Abstract
Kinship plays an important role in the social behavior of many primate species, including patterns of intra-group affiliation and cooperation. Within social groups, kinship is strongly affected by dispersal patterns, with the degree of relatedness among group-mates expected to decrease as the tendency to disperse increases. In primate species characterized by bisexual dispersal, relatedness among adult group-mates is predicted to be low, with social interactions shaped largely by factors other than kinship. To date, however, few studies have examined the role of kinship in social interactions in bisexually dispersing species. Accordingly, we collected genetic, spatial and behavioral data on all adult members (three males, six females) in a group of free-ranging mantled howler monkeys (Alouatta palliata) — a bisexually dispersing species of atelid primate — from Barro Colorado Island (BCI), Panama. Analyses of microsatellite variation revealed that relatedness was greater among adult males in this group (mean pairwise relatedness = 0.32 for males versus 0.09 for females). Relatedness among individuals, however, was not associated with either spatial proximity or frequency of social interactions. Instead, sex was a better predictor of both of these aspects of social behavior. While relatedness among adults had no discernible effect on the intra-group social interactions documented in this study, we postulate that kinship may facilitate affiliative and cooperative behaviors among male group-mates when interacting competitively with neighboring howler groups over access to food or potential mates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social interactions are a fundamental component of the lives of group-living primates (Smuts et al. 1987). In many species, kinship among group-mates appears to play a critical role in shaping social behavior, with related individuals being more likely to display affiliative and cooperative behavior and less likely to engage in agonistic interactions with one another (Hamilton 1964; Greenwood 1980; Hinde 1983; Trivers 1985; Chapais and Berman 2004). Kinship within social groups is strongly affected by dispersal patterns (Melnick and Pearl 1987; Pusey and Packer 1987; Smuts 1987), with the degree of relatedness among individuals expected to decrease as the tendency to disperse increases (Melnick and Hoelzer 1996). Most social mammals, including most primates, are characterized by male-biased dispersal (Greenwood 1980; Dobson 1982; Pusey and Packer 1987; Clutton-Brock 1989), leading to the expectation that kinship and hence affiliative behavior should be greater among female group-mates.
In contrast, comparatively little is known about patterns of kinship and social affiliation in primate species characterized by bisexual dispersal, in which most male and female offspring leave the natal group. Although bisexual dispersal is common in monogamous primates, it appears to be relatively rare among polygynous or polygynandrous primate species living in social groups composed of multiple adult males and females (Glander 1992). Among the non-monogamous primate taxa believed to display bisexual dispersal are multiple species of howler monkeys (genus Alouatta). Evidence for bisexual dispersal in howler monkeys includes direct observation of emigration and inter-group transfers by members of both sexes, i.e., A. palliata (Glander 1980, 1992; Jones 1980, 1999; Crockett 1984; Crockett and Pope 1993; Clarke and Glander 2004, 2008), A. arctoidea (Pope 1992, 1998; Crockett and Pope 1993), A. pigra (Brockett et al. 2000; Ostro et al. 2001; Kitchen 2004; Van Belle and Estrada 2008; Van Belle et al. 2014a), A. guariba (Miranda and Passos 2005), A. caraya (Rumiz 1990; Calegaro-Marques and Bicca-Marques 1996; Oklander et al. 2010), as well as observations of aggressive interactions leading to the forced eviction of individuals of one or both sexes from the natal group, i.e., A. palliata (Glander 1992), A. arctoidea (Crockett 1984; Pope 1998), A. pigra (Brockett et al. 2000), A. caraya (Rumiz 1990; Calegaro-Marques and Bicca-Marques 1996). Accordingly, within social groups of Alouatta, adults are generally not expected to be closely related (Glander 1980; Pope 1996), leading to the prediction that kin-based affiliative interactions, particularly among same-sex adults, should be relatively rare (Glander 1980, 1992; Zucker and Clarke 1998; Bezanson et al. 2008; Clarke and Glander 2008).
