Abstract
The past few decades have seen a burgeoning of scientific studies on great apes’ use of nests for sleeping in the wild, as well as their nesting behavior and sleep in captivity. We review recent advances in knowledge of these topics, with the aim of promoting information exchange between people working in the field and with captive great apes. We trace developments in research into nest-building techniques in adults and immatures, factors that influence selection of general sleeping sites and specific locations, social aspects of sleep, postures, and nighttime activities. We argue that exchanges of information deriving from studies of captive and wild apes are valuable for obtaining a better understanding of sleep-related adaptations in our nearest evolutionary neighbors, and conclude by making some recommendations regarding sleeping arrangements in captivity from a welfare perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nightly construction of a “platform”, “bed”, “shelter”, or “nest” for sleeping on is a behavioral characteristic that distinguishes great apes from monkeys and the so-called “lesser” apes. Whereas monkeys and gibbons typically sleep at night in semi-upright or crouched postures, the larger-bodied chimpanzees, bonobos, gorillas, and orangutans sleep lying down, usually in the nest (the most commonly used term) that they have made from tree branches and leaves or terrestrial vegetation, depending on the nest location. For several decades after the first scientific descriptions of chimpanzee nests in western Africa by Nissen (1931) knowledge about great apes’ nesting activities accumulated slowly; early literature consisted mainly of simple descriptive accounts of nesting and sleep-related behavior, and using nest counts to estimate population densities. Over the last two decades, however, research on both wild and captive apes has progressed to the point where specific hypotheses are increasingly proposed and tested, for example about the accuracy of nest groups as indicators of population size/density, the precise functions of nests, why nests are used at all as opposed to simply sitting on or draping across branches, choice of nesting sites in relation to feeding sites, and factors that influence use of specific sites and locations (see Fruth et al. 2018 for a historical review of field observations).
Given their evolutionary closeness, great apes and humans are likely to share many if not most of the biological functions and correlates of sleep. For example, although humans are outliers in having reduced total sleep duration and a higher proportion of REM sleep than predicted, great apes show a resemblance in the latter characteristic (Nunn and Samson 2018). The importance of sleep for mental and physical health in humans is becoming clearer (Banks and Dinges 2007; Landolt et al. 2014), and comparative research is valuable for addressing many biological and medical questions concerning the evolution of sleep, its mechanisms, functions, and associated behaviors (Joiner 2016; McNamara et al. 2010). The aim of this paper is to review developments in research on nesting, nest use, and nighttime activities in great apes, underlining the complementarity of fieldwork and studies in captivity. Specifically, we consider the following topics comparing data from relevant fieldwork and research in captivity: development of nest-building skills in young individuals, factors influencing selection of nest sites and within-site nest locations, and the relationship between nighttime nesting arrangements and daytime social associations. We conclude by proposing that knowledge about nesting patterns and sleeping arrangements in the wild should feed into designs of housing quarters for great apes in captivity. Furthermore, greater integration of findings from studies in the field and captivity should help to strengthen the database for reconstructing the evolutionary history of hominin sleep profiles.
Natural nest building in great apes: adults and immatures
All normal, adult great apes living in a natural environment are likely to be skillful nest builders. However, young infants are not. Competent nest construction requires learned, complex behavioral sequences combined with sufficient strength to break and bend branches and twigs to form a platform that will safely accommodate a large-bodied ape. Reynolds (1965) gives the following example of nest construction by an adult male chimpanzee in the Budongo Forest (Uganda):
“Site selection was fascinating to watch: one chimp looked up into a sapling, climbed it, climbed down again, went up a bigger tree and out along a branch, and there decided to build. Sitting on the main branch, he pulled in surrounding leafy branches, working his way round in a circle and intertwining the branches, holding them in place beneath his feet. He did not break them off completely but just snapped them half through so that their resilience was gone but they were still firmly attached to the tree. After he had used every available branch at the site, his nest was still patchy, so he collected more branches from near by, breaking them off this time, and laid them on top, or wove them in a little, finally adding leafy twiglets to complete the big, firm structure in which he was going to sleep. He did this in about three or four minutes” (p. 128).
Reflecting the strong bond that exists between them, infant chimpanzees sleep with their mother in her nest every night. Goodall (1962) reported that juveniles at Gombe (Tanzania) were still sleeping in their mothers’ nests at 2.5 years of age; this was later pushed back to 3–4 years (van Goodall Lawick 1968). The youngest individual to try to make some kind of nest—playfully, on the ground—was 8 months old. One-year-olds made rudimentary nests in trees and sometimes briefly lay down in them, but they also often jumped up and down, resulting in the nests breaking. This sequence would sometimes be repeated—good practice for learning how sturdy nests should be when the youngsters eventually slept in their own, at 4 or 5 years of age.
According to Kano (1983), nest building by bonobos was “basically the same” as that described for chimpanzees by Goodall (1962). In the first detailed study of nest building in wild bonobos, Fruth and Hohmann (1993) reported that basic night nests were completed in approximately 4 min, although adjustments could be made even several hours after initial completion. At Lomako, immature bonobos initially attempted to make nests by bending and folding vegetation in front of them, rather than around them (Fruth and Hohmann 1994). These authors drew parallels between weaning and a mother denying an infant access to her nest, eventually resulting in independent nesting by the latter.
Adult gorillas typically finish building their night nests within a few minutes. In contrast to reports of complex intertwining of materials in some early accounts of chimpanzees’ nest construction, Schaller (1965) reported no “interlacing, knot-tying, or other involved manipulation” in nest making by mountain gorillas (p. 357). He described construction of a nest by a juvenile as follows:
“A juvenile sits at the base of a tree and bends four to five handfuls of small herbs toward its left side with the right hand. It then stands on two legs, grabs the top of a mass of Senecio trichopteryguis heavily overgrown with Galium, and pulls it in. It sits and breaks or bends the tips of the herbs to fit in a semicircle around its body before pressing the mass down with both hands. Standing on three legs, it reaches far out and breaks two to three more Senecio stalks off at the base and pulls them in. After placing these individually along the edge of the nest, it breaks their protruding tops to fit the rim. It sits, turns around, and sits again. The time required for building was about one minute” (p. 357-8).
