarsus: Sex × Experiment × Year, F 1,840=4.49, P=0.034, Experiment × Year, F 1,66.5=4.93, P=0.030; body mass: Experiment × Year, F 1,49.1=4.01, P=0.051, Sex × Year, F 1,856=5.11, P=0.024, Experiment × Sex × Year, F 1,853=5.44, P=0.020; wing: Experiment × Year, F 1,46.9=6.81, P=0.012, Experiment × Sex × Year, F 1,869=3.59, P=0.058). We therefore analysed the 2 years separately.
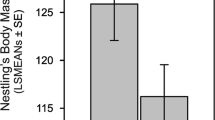
The analyses of each year separately are presented in Fig. 1 and Table 1. There were significant Pair by Experiment interactions for all traits, indicating that the effect of the brood size manipulation differed between nest box pairs, but the other interactions involving random effects were not significant in any case. Tarsus length and body mass were sexually dimorphic and strongly affected by the experimental treatment in both years. Wing length was dimorphic and affected by the experiment in 2001. Most importantly, in 2001, there were Experiment by Sex interactions for body mass and wing length (and marginally significantly for tarsus length), such that females were more affected by poor environmental conditions than males.
Tarsus length, body mass and wing length on day 14 after hatching (LS means ± SE from models in Table 1) of male and female blue tit nestlings in enlarged and reduced broods. Sample sizes are given as number of nestlings in enlarged broods + number of nestlings in reduced broods for each sex. ns not significant, †P<0.10, *P<0.05, **P<0.01, ***P<0.001. See Table 1 for details of statistical analyses
Nestling mortality
The mean fledging rate per nest box differed between years (2000: 0.929±0.024, N=47; 2001: 0.838±0.022, N=54; F 1,98=8.35, P=0.0038) and experimental treatments [enlarged: 0.810±0.023, N=51; reduced: 0.958±0.023, N=50; F 1,98=20.8, P<0.0001; non-significant (P=0.15) interaction removed].
Fledgling sex ratio
If the sexes differ in sensitivity to environmental conditions, one could expect that the experimental treatment should affect the sex ratio at fledging. Furthermore, given the apparent difference in breeding conditions between the two study years as revealed by the difference in fledging rate and morphology, one could also expect that the fledgling sex ratio should differ between the 2 years. However, there was no difference in sex ratio (proportion of males) at fledging between experimental treatments (enlarged: 0.486±0.026, N=51; reduced: 0.498±0.026, N=50; F 1,98=0.22, P=0.64), and no difference between years [2000: 0.513±0.027, N=47; 2001: 0.471±0.025, N=54; F 1,98=1.68, P=0.19; non-significant interaction (P=0.14) removed].
Post-fledging survival
Selection coefficients are given in Table 2. The univariate analyses (selection differentials; i) showed significant positive directional selection on body mass and wing length in females, and on body mass, tarsus and wing length in males. In the univariate analyses with both sexes combined, there was significant selection on all three traits. The multivariate analysis (selection gradients; β) with both sexes combined revealed that both body mass and wing length were direct targets of selection, while tarsus length was subject to indirect selection. We found no indication of an interaction between Sex and any of the morphological traits (P≥0.68), thus the strength of selection did not differ significantly between males and females. There was no evidence of non-linear or correlational selection (not shown).
Discussion
We found that poor environmental conditions, as a result of experimentally enlarged brood size, affected the body size of females more than males, although only in one out of 2 years. As judged from overall nestling size and survival, this year was a relatively unfavourable year for blue tit reproduction. The fact that females suffered more than males is most easily explained by that they, being the smaller sex, have a disadvantage in competition for food delivered by the parents. We found, however, no difference in fledgling sex ratio between enlarged and reduced broods. Thus, there was no evidence that the competitive disadvantage of females increased their pre-fledging mortality. Note, however, that the statistical power of this test might have been low because of variation among broods in hatchling sex ratio.
The selection analyses showed that all three morphological traits were positively related to post-fledging survival (although tarsus length only indirectly so). Hence, the smaller body size attained by birds in enlarged broods reduced their survival prospects. The strength of selection was similar in males and females, and the analyses with both sexes combined showed no interaction between sex and fledgling morphology. Hence, there was no evidence of sex-specific selection on fledgling body size. Similar results were obtained in a study on pied flycatchers Ficedula hypoleuca (Potti et al. 2002). However, a sex difference in the relationship between fledgling body size and post-fledging mortality has been found in two other species. In a brood size manipulation experiment with captive zebra finches Taeniopygia guttata, de Kogel (1997) found a stronger positive correlation between fledgling body size and post-fledging survival in females than in males. Similarly, Merilä et al. (1997) found positive directional selection on fledgling body size in female collared flycatchers Ficedula albicollis, but negative in males. Clearly, more studies are needed before it is possible to discern any general patterns of sex-specific selection on body size.