Although genetic analyses of relatedness have been completed for several species of howler monkeys, available data provide potentially contradictory information regarding patterns of kinship among adult group-mates. For example, genetic data from Venezuelan howler monkeys (A. arctoidea) indicate that within each sex, adult group-mates are often closely related to one another (e.g., father-son or mother-daughter; Pope 1990, 1992; Crockett and Pope 1993). In contrast, in Mexican black howler monkeys (A. pigra), adult female group-mates are more closely related than are adult males, with opposite-sex kin rarely residing in the same group (Van Belle et al. 2014a). Among black-and-gold howler monkeys (A. caraya) from northern Argentina, genetic patterns of kinship appear to vary with habitat type; while animals in continuous forest habitat exhibit no close kinship among adult group-mates of either sex, animals living in more fragmented habitat display close intra-group kinship among females (Oklander et al. 2010). Thus, available data reveal no consistent relationship between bisexual dispersal and kinship among adults within social groups of howler monkeys.
The population of mantled howler monkeys (A. palliata) on Barro Colorado Island (BCI), Panama, provides a particularly valuable opportunity to examine relationships among patterns of dispersal, kinship and social interactions in a bisexually dispersing species of Alouatta. The BCI population has been the subject of extensive behavioral and ecological research (e.g., Carpenter 1934, 1964; Hladik 1978; Smith 1977; Milton 1980, 1996). While most species of Alouatta tend to live in small groups that contain only a single adult male and one to a few adult females (Chapman and Balcomb 1998; Di Fiore et al. 2011), A. palliata routinely lives in larger social groups (≥10 individuals: Chapman and Balcomb 1998). On BCI, group sizes tend to be particularly large, with a mode of ~19 animals per group, including multiple adults of both sexes (Carpenter 1934, 1964; Milton 1982, 1996; Wang and Milton 2003; Ryan et al. 2008). Dispersal between groups has been recorded for males; although direct evidence of dispersal by females is limited, population-level analyses of genetic variation indicate that females routinely disperse among groups (Milton et al. 2009), leading us to predict that adult group-mates should not be closely related (Carpenter 1934; Milton 1980). Social interactions among adult group-mates, however, are generally amicable (Milton 1980), a pattern that is more typically associated with groups composed of kin. Collectively, these data suggest that the BCI population of mantled howlers offers a particularly rich opportunity to examine the role of kinship in social interactions within a species of bisexually dispersing primate.
To explore relationships among dispersal, kinship, and social behavior in mantled howler monkeys, we used molecular data to assess genetic relatedness among all adult members of a group of A. palliata resident on BCI. Although a previous study of the BCI population had revealed that, at the population level, genetic relatedness was greater among males than females (Milton et al. 2009), no analyses have been conducted that characterize kinship among all adults in the same social group. Estimates of genetic relatedness were analyzed in conjunction with records of spatial and behavioral interactions to assess the role of kinship in shaping social relationships among adult group-mates. Specifically, we sought to test the hypothesis that apparent bisexual dispersal in this population is associated with low levels of kinship among same-sex adults and, thus, that kinship is not a critical predictor of intra-group social interactions. Despite extensive research on the ecology and foraging behavior of howler monkeys, this study is one of the first to use independent genetic estimates of kinship to examine the factors that shape intra-group spatial and social relationships in the genus Alouatta.
Methods
Study Site
The study was conducted on Barro Colorado Island (BCI) Panama from 10 June to 27 August 2007, which represents the mid-rainy season at this location (Milton 1980; Leigh et al. 1982). BCI is a densely forested 1500-ha nature reserve located in Lake Gatun, a large freshwater lake that serves as the principal water source for the Panama Canal. Detailed descriptions of the climate and topography of the island and of its flora and fauna can be found in Leigh et al. (1982). The island is the site of a permanent field research station maintained by the Smithsonian Tropical Research Institute (STRI).
Study population
The population of mantled howler monkeys (Alouatta palliata) on BCI was established in 1914 when an unknown number of individuals already resident at this location became isolated from the surrounding mainland by the damming of the Rio Chagres to create Lake Gatun (Carpenter 1934; Milton 1980, 1996; Leigh et al. 1982). On BCI, howler groups contain an average (±1 SD) of 3.1 (±1.2) adult males and 8.6 (±3.1) adult females (Milton 1996), with total group size (including juveniles and infants) averaging 19.4 (±6.3) individuals (Milton 1996; see also Carpenter 1934, 1964; Milton 1982; Wang and Milton 2003; Ryan et al. 2008; Di Fiore et al. 2011). Currently, the BCI howler population is estimated at ~1200 individuals distributed more or less uniformly across the island in 60–70 social groups (Milton 1982, 1996; Milton et al. 2009).