Schaller (1965) noted that arboreal gorilla nests were usually more solidly built than ground nests, with more attention given to the bottom of arboreal nests when necessary. He also reported that two juvenile gorillas might occupy the same nest, although whether they actually slept together overnight was not confirmed. Like their chimpanzee counterparts, infant gorillas start to practice making nests before their first birthday, and they share their mother’s nest until she next gives birth, after which independent nesting and sleeping rise sharply, at around 3 years of age (Fossey 1979). The generality of reported gourd-shaped nests—consisting of a large nest (the mother’s) attached to a smaller nest (the juvenile’s) before the juvenile finally nests fully apart from the mother (Kawai and Mizuhara 1959) remains to be established.
Orangutans are the most arboreal of the great apes. Unlike chimpanzees (sometimes) and gorillas (frequently), they almost never make their night nests on the ground. van Casteren et al. (2012) described a typical nest-building episode by an orangutan as follows:
“After choosing a nest location on a lateral branch, or branches, the orangutan will bend and break branches inward toward a central point, weaving and twisting the branches to lock them into the basic nest structure. Layers are then generally added on top of this basic structure, in the form of smaller branches, bent, broken, and woven, forming a “mattress” or “rim.” Leafy branches are detached, usually from the surrounding area, and placed on top of the base structure as a lining. Extra features, such as a roof, “pillow,” or “blanket,” are then constructed and added if required by the individual… Orangutan nests have been described as sturdier, more complex and elaborate, and as lasting longer in the forest canopy, than those of African apes” (p. 6873).
In agreement with the suggestion of more complex nest constructions by orangutans compared to African apes, van Schaik (2004) reported that it may take up to 10 min for an orangutan to finish making a nest, almost twice as long as chimpanzees. Also, orangutans often make a roof-like structure above their night nest (especially in rainy conditions) and add a “pillow” of leafy twigs to the nest (MacKinnon 1974; Prasetyo et al. 2009). Like their African counterparts, 1-year-old orangutans play at nest building; they also watch their mothers’ nest building and may add some small pieces of vegetation to her nest (van van Noordwijk 2009). Although capable of building functional nests, 4- or 5-year-old juveniles may continue to sleep with their mother and her new baby (van Noordwijk and van Schaik 2005). Also like in chimpanzees (Clark 1977), refusals by an orangutan mother to accept her older juvenile in her nest may lead to tantrums in the latter (Horr 1977).
The descriptions above show some fundamental similarities but also differences in the making of night nests in great ape species, and differences between the crude initial structures made by youngsters and the finished items made by older, more experienced individuals. It is noteworthy, however, that detailed longitudinal studies of the ontogeny of nest-building skills are lacking for all great ape species, as are quantitative cross-species comparisons of nest architecture.
Responses to nesting materials in captive great apes
In a study of what captive chimpanzees did with nesting materials, Videan (2006a) described three nest-building techniques of increasing complexity: (1) arrange-and-tuck—individuals picked up handfuls of hay or browse and placed it around themselves, (2) outside-in—they took small amounts of hay from the outside edge and placed them on the inside edge of the nest, (3) bend-and-weave—they bent some of the nesting materials and “wove” them together. Videan observed that wild-born nest builders were more likely than captive-born counterparts to use all three techniques.
A group of chimpanzees at Edinburgh Zoo was given the following four pairs of potential nesting materials over a 2-month period: straw and eucalyptus branches (the standard materials), wood wool and cotton textiles (blankets, towels, clothes), hessian sacks and browse (locally collected branches and foliage), hay and paper sacks. Adequate amounts of each material were distributed throughout the indoor areas for each chimpanzee to make a nest and left for 1 week, with replenishment as necessary. Lock (2012) distinguished two basic nest-building techniques: simple transport or manipulation of materials then either simply lying down or making a rough mattress with no discernable shape, and a more complex technique involving gathering materials around the legs/lower torso, and pressing/tucking material around the body to form an oval or circular shape with a clear “cup” (center of nest) and “rim” (outside edge). “Complex nest” builders often formed the structure while turning around inside it, or tossed material over their head to add a soft layer or pillow. Lock (2012) reported individual differences in frequency of nest-building and specific techniques used. Table 1 shows the total number of nests that incorporated each of the materials provided; clear preferences emerged. Notably, no nests were made using only eucalyptus branches or cotton textiles, but these materials were often combined with the more preferred materials—straw and wood wool, respectively. These findings are similar to Videan’s (2006a) observations of captive chimpanzees making nests especially from hay, but rarely from paper or browse.
Lukas et al. (2003) described two components of nest building by captive lowland gorillas: gathering materials (again, only hay was used to make nests), and manipulating them. However, manipulation was subdivided into categories including, in descending order of occurrence: tucking beneath the body, fluffing up, parting on the ground with hands or legs, placing on head or body.
Samson and Shumaker (2015a) used infrared cameras to record five captive orangutans’ responses to nesting materials over 47 nights. Materials included straw, cardboard, shredded and full newspaper sheets, and textiles. Over a 15-month period, the orangutans used an average of 2.4 techniques (mostly “arrange” and “flatten”) and 7.5 different actions (mostly sit, lie, and stand on) to make a nest (or “platform”). The average time taken to complete a nest was 10.1 min, with individual differences in building time and nest complexity. All orangutans started by arranging a base of straw, to which a layer of cardboard/paper was often added, and then further materials might be tucked into form a pillow. In marked contrast to these apes, baboons housed in similar conditions were never seen to manipulate materials in any way that could be construed as making a nest (Samson and Shumaker 2015b).
The role of early experience: insights from captivity
Complementing reports from the field, observations in captivity have clarified some aspects of the ontogeny of nesting in great apes. At least four factors appear to contribute to developing competence in nest building: an inborn tendency to manipulate and bring objects toward the body, increasing physical strength to break and twist sturdier or more resistant vegetation, observational learning, and individual experience, i.e., trial and error. Schaller (1965) suggested the existence of an innate tendency to pull objects toward one’s own body, citing the case of a human-reared infant lowland gorilla named Goma. At almost 1 year of age, despite never having seen other apes making nests, Goma pulled down branches one by one, stood on them, and patted them down under and around her, similar to young apes in the wild (Lang 1962). An isolation-reared orangutan similarly arranged objects around himself and patted down materials with his hands (Lethmate 1977). However, some apes reared without access to nest-building models may never build nests. Bernstein (1962) provided wild- and captive-born adult chimpanzees with three sets of potential nesting materials. He reported that all wild-born chimpanzees made “good”-quality nests. In contrast, over 50% of captive-born chimpanzees never made nests, and most nests captive-borns did make were “crude”. The wild-born chimpanzees took on average 16 min to construct their good nests, considerably longer than reported in naturally free-ranging chimpanzees; this may be because the various materials provided were dispersed throughout the enclosure, meaning that the chimpanzees had to transport them to their chosen sleeping locations.
