Abstract
Inorganic fertilisers need to be applied only when the inherent soil fertility alone cannot supply the plant nutrient demand for rice. When managing such systems, identification of the most sensitive morphological and/or physiological characteristics of a rice plant and the growth stage at which those responses appear when soil phosphorus (P) and potassium (K) availabilities have declined are important. Such a practice will increase fertiliser-use efficiency and enhance environmental sustenance. Experiment was conducted in a field differing in initial soil P and K availabilities due to the application of four fertiliser treatments for three consecutive seasons. Observations in this experiment were made in the fourth season. Four fertiliser treatments were the application of (i) both P and K (P1K1), (ii) only P (P1K0), (iii) only K (P0K1), and (iv) no P and K (P0K0). Rice variety Bg300 was grown. Shoot samples were obtained at two-week intervals, while root and soil samples were collected using a soil core up to 80 cm depth at physiological maturity. At physiological maturity, root length, diameter, and root length density were not responsive to the declined soil P and K availability, whereas the total above-ground dry weight (DW) reduced in P- and K-deficient plots. Shoot physiological responses [i.e. reduced green leaf P and K concentrations, and increased phosphorus-use efficiency (PUE) and potassium-use efficiency (KUE)] were more prominent than shoot morphological responses (i.e. plant height, number of tillers, and total above-ground DW), throughout the growth cycle. The intensity (i.e. statistical significance) and duration of the appearance of K deficiency symptoms were lower than those of P. The most sensitive growth stage of rice to slight deficiencies of P and K was the tillering stage. This knowledge on the morphological and physiological shoot and root responses that can be observed during the lifecycle of a rice plant, and the growth stage(s) at which those responses are prominent in response to declining soil P and K availability can be used when identifying the development of soil P and K limitations hindering the optimal growth of rice plant, and sustaining rice cropping systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) provides staple food for more than half of the world’s population (Ainsworth 2008; Shimono 2011), and most of the rice fields in Asia are supplied with inorganic fertilisers (Rose et al. 2010). Total fertiliser application for rice is estimated to be 27.3 million t in 2015 and is expected to increase further up to 29.3 million t by 2030 (FAO 2000). The efficiency of fertilisers applied is 50% or less for nitrogen (N), less than 10% for phosphorus (P), and 40% for potassium (K) (Baligar et al. 2001). Due to the continuous and/or excess application of inorganic fertilisers, most of the lowland rice soils are rich in P and K (Ando 1983; Swarup and Chhillar 1986; Lee et al. 2004). Therefore, those rice crops do not show P and/or K deficiency symptoms at present (Lee et al. 2004; Vinod and Heuer 2012). Moreover, long-term application of high rates of inorganic fertilisers to rice fields has caused undesirable environmental consequences (Ju et al. 2009; Ye et al. 2014).
Even though farmers apply high rates of inorganic P and K fertilisers at present, due to the expected increase in fertiliser prices and reduction in their quality, farmers may not be able to continue the current rates of P and K fertiliser application in future (Fixen 2009; Pame et al. 2015; Shepherd et al. 2015). Thus, the soil P and K fertility would decline gradually. However, the productivity of lowland rice cultivation has to be increased or maintained at the current rate to satisfy the growing demand for rice. Therefore, farmers should strategically design P and K fertiliser application plans, i.e. application of P and K fertilisers only when the inherent soil fertility is not sufficient enough to maintain or increase the rice crop productivity (Kirk et al. 1998). In such instances, it is important to know the critical soil P and K concentrations that begin to hinder the plant growth and yield. Together, knowledge on initial responses of a rice plant (i.e. at early growth stages), and the growth stage(s) at which those responses occur in response to the reduction in soil P and K availability are required. This understanding will have important implications to farmers, managers, and researchers when deciding the P and K fertiliser application plans, only in required quantities and times. Moreover, this practice will enhance the use efficiency of P and K fertilisers applied, while minimising the negative impacts to the environment.
Reduction in plant height, shoot dry weight, grain yield, root diameter, number of roots and tillers, shoot: root DW ratio, tissue P and K concentrations, and the amount of P and K taken up are known to be the widely observed morphological and physiological responses of rice to the reduction in soil P and/or K availability (Li et al. 2009; Suriyagoda et al. 2014; Wu and Cheng 2014). However, to date the most sensitive morphological and/or physiological response(s) of a rice plant that can be considered as an indicator to the declining soil P and/or K availability (i.e. below the optimal level causing deficiency), and the growth stage at which those responses can be observed are not known.
Root plasticity is known to be a key trait for adaptation to nutrient deficiency (Lafitte et al. 2001; Kano et al. 2011; Somaweera et al. 2015; Weerarathne et al. 2015). However, adaptive responses of rice roots to declining soil P and K availability, relative importance of root responses in comparison to shoot responses, and adaptive responses that appear in early growth stages of a rice plant are not well known (Somaweera et al. 2015).
