Abstract
One of the most important agricultural areas in Iran is Golestan Province. In this study, the suitability of groundwater for irrigation purposes was assessed. To determine hydro-geochemical classification of the station and assessment of spatial modeling of the study area, 12 groundwater stations were selected and an average of the last 5 years of sampling (2011–2015) has been considered. Suitability of groundwater for irrigation was assessed based on Sodium Adsorption Ratio (SAR), Residual Sodium Carbonate (RSC), Na%, Permeability Index (PI), Magnesium Adsorption Ratio (MAR), Kelley’s Ratio (KR), Corrosion Ratio (CR), Residual Sodium Carbonate (RSC), Potential Soil Salinity (PSS), Chloro-Alkaline Indices (CAI), Meteoric genesis (Met. gen) and Wilcox classification. The abundance of major ions followed a HCO3 > SO4 > Na > Ca > Mg > Cl > K trend. CAI results indicate reverse cation exchange for this area, and Wilcox classification indicates that only Kalou, Kafshgari, and Kia stations are appropriate for irrigation. The spatial distribution of groundwater quality demonstrates a decreasing trend from SE to NW based on CAI, Cl, CR, EC, TSS, and TDS and shows reverse trends for KR, MAR, Met. gen, Mg/Ca, Na%, pH, PI, and RSC. In addition, the results of hydro-geochemical classification of irrigation parameters have categorized the irrigation water of this area into three different classes. Based on EC, MAR, Mg/Ca, Cl and CR, this area is suitable, but based on PSS, KR, and TH, all stations are unsuitable and according to SAR, TDS, Na% and PI, this area’s groundwater is moderate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh water is confined, but demand is increasing every day (Ravikumar et al. 2011). Pressure on every natural resource has been increased by enhancing the human population to produce enough food and raw materials for demand (Smil 1999). Groundwater is vital for human life, and in Asia alone approximately one billion people are directly related to this resource (Foster 1995). Groundwater is the major source of water for domestic, agricultural, and industrial purposes in many countries (Ramesh and Elango 2012). Groundwater is an important part of the total water resource and plays a significant role in supplying drinking, agricultural and industrial water (Edmunds 2003).
Although the amount and quality of water existing for irrigation is changeable from place to place, many groundwater exploitation schemes have been designed without due consideration to quality issues (Ravikumar et al. 2011). The quality of water irrigation can affect the soil fertility and productivity (Haritash et al. 2008). Safe and adequate crops are dependent on the availability and quality of water for irrigation (Sharifi and Safari Sinegani 2012). Irrigation with inferior water may bring problems. For instance, based on the FAO guideline, the salinity of water for irrigation may cause permeability possibilities (Rasouli et al. 2012).
Agriculture is one of the most important parts of the economic development of Iran. Figure 1 shows gross domestic product (GDP) of Iran during the recent years. Based on trading economic reports, the proportion of agriculture in the GDP of Iran is between 10 and 20% and it shows the significant role of agriculture for Iran. As groundwater is one of the main sources for irrigation in order to supply the required water for agriculture, quality of irrigation water has been considered for this study.
There are many cases in groundwater that have been affected by human activity such as uncontrolled fertilizer like nitrate and phosphate contamination in Iran (Sharifi and Safari Sinegani 2012). Water scarcity and salinity are major problems in the reduction of crop production in the arid and semiarid regions of Iran (Kiani and Abbasi 2009). Golestan Province is the third largest cereal producer in Iran. Increasing scarcity of freshwater resources is driving countries toward using marginal quality water for irrigation purposes (Srinivasan and Reddy 2009).
Water quality issues and its management need more attention in developing countries. Realizing the hydrochemical properties and quality of water is becoming critical for groundwater management (Alaya et al. 2014). Hydrochemical properties of groundwater depend on some parameters such as geology, chemical weathering rocks, quality of recharge water, and water– rock interaction (Trabelsi et al. 2012).
Many studies around the world have been presented on groundwater quality regarding drinking and irrigation purposes such as Durvey et al. (1991), Majumdar and Gupta (2000), Sujatha and Reddy (2003), Pulle et al. (2005), Rao (2006), Kumar et al. (2007), Haritash et al. (2008), Ravikumar et al. (2011), Sharifi and Safari Sinegani (2012), Ağca (2014), Jassas and Merkel (2015).
In order to achieve desirable water for irrigation and subsequently proper crop yield, monitoring and assessment of quality of water is necessary. Hence, the objectives of the present study are to assess the groundwater quality and to evaluate its suitability for irrigation purposes in Golestan Province, north of Iran. In addition, in order to assess the impact of the water discharge on soil in agriculture, especially the effect of irrigation water on crops which is cultivate in farmlands of the study area such as wheat, sugar beet, barley, rice, potato, cotton, and rapeseed, it is important to figure out the hydrogeochemistry of the chemical–soil–groundwater interactions. In order to assess the water quality, 12 stations in Golestan Province have been selected. The results presented in this study are based on physicochemical water quality parameters determined in the Qareh Sou basin according to results of Regional Water Organization of Golestan Province.