Social structure and dispersal patterns
Home range sizes for social groups of howler monkeys on BCI average ~31 ha (Milton 1980). Although home ranges for neighboring groups overlap extensively, groups typically exhibit strong mutual antipathy (Carpenter 1934; Milton 1980). On BCI, the characteristic sonorous howling vocalization produced by adult males appears to function primarily to announce group location, maintain spatial segregation of groups, defend group access to contested food resources and discourage encroachment by extra-group males (Carpenter 1934; Milton 1980; Hopkins 2013). Dispersal in this population has been characterized as bisexual based on genetic analyses of relatedness that suggest regular movement among social groups by males and females (Milton et al. 2009). Consistent with this, dispersal by males has been observed frequently on BCI (Carpenter 1934; Milton 1980; Milton et al. 2009). In contrast, however, despite hundreds of hours of observations — and in striking contrast to observations of A. palliata in Costa Rica (Glander 1992; Clarke and Glander 2004), A. arctoidea in Venezuela (Pope 1992, 2000), A. caraya in Argentina (Oklander et al. 2010) and A. pigra in Mexico (Van Belle et al. 2014a) — dispersal by females has not been observed, nor have lone female howlers been detected in the BCI population. Thus, although dispersal in this population is considered bisexual, the prevalence of this behavior may differ between males and females.
Focal study group
Although a previous population-wide study of genetic variability among BCI howler monkeys (Milton et al. 2009) generated genotypes for individuals distributed among multiple social groups on the island, no previous studies of these animals have examined genetic structure and relatedness among all adults within the same social group. Thus, to examine the role of kinship in social relationships among adult group-mates, it was necessary to integrate detailed behavioral and genetic data for the same animals. To maximize the behavioral data obtained, the group selected for study was chosen for its proximity to the research station on BCI. Approximately seven groups are known to use this portion of the island; we selected the first group that we encountered at the beginning of the study, which differed from the group monitored by Wang and Milton (2003).
Initially, the focal study group contained three adult males, six adult females, four juveniles and two infants; no emigration from or immigration to the focal group was observed during the study, although one infant was born during this period, raising the total number of immature animals to seven. To explore the potential effects of natal dispersal on kinship and social behavior, we focused our data collection efforts on fully adult individuals. Prior to beginning behavioral observations, the animals to be monitored were captured (see below) and individually marked with a PIT tag implanted at the nape of the neck. For rapid visual identification, each animal was fitted with a stainless steel earring containing a uniquely colored plastic bead and distinctive patches of its pelage were bleached using a commercially purchased human hair product. All work was conducted in compliance with national and institutional regulations and adhered to the American Society of Mammalogists’ guidelines for research involving live mammals (Sikes and Gannon 2011) and the legal requirements of the Republic of Panama.
Spatial associations
Bezanson et al. (2008) noted that although mantled howler monkeys live in large, cohesive multi-male-multi-female groups, direct social interactions among adult group-mates are relatively rare, accounting for <2 % of the daily activity budget of these animals. Given the rarity of direct social interactions, these authors suggested that for howler monkeys, spatial relationships provide the best means of assessing associations among group-mates (Crockett and Eisenberg 1987; Bezanson et al. 2008). The spatial placement of animals within a group is indicative of the nature and strength of social relationships among individuals, with greater proximity typically signaling a stronger social bond (White and Chapman 1994). To obtain data on spatial associations, scan sampling (Altmann 1974) was used to characterize proximity among adults in the focal study group. At 10-min intervals, for each marked individual that was visible, we recorded the identity of its nearest neighbor (McCann and Rothman 1999) and the estimated distance category between that pair of animals. For convenience, inter-individual distances were recorded as belonging to one of the following categories: 0 (animals in physical contact with each other), 1 (≤1 m apart), 2 (>1 to ≤5 m apart), 3 (>5 to ≤10 m apart), 4 (>10 to ≤15 m apart), or 5 (no other marked individual within 15 m; i.e., no nearest neighbor). Thus, individuals observed during a scan but located >15 m from any adult group-mate were recorded as category 5. Scan sampling of nearest neighbor distances was conducted between 0600 and 1800 throughout each week of the study, often in conjunction with focal animal observations (see below).