Patterns of nutrient uptake and biomass increment may vary along the life cycle of a rice plant (García et al. 2003; Somaweera et al. 2015). Nevertheless, the uptake pattern, use efficiency, and partitioning of P and K among different tissues (i.e. flag leaves, green leaves, dead leaves, stems, and panicles) during the life cycle of a rice plant grown in lowland flooded field conditions under different P and K availabilities have received less attention (Dobermann et al. 1998; Li et al. 2014; Somaweera et al. 2015). Therefore, periodic uptake, accumulation, and partitioning of P and K to different tissues of a rice plant under variable soil P and K availabilities in flooded lowland soil conditions should receive urgent attention. This understanding would assist researchers, agronomists, and managers to implement efficient and effective P and K nutrient management strategies to ensure sustainable rice production (Buresh et al. 2010; Ye et al. 2014; Somaweera et al. 2015). Data generated from pot experiments on adaptive responses cannot be directly extrapolated to field level plant responses as the volume of a pot, density, water and nutrient management, movement and availability of nutrients in soil, and intra-specific competition may influence those adaptive responses greatly (Poorter et al. 2012; Suriyagoda et al. 2012; Rose et al. 2013). Therefore, this experiment was conducted in a lowland rice field containing plots differing in their availability of P and/or K. The objectives of the present experiment were to identify the most responsive morphological and/or physiological characteristics of a rice plant, and the growth stage(s) at which those responses appear when the soil P and K availabilities began to decline causing deficiency. We hypothesised that (i) root system of rice would be more sensitive to slight reduction in soil P and/or K availability and therefore would show early adaptive morphological responses (i.e. root length and root DW would be higher and root diameter would be lower, particularly in surface soil layers) than shoot responses, (ii) shoot physiological responses of rice (i.e. reduction in tissue P and K concentrations and increase in P and K-use efficiencies) are better indicators of reduction in soil P and/or K availability than shoot morphological responses (i.e. reduction in height, tillering and DW), and (iii) responses in rice plant to reduction in soil P and/or K availability would be prominent during the flowering stage as both vegetative (e.g. appearance of new tillers) and reproductive processes demand more P and K than early growth stages.
Materials and methods
Establishment and management of the field experiment
Field experiment was conducted at the Rice Research and Development Institute, Bathalagoda (07°31′32.65′′N, 80°26′20.75′′E), Sri Lanka during the period from October 2013 to February 2014 as a randomised complete block design with four blocks (Fig. S1). Each block consisted of four fertiliser treatments: P1K1—both P and K fertilisers were applied; P0K1—only K fertiliser was applied; P1K0—only P fertiliser was applied; and P0K0—both P and K fertilisers were not applied (Fig. S1). Application of P and K fertilisers was made as recommended by the Department of Agriculture (DOA), Sri Lanka using triple superphosphate and muriate of potash, respectively (Somaweera et al. 2015). These experimental plots had previously been used for three consecutive seasons with the application of same fertiliser treatments as explained above for a different objective. As the current experiment was conducted on those same plots, the initial P and/or K concentrations among plots differed. Initial soil characteristics before the beginning of the present experiment and applying inorganic fertilisers are given in Table 1. As expected, P and K concentrations were lower in the deficient range in plots which did not receive P and K, respectively. Each plot had dimensions of 6 × 3 m2 in length and width, respectively. Drains were established around each plot to avoid the movement of fertilisers among plots. For all the plots, both nitrogen (N- as urea) and zinc (Zn- as zinc sulphate) fertilisers were applied as recommended by the DOA (Somaweera et al. 2015). Rice variety Bg300 was used as the test variety as it is one of the widely grown rice variety in the country. Ten-day-old Bg300 seedlings were transplanted at a spacing of 15 cm × 15 cm using one seedling per hole. Insect pests, diseases, and weeds were managed as recommended by the DOA when required. Throughout the cropping season, plots were maintained under flooded condition through irrigation unless rained. Irrigation water was collected at the inlet to the field at two-week intervals for the determination of P and K concentrations in irrigation water. Three plants selected randomly from the central 5 × 2.5 m2 area of each plot were used to measure the height from the base of the plant to the tip of the highest leaf at two-week intervals. Those three plants per plot were cut at the base and transferred immediately to the laboratory. Plants from each plot were partitioned to green leaves, dead leaves, stems, flag leaves (only after flowering), and panicles (only after flowering) to measure the dry weight (DW), P and K concentrations of each component separately. Physiological maturity (i.e. time of harvest) was determined when green-coloured grains in an erect panicle were filled, became golden colour, and the panicle turned down (Suriyagoda et al. 2014).