Materials and methods
Study area
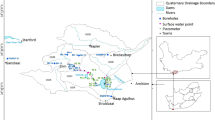
This study focuses on the southern to western part of Golestan Province, from Ziarat jungle to Qareh Sou. Golestan Province covers an area of 20,893 km2 (Semnani et al. 2010). This area is located in the northern mountainside of the Alborz Mountains and is regarded as having the most important nutrients of Golestan Province. Golestan Province also has the third largest cereal yield among the 31 provinces of Iran. The majority of the male population are farmers. The total area of arable soils in this region is about 5600 km2 (Keshavarzi et al. 2012). Golestan Province contain several land uses such as forest, industrial, agricultural, uncultivated lands and urban. Industrial, Agricultural, and urban zones are located mostly in the central and western parts of Golestan Province (Mirzaei et al. 2014). This study has been focused on central and western parts of province where agricultural activities are most important activities. By the way, recently some studies have been done about land use and land use change in some part of Golestan Province. For example, Ayoubi et al. (2011) studied the land use of Gorgan city and around the Gorgan which contain most of important part of this study (central part). According to soil classification, they divided the land use of this area into 4 group: Natural Forest, Cultivated land, Reforested (Olea), Reforested (Cupressus). Moreover, based on available data on southern and southwestern part of study area, in terms of land use, the portion of dry farming is 23%, irrigated farming 1%, horticulture 2%, dead forest 5% and about 69% is the forest.
Presence of dense forest and grasslands in the Golestan Province caused the rock do not have enough outcrops. The southern part of the province is located in Alborz tectonic units which known as Gorgan–Rasht units. The oldest rocks in this area related to Precambrian era. According to the geological map, central and western part of Golestan Province, especially around the sampling station, consist of regional metamorphic rocks, sandstones, argillic limestone, red sandstone with shale, clay, swamp and alluvial and conglomerate. The geological map of the study area is shown in Fig. 2. Moreover, geological formation around the Omidvar, Bandar-e-Gaz and Damdari consists mainly of young alluvium, young terraces, and gravel fans. In term of geology, around Bagho, Frozanfar and Kalou consists mainly of alternating dark gray to greenish shale and sandstone, thick bedded sandstone with plant remains and coal seams, thin to thick bedded fossiliferous limestone with gray shale, thick bedded and unconsolidated sandstone and conglomerate, clay and silt. Around Ziarat, Alang, Sarkalateh and Kurdkoy, the geological formation consists of two stratigraphic units, the Precambrian and Mesozoic sediments, and plays major roles in its lithology. The Precambrian sediments consist mainly of dark green metamorphic schist (mica schist, chlorite schist, quartzite, marble and slate) and the bright green Gorgan green schist. The Mesozoic sediments are mostly limestone and dolostone with layers of marl in the upper Jurassic. In some places, there are loose sandy Quaternary sediments.
Some of the hydrological data, such as flow velocity, hydraulic radius and bed slope of the major river in the study area based on Dehghani et al. (2014), are presented in Table 1. In terms of permeability of the study area, most part of this area can be consider as class C (infiltration rate between 12.5 and 25 mm/h) and several part in class B (infiltration rate between 2.5 and 12.5 mm/h) of hydrology of soil.
Sampling and analysis
In order to study the physiochemical parameters of groundwater quality, 12 stations for which the hydrometric characterization is shown in Table 2 in the study area have been selected. As shown in Table 2, name, type and coordination of selected station have been shown. In addition, the location of sampling point and study area is illustrated in Fig. 3. To examine the hydro-geochemical classification, physiochemical parameters were analyzed. TDS, pH and EC were measured by a water checker portable hatch model HQ40D53000000. The bicarbonate (HCO3 −) had been measured by the Alkalinity measurements method. K+, Na+, Cl−, Mg2+, Ca2+, and SO4 2− were measured by furnace 4100 atomic absorption using standard methods (APHA 1998). To determine hydro-geochemical classification of the station and assessment of spatial modeling of the study area, an average of the last 5 years of sampling (2011–2015) have been considered.