Social interactions
Focal animal observations (Altmann 1974) were used to characterize social interactions among members of the focal study group. The activities recorded were defined by Wang and Milton (2003) and included displacements of an animal (i.e., one individual moves toward another, causing the first individual to relocate) as well as other agonistic interactions such as grabs, pushes, slaps, and bites. Affiliative social interactions consisted of embracing and grooming. Sexual activities (e.g., solicitations, copulations) were not included in our analyses. Focal animals were chosen opportunistically but according to these criteria: no more than two full-length (1-h) samples on the same animal were completed during any single day and at least 4 h elapsed between successive follows of the same animal. Focal follows were discarded if the animal disappeared from view for >15 consecutive min or if the total observation time for an animal (after 1 h of following) was <30 min. We did not use a predetermined sequence to select focal individuals on each day of data collection, but attempted to balance the total observation time for each animal on a monthly basis.
Samples for genetic analyses
Blood samples for genetic analyses were obtained from all adults in the focal study group. Two of the adult males in the group had been captured and sampled in 2000 as part of a previous study of the genetic structure of this population (Milton et al. 2009). As part of the present study, the remaining individuals were anesthetized following the procedure of Glander et al. (1991). After an animal was immobilized, 10 ml of whole blood was drawn from a femoral blood vessel. Blood samples were stored in lysis buffer (Longmire et al. 1988; 1:1 ratio blood:buffer) at 4 °C for 1–2 weeks prior to transport to the Berkeley campus. Subsequently, the samples were frozen at −20 °C until analysis. Genomic DNA was extracted from each sample using the DnaEasy Kit (Qiagen), after which variation at ten microsatellite loci was assessed following the procedures outlined in Milton et al. (2009).
Analyses of genetic data
To assess genetic relatedness among members of the focal study group, it was first necessary to determine overall microsatellite allele frequencies within the BCI population of howler monkeys. To do this, genetic data for the nine adults monitored during this study were added to an existing database of genotypes for 91 members of the BCI population; all previous genotypes had been generated using the same ten microsatellite loci employed here (Milton et al. 2009). This larger, combined data set included animals of both sexes and all age classes drawn from a minimum of ten social groups distributed across the island, thereby providing robust estimates of allele frequencies for each microsatellite locus examined. To avoid potential biases in these estimates, members of the focal study group were excluded from calculations of overall allele frequencies.
Coefficients of relatedness (r) were estimated for each adult dyad in the focal study group using the maximum likelihood procedures in ML-RELATE (Kalinowski et al. 2006). ML-RELATE was also used to assess the presence of null alleles at each locus, which can affect estimates of r (Wagner et al. 2006); differences between corrected and uncorrected estimates of relatedness were negligible. Because the focal group contained more adult females than males, we examined the sensitivity of these estimates to sample size by performing 1000 Monte Carlo rarefactions of the data from females; for each rarefaction, we randomly selected three adult females from the focal group and calculated the mean pair-wise relatedness among this subset of animals. Rarefaction analyses revealed these values to be robust to differences in sample sizes for males versus females. Van Horn et al. (2008) cautioned that genetic estimates of r may provide poor estimates of pedigree relationships. While we agree that caution may be warranted when interpreting the precise values of such measures, we believe that consistent directional differences in genetic estimates are informative regarding relative kinship among different categories of individuals. To examine the significance of the difference in mean r values between male–male and female–female dyads, we used the permutation approach described by Di Fiore and Fleischer (2005): the sexes of the nine adults examined were randomly reassigned (three males, six females) 10,000 times and the difference between mean male–male relatedness, mean female–female relatedness, and mean male–female relatedness was calculated each time to generate a non-parametric reference distribution of differences in mean kinship values. Because errors in estimating r may have reduced the power of our statistical analyses, we were conservative in accepting the null hypothesis (i.e., no impact of kinship) in the absence of a statistical effect of relatedness.