Soil and root sample collection
Before the beginning of the experiment, three soil samples were collected from the top 10-cm soil layer of each plot in the central 4 × 2 m2 area. The three samples from each plot were combined to make a composite sample and used for the determination of initial soil characteristics. At physiological maturity, four randomly selected plant bases from the central 4 × 2 m2 area of a plot were used to obtain soil and root samples. An auger with a diameter of 2.5 cm was inserted at the base of the plant down to 80 cm depth in the soil profile. Soil column with 80 cm length was partitioned into four segments, 20 cm each, and labelled as 0–20, 20–40, 40–60, and 60–80 cm from the soil surface. Soil samples obtained from similar depth classes of two plant bases (out of four) were combined to make a composite sample. Those soil samples were immediately transferred to the laboratory for chemical analyses (i.e. determination of soil solution P and K, available P and exchangeable K concentrations). Soil samples obtained from similar depth classes of the other two plant bases of a plot were combined to make a composite sample to extract roots for the determination of root length, surface area, diameter, and DW. Roots from each soil layer were extracted by sending the soil through a series of sieves as explained by Suriyagoda et al. (2014). Root length, surface area, and diameter were determined using a root scanner (Regent Instruments Inc. Quebec, Canada, 2000, with the software package WinRHIZO 4.1). Each root sample was then dried at 70 °C for one week (Memmert and PRECISION PS Scientific Co. model17) for the determination of root DW at each soil layer separately. Root length density (RLD) at each soil layer was calculated as the ratio of root length and soil volume (cm cm−3).
Laboratory analyses
The DWs of shoot components were determined separately after oven drying at 70 °C for one week (Memmert and PRECISION PS scientific Co., model17) and expressed per plant. To determine P and K concentrations, stems, green leaves, flag leaves, dead leaves, and panicles of each plot were ground separately. For the determination of tissue P concentration, approximately a 100 mg subsample was taken, digested in nitric/perchloric acid, and analysed using the molybdo-vanado-phosphate method (Kitson and Melon 1944). For the analysis of K concentration in the plant tissues, samples were digested in nitric acid and tested using a flame atomic absorption spectrophotometer (GBC model 932AA) (Van Ranst et al. 1999). The amount of P and K taken up by a rice plant was calculated by multiplying the corresponding DW and tissue concentrations. The phosphorus-use efficiency (PUE) was calculated as the ratio of shoot DW and P content in the shoot (g DW g−1 P). Similar approach was used when calculating K-use efficiency (KUE).
Soil samples collected from the top 10-cm soil layer before the experiment began and from four depth classes of each plot at harvest were used to determine the plant-available and soil solution P concentrations, exchangeable K concentration, total N concentration, pH, and organic matter content (%). Plant-available P concentration in soil was determined as explained by Olsen et al. (1954). Total soil P concentration was analysed by using the dry ash method—drying the sample in a muffle furnace (THERMOLYNE 62700) at 450–550 °C for 2 h followed by the general method/colorimetric method (Allen 1940). Soil N concentration was measured using the Kjeldahl method and organic matter by Walkley and Black method (Walkley and Black 1934). The pH was measured in 1:5 water extracts (HM 20S, TOA Electronics Ltd, Japan). Exchangeable K was determined after extracting in ammonium acetate (NH4OAc) and testing using flame photometry (Kitson and Melon 1944).
Statistical analyses
Data collected from shoots were subjected to two-way analysis of variance in SAS/STAT software Version 6.1 (SAS Institute Inc., Cary, NC, USA) to examine the impact of ‘fertiliser treatments’, ‘harvesting time’, and their interactions using PROC MIXED (SAS 1995). As the interaction effect was significant for most of the variables studied, subsequent analyses were conducted for each ‘harvesting time’ event separately to find the difference between ‘fertiliser treatments’ (Table 2). Similarly, data collected from soil and root characteristics at physiological maturity were subjected to two-way analysis of variance to examine the impact of ‘fertiliser treatments’, ‘soil profile depth class’, and their interactions using PROC MIXED. As the interaction effect was significant for most of the variables studied, subsequent analyses were conducted for each ‘soil profile depth class’ separately to find the difference between ‘fertiliser treatments’ (Table 3). No data transformations were needed to meet ANOVA assumptions. Comparisons between means were made using Tukey’s honest significant difference procedure. Means were presented with standard error and significance is expressed at α = 0.05.