Other reports support the view that relevant experience during an early sensitive period increases the likelihood of developing nest-building skills. Carpenter (1937/1964) reported that two wild-captured juvenile gorillas transported to an American zoo “invariably” made night nests from straw, by pulling in and tucking the material under their bodies and then lying down in the resulting pile. Young orphaned, rehabilitant great apes made some nests but these were relatively unsophisticated, and often unusually close to or on the ground (e.g., Harrisson 1969; Carter 1981); the presence of an older, more experienced role-model led to improvements in released orphan chimpanzees’ nesting behaviors (Brewer 1978).
Drawing on Bernstein’s (1962) report of more and higher-quality nest building in wild- than captive-born chimpanzees, Videan (2006a) further explored the influence of social learning and early experience. She predicted that captive-born adult chimpanzees raised for at least 2 years by wild-born mothers would show more nest building and nest use than adults raised by humans for the same length of time. Studying 73 adult chimpanzees in two captive facilities, Videan (2006a) found that 85% of wild-born adults made nests within 5 min of receiving hay (fewer did so when paper or natural browse was provided instead). Overall, wild-born adults made and used nests more than captive-born adults. Among the latter, those raised by their biological mother for at least 2 years showed more nest building and use than those raised by humans. The most complex technique identified—“bend-and-weave”—was more typical of wild—than captive-born builders. Videan (2006a) raised the question of whether there might be a critical or sensitive period for learning how to make nests during the first few years of life. Such a period has been proposed for other aspects of great ape behavior, including chimpanzees’ learning how to crack open nuts with stone or wooden tools (Biro et al. 2003), and gorillas’ learning of complex feeding techniques (Byrne 2016).
Nesting sites and nest locations
In nature
Where free-ranging, diurnal primates sleep at night is determined by multiple factors, including staying safe from nocturnal predators, proximity to the last and the next day’s projected first feeding sites, visibility of the surrounding countryside, shelter from inclement weather and wind sway, likelihood of disturbance by other animals, and exposure to late afternoon and early morning sun (see Anderson 1984, 1998; Fruth and Hohmann 1996; Hernandez-Aiguilar et al. 2013; Stewart and Pruetz 2013). Increasing emphasis is also being placed on physical comfort in great apes’ choice of specific nest locations and the materials used (e.g., Fruth and Hohmann 1993; Stewart et al. 2007, 2018; Koops et al. 2012a, b; Zamma and Ihobe 2015). Leafy nests probably help to conserve body heat during the night, and in some environments nests may be built at heights or in specific tree species to reduce the chances of getting bitten by insects (Stewart 2011; Samson et al. 2013). Re-use of specific locations within trees may result in creation of especially suitable places to make nests as a result of permanent structural modifications from the breaking and deforming of branches (Stewart 2011).
Gorillas sleep on the ground more than other great apes; indeed in some areas terrestrial nests outnumber arboreal nests (e.g., Willie et al. 2014). Some gorillas sleep on patches of bare earth without making anything resembling a nest (e.g., Mehlman and Doran 2002). The latter authors emphasized the flexibility of gorillas’ nesting behavior and suggested possible “regional traditions.” Contrary to the previously accepted view that chimpanzees failed to make arboreal night nests only if they were injured, sick, or foraged for longer than normal, terrestrial night nesting has now been reported at several chimpanzee field sites. Terrestrial nesting appears more likely where predation risk is low (e.g., Pruetz et al. 2008), but this is not the only influencing factor; for example, some nests may be made on the ground for thermoregulatory reasons (Samson and Hunt 2012). Confirmation of ground nesting in Cameroon (Last and Muh 2013; Tagg et al. 2013) means that the phenomenon has now been reported in all three widely recognized subspecies. It is conceivable that ground-nesting has become part of some chimpanzee populations’ sleep-related culture, occurring habitually (e.g., Koops et al. 2007, 2012a, b). The recent description of some nests that appeared to be fixed by vegetation to the main tree for added support provides another tantalizing example of how flexible nesting-related behaviors in wild great apes may be (McLennan 2018).
In captivity
When given the choice, like their wild counterparts, captive great apes show preferences in where they sleep at night. Riss and Goodall (1976) studied a captive group of wild-born adolescent chimpanzees. Over a 3-month summer period, the apes’ locations were noted 15 min after the last one had apparently settled down for the night, again at 22.00 h and 24.00 h, and then again the following morning before they arose. This observation schedule spanned four consecutive phases: (1) three cages each with an elevated hutch were available, adjacent to the outdoor enclosure (the standard condition; 12 nights); (2) only the outdoor enclosure was available day and night (17 nights); (3) only one cage was available (5 nights); (4) three cages were again continuously available (17 nights). The authors reported that the chimpanzees were usually found in the morning in the same location where they had settled down the previous evening, always on elevated platforms, either outside or inside. In phase 2, when the chimpanzees had no access to the inside cages, they all settled down about 80 min later than normal. In phase 3, all six apes crowded into the single indoor cage that was available, even though the space available for sleeping was more restricted.
A group of four adult chimpanzees studied by Lock (2012) also always slept on indoor elevated platforms; the only exceptions occurred when an elderly female who was too weak to climb simply nested on the floor, and her group-mates did likewise (Anderson et al. 2010). In contrast, most of the chimpanzees studied by Videan (2006a, b) slept on the floor rather than on elevated shelves. She suggested that the front of the enclosure afforded greater visibility of the surrounding environment, hence the preference for the floor instead of the elevated shelves at the back of the enclosures. She also reported that two-thirds of nests were made in corners of the enclosure.
Lock and Anderson (2013) identified five potential general sleeping areas available to a zoo-housed group of chimpanzees; all were indoors, but varied in terms of physical features (amount of available space, presence of multiple floor levels and elevated sleeping baskets, elevated connecting tunnels, etc.). Over 29 nights, ten of the 11 chimpanzees showed highly significantly preferred sleeping sites, and several showed strong within-site location preferences. For example, individuals returned to a specific elevated basket or a specific spot on the floor each night (always on the highest of several levels within a room). These spatial preferences outranked social preferences in determining where the chimpanzees slept.