Relational network analysis
To quantify relative interaction rates for dyads in the focal study group, relational networks were generated from data on kinship, nearest neighbor distances, and the occurrence of agonistic and affiliative interactions among pairs of individuals. Because these data violate assumptions of independence underlying many statistical tests, we used a quadratic assignment procedure (QAP; Krackhardt 1987, 1988) to regress one network variable on another. This method is commonly employed in social network analysis and controls for non-independence by comparing the magnitude of regression coefficients calculated from the observed data against a distribution of coefficients generated by permuting the rows and columns of the data matrices and then recalculating the regression coefficients. Further, although count data are ordinarily expected to follow a Poisson distribution, they are also prone to over-dispersion. Quasi-Poisson regression circumvents this problem by including a dispersion parameter that relaxes the strict Poisson assumption that the mean and variance of the distribution are equal, and adjusts the standard errors accordingly. We performed QAP-permuted quasi-Poisson matrix regressions using a modified version of the netlogit command from package sna (Butts 2010) for R (version 3.1.2) (R Development Core Team 2014), designed to call the glm function with a quasi-Poisson family specification. We employed the semi-partialling plus QAP permutation procedure (Dekker et al. 2003, 2007). A total of 10,000 permutations were performed, generating a non-parametric reference distribution. The resulting p values represent the proportion of permutations that generated a regression coefficient at least as large as the observed coefficient and provide a test of the significance of each individual coefficient. Although we report the quasi-Poisson standard errors of estimates in our results, we emphasize that these are not the criteria by which the significance of coefficients were judged, as these standard errors may be biased by non-independence of values in the data set.
Results
Genetic relatedness among group members
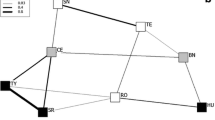
Analyses of microsatellite genotypes revealed that the mean pairwise relatedness among adult males in the focal study group was 0.32 ± 0.16 SD (range 0.21–0.50, N = 3 dyads; Fig. 1). In contrast, mean pairwise relatedness among the adult females in the group was 0.09 ± 0.11 SD (range 0.00–0.32, N = 15 dyads) and mean relatedness among male–female pairs of adults was 0.18 ± 0.16 SD (range 0.000–0.35, N = 18 dyads; Fig. 1). The permutation procedure applied to these data revealed that members of male–male dyads were significantly more related than were members of female–female dyads (observed difference = 0.23; p < 0.05), but that neither male–male dyads nor female–female dyads differed significantly from mixed-sex dyads. This between-sex difference was consistent with the apparent prevalence of unrelated female dyads in the focal study group; while all pairwise r-values for males were >0.20, only 3 pairs (20 %) of females exceeded this value and 6 pairs (40 %) of females were characterized by r = 0.00. Collectively, these data suggest that adult males in the study group were more closely related to each other than were adult females.
Relational networks for adult kinship, spatial and social affiliations within the focal study group. Circles indicate females; squares indicate males. Estimated ages for females ranged from 5 to 7 years; females 107 and 112 had associated older infants during the study, female 111 had an associated new infant a few weeks into the study while female 108 had an associated young juvenile. Estimated ages for males 25, 29, and 115 were 20, 15, and 7 years, respectively. The three infant and four juvenile group members are not plotted. Node size is proportional to the sum of values originating from a node. Line width is proportional to tie value for undirected relations (relatedness) or to the sum of both tie values between nodes for directed relations (all others). In the lower panels, arrowhead size is proportional to the value of the tie directed to the node. For clarity, in the upper two panels only the top 50 % of ties are displayed (those ties equal to or greater than the median tie value)
Spatial associations and genetic relatedness
Scan sampling was conducted on 55 different days, with an average of 42.1 ± 19.1 scans completed per day. Sampling was divided roughly equally between the morning (0600–1200; mean = 21.2 ± 11.5 scans per day) and the afternoon (1200–1800; mean = 20.9 ± 12.2 scans per day). A total of 3979 nearest neighbor associations was recorded during this study. These interactions included all possible dyads (N = 72) that could be formed by the 9 adult monkeys in the focal study group. Visual inspection of these data revealed that, typically, all possible dyads of adults displayed nearest neighbor associations on >10 different days of data collection, with some dyads exhibiting such associations on nearly all days on which scan sampling occurred. Thus, the results of our analysis of spatial relationships among the study animals did not appear to be an artifact of a few, persistent spatial relationships among members of the focal group.