Hydro-geochemical indices
In order to determine the suitability of groundwater for irrigation, understanding the quality is necessary (Kumar et al. 2007). To assess water quality for irrigation most popular criteria like Sodium Adsorption Ratio (SAR), Residual Sodium Carbonate (RSC), Na%, Permeability Index (PI), Magnesium Adsorption Ratio (MAR), Kelley’s Ratio (KR), Corrosion Ratio (CR), Residual Sodium Carbonate (RSC), Potential Soil Salinity (PSS), Chloro-Alkaline Indices (CAI), and Meteoric genesis (Met. gen) have been considered. For current irrigation water quality assessment, the following indices were calculated.
Chloro-Alkaline Indices (CAI), which was calculated using Eq. 1, present an indication that shows possible ion exchange reactions between ions in the groundwater and their host rock (Sastri 1994).
Corrosion Ratio is defined as alkaline earth and alkaline and calculated using Eq. 2 (Tripathi et al. 2012).
The Kelly’s ratio calculated using Eq. 3 (Ravikumar et al. 2011) which described as:
The Magnesium Adsorption Ratio (MAR) was suggested by Raghunath (1987) and calculated using Eq. 4.
Meteoric genesis (Met. gen) is one of the chemical relations, which has been suggested by Abdel Moneim (1988) in order to study the source of meteoric groundwater and calculated using Eq. 5.
In other word, to study the sodium hazard, the percent of sodium (Na%) was calculated by Wilcox (1948) and defined as Eq. 6.
Doneen (1964) suggested Permeability Index (PI) for classification of irrigation water and defined as Eq. 7.
The potential of soil salinity of the water is suggested by Doneen (1962). PSS is calculated using Eq. 8.
Residual alkalinity of water is presented by Residual Sodium Carbonate (RSC) which is calculated by using Eq. 9 (Richards 1954).
The Sodium Adsorption Ratio (SAR) is an index which expresses the alkali hazard and is calculated by Eq. 10 (Richards 1954).
Results and discussion
Table 2 presents a statistical summary of the physicochemical parameters analyzed and popular criteria of irrigation water for this study. EC of groundwater of this area ranged from 1362.8 to 1998.7 µS/cm with an average value of 1449.4 µS/cm. This high average value of EC may be related to effect of compounds that dissolve into water from dissolved salts and inorganic materials. Also, results of flooding and runoff can cause increase in the EC level.
One of the main sources of alkalinity is bicarbonate, and it shows the capacity of neutralization (Kumar et al. 2007). The bicarbonate of samples ranged from 344.54 to 361.73 ppm.
Naturally, since rocks which have potassium are almost non-susceptible to weathering, K occurs in low concentrations (Chapman 1996). Generally, the main natural sources of potassium in groundwater can be feldspars (orthoclase, microcline), clay minerals, and some mica. In this study, potassium ranged from 5.98 to 22.23 ppm. According to the geology of the study area, the possible source of potassium might be clay, alluvial and metamorphic rocks.
Sulfate concentrations in natural waters are usually (globally) between 2 and 80 ppm (Chapman 1996) and based on UNEP (1990), ranges from 0 to 230 mg/l in groundwater. In this study, sulfate ranged from 185.5 to 334.6 ppm, and this excess may be related to the industrial or sulfate minerals effect.
Based on the mean concentrations, the dominant major ions in groundwater are in the following order HCO3 > SO4 > Na > Ca > Mg > Cl > K. As shown, the dominant ion is bicarbonate and sulfate. Presence of bicarbonate probably related to geology of the study area but for sulfate may be come from geology (gypsum) or industrial pollution. Also based on this order, the water types of groundwater may be HCO3–SO4–Na.
Hydro-geochemical classification
In order to carry out a hydro-geochemical classification, some popular criteria of irrigation water are calculated and each station has been classified based on the limits of indices.
If cation exchange occurs between Ca and Mg in the host rock and K and Na in water, CAI is positive. However, if CAI is negative, it shows a reverse cation exchange (Schoeller 1965; Kurdi et al. 2014). Reverse ion exchange can be considered as an important process controlling of groundwater chemistry especially in the arid area. Reveres ion exchange could lead to increasing trend of salinity and depletion of Na (Zaidi et al. 2015). These parameters can effect on the quality of irrigation water, and also the changing of Na could change the soil texture and permeability. In addition, increasing the salinity has many problems for farming soil and crops and many of crops are sensitive about the salinity of irrigation water.
Classification of these stations is shown in Fig. 4a. As shown, all CAI in the station are negative which have been shown in violet, so reverse cation exchange has occurred (Table 3).