A zoo-housed group of 16–21 lowland gorillas showed stable individual preferences among the seven indoor connected rooms in which they slept at night (Weiche and Anderson 2007). Larger rooms were associated with larger mean nest clusters than smaller rooms: 4 and 2, respectively. The authors suggested that where the gorillas settled down for the night reflected strategic decisions that took into account comfort (climate), and visual access to the environment and other members of the group. In five captive gorilla groups studied by Lukas et al. (2003), nests were more likely to be on elevated structures than on the floor, regardless of age-sex class or rearing history of the individuals. In the same group, nesting off the ground increased in colder weather.
In contrast to the almost exclusively arboreal nesting reported for wild orangutans, the zoo-housed orangutans studied by Samson and Shumaker (2015a) made most of their night nests (77%) on the ground. Four of five individuals significantly preferred the ground, whereas the fifth preferred an elevated site; sleeping site preferences were generally stable. Although limited, observations of captive great apes provide further evidence of flexibility in aspects of sleep-related behavior; there is no single species- or genus-wide pattern for nest-building and nest use.
Nests and social dynamics
In nature
The number of nests at a sleeping site often but not always corresponds to the number of independent individuals traveling together during the day. Mountain gorilla groups were described as being more compact at night than during the day (Schaller 1963). However, given chimpanzees’ and bonobos’ fission–fusion social organization, these apes’ night-nest group sizes appear more variable (Goodall 1962; van Goodall Lawick 1968; Fruth and Hohmann 1994; Mulavwa et al. 2010). Orangutan nests are most frequently encountered alone, although independent juveniles traveling with their mother make their nests near to hers. In contrast to the popular earlier view that larger nest clusters of orangutans could arise from several adults converging on a prized feeding site, most clusters are now thought to arise from just one or two individuals remaining in the area for several days. Consort pairs typically nest close together: males nest below females, and unflanged males may also nest nearby (Prasetyo et al. 2009).
Apart from the closeness of juveniles’ nests to their mothers’ and between adult males and estrus females (Goodall 1962; Fruth and Hohmann 1993; van Schaik 2004; Prasetyo et al. 2009), there is little evidence of any regular patterns in the spatial layouts of ape night-nest groups. Schaller (1965) stated that most members of a mountain gorilla group nested near the silverback male, though not necessarily within sight. Like Schaller, other researchers have plotted the positions of gorilla nests to explore possible links with social organization (Kawai and Mizuhara 1959; Casimir 1979), but except for a tendency in younger blackback males to nest away from the main group, no overall clear picture has emerged. How social relationships might be reflected in the spatial layout of nests of sleeping parties needs further study in wild great apes.
In captivity
In Riss and Goodall’s (1976) study of six adolescent chimpanzees, two or more chimpanzees usually slept together in one cage, often though not always in physical contact with each other. The authors attributed such co-sleeping to the absence of rejection by their mothers when they were juveniles. Riss and Goodall (1976) reported that time of retiring for the night was highly synchronized in the group: only 5 min elapsed between the first and last individual to settle down; this was also generally true for leaving the sleeping location the next morning. Notably, sleeping partner preferences did not strongly correlate with daytime grooming or play partner preferences, and the dominant male slept near estrus females, as has been reported in the wild.
Similar to Riss and Goodall (1976) but unlike Videan (2006b), Lock and Anderson (2013) found no relationship between daytime social associations and sleeping associations (i.e., sleeping in the same general area) among females in a captive group of 11 chimpanzees. For males, however, there was a strong relationship, with the two highest-ranking adult males usually associating with each other both during the day and when they settled down for the night. Except for one mother–adult daughter pair there was no overall strong effect of kinship on the likelihood of occupying the same general sleeping site. Interestingly, however, each evening the group dispersed over the five available sleeping sites, with a maximum of six individuals occupying the same site, reminiscent of nest group sizes in the wild.
In contrast to the lack of continuity between daytime and nighttime associations in captive chimpanzees (Lock and Anderson 2013), continuity was observed in a captive group of lowland gorillas (Weiche and Anderson 2007). This was especially the case within matrilines and in two adult female–female dyads. In the same group, over the 5-year study period both the silverback and blackback male slept alone on many occasions.
Sleeping postures
Due to the lack of good nighttime observations of wild apes in their nests, little is known about preferred sleeping postures, typical number of awakenings, responses to disturbances, etc. Great apes often move around in their nest (Goodall 1962), and they may sit up before finally settling down to sleep, for example to huddle together (juvenile orangutans: Harrisson 1969), to hunch up in rain (chimpanzees: Goodall 1962), or for social grooming, play, or sex (bonobos: Fruth and Hohmann 1993, 1994). When asleep, a great ape usually lies recumbent in the nest. A supine posture is common shortly after settling (e.g., orangutans: van Schaik 2004), but this does not appear to the preferred sleeping posture (chimpanzees: Goodall 1962; mountain gorillas: Schaller 1963). Zamma and Ihobe (2015) suggest that lying supine may be associated with shallower sleep—wild chimpanzees moved more in this position than when lying on one side. Lying face down or on one side and drawing the limbs close to the body may help to conserve body heat as nighttime temperatures drop (Nissen 1931; Goodall 1962).
Because it is easier to get all-night behavioral records from video, better information about nighttime postures and postural changes is available for captive than for wild apes. In an early project, Freemon et al. (1970, 1971) reported similarities in biotelemetrically monitored sleep stages between juvenile chimpanzees and adult humans; they also noted that chimpanzees usually slept on their side or face down in a crouching-like position. Videan (2006b) confirmed that adult chimpanzees prefer lying on one side, but prone was less common than supine; the discrepancy between Videan’s (2006b) and Freemon et al.’s (1970) posture-related findings might reflect an age-related effect, a question for future study. Taken together with information from the wild, these observations suggest that adult chimpanzees do not typically spend most of the night lying supine.
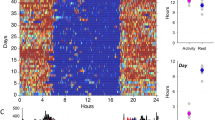
In a 29-night video study of nighttime behaviors in a captive group of four adult chimpanzees, Lock (2012) recorded between 2.5 and 6.5 postural changes between 17.00 h and 24.00, and 1.3 and 3.9 changes between midnight and 07.00 h. Lock’s data also appear to confirm a preference for sleeping while lying on one side (Table 2; Fig. 1); they also suggest possible lateral biases.