The sex difference in sensitivity of growth to environmental conditions that we observed in this blue tit population has several potential consequences: (1) In most species, SSD is reduced under poor environmental conditions, because the larger sex (usually males) is more sensitive (Badyaev 2002). In contrast, the greater environmental sensitivity of females in the blue tit will result in SSD in this species being reinforced by poor environmental conditions. (2) Because female body size was more affected by brood enlargement than male body size, and body size at fledging predicted post-fledging survival, poor environmental conditions could result in a male-biased sex ratio in the breeding population. (3) In species with SSD, females have been shown to bias the order of sons and daughters within the laying sequence, and hatch the eggs asynchronously, to mitigate the competitive disadvantage of the smaller sex (Bortolotti 1986; Badyaev et al. 2003). Thus, female blue tits can be expected to produce female eggs in the beginning of the laying sequence and male eggs towards the end, especially during poor environmental conditions.
Our finding—that the smaller sex is most sensitive to poor environmental conditions—is in accordance with the results of Oddie (2000). Oddie manipulated the degree of hatching asynchrony in great tit Parus major broods, and found that late-hatched females (the smaller sex) suffered more, in terms of growth, than late-hatched males. Similarly, in a study of Tengmalm’s owls Aegolius funerues (where males are the smaller sex), Hipkiss et al. (2002) found that supplementary feeding enhanced the survival of male nestlings, but not female nestlings. Still, most studies of sex differences in environmental sensitivity of altricial birds have found that the larger sex is more sensitive (see Table 3). The different results across species raise the question, what determines whether the smaller or larger sex suffers the most under poor conditions?
In an attempt to answer this question, we gathered data on some potentially important factors, viz., degree of SSD [(body mass of larger sex − smaller sex)/smaller sex], brood size, length of nestling period, and hatching asynchrony (expressed as hatching spread/nestling period) for the species in Table 3. We then coded the relative sensitivity of the sexes (smaller sex most sensitive=1; no difference in sensitivity=2; larger sex most sensitive=3), and calculated Spearman rank correlations between relative sensitivity and the four factors above. Using each study as an independent data point, there was a negative correlation between sensitivity and brood size (r s =−0.72, n=14, P=0.0034). In other words, the larger sex is most sensitive in species with small broods, whereas the smaller sex is most sensitive in species with large broods. There was also a positive correlation between sensitivity and SSD (r s =0.54, n=14, P=0.046). Hence, the smaller sex is most sensitive in species with slight SSD, whereas the larger sex is most sensitive in species with large SSD. In contrast, neither the degree of hatching asynchrony nor the nestling period predicted whether the larger or smaller sex was most sensitive to poor conditions (P>0.10 in both cases). However, there are two problems with these analyses. First, brood size and SSD are themselves correlated, which makes it difficult to disentangle the relative importance of these two factors. Second, the emerging patterns are clearly confounded by phylogeny; for example, three of the species showing greater sensitivity of the larger sex are from the family Icteridae. To control for possible phylogenetic artefacts, we calculated the mean sensitivity, brood size and SSD within each family. When using these family means as data points, only the correlation between sensitivity and brood size remained significant (brood size: r s =−0.86, n=8, P=0.0067; SSD: r s =0.64, n=8, P=0.088). We thus tentatively conclude that the currently available data suggests that brood size plays a role as determinant of whether the larger or smaller sex suffers most under adverse conditions.
How can brood size affect the relative sensitivity of the sexes? A potential explanation is that a large brood size reinforces the dominance advantage of the larger sex. This occurs even though parents try to win the parent–offspring conflict over brood size by distributing food as equal as possible among their young ones (Stamps et al. 1985; Nilsson and Gårdmark 2001), because the larger the brood size, the less control do the parents have over how food is distributed (Nilsson and Gårdmark 2001). Thus, a nestling’s position in the dominance hierarchy will have a greater impact on the relative amount of food it gains in large as compared to small broods. At a certain brood size, the competitive advantage of being the larger sex will offset the disadvantage of increased food requirements. This could result in increased sensitivity of the smaller sex in species with large broods, and decreased sensitivity of the smaller sex in species with small broods.
Besides the effect of brood size, the great tit studies also suggest that the relative sensitivity of the sexes depends on the type of environmental stress. While Oddie (2000) found that female nestlings suffered most in broods with experimentally increased hatching asynchrony, Tschirren et al. (2003) found that males suffered most from hen flea parasitism. Furthermore, in the zebra finch, which does not show SSD, females are more sensitive (de Kogel 1997), even in the absence of competition (Martins 2004), indicating an intrinsic sex difference in environmental sensitivity. Clearly, more studies of what determines the relative sensitivity of males and females are needed.
