Abstract
A growing body of research focuses on how anthropogenic factors affect the behavior and ecology of primates and their ecosystems. Infrastructural development, such as roads, is an increasingly pervasive anthropogenic impact that destroys primate habitat, affects the distribution and dispersal of primates, and facilitates human–primate interactions. At our field site in Bantimurung-Bulusaraung National Park, Sulawesi, Indonesia, a major road bisects the habitat of the endangered moor macaque (Macaca maura). Beginning in 2015, we observed a behavioral shift by our main study group: they began spending more time along the road foraging in trash pits and waiting for provisions from vehicles. Our objective in this study was to examine how access to anthropogenic foods has affected the group’s ranging behavior by comparing ranging data collected before (2010–2011) and after the shift (2016–2017). In contrast to what we expected, home ranges were significantly larger and daily travel distance was significantly longer after the shift compared to before. As predicted, mean distance to the road decreased after the shift. These results likely reflect the irregular and spatially dispersed nature of provisioning at this site. The macaques appear to be attracted to the road because it presents opportunities to obtain palatable and energy-dense foods. Our results indicate that moor macaques are able to flexibly adjust their ranging behavior in response to anthropogenic impacts. However, given the risks of being in proximity to roads and humans, management of this emerging human–macaque interface is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the current era, in which many primate communities live in close proximity to humans and/or whose habitats are altered by human activities, examining how anthropogenic factors affect primate behavior and ecology has also become an important objective (Dore et al. 2017; McLennan et al. 2017; Behie et al. 2019). These factors are diverse in type and in impact, ranging from the selective removal of forest products (Srivastava et al. 2001) and the conversion of habitat for agricultural land (Estrada et al. 2012), to tourism sites where people encounter primates (Berman et al. 2007), and major infrastructural development, such as road networks (Estrada et al. 2017). While roads facilitate economic growth and social integration, they are also linked with a suite of environmental impacts (Laurance et al. 2009). Roads typically fragment and/or destroy primate habitat (Singh et al. 2011; Estrada et al. 2017). Moreover, roads and their environmental effects act as agents of selection (Brady and Richardson 2017), in that they alter ecological dynamics, affect abundance, distribution, and dispersal patterns, and are a major cause of mortality (Forman and Alexander 1988; Pragatheesh 2011; Al-Razi et al. 2019). Roads also facilitate another type of anthropogenic impact—provisioning (Sha and Hanya 2013; Sengupta and Radhakrishna 2018).
Provisioning—the deliberate offering of food, typically human foods, to animals—is widespread across areas where primates are sympatric with humans, and has been shown to influence primate behavior, ecology, and health (Sinha et al. 2005; McKinney 2011; Sha and Hanya 2013; Maréchal et al. 2016; Ilham et al. 2018). Anthropogenic foods are typically palatable, easily digestible, energy-dense, and spatially concentrated, thereby offering energetic advantages over wild foods (Strum 2010; Riley et al. 2013). Behavioral ecological theory predicts that animals will adaptively manage their time and energy (e.g., energy-maximizing or energy-conserving strategy) in response to fluctuations in food availability (Stephens and Krebs 1987). For example, during periods of lower food abundance, some primates increase their home range size and daily travel distances to locate high-quality foods (e.g., lion-tailed macaques, Macaca silenus; Santosh et al. 2015), while others will travel less and spend more time consuming lower-quality foods (e.g., Sichuan snub-nosed monkey; Rhinopithecus roxellana; Li et al. 2000). In other cases, primates may shift back and forth between these strategies as environmental conditions fluctuate (e.g., pig-tailed macaques; Macaca leonina; Albert et al. 2013). Because primates do not distinguish between “natural” and anthropogenic sources of environmental disturbances (Strum 2012), we should expect primates to assess the tradeoffs that emerge in anthropogenic contexts, such as provisioning sites, just as they would in “natural” environments. For example, in terms of energetic tradeoffs, a number of studies have found that primates that are provisioned or otherwise food-enhanced show smaller home ranges and shorter daily travel distances, reflecting reduced travel costs associated with food resources that are abundant and spatially concentrated (Saj et al. 1999; Hoffman and O’Riain 2012; Sengupta et al. 2015; Hansen et al. 2020).
Field studies conducted across the primate order have elucidated the key intrinsic and extrinsic factors that influence ranging behavior, including body size (Milton and May 1976), diet (Goldsmith 1999), the distribution and availability of food (Reyna-Hurtado et al. 2018), climate (Johnson et al. 2015), group size (Dias and Strier 2003), group composition (Bartlett et al. 2016), and the presence of neighboring groups (Gibson and Koenig 2012). Understanding the impact of these factors as well as how primates move across and use space in anthropogenically modified areas enables us to understand species’ ecological requirements, their potential for ecological and behavioral flexibility, and how to better conserve them (Hockings et al. 2015; Estrada et al. 2017). For example, examining whether and how primates use disturbed areas and where primates concentrate their feeding in and around those areas enables us to determine which resources to protect and to push for the inclusion of anthropogenically modified landscapes in conservation planning (Meijaard et al. 2010; Santhosh et al. 2015). While anthropogenic impacts on ranging behavior have been well-studied for other synanthropic primates (e.g., Macaca fascicularis; Sha and Hanya, 2013; Klegarth et al. 2017; Papio ursinus; Pebsworth et al. 2012; Fehlmann et al. 2017), there has been comparatively less research done on the Sulawesi macaques (but see Riley 2008).
In this study, we took advantage of an emerging shift at our study site to better understand the behavioral flexibility of the endangered Sulawesi moor macaque (Macaca maura). At Bantimurung-Bulusaraung National Park, South Sulawesi, Indonesia, a major road traverses through 11 km of the park, bisecting the habitat of resident fauna, including the moor macaque. This road, which was formally established in 1930, and hence, existed prior to the National Park designation in 2004, is economically important and heavily trafficked because it is the primary path for land travel from the provincial capital of South Sulawesi, Makassar, to the major port city of Bone, on the eastern side of the peninsular province. This road has long bisected the home ranges of moor macaque groups in the area (Matsumura 1991; Albani et al. 2020), but after crossing the road on the ground or in the canopy, groups retreat back into the forest (Author, personal observation). Beginning in 2015, however, we observed a shift at this site, whereby the main habituated study group (group B) began spending more time along the road, which in turn made them more visible to people passing in cars, at which point people began feeding them (Fig. 1). Prior to this point, no provisioning of the macaques occurred along the road. The exact cause of the shift is unknown, as there were no changes, to our knowledge, to the road and the surrounding forest that would have impacted the abundance of natural foods, and traffic patterns were consistently heavy across the two time periods. The timing of the shift did, however, coincide with an increase in the number of vendor stalls along the road where people sell forest products such as honey, suggesting that the macaques may have been attracted to the food remains and trash that accumulates near these stalls. By 2016, it was estimated that group B spent approximately 20% of scans (N = 1170) along the road, foraging in trash pits and waiting for provisions from passing motorists (Morrow et al. 2019). By 2018, we observed additional, unhabituated groups waiting on the side of the road for provisions along the 11-km stretch through the park (Author, personal observation).