Nocturnal activities
Although sleep is the main nighttime business, wakeful activities do not entirely cease. Video traps recorded nocturnal terrestrial activities in 18 of 22 chimpanzee field sites, particularly where human activity levels were low, daytime temperatures were high, and forest predominated (Tagg et al. 2018). Nocturnal arboreal activities also occur. For example, wild apes may make nest adjustments or even new nests (Zamma and Ihobe 2015); urination and defecation occur, chimpanzees may sporadically exchange loud vocalizations during the night (Izawa and Itani 1966; Goodall 1962), and adult male gorillas may chest beat (Schaller 1963). Some feeding, mating, and traveling have been recorded in chimpanzees (van Goodall Lawick 1968; Nishida 1996). Zamma (2014) reported some activities every night in a 5-night study of Mahale chimpanzees; vocal exchanges were especially common, and were sometimes associated with sounds of locomotion. Recordings made over 250 days revealed that nocturnal calling rate in Issa Valley chimpanzees varied with nesting party size (Piel 2018). Zamma (2014) suggested that nighttime pant hoots might be used to maintain spatial contact with other chimpanzees if some individuals moved around at the night.
Like other primate species, chimpanzees may be more active in moonlight (Anderson 1984; Pruetz and Bertolani 2009; Pruetz 2018); activities include locomotion, foraging, and making use of nearby water (the latter behavior recorded by camera trap: Boyer-Ontl and Pruetz 2014 and direct observations: Pruetz 2018). However, Zamma (2014) reported considerable activity even during nights with no bright moonlight. Indeed, in another camera- and video-trap study, chimpanzees from one community at Kibale (Uganda) raided humans’ crop fields during darker nights rather than full-moon periods (Krief et al. 2014). It would be interesting to know if the chimpanzees returned to their night nests to eat the fruits of their raids.
Video recordings of small captive groups revealed that, like their wild counterparts, adult chimpanzees frequently woke up during the night—on average between 3 and 5 times (Videan 2006b). Waking periods, which accounted for an average of over 20 min each night, were associated with changing sleeping location, vigilance, foraging, drinking, occasional social grooming and even copulation. In the same study, unlike in humans, older adult chimpanzees slept for longer and had higher-quality sleep than younger adults. Videan (2006b) suggested that this human–chimpanzee difference might be related to relatively greater activity levels and social engagement in chimpanzees.
Lock (2012) reported large individual differences in frequency of leaving the nest and the elevated sleeping platforms in a small group of captive chimpanzees. All individuals sometimes left the sleeping platform before midnight and returned with more straw for their nest; such excursions and nest amendments declined markedly in the second half of the night. Among other nighttime activities analyzed by Lock (2012), self-grooming and self-manipulation (e.g., of the feet) occurred rarely, indeed not at all in the oldest female. The single adult male displayed aggressively on two consecutive nights, but without attacking anyone. Following both displays, the male groomed with his mother for several minutes before going back to sleep; other individuals also occasionally groomed each other.
Morimura et al. (2012) used overnight video recording to study three groups of adult male chimpanzees living in groups by day but separated in individual compartments at night. Marked individual differences in total sleep time and periods of wakefulness were evident, with notably disrupted sleep patterns in three males who were moved to another housing facility. In these latter three males, sleep returned to normal at 9, 11, and 21 days post relocation, respectively. Morimura et al. (2012) attributed the disruptive effect of relocation on sleep to stress. Another highly stressful event—the death of a core member of a small captive group of adult chimpanzees—resulted in delayed and disorganized nest building: the adult daughter of the deceased elderly female made only an unusually rudimentary nest, more than 90 min later than usual and near her mother’s body in a place where she never normally slept (Anderson et al. 2010). All three of the surviving chimpanzees showed above average numbers of postural changes during the night following the old female’s death, indicating disturbed sleep.
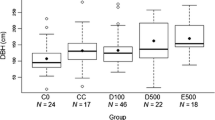
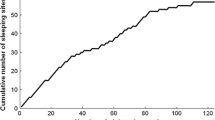
A possible influence of stress on nesting in zoo-housed lowland gorillas was reported by Ang (2012). Video recordings were used to compare the gorillas’ behavior at dusk and the following dawn after days of high vs. low visitor numbers. The author noted individual differences in nesting, and an almost total lack of nest making by the silverback, as in the wild. Concerning visitor influence, it took longer for adult females to finish making their nests at the end of days with high visitor numbers, especially nests made while sitting (Fig. 2). Furthermore, it took more time for all adults to settle down for the night following high vs. low visitor numbers (Fig. 3). These data suggest disrupted nesting following many daytime visitors, but more strikingly, changes in the adult females’ behavior were still detectable the following morning: while still in the nest they spent less time supine; they were also in the nest for less time, vacating the sleeping site earlier than on mornings following low visitor numbers.
Using infrared video recordings of four adults and one adolescent, Samson and Shumaker (2013) studied total sleep duration, sleep architecture, and overall quality of sleep in relation to nest complexity in captive orangutans. To do this, they focused on the apes’ eyes (open/closed) gross movements (present/absent), respiration (regular/irregular), eye movements (absent/present), vocalizations (absent/present) and muscle atonia/limb twitching (absent/present). Over a 1-year period, average total sleeping time was 9.1 h per night, composed of 8 h of non-rapid eye movement and 1 h of rapid eye movement sleep. Several nighttime activities were observed, including vocalizations (notably, long calls by the adult male, see Samson et al. 2014), vigilance, changes of location and even social behaviors. Lying on the left or right side was the most common sleep posture, followed by supine and ventral postures. Interestingly, measures of sleep fragmentation (number of awakenings per hour) and arousability (number of movements per hour) were negatively correlated with the complexity of the nest each orangutan had made to sleep in.
Great apes’ recumbent nighttime sleep postures contrast markedly with postures of monkeys: a comparison of captive orangutans and baboons showed that the latter slept mostly while sitting, their weight resting on the ischial callosities (Samson and Shumaker 2015b). Sleep was deeper and more efficient in the orangutans than the baboons. Furthermore, in total contrast to the orangutans, baboons usually slept huddling with other conspecifics (as do wild baboons, see Anderson and McGrew 1984). Overall, these data support the hypothesis that the emergence of nest building for achieving a comfortable night’s sleep was an important stage in the evolution of sleeping adaptations in large-bodied great apes.
Conclusions
As research on nesting and sleeping in great apes has increased in the last few decades, there has been a clear shift from general descriptions or using nest counts to estimate population densities, to systematic tests of specific hypotheses using both observational and experimentally derived data. A major development has been the introduction of some experiments in the field (e.g., testing the comfort of nests, their microclimates, or their insect-repellant properties), and more researchers are now working both in the field and with captive apes. This “crossing of boundaries” promises to deliver a more comprehensive understanding of sleep and sleep-related adaptations in humans’ nearest evolutionary relatives. In closing, we suggest how findings from captive studies might give new perspectives for field work, and vice versa, and consider the issue of sleeping arrangements as a welfare issue for captive great apes.