Regression analyses revealed that genetic relatedness was not a significant predictor of the tendency for dyads to associate at any of the distance categories considered (Table 1). QAP-permuted regression analyses revealed that genetic relatedness explained ~14 % of the variance in direct contact among individuals (distance category = 0), with relatedness having less explanatory power for each of the remaining distance categories. None of the coefficients generated by analyses of the frequency of nearest neighbor distances and relatedness were significant (all p > 0.05; Table 1). Further, visual comparison of relational networks for genetic relatedness and spatial and social interactions (Fig. 1) revealed that the three most closely related dyads of adult females (pairwise r > 0.20) did not tend to be more closely spatially associated than other, less related dyads of females.
Spatial associations and sex
Spatial relationships among members of the focal study group were influenced by the sex(es) of interacting adults. Because >91 % of nearest neighbor interactions fell within distance categories 0–2 (≤5 m between individuals), we restricted our analyses to this subset of spatial observations. Analyses of spatial relationships were completed for each of the following combinations: (1) male–male versus all other dyads, (2) female–female versus all other dyads, and (3) both male–male and female–female dyads versus mixed-sex dyads. Regression analyses revealed that male–male dyads were significantly less likely than other dyad types to be observed within 5 m of each other (Table 2). Male-male dyads accounted for 3.4 % of all recorded nearest-neighbor observations and explained 6.5 % of the model deviance (compared to an intercept-only null model). In contrast, female–female dyads did not differ from other dyad types with regard to the frequency of occurrence within 5 m (Table 2). Thus, in general, close spatial associations were not as common among males as among females, although all group members tended to remain in relatively close proximity to one another throughout the day, typically feeding or resting within the same tree. Although no significant effect of relatedness on spatial proximity was found for opposite-sex dyads, this analysis suggested that more closely related male–female pairs tended to be more closely associated spatially.
Social behaviors
A total of 89 social interactions were documented during 229 h of focal animal observations, yielding an overall rate of 0.39 interactions per hour. Variation in duration of observations among the nine focal individuals was minimal (mean 25.45 h, range 24.74–25.85 h, SD 0.41, CV 0.016). All adults in the study group were observed engaging in at least one of the categories of social interaction monitored. Social interactions typically included a clear initiator and clear recipient of the behavior; the mean number of interactions instigated per individual was 9.89 + 7.59 (range 2–23) and the mean number of animals that each individual targeted was 3.89 + 1.76 (range 2–7). Over half (58.4 %) of the social interactions observed were agonistic. Of these, displacements were most common, accounting for 33 (63.5 %) of the agonistic interactions recorded. Of the 37 affiliative interactions recorded, grooming was the most frequent (59.5 %), followed by embracing (40.5 %). Regression analyses revealed no discernable effect of genetic relatedness on the tendency of individuals to engage in either agonistic or affiliative interactions with adult group-mates (all p > 0.05; Table 3).
Social interactions and sex
Social interactions among members of the focal study group were influenced by the sex(es) of the interacting adults. Because social interactions are directional (i.e., are characterized by an initiator and a recipient), our regression models for these analyses included independent variables that captured the relative frequency with which males and females tended to initiate interactions as well as the relative frequency with which males and females directed interactions toward same- versus opposite-sex adults. For all analyses, the dependent variable was the total number of agonistic or affiliative interactions by an individual.
With regard to agonistic interactions, females were significantly more likely to be the recipients of these behaviors than were males (Table 4). Males and females did not differ, however, with respect to the frequency with which they initiated agonistic interactions (Table 4). Neither males nor females were significantly more likely to direct agonistic behaviors toward same-sex versus opposite-sex individuals (Table 4). Females directed agonistic behaviors towards males significantly less often than the overall frequency for mixed-sex dyads (Table 4). With regard to affiliative interactions, there were no significant differences between the sexes with regard to the frequency of either initiating or receiving these behaviors (Table 4). Although males did not direct affiliative behaviors towards each other more often than toward females, females were significantly more likely to direct affiliative behaviors toward other females (Table 4). Males directed affiliative behaviors towards females at a significantly lower frequency than the overall rate among all same-sex dyads (Table 4). Thus, despite clear differences in relatedness within the study group, sex appeared to be a more important determinant of spatial and social relationships among adult group-mates than genetic kinship.