Our objective in this study is to examine how the shift in the human–macaque interface at our study site, which resulted in access to anthropogenic foods along the road, affected moor macaque ranging behavior. To do so, we compared two ranging behavior data sets collected on the main study group (group B) at the site. As part of a separate study on the impact of human–macaque interactions on moor macaque social networks (Morrow et al. 2019), we collected 6 months of ranging data in 2016–2017; this data set constitutes the “after the shift” period. We used ranging data from comparable months that we collected for a different project on female reproductive ecology in 2010–2011 (Sagnotti 2013) as the “before the shift” data set. Following previous studies (Saj et al. 1999; Hoffman and O’Riain 2012; Sengupta et al. 2015; Hansen et al. 2020), we predicted that the group would have a smaller home range and shorter daily travel distance (DTD) after the shift (once roadside provisioning began). In addition, given that other behaviorally flexible, omnivorous cercopithecines have been shown to be attracted to locations in their range where anthropogenic foods are present (e.g., olive baboons, Papio anubis; Strum 2010), we predicted that, on average, the group would be in closer proximity to the road after the shift (compared to before). We discuss our findings in relation to what is known from other sites where humans provision macaques and explore the implications of our results for the conservation of the endangered moor macaque and the management of human–primate interfaces worldwide.
Methods
Study site
For the purpose of this study, we used two existing sets of ranging data collected during comparable months in two time periods (August 2010–February 2011 and August 2016–January 2017) in the Karaenta area of Bantimurung Bulusaraung National Park (TNBABUL), located in South Sulawesi, Indonesia (Fig. 2; latitude: 4° 42′ 49″–5° 06′ 42″ S, longitude: 119° 34′ 17″–119° 55′ 13″ E). Comprising 43,750 ha, TNBABUL was gazetted in 2004 to protect the region’s karst ecosystem and biodiversity. The Karaenta area, which is situated at approximately 300 m.a.s.l., consists of primary and secondary forest amidst and upon karst (limestone) tower formations that rise up to 70 m from the ground (Albani et al. 2020). This forest is dominated by several species of trees which produce edible fruit for moor macaques, including Ficus spp. Dracontomelon dao, Cananga odorata, Parartocarpus sp., Diospyros celebica, and Palaquium obovatum (Achmad 2011; Sagnotti 2013; Albani et al. 2020). Following the Schmidt and Ferguson (1951) rainfall type classification system, the study area is characterized as seasonal with six to nine wet months (dry/wet month ratio = 33.3–100%), whereby any month with less than 60 mm of rain is dry, months with 60–100 mm are moist, and months with more than 100 mm are wet (Whitmore 1984). Mean annual rainfall for the region, collected at the Maros/Balitjas Climate Station in Maros, South Sulawesi, was 4361 mm ± 262 (SD) for 2010–2011 and 3212 mm ± 177 (SD) for 2016–2017 (Fig. 3). The region shows an average temperature of 28–30 °C (Lubis et al. 2008).
Study species and group
The moor macaque is one of the seven macaque species endemic to Sulawesi, Indonesia (Fooden 1969). Moor macaques live in multi-male/multi-female, female philopatric social groups, and are characterized as socially tolerant (Matsumura 1998; Riley et al. 2014). They are primarily frugivorous, but also eat leaves, flowers, shoots and stems from trees, shrubs and herbaceous vegetation, with figs (Ficus spp.) comprising a large part of their diet, as well as insects (Matsumura 1991; Sagnotti 2013; Albani 2017). The estimated population density of moor macaques in the central part of Karaenta is 3.5 groups/km2 (Matsumura 1998).
The study group (group B) is a well-habituated group that has been the subject of intermittent field studies since the 1980s. Based on research conducted during the early years, group B’s home range was estimated to be 20 ha (Matsumura 1991). At that time, researchers heavily provisioned group B at a designated feeding site (1–2 times per day) in an effort to accelerate habituation, obtain group counts, and identify individuals (Watanabe and Matsumura 1996; Okamoto et al. 2000). Since the late 1990s until 2018, group B has been occasionally provisioned by park staff inside the forest for tourism and media purposes, except during field research. From 2010 to 2011, researchers provisioned the group at a set location in the forest as part of a separate study that required food baiting for training. There were no systematic field research studies conducted on the group from 2012 to 2014. Field research on the group by multiple teams commenced again in late 2014 (Albani 2017; Morrow 2018; Germani 2018). Based on ranging data collected between September 2014 and February 2015 (i.e., just prior to the shift), the home range of group B was estimated at 21.53 ha (95% kernel density estimation [KDE]) (Albani et al. 2020). Group size was the same for the two time periods of this study (N = 35), but group composition varied (Table 1).
Data collection
In order to accommodate the multiple research projects that were concurrently being conducted at the site during both time periods, the research teams divided observation days into 6-h blocks (either 0600–1200 or 1200–1800) so that only one research team was following the group at a time. Because of this compromise, we were unable to conduct full-day follows. We recorded the geocoordinates of the group’s approximate center using a handheld GPS unit every 30 min (2016–2017) or 40 min (2010–2011) during the 6-h blocks (Fig. 4). To increase accuracy, we only took GPS locations when error readings were less than 10 m (but generally errors fell between 3–8 m). We collected ranging data on consecutive days generally for 3–6 days per week per month, alternating morning and afternoon sampling periods, for a total of 104 days in 2010–2011 and 120 days in 2016–2017 (Table 1). The study site includes tower karst formations that are utilized by the macaques but are difficult for people to safely climb and traverse. We therefore acknowledge that we restricted spatial data collection to areas where the macaques were visible without having to climb the karst. Our home range estimates may therefore underestimate total home range, but this should not affect the analyses as the same safety protocol was employed in both study periods.