One topic for which captive studies have so far yielded higher-quality data than field studies is that of sleep postures. Thanks to infrared videography it has become clear that in captive great apes sleeping while lying on one side predominates, rather than supine or prone postures. This general finding remains to be confirmed in natural settings; to do so will probably require use of camera- or video traps positioned at great apes’ nighttime sleeping sites. It should also be noted that, to our knowledge, no study involving overnight recordings of either captive bonobos or gorillas has yet been published.
As described above, it is now established that nesting on the ground is common in some groups of chimpanzees, possibly associated with relaxed predator pressure and/or thermoregulatory factors. The observation that members of a captive group of chimpanzees nested on the ground to remain beside an elderly, ailing group member (Anderson et al. 2010) should alert field workers to another possibility: ground nesting as an empathy-based response to a sick or injured group-member’s inability to make an arboreal nest.
In accordance with the view that “lab and zoo workers should listen to field workers” (McGrew 1981 p. 139), several authors have argued that improvements in welfare might be achieved by applying information about nesting and sleeping in the wild to captivity (Anderson 1998; Lukas et al. 2003; Weiche and Anderson 2007; Lock and Anderson 2013; Samson and Shumaker 2015a). Multiple parameters now being studied in the wild might usefully be considered in the design of captive facilities: environmental disturbance levels, temperature variations, visibility, types of nesting materials and substrates, spatial layout of sleeping areas including vertical and horizontal dimensions, etc. However, we should not overlook an aspect of great ape nesting that is becoming increasingly clear: its flexibility. Despite probable innate influences on basic nest-building methods and site/location preferences, wild apes appear able to adapt their sleeping and nesting to a wide range of environmental and social conditions (see also McCarthy et al. 2017), although how sleep quality might vary as a function of sleeping environment remains unknown. Regarding captivity, however, it seems clear that multiple potential nest sites should be available. These should offer individuals a choice of sleeping at different heights, and the possibility of sleeping apart from others if desired. In the wild, nest site area size correlates positively with sleeping party size (e.g., gorillas: Mehlman and Doran 2002); therefore spatially restricted captive environments should be suitably equipped with screens or partitions that can serve as visual and “psychological” barriers at night as well as during the day.
Although some captive great apes readily sleep on the floor (e.g., orangutans: Samson and Shumaker 2015a), in one group of chimpanzees the preference was always for the highest floor area available in a multi-level enclosure (Lock and Anderson 2013). In view of the increasing number of reports of terrestrial nesting in wild African apes, we are quite relaxed about ground-sleeping in captivity. Indeed, in some facilities underfloor heating or deep litter, may make sleeping on the floor particularly attractive. Alternatively, depending on facility design there may be fewer drafts at ground level than higher up, or vice versa. Again, we emphasize choice. Some facilities provide elevated pods or baskets as potential sleep locations, with variable success: whereas some may be used regularly for nesting (see Fig. 1), others may be completely ignored. The pods shown in Fig. 4 were supposed to offer more naturalistic nesting opportunities to captive chimpanzees, but none has ever been used for sleeping in, probably because they are too small, too deep, and insufficiently firm. By contrast, the chimpanzees habitually sleep at night on the elevated, solid platforms that are also visible in Fig. 1 (Anderson et al. 2010). We suggest that there are now sufficient data available on nest architecture in the wild to improve the design of nest pods or baskets for captive apes, focusing on size, shape, sturdiness/softness, and thermoregulatory affordances.
Finally, despite numerous reports of preferences for particular kinds of nesting materials, captive great apes often construct nests that combine preferred materials with others (Videan 2006b; Lock and Anderson 2013; Lukas et al. 2003; Samson and Shumaker 2015a). We recommend that different combinations of potential nesting materials be provided with the proviso that at least one is known to be favored. Varying nesting materials can simulate the variety of vegetation types encountered in the wild, and might be considered as environmental enrichment if new behaviors are stimulated. Given the demonstrated relationship between nest complexity and sleep quality (Samson and Shumaker 2013), it would also be interesting to examine sleep quality in relation to daytime behaviors (normal and abnormal). For example, in view of the possible positive effects of sleep on great apes’ learning and retention of new information (see Martin-Ordas and Call 2011), future research could usefully ask about how the sleep environment and fluctuations in sleep quality impact on cognition and various other aspects of daily life, both in captivity and the wild.
References
Anderson JR (1984) Ethology and ecology of sleep in monkeys and apes. Adv Study Behav 14:165–229
Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46:63–75
Anderson JR, McGrew WC (1984) Guinea baboons (Papio papio) at a sleeping site. Am J Primatol 6:1–14
Anderson JR, Gillies A, Lock L (2010) Pan thanatology. Curr Biol 20:R349–R351
Ang MYL (2012) Visitor effects on crepuscular behaviour in a group of zoo-housed Western lowland gorillas (Gorilla g. gorilla). Unpublished dissertation, Veterinary School, University of Cambridge
Banks S, Dinges DF (2007) Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 3:519–528
Bernstein IS (1962) Response to nesting materials of wild born and captive born chimpanzees. Anim Behav 10:1–6
Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T (2003) Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim Cogn 6:213–223
Boyer-Ontl KM, Pruetz JD (2014) Giving the forest eyes: the benefits of using camera traps to study unhabituated chimpanzees (Pan troglodytes verus) in southeastern Senegal. Int J Primatol 35:881–894