Discussion
This study is one of the first to examine genetic, spatial and social relationships among adult group-mates in a polygynandrous, bisexually dispersing species of monkey. Although a previous study of the howler monkeys on BCI (Milton et al. 2009) surveyed population-wide levels of genetic variability among these animals, no previous analyses at this site have characterized genetic relatedness among all adult members of the same social group. Our analyses indicate that within the focal study group, genetic relatedness among adult males was greater than that among adult females. This finding is consistent with earlier data from Milton et al. (2009) indicating that across multiple groups of howlers on BCI, genetic relatedness tended to be higher among adult males. Although calculating precise estimates of genetic relatedness is challenging and the resulting dyadic estimates of kinship may be subject to some error, greater genetic relatedness among male group-mates has also been reported for A. palliata in Costa Rica (Ellsworth 2000) and A. arctoidea from Venezuela (Pope 1990). Thus, while genetic analyses of kinship in howler monkeys are still rare, close kinship among adult male group-mates does not appear to be limited to our focal study group of A. palliata.
Somewhat surprisingly, we found no evidence that genetic relatedness influenced spatial associations or social interactions among adult group-mates. Instead, sex provided a better predictor of spatial and social behavior within the focal study group. In particular, the timing and frequency of the majority of close male–female dyadic associations observed suggested that female sexual receptivity was an important contributing factor. Based on the four species of howler monkeys for which data are available (Alouatta palliata, A. pigra, A. caraya, A. arctoidea), the length of the ovarian cycle is estimated at 16–20 days with a 2–4 days periovulatory period (Van Belle et al. 2009; see also Glander 1980). During our study, a male–female pair would often be observed in prolonged, close spatial association for portions of 1 or 2 days, accompanied by copulation or solicitations for copulation. This spatial association would then end, but would often resume after a period of about 3 weeks, presumably due to renewed receptivity on the part of the female (Glander 1980; Van Belle et al. 2009). Among females, the presence of a new infant evoked considerable interest, with other adult females often approaching the mother to try and gain proximity to the infant (K. Milton, pers. obs.). Although further study is needed to confirm the roles of female reproductive status and the presence of young infants in shaping spatial and social interactions in the study population, our data clearly indicate that kinship is not a key predictor of these relationships.
Because our study examined only a single social group, it is possible that our findings do not reflect general patterns of social structure in A. palliata. In particular, the power to detect behavioral associations may have been low due to both the small number of dyads possible within our focal study group and the limited number of social interactions among males observed. However, previously published data on male–male behavior within a different group of A. palliata on BCI were similar in revealing little social interaction among male group-mates (Wang and Milton 2003) and studies of A. palliata from Nicaragua (Bezanson et al. 2008), A. arctoidea from Venezuela (Sekulic 1981; Saavedra 1984) and A. pigra from Mexico (Van Belle et al. 2008; Van Belle et al. 2014a) have also reported low rates of social interaction among male–male dyads relative to female–female or mixed sex dyads. Also consistent with our findings, studies of other howler monkey species have noted that close spatial proximity in mixed-sex dyads is often due to the reproductive status of the female (Sekulic 1981; Carpenter 1934; Kowalewski and Garber 2010). Collectively, these similarities across studies and species suggest that our findings are indicative of general patterns of howler monkey behavior.
Social tolerance among males
While other studies of howler monkey behavior have reported episodes of aggression among male group-mates (Saavedra 1984; Glander 1992; Clarke and Glander 2004; Van Belle et al. 2014a), our observations as well as previous studies of the howler monkeys on BCI suggest that male group-mates in this population are quite tolerant of one another, with agonistic interactions other than occasional displacements rarely observed (Milton 1980; Wang and Milton 2003; M. Hopkins, pers. comm. to KM). However, male group-mates in the BCI population typically display intense agonism toward non-group males. In particular, during inter-group encounters, two or more adult males from the same social group are often seen standing in contact with one another while displaying defense behaviors and howling at members of the other group. Male group-mates may also make coordinated charges toward members of the other group and physical altercations between males from different social groups can occur. Although not observed during our study, such encounters have been recorded multiple times on BCI (K. Milton, pers. comm.) The distinction between adult male behavior within versus among groups leads us to suggest that kinship among adult male howler monkeys at our site may be linked to coalition formation and coordinated defense of females rather than benefits arising from within-group social interactions (Pope 1990).