Results
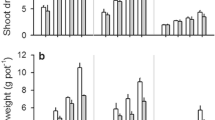
Plant height was similar among fertiliser treatments in most of the weeks in their growth cycle except for 4th and 10th week after establishment (Fig. 1a). At 4th and 10th weeks, height of P1K1 plants was higher than P0K1 and P0K0 plants. For all the fertiliser treatments, maximum tillering was reached at 6 weeks after crop establishment and then reduced until the 10th week (Fig. 1b). The number of tillers per plant was similar among fertiliser treatments except for 4, 6, and 8 weeks after establishment (Fig. 1b). During the period from 4 to 8 weeks, the number of tillers per plant by P1K1 was higher than that in P0K1 and P0K0 plants. Above-ground DW increased until maturity for all the fertiliser treatments (Fig. 1c). When comparing fertiliser treatments, above-ground DW of P1K1 was higher than that of P0K1 and P0K0 throughout the growth cycle. This resulted 11 and 10% higher DW in P1K1 at maturity than that in P0K1 and P0K0, respectively. However, P1K0 had an intermediate growth response.
Above-ground (i.e. shoot) P content of a rice plant increased until maturity (i.e. 14th week) in all fertiliser treatments (Fig. 2a). When comparing fertiliser treatments, above-ground P content of P1K1 and P1K0 plants was higher than that in P0K1 and P0K0 plants until the 8th week after establishment. At maturity, P content of all the fertiliser treatments was similar.
Above-ground (i.e. shoot) K content of rice plants increased until the 12th week from establishment and then decreased until maturity (i.e. 14th week) for all the fertiliser treatments (Fig. 2b). When comparing fertiliser treatments, above-ground K content of plants which received P1K1 was higher than that in plants received P1K0, P0K1, or P0K0 until the 6th week after establishment. However, from the 6th week of establishment onwards, tissue K content among fertiliser treatments was similar.
Phosphorus-use efficiency of a rice plant decreased until the 6th week from establishment and then maintained at a constant level until maturity at 14th week in all the fertiliser treatments (Fig. 3a). When comparing fertiliser treatments, PUE of rice plants in P1K1 and P1K0 was lower than that in P0K1 and P0K0 plants until the 6th week after establishment. The highest PUE was observed at 4th week in P0K0 plants. From the 6th week after establishment, PUE of P1K1-treated plants was lower than that in P0K1 plants, while P1K0 and P0K0 had an intermediate response.
Potassium-use efficiency of a rice plant decreased until the 10th week from establishment and then increased until maturity at 14th week in all the fertiliser treatments (Fig. 3b). When comparing fertiliser treatments, KUE of rice plants in P1K1 and P0K1 was lower than that in P1K0 and P0K0 during 4–6 weeks after establishment. From 10th to 14th week from establishment, KUE of all the rice plants increased.
In all fertiliser treatments, panicle DW increased until the 12th week and remained constant until the 14th week (Fig. 4). Panicle DW of P1K1 plants was higher than P0K1 and P0K0 plants during the period from 10th to 12th weeks after crop establishment. Stem could not be separated from green leaves until the 4th week. However, it increased from 4 to 8 weeks and then remained constant until maturity. Throughout the growing period, stem DW was similar among fertiliser treatments. In all fertiliser treatments, green leaf DW increased until the 6th week after crop establishment and then gradually decreased until the 12th week and remained constant until maturity. When comparing fertiliser treatments, plants in P1K1 and P1K0 had a higher green leaf DW than those in P0K1 and P0K0 during the period from 6th to 8th weeks after establishment. In all the fertiliser treatments, dead leaf DW increased from 6th week until maturity and was similar among fertiliser treatments. Flag leaf DW remained constant from the time of flowering to maturity and was similar among fertiliser treatments.
Panicle, stem, green leaves, dead leaves, and flag leaves dry weights of a rice plant from the establishment until physiological maturity under the application of both P and K (P1K1), absence of only K (P1K0), absence of only P (P0K1), and absence of both P and K (P0K0) fertilisers. Mean ± SE, n = 3
Panicle P concentration increased from 8 to 14 weeks after establishment in all fertiliser treatments (Fig. 5). When comparing fertiliser treatments, panicle P concentration among treatments was similar. Stem P concentration decreased from 8th week to maturity in all the fertiliser treatments. Moreover, when comparing fertiliser treatments, stem P concentration of plants which received P fertiliser was higher than those that did not receive P fertiliser until the 12th week. Green leaf P concentration remained at a low concentration until the 4th week, rapidly increased from 4 to 6 weeks, and then gradually decreased until the maturity for all the fertiliser treatments. When comparing fertiliser treatments, plants receiving P fertiliser had a higher green leaf P concentration throughout the growth period, except at maturity, in comparison to those that did not receive P fertiliser. Dead leaf P concentration decreased from 10th week until maturity in all fertiliser treatments. When comparing fertiliser treatments at the 8th week, dead leaf P concentration in plants receiving P fertiliser was higher than those in plants not receiving P fertiliser. Flag leaf P concentration decreased from 8th week until maturity and was similar among fertiliser treatments.