Brewer S (1978) The forest dwellers. Collins, London
Byrne RW (2016) Evolving insight. Oxford University Press, Oxford
Carpenter CR (1937/1964) An observational study of two captive mountain gorillas. In: Carpenter CR (ed) Naturalistic behavior of nonhuman primates. Pennsylvania State University Press, University Park, pp 106–121
Carter J (1981) A journey to freedom. Smithsonian 12:90–101
Casimir MJ (1979) An analysis of gorilla nesting sites of the Mt. Kahuzi Region (Zaïre). Folia Primatol 32:290–308
Clark CB (1977) A preliminary report on weaning among chimpanzees of the Gombe National Park, Tanzania. In: Chevalier-Skolnikoff S, Poirier FE (eds) Primate bio-social development: biological, social and ecological determinants. Garland Publishing, New York, pp 235–260
Fossey D (1979) Development of the mountain gorilla (Gorilla gorilla beringei): the first thirty-six months. In: Hamburg DA, McCown ER (eds) The great apes. Benjamin/Cummings, Menlo Park, pp 139–184
Freemon FR, McNew JJ, Adey WR (1970) Sleep of unrestrained chimpanzee: differences between first and last rapid eye movement periods. Folia Primatol 13:144–149
Freemon FR, McNew JJ, Adey WR (1971) Chimpanzee sleep stages. Electroenceph Clin Neurophysiol 31:485–489
Fruth B, Hohmann G (1993) Ecological and behavioral aspects of nest building in wild bonobos (Pan paniscus). Ethology 94:113–126
Fruth B, Hohmann G (1994) Comparative analyses of nest-building behavior in bonobos and chimpanzees. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (eds) Chimpanzee cultures. Harvard University Press, Cambridge, pp 109–128
Fruth B, Hohmann G (1996) Nest building behavior in the great apes: the great leap forward? In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge University Press, Cambridge, pp 225–240
Fruth B, Tagg N, Stewart F (2018) Sleep and nesting behavior in primates: a review. Am J Phys Anthropol 166:499–509
Goodall JM (1962) Nest building behavior in the free ranging chimpanzee. Ann NY Acad Sci 102:455–467
Harrisson B (1969) The nesting behaviour of semi-wild juvenile orang-utans. Sarawak Museum J 17:336–384
Hernandez-Aiguilar RA, Moore J, Stanford CB (2013) Chimpanzee nesting patterns in savanna habitat: environmental influences and preferences. Am J Primatol 75:979–994
Horr DA (1977) Orang-utan maturation: growing up in a female world. In: Chevalier-Skolnikoff S, Poirier FE (eds) Primate bio-social development: biological, social and ecological determinants. Garland Publishing, New York, pp 289–321
Izawa K, Itani J (1966) Chimpanzees in Kasakati Basin, Tanganyika (1) ecological study in the rainy season 1963–1964. Kyoto Univ Afr Stud 1:73–156
Joiner WJ (2016) Unraveling the evolutionary determinants of sleep. Curr Biol 26:R1073–R1087
Kano T (1983) An ecological study of the pygmy chimpanzees (Pan paniscus) of Yalosidi, Republic of Zaire. Int J Primatol 4:1–31
Kawai M, Mizuhara H (1959) An ecological study on the wild mountain gorilla (Gorilla gorilla beringei): report of the JMC 2nd gorilla expedition 1959. Primates 2:1–42
Koops K, Humle T, Sterck EHM, Matsuzawa T (2007) Ground-nesting by the chimpanzees of the Nimba Mountains, Guinea: environmentally or socially determined? Am J Primatol 69:407–419
Koops K, McGrew WC, de Vries H, Matsuzawa T (2012a) Nest-building by chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: antipredation, thermoregulation, and antivector hypotheses. Int J Primatol 33:356–380
Koops K, McGrew WC, Matsuzawa T, Knapp LA (2012b) Terrestrial nest-building by wild chimpanzees (Pan troglodytes): implications for the tree-to-ground sleep transition in early hominins. Am J Phys Anthropol 148:351–361
Krief S, Cibot M, Bortolamiol S, Seguya A, Krief J-M, Masi S (2014) Wild chimpanzees on the edge: nocturnal activities in croplands. PLoS One 9(10):e109925. https://doi.org/10.1371/journal.pone.0109925
Landolt HP, Sousek A, Holst SC (2014) Effects of acute and chronic sleep deprivation. In: Bassetti CL, Dogaš Z, Peigneux P (eds) ESRS European sleep medicine textbook, Ed. 1. European Sleep Research Society, Basel, pp 49–62
Lang EM (1962) Goma, the baby gorilla. Victor Gollancz Ltd, London
Last C, Muh B (2013) Effects of human presence on chimpanzee nest location in the Lebialem-Mone Forest landscape, Southwest Region, Cameroon. Folia Primatol 84:51–63
Lethmate J (1977) Nestbauverhalten eines isoliert aufgezogenen, junger Orang-Utans. Primates 8:545–554
Lock LC (2012) Nesting and nighttime behaviours of captive chimpanzees (Pan troglodytes). Unpublished PhD thesis, University of Stirling
Lock LC, Anderson JR (2013) Kin, daytime associations, or preferred sleeping sites? Factors influencing sleep site selection in captive chimpanzees (Pan troglodytes). Folia Primatol 84:158–169
Lukas KE, Stoinski TS, Burks K, Snyder R, Bexell S, Maple TL (2003) Nest building in captive Gorilla gorilla gorilla. Int J Primatol 24:103–124
MacKinnon J (1974) The behaviour and ecology of wild orang-utans (Pongopygmaeus). Anim Behav 22:3–74
Martin-Ordas G, Call J (2011) Memory processing in great apes: the effect of time and sleep. Biol Lett 7:829–832
McCarthy MS, Lester JD, Stanford CB (2017) Chimpanzees (Pan troglodytes) flexibly use introduced species for nesting and bark feeding in a human- dominated environment. Int J Primatol 38:321–337
McGrew WC (1981) Social and cognitive capabilities of nonhuman primates: lessons from the wild to captivity. Int J Stud Anim Prob 2:138–149
McLennan MR (2018) Tie one on: ‘nest tying’ by wild chimpanzees at Bulindi—a variant of a universal great ape behavior? Primates 59:227–233
McNamara P, Barton RA, Nunn CL (2010) Evolution of sleep: phylogenetic and functional perspectives. Cambridge University Press, New York
Mehlman PT, Doran DM (2002) Influencing western gorilla nest construction at Mondika Research Center. Int J Primatol 23:1257–1275
Morimura N, Fujisawa M, Mori Y, Teramoto M (2012) Environmental influences on sleep behavior in captive male chimpanzees (Pan troglodytes). Int J Primatol 33:822–829
Mulavwa MN, Yangozene K, Yamba-Yamba M, Motema-Salo B, Mwanza NN, Furuichi T (2010) Nest groups of wild bonobos at Wamba: selection of vegetation and tree species and relationships between nest group size and party size. Am J Primatol 72:575–586