During the time period when the “before the shift” data set was collected (2010–2011), as part of another study being conducted, the group was provisioned with approximately 2–3 kg of dry corn kernels once a week at a set location in the forest situated 57.3 m from the road (Fig. 6). No provisioning occurred along the road in 2010–2011. During the time period when the “after the shift” data set was collected (2016–2017), no provisioning from researchers or park staff occurred in the forest; the group was only provisioned by passing motorists on the road, and this provisioning occurred all along the road within group B’s home range (Table 2; Fig. 6). Roadside-provisioned items were high-energy-dense foods (e.g., bread, cookies, chips) and/or carbohydrate-rich foods (e.g., bananas, oranges, boiled corn cobs) (Morrow 2018).
One concern with comparing these data sets is the fact that provisioning also occurred in 2010–2011, and it occurred in the forest away from the road. Nonetheless, we are confident in our comparison and ability to assess the effect of the shift on the group’s ranging patterns for a number of reasons. First, in 2010–2011, provisioning in the forest only occurred one time per week for roughly 15–60 min, whereas in 2016–2017, provisioning along the road occurred regularly, often multiple times per day and for many hours throughout the day (Morrow 2018). Secondly, in 2010–2011, we did not collect ranging data during the forest provisioning events. Third, we descriptively compared available activity budget data from the two time periods. Based on data collected on adult females (i.e., the only data available for the 2010–2011 time period), more time was spent feeding, foraging, and moving and less time was spent resting and self-grooming in 2010–2011 compared to 2016–2017 (Sagnotti 2013; Morrow, unpublished data; Fig. 5). These differences suggest that forest provisioning did not provide sufficient nutritional benefit during the 2010–2011 period to substantially impact ranging patterns. Lastly, we assessed whether provisioning in the forest may have influenced daily path length and distance from the road in the 2010–2011 period by comparing these two variables on days the macaques were provisioned (N = 14) versus days when they were not (N = 90). We found that neither daily travel distance (Mann–Whitney U = 477.00, p = 0.145) nor mean distance from the road (Mann–Whitney U = 48,154.00, p = 0.894) showed significant differences between provisioned and non-provisioned days. We therefore used all data points from each time period in the comparative analysis.
Percentage of behavioral records spent by group B adult females in different activities in 2010–2011 (n = 4783 behavioral records) and 2016–2017 (n = 751 total behavioral records). Source: Sagnotti (2013); Morrow, unpublished data
Data analysis
While home range estimation is an essential and routine practice in ecological research, there continues to be debate concerning which methods are the most accurate and which are most appropriate for different types of data sets (Noonan et al. 2019). Kernel density estimation (KDE) is one of the most statistically efficient and accurate methods and has become ubiquitous in studies of animal movement since being introduced in 1989 (Worton 1989; Fleming and Calabrese 2017). However, because different methods for estimating home range utilization can produce widely different results (Boyle et al. 2009), we employed two methods to calculate home ranges: minimum convex polygon (MCP) and KDE. The MCP was calculated as the MCP enclosing 100% of GPS locations. We estimated the 95% and 50% KDE using a bandwidth determined by the plugin method. We used ArcGIS v. 10.3.1 and Geospatial Modeling Environment (GME) v. 0.7.4 for home range analysis. We generated daily travel distances (DTD) using the points-to-line tool in ArcGIS. Because behavioral data were primarily collected in 6-h blocks per day, we averaged morning travel distances and afternoon travel distances per month and then summed these to generate an average daily travel distance per month (cf. Gregory et al. 2014). We measured proximity to the road by calculating the mean distance each GPS location record was from the road using the near tool in the proximity toolbox of ArcGIS.
Statistical analysis
After using the Kolmogorov–Smirnov test to verify that home range data were normally distributed, we conducted a two-tailed paired t test to compare monthly home ranges using IBM SPSS Statistics for Macintosh, version 25.0. We considered results significant at p < 0.05.
To determine how daily travel distance (DTD) changed from the 2010–2011 study period to 2016–2017, we performed a generalized linear model (GLM) where the response variable was mean DTD per month, and monthly rainfall, study period (2010–2011 and 2016–2017), and the interaction between rainfall and study period were fixed effects. However, because the interaction term was not significant and did not improve model fit (likelihood ratio test χ2 = 0.05, p = 0.820), we did not retain it in the final model. Because we did not have data on forest fruit availability during these time periods, we used rainfall as a proxy for food availability, given that fruit and young leaves are expected to be more abundant in Southeast Asian tropical rainforests during the wettest months (Medway 1972), and previous research conducted at the study site documented a significant positive correlation between rainfall and fruit abundance (Germani 2018). Before fitting the model, we verified the assumptions of normal distribution of residuals by visual inspection of Q–Q plots. Data exploration and residuals revealed no violations of the assumptions of the model. We used a Gaussian distribution with a log link function for the model using the GLM function in R. We compared the significance of the full final model to the corresponding null model using a likelihood ratio test (R function ANOVA) and used Wald chi-square tests to assess the significance of individual effects.
To compare the extent to which macaques changed their ranging behavior around the road from 2010–2011 to 2016–2017, we performed a generalized linear mixed model (GLMM) where the response variable was mean distance of the macaque group from the road at 30-min (2016–2017) and 40-min (2010–2011) intervals. We incorporated monthly rainfall, study period (2010–11 versus 2016–17), and the interaction between rainfall and study period as fixed effects. However, because the interaction term was not significant and did not improve model fit (likelihood ratio test χ2 = 1.31, p = 0.250), we did not retain it in the final model. To account for nonindependence among scans throughout the day, we included date as a random effect. We checked homoscedasticity, model stability, and the assumptions of normal (Gaussian) distribution of residuals by visual inspection of Q–Q plots. These revealed no violations of the assumptions of the GLMM. We used a Gaussian distribution with a log link function for the model using the function lme of the R package nlme (Pinheiro et al. 2019). We compared the significance of the full final model to the corresponding null model using a likelihood ratio test (R function ANOVA). We assessed the influence of the individual fixed effects on the response variable using the t and p values (R function lme) and used the models to estimate marginal means for daily travel distance and distance to the road. In our results, we report both the raw means (Table 3; Fig. 7) and the estimated marginal means for these response variables. We fitted both the GLM and GLMM using R 3.1.3 (R Core Team 2017).