Panicle, stem, green leaves, dead leaves, and flag leaves P concentrations of a rice plant from the establishment until physiological maturity under the application of both P and K (P1K1), absence of only K (P1K0), absence of only P (P0K1), and absence of both P and K (P0K0) fertilisers. Mean ± SE, n = 3
Panicle K concentration increased from 8th to 10th week after crop establishment in all the treatments (Fig. 6). However, the decrease in panicle K concentration occurred from the 10th week onwards in plants receiving K, whereas it was delayed until the 12th week in plants not receiving K (Fig. 6). When comparing fertiliser treatments, panicle K concentration among treatments was similar on all dates of measurement. Stem K concentration increased from 8th to 12th week and this response was prominent in plants receiving K fertiliser. When comparing fertiliser treatments, stem K concentration of plants which received K fertiliser was higher than plants which did not receive K fertiliser at the 10th to 12th week. Green leaf K concentration remained at a constant level from the crop establishment until maturity for plants which did not receive K. However, for P1K1 and P0K1, green leaf K concentration increased until 10th and 8th week after crop establishment, respectively, and then decreased until maturity. Dead leaf K concentration decreased from 10th week until maturity for all the plants and was similar among fertiliser treatments. Flag leaf K concentration decreased from the 8th week until maturity for all the fertiliser treatments and was similar among fertiliser treatments.
Panicle, stem, green leaves, dead leaves, and flag leaves K concentrations of a rice plant from the establishment until physiological maturity under the application of both P and K (P1K1), absence of only K (P1K0), absence of only P (P0K1), and absence of both P and K (P0K0) fertilisers. Mean ± SE, n = 3
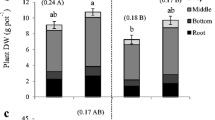
Root DW, RLD, root diameter, and root surface area in the top 0–20, middle 20–40, and bottom 40–60 cm in the soil profile were similar among fertiliser treatments (Table 3). Roots were not present below the 60-cm depth in the soil profile. More than 90% of the roots were located in the top 20-cm soil profile, and root diameter did not change with the soil profile depth (Table 4).
Discussion
Opportunities are limited for breeding rice varieties that acquire more P, and K from soil or have higher internal nutrient-use efficiencies, and therefore, long-term management strategies must focus on maintaining adequate nutrient balances in the topsoil layer (Dobermann et al. 1998; Suriyagoda et al. 2012). Therefore, identification of the most sensitive morphological and/or physiological characters of a rice plant, and the growth stage(s) at which those responses appear when soil P and K availabilities are declined are of prime importance when improving system productivity, increasing fertiliser-use efficiency, and enhancing environmental sustenance. In order to address these uncertainties, three hypotheses were tested.
The first hypothesis that root system of a rice plant would be more sensitive to reduction in soil P and/or K availability and therefore, would show early adaptive morphological responses than shoot responses was not supported. Even though reductions in soil P and/or K concentrations in plots which did not receive P and/or K fertilisers for four consecutive seasons were observed, root length, root DW, RLD, and the percentage of roots present in the top soil layer did not increase, and root diameter did not decrease in plots deficient in P and/or K at physiological maturity. However, shoot DW of P0K1- and P0K0-treated plots was reduced in comparison to that in P1K1 indicating that shoot growth was more sensitive to reductions in soil P and/or K availabilities than root responses. Due to practical difficulties, root system of plants harvested from 2 to 12 weeks after establishment could not be extracted in the present experiment. Moreover, instead of extracting the total root system of a rice plant, only the core samples were obtained in the present study at physiological maturity. Therefore, expansion of root length in the horizontal direction, away from the base of a plant, total root length and root DW of a plant could not be studied. Even though root system did not show any morphological response to declined soil P and/or K status at physiological maturity, rice roots may have adaptive responses at its early growth stages (Somaweera et al. 2015). Therefore, the root growth responses to the declined soil P and/or K availability at early growth stages have to be studied further. As more than 90% of the root length is located within the top 0–20 cm soil layer, long-term strategies for P and K management in intensive rice growing systems must focus on sustaining soil fertility in this layer (Dobermann et al. 1998; Suriyagoda et al. 2014). Therefore, more emphasis should be given to the study of root system plasticity in the top 20-cm soil layer throughout the life cycle to identify rice plant’s responses to declined P and/or K availability in comparison to taking core samples from deeper soil layers and only at physiological maturity.