In this study, Cl ranged from 20.1 to 169.3 ppm with an average value of 33.9 ppm. The chlorinity index of the groundwater has been calculated by the chloride concentration. Chloride ion up to 70 ppm is acceptable and suitable, but if it exceeds 350 ppm, it can be harmful for crops (Hopkins et al. 2007). The chlorinity has relationship with salinity, and study of this index could be useful guidance for considering the salinity tolerance of crops. For example, in this study, as mentioned, most important crops are wheat, sugar beet, barley, rice, potato, cotton, and rapeseed which are moderately tolerant, tolerant, moderately tolerant, sensitive, moderately sensitive, tolerant and tolerant regard salinity. As shown in Fig. 4b, all stations except Ziarat are in a suitable situation. Ziarat spring is vulnerable, but it is not harmful. The chloride is an anion which found and occur naturally in water but increasing in chloride could be result of anthropogenic source or geological formation. In the Ziarat station, most important anthropogenic factor which effect on Cl may be related to fertilizer. As animal waste consists high concentrations of Cl and even small concentrations of livestock can make a local problem for groundwater (Kelly et al. 2012), another probably source of Cl in the Ziarat can be animal waste because near the Ziarat spring rural people with livestock are living.
If CR < 1, water is considered safe for transporting by any type of pipes, but CR > 1 indicates corrosive water and it is not safe for metal pipes (Raman 1983). Classification of the study stations based on CR is shown in Fig. 4c. As shown, all stations except Ziarat are safe and non-corrosive, but Ziarat spring is corrosive. As regards chloride is a corrosive agent and also chloride ion concentration in Ziarat station is high, so the probable reason for being corrosivity of Ziarat is Cl ion.
The salinity hazard is one of the most important parameters that can be harmful for crop fertility. EC can be considered as the salinity hazard (Johnson and Zhang 1990). Based on Ayers and Westcott (1985), groundwater can be classified as “excellent” if EC values are less than 700 µS/cm and “good” if the EC ranges between 700 and 3000 µS/cm. If EC exceeds 3000 µS/cm, it will be harmful for crop productivity. As Fig. 4d indicates, all samples of this study can be termed as good which have been shown in dark yellow in the Figure.
Kelley (1963) suggested that KR for irrigation water should not exceed one (KR < 1). The Kelly’s ratio of more than one demonstrates an abundance of sodium in groundwater (Ravikumar et al. 2011). Also, based on the equation, depletion of Ca or Mg can lead to an excess level of Kelly’s index. Hence, waters with a Kelly’s index more than 1 are unsuitable. Figure 4e shows that KR in all the samples is greater than one which has been shown in red. This suggests that the groundwater of this area based on the Kelly index is unfit for irrigation. In this study, with regard to the presence of limestone in geology of this area, the presence of sodium more than calcium and magnesium probably effected on Kelly ratio in which this increasing of sodium may be related to overuse of Na-fertilizers in farm lands.
The Magnesium Adsorption Ratio (MAR) was suggested by Raghunath (1987). High magnesium adsorption (MAR > 50%) has a harmful result for crops, especially in alkaline soil. According to Alperovitch et al. (1981), magnesium has a specific effect on clay dispersion and loss of hydraulic conductivity of non-calcareous soils. Results of classification of this area based on MAR show that station samples are lower than 50% and are suitable for irrigation which have been shown in green. The results of classification based on MAR are shown in Fig. 4f. In addition, the Kelly ratio and MAR suggest that in the study area magnesium concentration is normal.
If Meteoric genesis <1, the water is related to deep meteoric water percolation but Met. gen >1 due to shallow meteoric water percolation type. As, most of groundwater are meteoric, the Meteoric genesis index demonstrates that groundwater sources belong to a shallow meteoric water percolation type or deep meteoric water percolation which can be useful for determining the source of ion whether related to surface pollution or deep geological setting of stations. In addition, Met. gen can be indicative about the weathering zone. Figure 5a demonstrates that Met. gen value in all stations is below one. Hence, the water type of this area is related to deep meteoric water percolation. The percolation has relationship with porosity and can influence to unstable mineral and dissolution process (Kleinspehn and Paola (2012).
Kumar et al. (2007) suggested that we can use Mg/Ca ratio in order to classify irrigation water. Based on this classification, if Mg/Ca > 3, the water is unsuitable, Mg/Ca < 1.5, the water is suitable, and if it ranges from 1.5 to 3 the water will be moderate. As most part of the study area have been composed limestone, evaluating the Mg/Ca ration can be important. In addition, according to Yadav and Girdhar (1981), the crop (e.g. wheat) yield will decline with increasing the Mg/Ca ratio. As shown in Fig. 5b, all the samples are suitable for irrigation in this area.
In order to assess the sodium available of soil, Sodium percentage (Na%) has been calculated. While the concentration of Na in water is high, sodium ions tend to be absorbed by clay and displacing Ca and M and the permeability of soil will decrease (Saleh et al. 1999). The classification of this index is the following order: 0–20 is excellent, 20–40 is good, 40–80 is fair, and 80–100 is poor for irrigation. Based on this classification, all samples fall in the fair category as shown in Fig. 5c. It means the water is useable for irrigation, but using this water may reduce the permeability of soil during the time and it needs careful monitoring.