Results
Home range size and daily travel distance
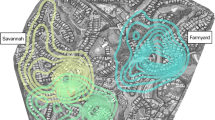
The analyses indicate that group B’s overall home range and core area were larger in 2016–2017 compared to 2010–2011 (Table 3; Fig. 6). Mean monthly home range was significantly larger after the shift compared to before (paired t test: t = −3.471, df = 5, p = 0.018). Group B also expanded their home range to the south in 2016–2017 (Fig. 6). The GLM using rainfall and study period to estimate daily travel distance was significant compared to the null model (χ2 = 64.98, p < 0.001), and study period was a significant predictor, but rainfall was not (Table 3). These results indicate that in contrast to what we predicted, group B significantly increased their daily travel distance after the shift (2016–2017) compared to before (2010–2011) (Tables 3 and 4; Fig. 7), with estimated marginal mean travel distances of 1406.29 (CI = 1324.00–1497.50) and 1109.44 m (CI = 1025.72–1184.51), respectively.
Proximity to the road
The GLMM using study period and rainfall to predict the distance macaques were observed from the road was significant compared to the null model (χ2 = 18.41, p < 0.001). As with daily travel distance, study period was a significant predictor of distance from the road, but rainfall was not (Table 4). These results indicate that, as predicted, group B was in greater proximity to the road after the shift compared to before (Tables 3 and 5; Fig. 7). Specifically, estimated marginal mean distance from the road decreased from 149.62 m (CI = 141.05–158.20) during the 2010–2011 study period to 124.85 m (CI = 117.54–132.16) during the 2016–2017 study period.
Discussion
Following a shift in the human–macaque interface at our study site, whereby roadside provisioning provided a new location for daily access to anthropogenic foods, our primary study group exhibited changes in their movement patterns and home range area. Previous studies on food-enhanced primates have found that greater accessibility of anthropogenic foods results in smaller home ranges and shorter daily travel (e.g., Saj et al. 1999; Sengupta et al. 2015; Klegarth et al. 2017; Hansen et al. 2020). Our results show the opposite pattern: the study group had a larger overall home range (and mean monthly home range) and a longer mean daily travel distance after the shift compared to before. However, our prediction that the group would be in closer proximity to the road was supported.
These results suggest that moor macaques are attracted to the road because it presents opportunities for them to obtain palatable and energy-dense foods (Ilham et al. 2018; Sengupta and Radhakrishna 2018). Because habituation, more generally, and provisioning, more specifically, involves animals accepting or tolerating humans in their environment (Asquith 1989; Hanson and Riley 2018), it is possible that group B perceived human encounters along the roadside to be low risk, positive associations given the fact that the group is well-habituated to humans and has experienced a long history of provisioning. It is also likely that the perceived risk of injury from passing vehicles is outweighed by the perceived benefit of receiving provisions from said vehicles (cf. Waterman et al. 2019).
The expanded home range size and longer travel distances we observed in this study likely reflect the nature of provisioning at this site. At some sites where humans and macaques interface, direct human provisioning is regular and spatially concentrated (e.g., Bali, Indonesia, and Gibraltar; Fuentes et al. 2007). At our site, while refuse piles along the road provide spatially concentrated areas in which the macaques can forage, the locations of these piles vary as they are not sanctioned refuse bins. Furthermore, roadside provisioning was not stationary. Instead, it typically occurred from moving vehicles or vehicles that only stop momentarily, thereby resulting in a more dispersed distribution of the food. Provisioning is also irregular as not all vehicles that pass offer food. These findings are similar to what Sha and Hanya (2013) observed among long-tailed macaques (M. fascicularis) in Singapore: the “high anthropogenic” group, which was observed more often in urban areas feeding on anthropogenic foods, had a significantly larger overall home range and mean monthly home range.
During the 2016–2017 study period, we often observed the macaques following vehicles that slowed in their proximity. This behavior has been previously observed at sites where macaques are similarly provisioned by moving modes of transport (e.g., provisioning of rhesus macaques from boats along the Silver River, Florida; Riley and Wade 2016). Accordingly, this following behavior may account for the greater mean daily travel distance and expanded home range size in 2016–2017 compared to 2010–2011. The difference in DTD could also reflect increasing group spread, as individuals space themselves out to reduce within group competition that can be amplified in provisioning contexts (e.g., Ram et al. 2003; Ilham et al. 2018). Although previous research has shown that moor macaques demonstrate social tolerance when feeding (Matsumura 1998), future work systematically examining whether group spread and levels of within-group competition shift when in proximity to the road would further illuminate the socioecological consequences of provisioning.
In the “after the shift” study period when roadside provisioning was firmly in place (2016–2017), we observed a southward extension of the home range. The area that contributes to this extended home range is situated along the road (Fig. 6), specifically a section where “shoulders” exist, allowing vehicles to pull over and facilitating provisioning. Easier access to provisioned foods, which in turn may offset additional foraging costs, coupled with following behavior, may explain this extension of the home range. Barbary macaques (M. sylvanus) in Gibraltar exhibited a similar pattern of extending the home range to include areas that offer nutritional benefits for the extra foraging effort (Unwin and Smith 2010). Although roads generate numerous negative ecological effects (Forman and Alexander 1998), the edge effects they create enable colonization by plant species that may actually be favored by wildlife, thereby increasing the use of edge habitat (Brodie et al. 2015). It is possible that at our study site the macaques are attracted to edge habitat along the road for both wild and anthropogenic foods. For example, we noted that there were two large fig trees (Ficus spp.) along the road that were fruiting when the home range extension was observed (December 2016 and January 2017).