References
Anderson DJ, Budde C, Apanius V, Gomez JEM, Bird DM, Weathers WW (1993) Prey size influences female competitive dominance in estling American kestrels (Falco sparverius). Ecology 74:367–376
Badyaev AV (2002) Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol 17:369–378
Badyaev AV, Hill GE, Beck ML (2003) Interaction between maternal effects: onset of incubation and offspring sex in two populations of a passerine bird. Oecologia 135:386–390
Bensch S (1999) Sex allocation in relation to parental quality. In: Adams N, Slotow R (eds) Proceedings of the 22nd Int. Ornithol. Congr., Durban BirdLife South Africa, Johannesburg, pp 451–466
Blank JL, Nolan V (1983) Offspring sex-ratio in red-winged blackbirds is dependent on maternal age. Proc Natl Acad Sci USA 80:6141–6145
Blondel J, Perret P, Anstett MC, Thebaud C (2002) Evolution of sexual size dimorphism in birds: test of hypotheses using blue tits in contrasted Mediterranean habitats. J Evol Biol 15:440–450
Bortolotti GR (1986) Influence of sibling competition on nestling sex-ratios of sexually dimorphic birds. Am Nat 127:495–507
Brodie ED III, Moore AJ, Janzen FJ (1995) Visualizing and quantifying natural selection. Trends Ecol Evol 10:313–318
Clutton-Brock TH, Albon SD, Guinness FE (1985) Parental investment and sex differences in juvenile mortality in birds and mammals. Nature 313:131–133
Dhondt AA (1970) The sex ratio of nestling great tits. Bird Study 17:282–286
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hipkiss T, Hornfeldt B, Eklund U, Berlin S (2002) Year-dependent sex-biased mortality in supplementary-fed Tengmalm’s owl nestlings. J Anim Ecol 71:693–699
Howe HF (1977) Sex-ratio adjustment in common grackle. Science 198:744–746
de Kogel CH (1997) Long-term effects of brood size manipulation on morphological development and sex-specific mortality of offspring. J Anim Ecol 66:167–178
Littell R, Milliken G, Stroup W, Wolfinger R (1996) SAS system for mixed models. SAS Institute Inc, Cary
Martins TLF (2004) Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behav Ecol 15:174–180
Merilä J (1996) Genetic variation in offspring condition: an experiment. Funct Ecol 10:465–474
Merilä J, Sheldon BC, Ellegren H (1997) Antagonistic natural selection revealed by molecular sex identification of nestling collared flycatchers. Mol Ecol 6:1167–1175
Nager RG, Monaghan P, Houston DC, Genovart M (2000) Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus). Behav Ecol Sociobiol 48:452–457
Newton I, Marquiss M (1979) Sex-ratio among nestlings of the european sparrowhawk. Am Nat 113:309–315
Nilsson J-Å, Gårdmark A (2001) Sibling competition affects individual growth strategies in marsh tit, Parus palustris, nestlings. Anim Behav 61:357–365
Oddie KR (2000) Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912
Potti J, Davila JA, Tella JL, Frias O, Villar S (2002) Gender and viability selection on morphology in fledgling pied flycatchers. Mol Ecol 11:1317–1326
Richner H (1992) The effect of extra food on fitness in breeding carrion crows. Ecology 73:330–335
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a laboratory manual. Cold Spring Harbour Laboratory Press, Cold Spring Harbour, NY
SAS Institute (1999) SAS OnlineDoc, V8. SAS Institute, Cary
Sayce JR, Hunt GL (1987) Sex-ratios of prefledging western gulls. Auk 104:33–37
Sheldon BC, Merilä J, Lindgren G, Ellegren H (1998) Gender and environmental sensitivity in nestling collared flycatchers. Ecology 79:1939–1948
Slagsvold T, Røskaft E, Engen S (1986) Sex-ratio, differential cost of rearing young, and differential mortality between the sexes during the period of parental care—fisher theory applied to birds. Ornis Scand 17:117–125
Smith HG, Källander H, Nilsson J-Å (1989) The trade-off between offspring number and quality in the great tit Parus major. J Anim Ecol 58:383–401
Stamps J, Clark A, Arrowood P, Kus B (1985) Parent–offspring conflict in budgerigars. Behaviour 94:1–40
Teather KL, Weatherhead PJ (1989) Sex-specific mortality in nestling great-tailed grackles. Ecology 70:1485–1493
Torres R, Drummond H (1997) Female-biased mortality in nestlings of a bird with size dimorphism. J Anim Ecol 66:859–865
Trivers RL, Willard DE (1973) Natural-selection of parental ability to vary sex-ratio of offspring. Science 179:90–92
Tschirren B, Fitze PS, Richner H (2003) Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J Anim Ecol 72:839–845
Acknowledgements
We thank Bengt Hansson and Henrik Smith for statistical advice, and Katrin Böhning-Gaese and anonymous reviewers for constructive comments on the manuscript. The study was financially supported by the Royal Swedish Academy of Science (to LR), Lunds Djurskyddsfond (to LR & MS), and the Swedish research council (to JÅN). The study was approved by the ethical committee for animal research, Malmö/Lund, Sweden (#126–00).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katrin Böhning-Gaese
Rights and permissions
About this article
Cite this article
Råberg, L., Stjernman, M. & Nilsson, JÅ. Sex and environmental sensitivity in blue tit nestlings. Oecologia 145, 496–503 (2005). https://doi.org/10.1007/s00442-005-0133-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0133-1