In the stations, the pH of samples ranged from 6.87 to 7.43 with an average value of 7.35. The groundwater of most of the sampling stations is slightly alkaline in nature. The pH value of all stations was alkaline except for Ziarat spring that may be related to this spring’s activity which can be result of leaching of acidic minerals (sulfuric or hydrochloric) by water. Because these minerals tend to be soluble in groundwater, the pH of this station may be result of dilution of these minerals.
Doneen (1964) suggested that the Permeability Index (PI) for classification of irrigation water considering long-time irrigation is affected by Mg, Ca, Na, and HCO3 of the soil. Classification based on PI is categorized as suitable (0–25), moderate (25–75) and harmful (75–100). According to Fig. 5e, all stations of this study area have fallen in the moderate group. Based on the result of this index, water of all station can be useful for irrigation, but it needs more monitoring about the future effect of using water on farmlands.
The potential of soil salinity of the water index classifies irrigation water into three groups: suitable (<5), moderate (5–15) and unsuitable (>15). As Fig. 5f shows, the water of this area is unsuitable for irrigation based on the PSS index. The most important reason for highly potential of soil salinity can be due to chlorine or sulfate. PSS value of water in the study area has been affected by increasing the Cl concentration (due to anthropogenic or geological formation) or Increasing the concentration of sulfate (due to industrial or geological source), change the quality of water to unsuitable for irrigation.
The RSC impact on the suitability of irrigation water because it causes dissolution of organic matter in the soil and will be harmful to the physical properties of soils (Kumar et al. 2007). Based on the classification, if RSC > 2.5, the water is unsuitable, RSC < 1.25, the water is suitable, and if it ranges 1.25–2.5, the water will be moderate. Figure 6a indicates that all samples of the study area fell in the unsuitable category.
Sodium concentrations of groundwater in this study varied from 158.03 to 213.67 ppm, with a mean value of 170.26 ppm. SAR categorizes irrigation water into four groups (Todd 1980): excellent (<10), good (10–18), fair (18–26) and poor (>26). Figure 6b examines all samples of the study area as fair and vulnerable. Same as Na% and PI results, it needs more monitoring about the future effect of using water on farmlands.
Salts in a water sample can be measured by the total dissolved solids (TDS) in order to assess the salinity hazard. Salinity hazard related to the dissolved salts in the irrigation water and by accumulate salts especially in the root zone will prevent growth of most crops. Irrigation water is classified by its salt hazard into five groups: very low hazard (0–160), low hazard (160–480), medium hazard (480–1280), medium–high hazard (1280–1920) and high hazard (>1920) (Hopkins et al. 2007). Based on this classification, as shown in Fig. 6c, in the study area all stations have fallen into the moderate group which means, as some indices presented, the water is useable, but it needs more careful attention and monitoring about the results of farming with this water. The sources of dissolved solids may be related to the natural sources as minerals in soils and anthropogenic results.
The hardness of water depends largely on the presence of calcium and magnesium. The overall quantity of these salts is called total hardness (TH) (Chapman 1996). In this study, TH varied from 356.9 to 537.8 mg/l with an average of 387.4 mg/l. According to Sawyer and McCarty (1967), the classification for TH, total hardness, as CaCO3 (mg/l) can be divided into 4 classes: soft (0–75), moderately high (75–150), hard (150–300) and very hard (>300). The classification of groundwater of the study stations based on total hardness indicates that all of the samples fall in the very hard water class (Fig. 6d). As total hardness related to concentration of calcium and magnesium, the geological formation of the study area especially presence of limestone formation plays the most important roles for exceeding the permissible limit. However, total hardness is not considered as a decisive factor, and using water with high amount of hardness could be considered as a negative factor.
One of the most important groundwater classifications for irrigation purposes (for local crop which mentioned before) is suggested by Wilcox (1948), which is due to correlating Na% and EC. This classification divided irrigation water into six groups. The Wilcox diagram for the study is shown in Fig. 7. As shown, the Kalou, Kafshgari, and Kia station have fallen in good; Ziarat, Kurdkoy, Sarkalateh and Damdari have fallen in fair; Frozanfar and Bandar-e-Gaz have fallen in poor, and Alang station has fallen in unsuitable for irrigation. As this classification categorized groundwater samples based on the effect of interaction between the two parameters (Na% and EC), the results are different with classification based on Na%. For instance, the Na% of Ziarat, Kalou, Kia and Kafshgari almost are same but because of higher EC value of Ziarat, this station has been fallen in fair. Different classifications of stations may be related to effect of compounds that dissolve into water from dissolved salts or changing the EC level by flooding and runoff and also usage of Na-fertilizers in farm lands.