Seasonality can influence home range size and daily travel distances, but the effects have been shown to vary by species and context (Santhosh et al. 2015). For example, northern pig-tailed macaques (Macaca leonina) in Khao Yai National Park, Thailand, where some provisioning occurs, decreased their daily travel distance and monthly home range size and remained close to human settlements during the dry season, when forest fruit availability was lower (Albert et al. 2013). In contrast, heavily provisioned Barbary macaques in Gibraltar increased their daily travel distance during the dry season (Klegarth et al. 2017). Because rainfall has been shown to positively correlate with food abundance in primate habitats (Vedder 1984; Barton et al. 1992; Stone 2007), including at our study site (Germani 2018), it is often used as a proxy when food availability data are unavailable (e.g., Okamoto and Matsumura 2002; Hill et al. 2003; Reyna-Hurtado et al. 2018). In this study, although we found no significant effect of rainfall on daily travel distance, we acknowledge that it is possible that there were inter-year differences in wild food availability that might account for the ranging patterns we observed (Hill and Agetsuma 1995; Tsuji and Takasuki 2009). That said, some studies have found that primates are attracted to anthropogenic foods, such as agricultural crops (e.g., maize; Naughton-Treves et al. 1998; papaya and cacao; Riley 2007; banana; Seiler and Robbins 2016), despite the concurrent availability of wild foods, and spend less time eating wild foods when provisioned foods are available (Koirala et al. 2017), suggesting that provisioned foods may be preferred over wild foods (Sengupta and Radhakrishna 2018). Ranging data collected across the year, coupled with monitoring of forest fruit availability and foraging behavior, will more fully contribute to our understanding of how the availability and distribution of wild food and provisioned food intersect to shape moor macaque behavior.
While limited provisioning of the group in the forest occurred during the 2010–2011 study period, several factors suggest that it does not account for the observed differences in ranging patterns. First, the forest provisioning site was located in close proximity to the road (57.3 m), meaning that access to the provisioned food in the forest in 2010–2011 did not require the group to travel great distances away from the road. Second, the observed home range size in 2010–2011 (25 ha, 95% KDE) is more similar to the home range size estimated from another study conducted prior to roadside provisioning (i.e., 21.53 ha, 95% KDE; Albani et al. 2020) compared to the 2016–2017 estimate (36 ha) once roadside provisioning began, thereby suggesting that the forest provisioning had a marginal if any impact on home range size for the study group. Lastly, the allocation of time across different activities during the 2010–2011 study period (Sagnotti 2013) does not align with what the expected pattern would be if the forest provisioning provided sufficient nutritional benefit to affect ranging behavior.
While this study predominantly focuses on ecological and anthropogenic factors influencing ranging behavior, it is important to also consider the effects of intrinsic factors, such as within-group and between-group social factors (Doran-Sheehy et al. 2004). In terms of within-group factors, group size cannot account for the differences in home range size and daily travel distance as it was the same across the study periods. Group composition, however, did vary, with there being more adult males in the group in the latter period. Males are generally expected to engage in behaviors of higher risk (Santillán-Doherty et al. 2010), such as road crossing and foraging near a road, and hence, could be the impetus for the group moving closer to the road. However, because we observed members from all age and sex classes in proximity to the road (Morrow et al. 2019), and the finding that in socially tolerant species, such as moor macaques, movement decisions are made by many individuals in the social group (Sueur and Petit 2008), it is unlikely that variation in group composition explains increased proximity to the road and the differences in ranging behavior observed in this study.
Although the presence of neighboring social groups is another important variable known to influence ranging behavior (Doran-Sheehy et al. 2004; Gibson and Koenig 2012), we did not explore the effect of intergroup interactions for two reasons. First, previous research conducted at the study site found that intergroup encounters were more frequently characterized by tension compared to aggression or fleeing and that intergroup interactions did not increase during the rainy season when fruit abundance was expected to be higher, thereby suggesting that intergroup interactions may be less important than other factors (Okamoto and Matsumura 2002). Second, the validity of intergroup interaction data hinges on a sufficient level of habituation of the social groups involved. In 2010–2011, only group B was habituated to observer presence, so we were unable to accurately collect intergroup encounter data. Further research examining how shifting ranging patterns influence intergroup encounters and dispersal patterns will require habituation and simultaneous study of multiple macaque groups. However, pursuing this research direction will mean carefully considering whether the benefits of habituating additional groups outweigh the risks, given the endangered status of the moor macaque (Riley and Bezanson 2018).
In conclusion, our results indicate that moor macaques are able to flexibly adjust their ranging behavior in response to emerging anthropogenic impacts, such as a new source of energy-dense food from roadside provisioning. While this capacity for flexibility may enhance moor macaques’ ability to survive in anthropogenic settings, there are also concomitant risks that could negatively affect the population in the long term. Previous research has shown that provisioning can be both beneficial and detrimental to primates at multiple levels. For example, the nutritional advantages gained from consuming anthropogenic food result in faster growth rates, earlier ages of sexual maturity, and increased birth and survival rates (Asquith 1989). Provisioning has also been shown to reduce time feeding on wild foods, resulting in more time for other activities, such as resting and socializing (Koirala et al. 2017; Ilham et al. 2018). On the other hand, interactions with humans, including provisioning, can result in negative social effects, such as reduced social grooming (Kaburu et al. 2019) and more frequent agonistic behavior (Ilham et al. 2018), as well as negative ecological effects, such as reduced frugivory and the disruption of seed dispersal (Sengupta et al. 2015). In addition, at sites like ours, where provisioning occurs along a major road, the risk of injury and/or death increases (Pragatheesh 2011; Fig. 8). The fact that there are now multiple groups of moor macaques descending to the road awaiting provisions all along the 11-km stretch of the road that bisects the national park makes this emerging human–macaque interface a population-level issue in need of dedicated management.
The implications of our findings are also broadly applicable to other contexts in which primates and people overlap and share resources. Because provisioning is a sociocultural tradition in many areas throughout South and Southeast Asia (Loudon et al. 2006; Sengupta et al. 2015), including Indonesia, all-out bans on provisioning are often difficult to implement. Park staff and researchers should role model appropriate ways to interface with primates, such as maintaining appropriate distances, not provisioning them, and avoiding overhabituation, as an important and ethically informed first step toward the management of these types of interfaces (Riley and Bezanson 2018; Lappan et al. 2020). In addition, a management approach grounded in conservation education outreach to the broader public that emphasizes the important role primates play in forest regeneration (Tsuji and Su 2018) and the negative effects of provisioning, thereby instilling empathy, may be a productive way to bring about human attitudinal and behavioral changes that will facilitate human–primate coexistence moving forward.
Data availability
The data sets analyzed for the current study are available from the corresponding author on reasonable request.