Nishida T (1996) Review of recent findings on Mahale chimpanzees: implications and future research directions. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (eds) Chimpanzee cultures. Harvard University Press, Cambridge, MA, pp 373–396
Nissen HW (1931) A field study of the chimpanzee: observations of chimpanzee behavior and environment in western French Guinea. Comp Psychol Monogr 8(36):1
Nunn CL, Samson DR (2018) Sleep in a comparative perspective: investigating how human sleep differs from sleep in other primates. Am J Phys Anthropol 166:601–612
Piel AK (2018) Temporal patterns of chimpanzee loud calls in the Issa Valley, Tanzania: evidence of nocturnal acoustic behavior in wild chimpanzees. Am J Phys Anthropol 166:530–540
Prasetyo D, Ancrenaz M, Morrogh-Bernard HC, Utami Atmoko SS, Wich SA, van Schaik CP (2009) Nest building in orangutans. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 269–277
Pruetz JD (2018) Nocturnal behavior by a diurnal ape, the West African chimpanzee (Pan troglodytes verus), in a savanna environment at Fongoli, Senegal. Am J Phys Anthropol 166:541–548
Pruetz JD, Bertolani P (2009) Chimpanzee (Pan troglodytes verus) behavioral responses to stresses associated with living in a savanna-mosaic environment: implications for hominin adaptations to open habitats. PaleoAnthropology 2009:252–262. https://doi.org/10.4207/PA.2009.ART33
Pruetz JD, Fulton SJ, Marchant LF, McGrew WC, Schiel M, Waller M (2008) Arboreal nesting an anti-predator adaptation by savanna chimpanzees (Pan troglodytes verus) in southeastern Senegal. Am J Primatol 70:393–401
Reynolds V (1965) Budongo: a forest and its chimpanzees. Methuen & Co., Ltd, London
Riss D, Goodall J (1976) Sleeping behavior and associations in a group of captive chimpanzees. Folia Primatol 25:1–11
Samson DR, Hunt KD (2012) A thermodynamic comparison of arboreal and terrestrial sleeping sites for dry-habitat chimpanzees (Pan troglodytes schewinfurthii) at the Toro-Semliki Wildlife Reserve, Uganda. Am J Primatol 74:811–818
Samson DR, Hurst D (2014) Shumaker RW (2014) Orangutan night-time long call behavior: sleep quality costs associated with vocalizations in captive Pongo. Adv Zool 2014. https://doi.org/10.1155/2014/101763
Samson DR, Shumaker RW (2013) Documenting orang-utan sleep architecture: sleeping platform complexity increases sleep quality in captive Pongo. Behaviour 150:845–861
Samson DR, Shumaker R (2015a) Pre-sleep and sleeping platform construction behavior in captive orangutans (Pongo spp.): implications for ape health and welfare. Folia Primatol 86:187–202
Samson DR, Shumaker RW (2015b) Orangutans (Pongo spp.) have deeper, more efficient sleep than baboons (Papio papio) in captivity. Am J Phys Anthropol 157:421–427
Samson DR, Muehlenbein MP, Hun KD (2013) Do chimpanzees (Pan troglodytes schweinfurthii) exhibit sleep related behaviors that minimize exposure to parasitic arthropods? A preliminary report on the possible anti-vector function of chimpanzee sleeping platforms. Primates 5:73–80
Schaller G (1963) The mountain gorilla: ecology and behavior. University of Chicago Press, Chicago
Schaller GB (1965) The behavior of the mountain gorilla. In: DeVore I (ed) Primate behavior: field studies of monkeys and apes. Holt Rinehart and Winston, New York, pp 324–367
Stewart FA (2011) Why sleep in a nest? Empirical testing of the function of simple shelters made by wild chimpanzees. Am J Phys Anthropol 146:313–318
Stewart FA, Pruetz JD (2013) Do chimpanzee nests serve an anti-predatory function? Am J Primatol 76:593–604
Stewart F, Pruetz J, Hansell M (2007) Do chimpanzees build comfortable nests? Am J Primatol 69:930–939
Stewart FA, Piel AK, Azkarate JC, Pruetz JD (2018) Savanna chimpanzees adjust sleeping nest architecture in response to local weather conditions. Am J Phys Anthropol 166:549–562
Tagg N, Willie J, Petre C-A, Haggis O (2013) Ground night nesting in chimpanzees: new insights from central chimpanzees (Pan troglodytes troglodytes) in south-east Cameroon. Folia Primatol 84:362–383
Tagg N, McCarthy M, Dieguez P, Bocksberger G, Willie J et al (2018) Nocturnal activity in wild chimpanzees (Pan troglodytes): evidence for flexible sleeping patterns and insights into human evolution. Am J Phys Anthropol 166:510–529
van Casteren A, Sellers WI, Thorpe SKS, Coward S, Crompton RH et al (2012) Nest-building orangutans demonstrate engineering know-how to produce safe, comfortable beds. Proc Natl Acad Sci USA 109:6873–6877
van Goodall Lawick J (1968) The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav Monogr 1:161–311
van Noordwijk MA (2009) Development of independence. In: Wich SA, Utami Atmoko SS, Mitra Setia T, van Schaik CP et al (eds) Orangutans: geographic variation in behavioral ecology and conservation. Oxford University Press, Oxford, pp 189–203
van Noordwijk MA, van Schaik CP (2005) Development of ecological competence in Sumatran orangutans. Am J Phys Anthropol 127:79–94
van Schaik C (2004) Among orangutans: red apes and the rise of human culture. Harvard University Press, Cambridge
Videan EN (2006a) Bed-building in captive chimpanzees (Pan troglodytes): the importance of early rearing. Am J Primatol 68:745–751
Videan EA (2006b) Sleep in captive chimpanzee (Pan troglodytes): the effects of individual and environmental factors on sleep duration and quality. Behav Brain Res 169:187–192
Weiche I, Anderson JR (2007) Influence of social and environmental factors on nesting behaviour in captive gorillas (Gorilla gorilla gorilla). Folia Primatol 78:154–165
Willie J, Tagg N, Petre C-A, Pereboom Z, Lens L (2014) Plant selection for nest building by western lowland gorillas in Cameroon. Primates 55:41–49
Zamma K (2014) What makes wild chimpanzees wake up at night? Primates 55:51–57
Zamma K, Ihobe H (2015) Bed making and nocturnal behavior. In: Nakamura M, Hosaka K, Itoh N, Zamma K (eds) Mahale chimpanzees: 50 years of research. Cambridge University Press, Cambridge, pp 583–598
Acknowledgements
All studies cited and conducted by the authors of this article received ethical approval from relevant committees and adhered to the IPS International Guidelines for the Acquisition, Care, and Breeding of Nonhuman Primates, Second Edition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Anderson, J.R., Ang, M.Y.L., Lock, L.C. et al. Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 60, 321–332 (2019). https://doi.org/10.1007/s10329-019-00723-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-019-00723-2