Plot of sodium percent-Electrical conductivity in study area (after Wilcox 1955)
Distribution model and spatial trend
One of the most significant aims of a groundwater quality assessment scheme is to obtain a general view of the spatial distribution of groundwater quality (Wilkinson and Edworthy 1981). In order to examine spatial distribution, contour maps of the irrigational quality index were created using the Surfer-11. Figures 8, 9 and 10 show spatial distribution in the study area. As shown, from the southeast to northwest of the study area (from Ziarat to Kia, Damdari and Bandar-e-Gaz station), irrigation water parameters examine slight variation. Some parameters such as CAI, Cl, CR, EC, PSS, and TDS were reduced from SE to NW by decreasing the altitude of stations. In addition, TH distribution shows the same trend, but in the Bagho station, the concentration of TH is high and shows almost a south to north trend. Irrigation water parameter values like KR, MAR, Met. gen, Mg/Ca, Na%, pH, PI, and RSC illustrate a reverse trend (from NW to SE). In these stations, by reducing the altitude, irrigation water parameter values were increased. Moreover, the spatial distribution of SAR was different from the other and did not pursue any of the trends.
The most important factors which effect on the variation of the water quality from SE to NW can be divided into three groups. First group is related to geological condition of sampling stations. From SE to NW, the rock formation changes from sandstone and limestone in the SE to alluvial, clay and bogs in the NW.
The second group may be related to the geographical condition of sampling stations like the altitude. As mentioned, along the SE to NW trend, most of index present different trend which may be related to level of groundwater of aquifer.
The third group can be related to land use of stations. Using different fertilities can make different pollution for groundwater. As mentioned before, using some fertilities can release some ion in groundwater like Ca, Mg and Na. In addition to agriculture, municipal and industrial activities can change the concentration of water and make it pollution.
Besides these reason, the type of sampling station should be consider as key point for this variation trend from SE to NW. As mentioned in Table 2, the station which is located in the SE (Ziarat and Frozanfar) is spring and this difference makes some especial trend for spatial distribution in the study area.
By the way, it should be consider that in this case of study regarding to the distribution of sampling location, we face to sparse data. Various methods exist to determine the spatial distribution for interpolation, but in order to achieve the spatial distribution in this study Kriging method has been used. Kriging has been conducted on a set of points, and Kriging mathematics can be used to project where the “next” sampling point or points should be to reduce uncertainty in the model. Although it is possible to have too many observations for Kriging, Kriging presents best results for sparse sample.
Summary
Agriculture in Golestan Province is one of the main occupations and sources of income for many families. The productivity of crops has a direct relation to the quality of irrigation water. Groundwater is one of the main sources for irrigation water. This study focused on the assessment of groundwater quality and evaluated the suitability of water for irrigation purposes. In order to achieve this goal, hydro-geochemical classification and spatial distribution modeling based on irrigation quality indices are used. The results of this study can be useful for the stakeholders involved in irrigation and experts who are interest in this field. The following main conclusions of this study demonstrated that:
-
The dominance of major ions in the groundwater in this study was in the following order HCO3 > SO4 > Na > Ca > Mg > Cl > K.
-
Chloro-Alkaline Indices classification showed that reverse cation exchange occurred in this area.
-
The classification-based chlorinity index indicated that all stations except Ziarat were suitable and Ziarat spring was vulnerable but it was not harmful.
-
The classification of groundwater based on corrosion ratio indicated that all stations except Ziarat were suitable and non-corrosive but Ziarat spring was corrosive.
-
Electrical conductivity classification as a salinity hazard agent showed that all samples of this area had good conditions but not excellent.
-
Categorization based on Kelley’s Ratio indicated that the groundwater of this area was not proper for irrigation.
-
The Magnesium Adsorption Ratio classification demonstrated that the groundwater of this area was suitable for irrigation even in alkaline soil.
-
Based on the Meteoric Genesis index, the source of water in this area may be related to deep meteoric water percolation.
-
Classification of groundwater of this area based on the Mg/Ca ratio indicated that this water is suitable for irrigation.
-
Classification of samples based on sodium percentage and the Permeability Index showed that all samples of the study fell in a fair category and long-time irrigation by this water may decrease the soil permeability.
-
Assessment of the potential of soil salinity of water in the study area demonstrated that the groundwater of all samples were not proper for irrigation.
-
All samples of the study fell in an unsuitable category based on the Residual Sodium Carbonate index.
-
Sodium Adsorption Ratio as an agent of sodium concentrations of groundwater showed that all stations of the study area were vulnerable.