Change history
24 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10329-021-00941-7
References
Achmad A (2011) Rahasia ekosistem hutan bukit kapur. Brilian Internasional, Surabaya
Albani A (2017) Ecology and habitat use of karst forest-dwelling Macaca maura (H.R. Schinz, 1825), endemic endangered primate of South Sulawesi (Indonesia). Roma Tre University, Rome
Albani A, Cutini M, Germani L, Riley EP, Ngakan PO, Carosi M (2020) Activity budget, home range, and habitat use of moor macaques (Macaca maura) in the karst forest of South Sulawesi, Indonesia. Primates. https://doi.org/10.1007/s10329-020-00811-8:1-12
Albert A, Huynen M-C, Savini T, Hambuckers A (2013) Influence of food resources on the ranging pattern of northern pig-tailed macaques (Macaca leonina). Int J Primatol 34:696–713
Al-Razi H, Maria M, Muzaffar SB (2019) Mortality of primates due to roads and power lines in two forest patches in Bangladesh. Zoologia (Curitiba) 36:e33540
Asquith PJ (1989) Provisioning and the study of free-ranging primates: History, effects, and prospects. Am J Phys Anthropol 32:129–158
Bartlett TQ, Light LE, Brockelman WY (2016) Long-term home range use in white-handed gibbons (Hylobates lar) in Khao Yai National park, Thailand. Am J Primatol 78:192–203
Barton R, Whiten A, Strum S, Byrne R, Simpson A (1992) Habitat use and resource availability in baboons. Anim Behav 43:831–844
Behie AM, Teichroeb JA, Malone N (eds) (2019) Primate research and conservation in the Anthropocene. Cambridge University Press, Cambridge
Berman CM, Li J, Ogawa H, Ionica C, Yin H (2007) Primate tourism, range restriction, and infant risk among Macaca thibetana at Mt. Huangshan, China. Int J Primatol 28:1123–1141. https://doi.org/10.1007/s10764-007-9199-4
Boyle SA, Lourenço WC, Da Silva LR, Smith AT (2009) Home range estimates vary with sample size and methods. Folia Primatol (Basel) 80:33–42
Brady SP, Richardson JL (2017) Road ecology: shifting gears toward evolutionary perspectives. Front Ecol Environ 15:91–98
Brodie JF, Giordano AJ, Ambu L (2015) Differential responses of large mammals to logging and edge effects. Mamm Biol 80:7–13
Dias LG, Strier KB (2003) Effects of group size on ranging patterns in Brachyteles arachnoides hypoxanthus. Int J Primatol 24:209–221
Doran-Sheehy DM, Greer D, Mongo P, Schwindt D (2004) Impact of ecological and social factors on ranging in western gorillas. Am J Primatol 64:207–222. https://doi.org/10.1002/ajp.20075
Dore KM, Riley EP, Fuentes A (eds) (2017) Ethnoprimatology: A practical guide to research at the human-nonhuman primate interface. Cambridge University Press, Cambridge
Estrada A, Raboy BE, Oliveira LC (2012) Agroecosystems and primate conservation in the tropics: a review. Am J Primatol 74:696–711
Estrada A et al (2017) Impending extinction crisis of the world’s primates: why primates matter. Sci Adv 3:e1600946. https://doi.org/10.1126/sciadv.1600946
Fehlmann G, O’Riain M, Kerr-Smith C, King AJ (2017) Adaptive space use by baboons (Papio ursinus) in response to management interventions in a human-changed landscape. Anim Conserv 20:101–109
Fleming CH, Calabrese JM (2017) A new kernel density estimator for accurate home-range and species-range area estimation. Methods Ecol Evol 8:571–579
Fooden J (1969) Taxonomy and evolution of the monkeys of Celebes. Karger, Basel
Forman RT, Alexander LE (1998) Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–231
Fuentes A, Shaw E, Cortes J (2007) Qualitative assessment of macaque tourist sites in Padangtegal, Bali, Indonesia, and the Upper Rock Nature Reserve, Gibraltar. Int J Primatol 28:1143–1158
Germani L (2018) Female ano-genital swelling as a complex sexual signal: Morphological, behavioral, and hormonal correlates in wild Macaca maura. Roma Tre University, Rome
Gibson L, Koenig A (2012) Neighboring groups and habitat edges modulate range use in Phayre’s leaf monkeys (Trachypithecus phayrei crepusculus). Behav Ecol Sociobiol 66:633–643. https://doi.org/10.1007/s00265-011-1311-2
Goldsmith ML (1999) Ecological constraints on the foraging effort of western gorillas (Gorilla gorilla gorilla) at Bai Hoköu, Central African Republic. Int J Primatol 20:1–23
Gregory T, Mullett A, Norconk MA (2014) Strategies for navigating large areas: A GIS spatial ecology analysis of the bearded saki monkey, Chiropotes sagulatus, in Suriname. Am J Primatol 76:586–595
Hansen MF et al (2020) Comparative home range size and habitat selection in provisioned and non-provisioned long-tailed macaques (Macaca fascicularis) in Baluran National Park East Java, Indonesia. BRILL. https://doi.org/10.1163/18759866-bja10006
Hanson KT, Riley EP (2018) Beyond neutrality: The human–primate interface during the habituation process. Int J Primatol 39:852–877
Hill DA, Agetsuma N (1995) Supra-annual variation in the influence of Myrica rubra fruit on the behavior of a troop of japanese macaques in Yakushima. Am J Primatol 35:241–250
Hill R, Barrett L, Gaynor D, Weingrill T, Dixon P, Payne H, Henzi S (2003) Day length, latitude and behavioural (in) flexibility in baboons (Papio cynocephalus ursinus). Behav Ecol Sociobiol 53:278–286