The second hypothesis that shoot physiological responses of a rice plant are better indicators of reduction in soil P and/or K availability than shoot morphological responses was supported. Green leaf P concentration (from 2 to 12 weeks) and shoot P content (from 2 to 8 weeks) were reduced, and PUE (from 2 to 6 weeks) increased with a greater significance (P < 0.01) from the second week of crop establishment in P-deficient plots. In contrast, supporting the second hypothesis the reduction in plant height (at 4th week), tiller count (from 4 to 8 weeks), green leaf DW (from 6 to 8 weeks), panicle DW (10–12 weeks), and shoot total DW (from 2 to 14 weeks) in P-deficient plots had a lesser significance (P < 0.05) and began at a later growth stage than shoot physiological responses (Table 2; Fig. 7). Even though P concentration was determined in different leaf types, only the green leaf P concentration was reduced when grown in P-deficient plots. Therefore, the reduced P content and increased PUE was mainly due to the reduction in green leaf P concentration. This further indicates that dead leaves, flag leaves, or stems (until 10th week) are not suitable tissues to be used in determining early stages of soil P deficiency of a rice plant as a physiological measurement. Green leaf, dead leaf and panicle P concentrations, panicle K concentrations, above-ground P content per plant, PUE, and KUE observed in the present study at physiological maturity are comparable with previous observations (Dobermann et al. 1996; Witt et al. 1999; Haefele et al. 2003; Naklang et al. 2006; Ye et al. 2014; Somaweera et al. 2015; Xu et al. 2015). The most sensitive morphological character to reductions in soil P and/or K availability changed with plant age, i.e. plant height only at the fourth week, tiller count and green leaf DW at tillering stage, and panicle DW at grain filling stage. Due to the reduction in DW of different shoot components at different growth stages, plant total DW was reduced throughout the crop growth cycle. Therefore, depending on the growth stage at which observations are made to determine whether a soil has begun to limit P availability to a rice plant, the morphological character that requires to be studied is different.
For K, green leaf K concentration reduced and KUE increased in K-deficient plots from 4 to 6 weeks with a greater significance (P < 0.01) than shoot morphological responses (P < 0.05) which supported the second hypothesis. However, the significances of the reduction in shoot K content from 2 to 6 weeks and stem K concentration from 10 to 12 weeks were similar to the significance of shoot morphological responses (P > 0.05) which did not support the hypothesis. Even though different leaf types were studied to detect tissue K deficiency, only the green leaf K concentration was reduced in plots which did not receive K. This may have caused a reduction in shoot K content and an increase in KUE in those plots. Unlike P, the duration of appearance of K deficiency symptoms was narrower and limited only to the early tillering stage, except for stem K concentration at grain filling stage. Lack of rice plant response to K deficiency in comparison to P as observed in the present study was previously reported (Swarup and Chhillar 1986). Prevalence of such responses in most of the rice cropping systems may be due to the presence of high K concentration in irrigation water and/or supply from unexchangeable K pools in soil (Greenland 1997; Wihardjaka et al. 1999; Shen et al. 2004; Buresh et al. 2010).
The third hypothesis that responses of a rice plant to reduction in soil P and/or K availability would be more prominent at flowering stage due to the demand for P and K from both vegetative and reproductive processes than early or late growth stages was not supported. At flowering stage, only the number of tillers per plant, total above-ground DW, green leaf P concentration, and above-ground P content were reduced for plants grown in P- and K-deficient plots. Apart from the above responses, plant height, green leaf K concentration, and above-ground K content were also reduced, and PUE and KUE increased at the tillering stage indicating that rice plants were more sensitive to slight reductions in P and K availability at tillering stage than at other growth stages. Moreover, responses observed at tillering stage were more prominent (P < 0.01) than those observed at flowering (P < 0.05). This may be due to the rapid growth rate during the tillering phase. A rapid uptake of P and K during the tillering phase from rice plants treated with different water treatments was recently reported (Somaweera et al. 2015). The observed reduction in stem P and K concentrations at grain filling stage of rice plants grown in P- and/or K-deficient plots may be due to the reduced partitioning of P and K to stems or increased retranslocation from stems to grains. However, this requires further attention.
Even though Dobermann et al. (1998) compared the sensitivity of different plant tissues to K deficiency at tillering, flowering, and maturity stages, the K concentration reported in their study was lower than the values observed in the present study. This may be the reason for limited K deficiency-related responses observed in the present study and also only during a narrow window of the growth cycle. Moreover, as reported by Dobermann et al. (1998 and references there in) flag leaf was the most sensitive tissue to P deficiency at the time of flowering. However, such a response was not observed in the present study. This may also be due to the lower soil P concentration observed in their study than that reported in the present study, despite soil P concentrations in both studies being in the deficient range.