-
Classification based on TDS and total hardness indicated the groundwater of all samples were moderate and very hard, respectively.
-
Based on the Wilcox classification, Kalou, Kafshgari, and Kia station fell in good; Ziarat, Kurdkoy, Sarkalateh and Damdari fell in fair; Frozanfar and Bandar-e-Gaz fell in poor, and Alang station fell in unsuitable for irrigation.
-
Spatial distribution of groundwater quality indicated a decreasing trend from SE to NW for CAI, Cl, CR, EC, TSS, and TDS and shows a reverse trend for KR, MAR, Met. gen, Mg/Ca, Na%, pH, PI, and RSC which may be related to geological condition, geographical condition, land use and type of sampling station.
In conclusion, based on the multiple classifications, indices and regional applicability of the groundwater for irrigation purposes, the quality of groundwater of the study area for some crops was useful, however, for some crops which are more sensitive based on the chemical composition, was fair and vulnerable. This variation may be related to different index because the selected indices focused on some especial ion concentration. Because of this reason, we have different results. Stakeholders based on the kind of crops and requirement irrigation water can use the results of this study. By the way, it should be considered that the quality of the irrigation water may affect crop yields and soil physical conditions and also different crops require different irrigation water qualities. Although the interpretation of water quality for irrigation purposes is crop-specific, using the results of calculated indices can help to make better decision.
References
Abdel Moneim AA (1988) Hydrogeology of the Nile basin in Sohag Province (Doctoral dissertation, M. Sc. Thesis, Geology Department, Faculty of Science, Assiut University, Assiut, Egypt)
Ağca N (2014) Spatial variability of groundwater quality and its suitability for drinking and irrigation in the Amik Plain (South Turkey). Environ Earth Sci 72(10):4115–4130
Alaya MB, Saidi S, Zemni T, Zargouni F (2014) Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ Earth Sci 71(8):3387–3421
Alperovitch N, Shainberg I, Keren R (1981) Specific effect of magnesium on the hydraulic conductivity of sodic soils. J Soil Sci 32(4):543–554
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Port City Press, Maryland
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO Irrigation and drainage paper 29 Rev. 1. Food and Agricultural Organization, Rome
Ayoubi S, Khormali F, Sahrawat KL, Rodrigues de Lima AC (2011) Assessing impacts of land use change on soil quality indicators in a loessial soil in Golestan Province, Iran. J Agric Sci Technol 13:727–742
Chapman DV (ed) (1996) Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring. E & Fn Spon, London, p 626
Dehghani AA, Haddadchi A, Omid MH, Movahedi N (2014) Applicability of MEP and SEMEP for computing total sediment load (case study: Chelichay Catchment in Golestan Province). KSCE J Civil Eng 18(6):1912–1919
Doneen LD (1964) Notes on water quality in agriculture. Published as a water sciences and engineering. Department of Water Sciences and Engineering, University of California, paper, 4001
Doneen LD (1962). The influence of crop and soil on percolating waters. In: Proceeding of 1961 biennial conference on groundwater recharge (from Raghunath 1987)
Durvey VS, Sharma LL, Saini VP, Sharma BK (1991) Handbook on the methodology of water quality assessment. Rajasthan agriculture university, Bikaner, p 156
Edmunds WM (2003) Renewable and non-renewable groundwater in semi-arid and arid regions. Dev Water Sci 50:265–280
Foster SSD (1995) Groundwater for development: an overview of quality constraints. Groundwater quality, 17th Special Report. Chapman and Hall, London, pp 1–3
Haritash AK, Kaushik CP, Kaushik A, Kansal A, Yadav AK (2008) Suitability assessment of groundwater for drinking, irrigation and industrial use in some North Indian villages. Environ Monit Assess 145(1–3):397–406
Hopkins BG, Horneck DA, Stevens RG, Ellsworth JW, Sullivan DM (2007) Managing irrigation water quality for crop production in the Pacific Northwest. Oregon State University Extension Service, Covallis
Jassas H, Merkel B (2015) Assessment of hydrochemical evolution of groundwater and its suitability for drinking and irrigation purposes in Al-Khazir Gomal Basin, Northern Iraq. Environ Earth Sci 74(9):6647–6663
Johnson G, Zhang H (1990) Classification of irrigation water quality, Oklahoma cooperative extension fact sheets. http://www.osuextra.com
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95(6):385–391
Kelly WR, Panno SV, Hackley K (2012) The sources, distribution, and trends of chloride in the waters of Illinois. In: Illinois state water survey bulletin 74, p 15