Hockings KJ et al (2015) Apes in the anthropocene: Flexibility and survival. Trends Ecol Evol 30:215–222
Hoffman TS, O’Riain JS (2012) Troop size and human-modified habitat affect the ranging patterns of a chacma baboon population in the Cape Peninsula, South Africa. Am J Primatol 74:853–863
Ilham K, Rizaldi NJ, Tsuji Y (2018) Effect of provisioning on the temporal variation in the activity budget of urban long-tailed macaques (Macaca fascicularis) in West Sumatra, Indonesia. Folia Primatol (Basel) 89:347–356
Johnson C, Piel AK, Forman D, Stewart FA, King AJ (2015) The ecological determinants of baboon troop movements at local and continental scales. Mov Ecol 3:14
Kaburu SS et al (2019) Rates of human–macaque interactions affect grooming behavior among urban-dwelling rhesus macaques (Macaca mulatta). Am J Phys Anthropol 168:92–103
Klegarth AR et al (2017) Urban primate ranging patterns: GPS-collar deployments for Macaca fascicularis and M. sylvanus. Am J Primatol 79:e22633
Koirala S, Chalise MK, Katuwal HB, Gaire R, Pandey B, Ogawa H (2017) Diet and activity of Macaca assamensis in wild and semi-provisioned groups in Shivapuri Nagarjun National Park, Nepal. Folia Primatol (Basel) 88:57–74
Lappan S, Malaivijitnond S, Radhakrishna S, Riley EP, Ruppert N (2020) The human–primate interface in the new normal: Challenges and opportunities for primatologists in the COVID-19 era and beyond. Am J Primatol 82:e23176. https://doi.org/10.1002/ajp.23176
Laurance WF, Goosem M, Laurance SG (2009) Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol 24:659–669
Li B, Chen C, Ji W, Ren B (2000) Seasonal home range changes of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in the Qinling Mountains of China. Folia Primatol (Basel) 71:375–386
Loudon JE, Howells ME, Fuentes A (2006) The importance of integrative anthropology: A preliminary investigation employing primatological and cultural anthropological data collection methods in assessing human-monkey co-existence in Bali, Indonesia. Ecol Environ Anthropol 2:2–13
Lubis MI, Endarwin E, Riendriasari SD, Suwardiansah, Ul-Hasanah AU, Irawan F, Aziz KH, Malawi A (2008) Conservation of herpetofauma in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia. Conservation Leadership Programme, Bogor, Indonesia
Maréchal L, Semple S, Majolo B, MacLarnon A (2016) Assessing the effects of tourist provisioning on the health of wild Barbary macaques in Morocco. PLoS ONE 11:e0155920
Matsumura S (1991) A preliminary report of the ecology and social behavior of moor macaques (Macaca maurus) in Sulawesi, Indonesia. Kyoto Univ Overseas Res Rep Stud Asian Nonhum Primates 8:27–41
Matsumura S (1998) Relaxed dominance relations among female moor macaques (Macaca maurus) in their natural habitat, South Sulawesi, Indonesia. Folia Primatol (Basel) 69:346–356
McKinney T (2011) The effects of provisioning and crop-raiding on the diet and foraging activities of human-commensal white-faced capuchins (Cebus capucinus). Am J Primatol 73:439–448
McLennan MR, Spagnoletti N, Hockings KJ (2017) The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int J Primatol 38:105–121
Medway L (1972) Phenology of a tropical rain forest in Malaya. Biol J Linn Soc 4:117–146. https://doi.org/10.1111/j.1095-8312.1972.tb00692.x
Meijaard E, Albar G, Rayadin Y, Ancrenaz M, Spehar S (2010) Unexpected ecological resilience in Bornean orangutans and implications for pulp and paper plantation management. PLoS ONE 5:e12813
Milton K, May ML (1976) Body weight, diet and home range area in primates. Nature 259:459
Morrow KS (2018) Risky business: Causes and conservation implications of human-moor macaque (Macaca maura) interactions in South Sulawesi. San Diego State University, San Diego
Morrow KS, Glanz H, Ngakan PO, Riley EP (2019) Interactions with humans are jointly influenced by life history stage and social network factors and reduce group cohesion in moor macaques (Macaca maura). Sci Rep 9:20162. https://doi.org/10.1038/s41598-019-56288-z
Naughton-Treves L, Treves A, Chapman C, Wrangham R (1998) Temporal patterns of crop-raiding by primates: linking food availability in croplands and adjacent forest. J Appl Ecol 35:596–606
Noonan MJ et al (2019) A comprehensive analysis of autocorrelation and bias in home range estimation. Ecol Monogr 89:e01344
Okamoto K, Matsumura S (2002) Intergroup encounters in wild moor macaques. Primates 43:119–125
Okamoto K, Matsumura S, Watanabe K (2000) Life history and demography of wild moor macaques (Macaca maurus): summary of ten years of observations. Am J Primatol 52:1–11
Pebsworth PA, MacIntosh AJ, Morgan HR, Huffman MA (2012) Factors influencing the ranging behavior of chacma baboons (Papio hamadryas ursinus) living in a human-modified habitat. Int J Primatol 33:872–887
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2019) Nlme: linear and nonlinear mixed effects models. R package version 3.1–143, https://cran.R-project.Org/package=nlme.
Pragatheesh A (2011) Effect of human feeding on the road mortality of rhesus macaques on national highway-7 routed along Pench Tiger Reserve, Madhya Pradesh, India. J Threat Taxa 3:1656–1662
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.Org/.