Concluding remarks
Identification of the most sensitive morphological and/or physiological characters of a rice plant, and the growth stage(s) at which those responses appear when soil P and K availabilities are declined are important when increasing fertiliser-use efficiency and enhancing environmental sustenance. Under the tested reduced soil P and K availabilities to a rice plant, root system was not responsive despite the reduction in shoot DW at physiological maturity. However, rice roots may have adaptive, plastic growth responses during the early stages of the life cycle and that has to be studied further. Shoot physiological responses such as green leaf P and K concentrations and shoot P content decreased, and PUE and KUE increased with a greater significance from the second week of crop establishment in P- or K-deficient plots than the shoot morphological responses (i.e. reduction in plant height, tiller count, green leaf DW, panicle DW, and shoot total DW). Therefore, shoot physiological responses were more sensitive to the reduction in soil P and K availabilities than shoot morphological responses. However, the intensity (i.e. based on the statistical significance presented in Table 2) and duration of K deficiency symptoms that appeared were lower than those of P. The most sensitive growth stage of a rice plant to deficiencies in soil P and K availabilities was the tillering stage as the plant height, number of tillers, dead leaf DW, total above-ground DW, green leaf P and K concentrations and above-ground P and K contents were reduced, and PUE and KUE increased for plants grown in P- and K-deficient plots. Knowledge on the morphological and physiological shoot and root responses, and the growth stage(s) at which those responses are prominent in response to declining soil P and K availability are of immense importance when identifying the development of soil P and K deficiencies hindering the optimal growth of rice, and sustainable fertiliser management of rice cropping systems. Therefore, tissue P and K concentrations tested at the tillering stage can be considered as the earliest measurements in rice plants to detect the occurrence of soil P and K deficiencies.
References
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Change Biol 14:1642–1650
Allen RJL (1940) The estimation of phosphorus. Biochem J 34:858
Ando J (1983) Future of phosphorus resources and suggested direction for Japan. Jpn J Soil Sci Plant Nutr 54:164–169 (in Japanese with English summary)
Baligar VC, Fageria NK, He ZL (2001) Nutrient use efficiency in plants. Commun Soil Sci Plant 32:921–950
Buresh RJ, Pampolino MF, Witt C (2010) Field-specific potassium and phosphorus balances and fertilizer requirements for irrigated rice-based cropping systems. Plant Soil 335:35–64
Dobermann A, Cassman KG, Sta Cruz PC, Adviento MAA, Pampolino MF (1996) Fertilizer inputs, nutrient balance, and soil nutrient-supplying power in intensive, irrigated rice systems: II. Effective soil K-supplying capacity. Nutr Cycl Agroecosyst 46:11–21
Dobermann A, Cassman KG, Mamaril CP, Sheehy JE (1998) Management of phosphorus, potassium, and sulfur in intensive, irrigated lowland rice. Field Crop Res 56:113–138
FAO (2000) Fertilizer requirements in 2015 and 2030. Food and Agriculture Organization of the United Nations (FAO), Rome, p 18
Fixen PE (2009) World fertilizer nutrient reserves-A view to the future. Better Crops 93:8–11
García AG, Neto DD, Basanta MV, Ovejero RFL, Favarin JL (2003) Logistic rice model for dry matter and nutrient uptake. Sci Agric 60:481–488
Greenland DJ (1997) The sustainability of rice farming, 1st edn. CAB International, Wallingford
Haefele SM, Wopereis MCS, Ndiaye MK, Barro SE, Isselmou MO (2003) Internal nutrient efficiencies, fertilizer recovery rates and indigenous nutrient supply of irrigated lowland rice in Sahelian West Africa. Field Crop Res 80:19–32
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci USA 106:3041–3046
Kano M, Inukai Y, Kitano H, Yamauchi A (2011) Root plasticity as the key root trait for adaptation to various intensities of drought stress water stresses in rice. Plant Soil 342:117–128
Kirk GJD, George T, Courtois B, Senadhira D (1998) Opportunities to improve phosphorus efficiency and soil fertility in rainfed lowland and upland rice ecosystems. Field Crop Res 56:73–92
Kitson RE, Melon MG (1944) Colorimetric determination of phosphorus as molybdovanadophosphoric acid. Ind Eng Chem 16:379
Lafitte HR, Champoux MC, McLaren G, O’Toole JC (2001) Rice root morphological traits are related to isozyme group and adaptation. Field Crop Res 71:57–70
Lee CH, Park CY, Park KD, Jeon WT, Kim PJ (2004) Long-term effects of fertilization on the forms and availability of soil phosphorus in rice paddy. Chemosphere 56:299–304
Li J, Xie Y, Dai A, Liu L, Li Z (2009) Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. J Genet Genomics 36:173–183