Keshavarzi B, Moore F, Najmeddin A, Rahmani F (2012) The role of selenium and selected trace elements in the etiology of esophageal cancer in high risk Golestan province of Iran. Sci Total Environ 433:89–97
Kiani AR, Abbasi F (2009) Assessment of the water–salinity crop production function of wheat using experimental data of the Golestan Province, Iran. Irrig Drain 58(4):445–455
Kleinspehn KL, Paola C (eds) (2012) New perspectives in basin analysis. Springer, Berlin
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53(3):553–574
Kurdi M, Hezarkhani A, Eslamkish T (2014) Assessment of chemical properties and hydro-geochemical coefficients at the Qareh Sou Basin, Golestan Province, Iran. Environ Earth Sci 72(9):3243–3249
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42(1):28–39
Mirzaei R, Ghorbani H, Moghaddas NH, Martín JAR (2014) Ecological risk of heavy metal hotspots in topsoils in the Province of Golestan, Iran. J Geochem Explor 147:268–276
Pulle JS, Khan AM, Ambore NE, Kadam DD, Pawar SK (2005) Assessment of ground water quality of Nanded City. Pollut Res 24(3):657
Raghunath HM (1987) Groundwater Wiley Eastern Ltd., New Delhi, India
Raman V (1983) Impact of corrosion in the conveyance and distribution of water. J Indian Water Works Assoc 15(1):115–121
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess 184(6):3887–3899
Rao NS (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49(3):413–429
Rasouli F, Pouya AK, Cheraghi SAM (2012) Hydrogeochemistry and water quality assessment of the Kor-Sivand Basin, Fars Province, Iran. Environ Monit Assess 184(8):4861–4877
Ravikumar P, Somashekar RK, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monit Assess 173(1–4):459–487
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78(2):154
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42(3):195–209
Sastri JCV (1994) Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling. Lecture notes—refresher course conducted by school of Earth Sciences. Bharathidasan University, Thiruchirapalli, Tamil Nadu, India
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. In: Chemistry for sanitary engineers. McGraw-Hill
Schoeller H (1965) Qualitative evaluation of groundwater resources. Methods and techniques of groundwater investigations and development. UNESCO, pp 54–83
Semnani S, Roshandel G, Zendehbad A, Keshtkar A, Rahimzadeh H, Abdolahi N et al (2010) Soils selenium level and esophageal cancer: An ecological study in a high risk area for esophageal cancer. J Trace Elem Med Biol 24(3):174–177
Sharifi Z, Safari Sinegani AA (2012) Arsenic and other irrigation water quality indicators of groundwater in an agricultural area of Qorveh Plain, Kurdistan, Iran. Am Eurasian J Agric Environ Sci 12(4):548–555
Smil V (1999) Long-range perspectives on inorganic fertilizers in global agriculture, 1999 Travis P Hignett lecture. Alabama, USA
Srinivasan JT, Reddy VR (2009) Impact of irrigation water quality on human health: a case study in India. Ecol Econ 68(11):2800–2807
Sujatha D, Reddy BR (2003) Quality characterization of groundwater in the south-eastern part of the Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44(5):579–586
Todd DK (1980) Groundwater hydrology. Wiley, New York, pp 10–138
Trabelsi R, Abid K, Zouari K (2012) Geochemistry processes of the Djeffara palaeogroundwater (Southeastern Tunisia). Quat Int 257:43–55
Tripathi AK, Mishra UK, Mishra A, Tiwari S, Dubey P (2012) Studies of hydrogeochemical in groundwater quality around Chakghat Area, Rewa District, Madhya Pradesh, India. Int J Mod Eng Res Technol 2(6):4051–4059
UNEP (1990) GEMS/Water data summary 1985–1987. Burlington, Ontario, Canada Centre for Inland Waters; United Nations Environment Programme, Global Environment Monitoring System, GEMS/Water Programme Office
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Depatment of Agriculture. Circ. 969, Washington, D.C
Wilcox LV (1948) The quality of water for irrigation use (No. 170282). United States Department of Agriculture, Economic Research Service
Wilkinson WB, Edworthy KJ (1981) Groundwater quality monitoring systems—Money wasted? Sci Total Environ 21:233–246
Yadav JSP, Girdhar IK (1981) The effects of different magnesium: calcium ratios and sodium adsorption ratio values of leaching water on the properties of calcareous versus noncalcareous soils. Soil Sci 131(3):194
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187(10):1–18
Acknowledgements
We are thankful to the Regional Water Organization of Golestan Province for providing the data and allowing its use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurdi, M., Eslamkish, T. Hydro-geochemical classification and spatial distribution of groundwater to examine the suitability for irrigation purposes (Golestan Province, north of Iran). Paddy Water Environ 15, 731–744 (2017). https://doi.org/10.1007/s10333-017-0587-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-017-0587-x