Ram S, Venkatachalam S, Sinha A (2003) Changing social strategies of wild female bonnet macaques during natural foraging and on provisioning. Curr Sci 84:780–790
Reyna-Hurtado R et al (2018) Primates adjust movement strategies due to changing food availability. Behav Ecol 29:368–376
Riley EP (2007) The human–macaque interface: conservation implications of current and future overlap and conflict in Lore Lindu National Park, Sulawesi, Indonesia. Am Anthropol 109:473–484
Riley EP (2008) Ranging patterns and habitat use of Sulawesi tonkean macaques (Macaca tonkeana) in a human-modified habitat. Am J Primatol 70:670–679
Riley EP, Bezanson M (2018) Ethics of primate fieldwork: Toward an ethically engaged primatology. Annu Rev Anthropol 47:493–512. https://doi.org/10.1146/annurev-anthro-102317-045913
Riley EP, Wade TW (2016) Adapting to Florida’s riverine woodlands: The population status and feeding ecology of the silver river rhesus macaques and their interface with humans. Primates 57:195–210. https://doi.org/10.1007/s10329-016-0517-3
Riley EP, Tolbert B, Farida WR (2013) Nutritional content explains the attractiveness of cacao to crop raiding Tonkean macaques. Curr Zool 59:160–169
Riley EP, Sagnotti C, Carosi M, Oka NP (2014) Socially tolerant relationships among wild male moor macaques (Macaca maura). Behaviour 151:1021–1044
Sagnotti C (2013) Diet preference and habitat use in relation to reproductive states in females of a wild group of Macaca maura inhabiting Karaenta forest in South Sulawesi. Hasanuddin University, Makassar
Saj T, Sicotte P, Paterson JD (1999) Influence of human food consumption on the time budget of vervets. Int J Primatol 20:977–994
Santhosh K, Kumara HN, Velankar AD, Sinha A (2015) Ranging behavior and resource use by lion-tailed macaques (Macaca silenus) in selectively logged forests. Int J Primatol 36:288–310
Santillán-Doherty AM et al (2010) Novelty-seeking temperament in captive stumptail macaques (Macaca arctoides) and spider monkeys (Ateles geoffroyi). J Comp Psychol 124:211
Schmidt FH, Ferguson JH (1951) Rainfall types based on wet and dry period ratios for Indonesia with western New Guinea. Verh. Djawatan Met. dan Geofisik Djakarta 42
Seiler N, Robbins MM (2016) Factors influencing ranging on community land and crop raiding by mountain gorillas. Anim Conserv 19:176–188
Sengupta A, Radhakrishna S (2018) The hand that feeds the monkey: Mutual influence of humans and rhesus macaques (Macaca mulatta) in the context of provisioning. Int J Primatol 39:817–830
Sengupta A, McConkey KR, Radhakrishna S (2015) Primates, provisioning and plants: Impacts of human cultural behaviours on primate ecological functions. PLoS ONE 10:e0140961
Sha JCM, Hanya G (2013) Diet, activity, habitat use, and ranging of two neighboring groups of food-enhanced long-tailed macaques (Macaca fascicularis). Am J Primatol 75:581–592
Singh M, Erinjery JJ, Kavana TS, Roy K, Singh M (2011) Drastic population decline and conservation prospects of roadside dark-bellied bonnet macaques (Macaca radiata radiata) of southern India. Primates 52:149–154
Sinha A, Mukhopadhyay K, Datta-Roy A, Ram S (2005) Ecology proposes, behaviour disposes: ecological variability in social organization and male behavioural strategies among wild bonnet macaques. Curr Sci 89:1166–1179
Srivastava A, Das J, Biswas J, Buzarbarua P, Sarkar P, Bernstein IS, Mohnot SM (2001) Primate population decline in response to habitat loss: Borajan reserve forest of Assam, India. Primates 42:401–406
Stephens DW, Krebs JR (1987) Foraging theory. Princeton University Press, Princeton
Stone AI (2007) Responses of squirrel monkeys to seasonal changes in food availability in an eastern Amazonian forest. Am J of Primatol 69:142–157
Strum SC (2010) The development of primate raiding: implications for management and conservation. Int J Primatol 31:133–156
Strum SC (2012) Darwin’s monkey: why baboons can’t become human. Am J Phys Anthropol 149:3–23
Sueur C, Petit O (2008) Shared or unshared consensus decision in macaques? Behav. Processes 78:84–92
Tsuji Y, Su H-H (2018) Macaques as seed dispersal agents in Asian forests: a review. Int J Primatol 39:356–376
Tsuji Y, Takatsuki S (2009) Effects of yearly change in nut fruiting on autumn home-range use by Macaca fuscata on Kinkazan Island, Northern japan. Int J Primatol 30:169–181. https://doi.org/10.1007/s10764-009-9336-3
Unwin T, Smith A (2010) Behavioral differences between provisioned and non-provisioned Barbary macaques (Macaca sylvanus). Anthrozoös 23:109–118
Vedder AL (1984) Movement patterns of a group of free-ranging mountain gorillas (Gorilla gorilla beringei) and their relation to food availability. Am J Primatol 7:73–88
Watanabe K, Matsumura S (1996) Social organization of moor macaques, Macaca maurus, in the Karaenta Nature Reserve, South Sulawesi, Indonesia. In: Shotake T, Wada K (eds) Variations in the Asian macaques. Tokai University Press, Toyko, pp 147–162
Waterman J, Campbell L, Maréchal L, Pilot M, Majolo B (2019) Effect of human activity on habitat selection in the endangered Barbary macaque. Anim Conserv. https://doi.org/10.1111/acv.12543
Whitmore TC (1984) Tropical forests of the far east. Clarendon Press, Oxford
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Acknowledgements
We thank the Kementerian Negara Riset dan Teknologi Republik Indonesia (RISTEK) for permission to conduct research in Indonesia, Taman Nasional Bantimurung-Bulusaraung (TNBABUL) for permission to work in the park, and Universitas Hasanuddin for supporting the research. We are grateful to Pak Haro, Hendra, and Amir for their invaluable research assistance. We also thank Kate Jameson for her assistance in the field, Hendra and Lavinia Germani for their images, and Chaeril (TNBABUL) for producing the map of the study site. Funding for the research was provided by San Diego State University’s President’s Leadership Fund and University Grants Program (PI: Erin P. Riley) and the Italian Ministry of Education, University and Research (MIUR; PI: Monica Carosi). We would also like to thank the handling editor and the anonymous reviewers whose insights and useful comments helped improve this manuscript.
Funding
This study was funded by a SDSU President’s Leadership Fund grant, an SDSU University Grants Program award, and by a grant from the Italian Ministry of Education, University and Research.
Author information
Authors and Affiliations
Contributions
EPR originally formulated the idea; EPR, KSM, and CS conducted fieldwork; CAS and JST conducted the spatial analysis; CAS and EPR performed statistical analyses; EPR wrote the manuscript with input from CAS, KSM, CS, JTS, MC, and PON.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We consulted the ASP/IPS Code of Best Practices for Field Primatology when designing and conducting this research. The research complied with protocols approved by the SDSU Institutional Animal Care and Use Committee, IACUC (APF #14-03-006R) and adhered to the legal requirements for foreigners conducting research in Indonesia.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Riley, E.P., Shaffer, C.A., Trinidad, J.S. et al. Roadside monkeys: anthropogenic effects on moor macaque (Macaca maura) ranging behavior in Bantimurung Bulusaraung National Park, Sulawesi, Indonesia. Primates 62, 477–489 (2021). https://doi.org/10.1007/s10329-021-00899-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-021-00899-6