Li Y, Šimůnek J, Jing LF, Zhang ZT, Ni LX (2014) Evaluation of water movement and water losses in a direct-seeded rice field experiment using Hydrus-1D. Agric Water Manag 142:38–46
Naklang K, Harnpichitvitaya D, Amarante ST, Wade LJ, Haefele SM (2006) Internal efficiency, nutrient uptake, and the relation to field water resources in rain fed lowland rice of northeast Thailand. Plant Soil 286:193–208
Olsen SR, Cole CV, Watanable FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular No. 939. U.S. Government Printing Office, Washington, DC
Pame AR, Kreye C, Johnson D, Heuer S, Becker M (2015) Effects of genotype, seed P concentration and seed priming on seedling vigor of rice. Expl Agric 51:370–381
Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct Plant Biol 39:839–850
Rose TJ, Hardiputra B, Rengel Z (2010) Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 326:159–170
Rose TJ, Impa SM, Rose MT, Pariasca-Tanaka J, Mori A, Heuer S, Johnson-Beebout SE, Wissuwa M (2013) Enhancing phosphorus and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding. Ann Bot 112:331–345
SAS Institute (1995) SAS/Stat User Guide, vol. 2, version 6.1. SAS Institute, Cary
Shen J, Li R, Zhang F, Fan J, Tang C, Rengel Z (2004) Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil. Field Crop Res 86:225–238
Shepherd JG, Kleemann R, Bahri-Esfahani J, Hudek L, Suriyagoda L, Vandamme E, van Dijk KC (2015) The future of phosphorus is in our hands. Nutr Cycl Agroecosys. doi:10.1007/s10705-015-9742-1
Shimono H (2011) Earlier rice phenology as a result of climate change can increase the risk of cold damage during reproductive growth in northern Japan. Agric Ecosyst Environ 144:201–207
Somaweera KATN, Suriyagoda LDB, Sirisena DN, De Costa WAJM (2015) Accumulation and partitioning of biomass, nitrogen, phosphorus and potassium among different tissues during the life cycle of rice grown under different water management regimes. Plant Soil. doi:10.1007/s11104-015-2541-2
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2012) Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 358:105–117
Suriyagoda L, De Costa WAJM, Lambers H (2014) Growth and phosphorus nutrition of rice when inorganic fertiliser application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 384:53–68
Swarup A, Chhillar RK (1986) Build up and depletion of soil phosphorus and potassium and their uptake by rice and wheat in a long-term field experiment. Plant Soil 91:161–170
Van Ranst E, Verloo M, Demeyer A, Pauwels JM (1999) Manual for the soil chemistry and fertility laboratory-analytical methods for soils and plants, equipment, and management of consumables. University of Gent, Gent
Vinod KK, Heuer S (2012) Approaches towards nitrogen- and phosphorus-efficient rice. AoB Plants 2012:pls028. doi:10.1093/aobpla/pls028
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Weerarathne LVY, Suriyagoda LDB, Marambe B (2015) Competitive effects of barnyard grass (Echinochloa crusgalli L. Beauv) on rice (Oryza sativa L.) is less when phosphorus is applied to deeper layers of a phosphorus-deficient soil irrespective of the soil moisture availability. Plant Soil 391:1–17
Wihardjaka A, Kirk GJD, Abdulrachman S, Mamaril CP (1999) Potassium balances in rainfed lowland rice on a light-textured soil. Field Crop Res 64:237–247
Witt C, Dobermann A, Abdulrachman S, Gines HC, Guanghuo W, Nagarajan R, Satawathananont S, Son TT, Tan PS, Tiem LV, Simbahan G, Olk DC (1999) Internal nutrient efficiencies in irrigated lowland rice of tropical and subtropical Asia. Field Crop Res 63:113–138
Wu W, Cheng S (2014) Root genetic research, an opportunity and challenge to rice improvement. Field Crop Res 165:111–124
Xu X, Xie J, Hou Y, He P, Pampolino MF, Zhao S, Qiu S, Johnston AM, Zhou W (2015) Estimating nutrient uptake requirements for rice in China. Field Crop Res 180:37–45
Ye Y, Liang X, Chen Y, Li L, Ji Y, Zhu C (2014) Carbon, nitrogen and phosphorus accumulation and partitioning, and C:N:P stoichiometry in late-season rice under different water and nitrogen managements. PLoS ONE 9(7):e101776. doi:10.1371/journal.pone.0101776
Acknowledgements
The authors appreciate the financial support from the National Research Council, Sri Lanka under the grant NRC-11-179, and technical assistance provided by G Wijesuriya and WMN Wanninayake. Authors appreciate the critical comments given by LW Galagedara, Memorial University of Newfoundland, Canada and the two reviewers to improve an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Somaweera, K.A.T.N., Sirisena, D.N., De Costa, W.A.J.M. et al. Age-related morphological and physiological responses of irrigated rice to declined soil phosphorus and potassium availability. Paddy Water Environ 15, 499–511 (2017). https://doi.org/10.1007/s10333-016-0567-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-016-0567-6