Implications for dispersal
Dispersal patterns in howler monkeys remain controversial. Although dispersal in Alouatta is widely characterized as bisexual, movement by individuals appears to be male-biased in A. arctoidea and A. pigra (Crockett and Pope 1993; R. Horwich, pers. comm. to KM), as well as in A. caraya in continuous forest habitat (Oklander et al. 2010). Although dispersal in A. palliata has been described as somewhat female-biased based on data from a Costa Rican population of this species (Glander 1992), direct observation of dispersal on BCI is limited to males (Carpenter 1934; K. Milton unpub. data). Thus, we had predicted that, within social groups on BCI, kinship would be greater among adult females.
Both our genetic analyses and previously published data from a population-wide survey of genetic variation among howlers on BCI (Milton et al. 2009) appear to contradict this expectation by revealing greater relatedness among adult males. Several factors may have contributed to the apparent discrepancy between observational and genetic evidence of dispersal. First, underestimating dispersal is common in studies of free-living vertebrates (Koenig et al. 1998), and it seems likely that young females in the BCI population are more mobile than observational records of inter-group movements reveal. Unpublished observations (K. Milton, pers. comm.) suggest that young adult female howler monkeys on BCI may transfer to a new group when in estrus, after which the immigrating female is quickly surrounded by new group-mates, making it difficult to detect such events. Second, it is possible that male group-mates disperse together or disperse to the same social group in close temporal sequence, resulting in the presence of related adult male group-mates. Although not observed directly in the BCI population, this pattern of male movement has been reported for ursine howler monkeys in Venezuela (Pope 1998) and black howler monkeys in Mexico (Van Belle et al. 2014a), as well as for several other species of primates (e.g., rhesus monkeys (Macaca mulatta, Meikle and Vessey 1981); vervet monkeys (Chlorocebus aethiops, Cheney and Seyfarth 1983); capuchin monkeys (Cebus capucinus, Jack and Fedigan 2004) and social vertebrates, e.g., lions (Panthera leo, Bygott et al. 1979; Packer et al. 1991). While our genetic analyses do not allow us to identify with confidence the specific kin relationships (e.g., brothers) among members of the study group (van Horn et al. 2008), the data presented here suggest that patterns of dispersal in mantled howler monkeys are more complex and more variable than previously realized.
Kinship and social structure in A. palliata
Kinship has been shown to significantly impact spatial and social associations among females in a number of primates characterized by female philopatry, including multiple species of Old World cercopithecines (Gouzoules and Gouzoules 1987; Smuts 1987; Silk 1987; Widdig et al. 2001; Kapsalis 2004; Silk et al. 2004; Xia et al. 2012) and New World cebids (Perry et al. 2008; Perry 2012; Van Belle et al. 2014a). In contrast, atelines and African apes tend to be characterized by male philopatry and female–biased dispersal (Nishimura 2003; Di Fiore and Fleischer 2005) suggesting that, in these species, it is male–male behavior that should be more strongly influenced by kinship. Where available, genetic or genealogical data support the expectation that in species with female-biased dispersal, social interactions among males tend to be affiliative, although the precise structure of male–male relationships varies among species (Strier et al. 2002; Di Fiore and Fleischer 2005; Langergraber et al. 2007). Thus, although the apparent lack of kin-based effects on spatial and social relationships among the adults in our study group was somewhat surprising, the general tendency for male group-mates to behave amicably toward one another is consistent with other primates characterized by related male group-mates.
It is possible that social interactions among members of our study group did not vary with degree of kinship per se but rather with the specific kin relationship between members of a dyad. For example, Langergraber et al. (2007) reported that interactions among male chimpanzees varied depending upon whether kinship was paternal or maternal in origin. Our data set did not allow us to determine the exact kin relationships (e.g., father-son) among individuals or to distinguish maternal from paternal kinship. As a result, it is possible that our analyses failed to detect important differences in behavior between specific combinations of kin. It is also possible that our failure to detect an effect of kinship on male behavior reflects the limited adaptive contexts in which our spatial and social data were obtained. For example, cooperative alliances among adult male group-mates appear to be important in a number of primate species, including several species of howler monkeys (Milton 1980; Sekulic 1982: Pope 1990, 1998; Crockett and Pope 1993; Kitchen 2004; Van Belle et al. 2008, 2014b), and it is possible that kinship among males facilitates these coordinated displays. As we did not have the opportunity to observe male–male interactions associated with response to external threats such as the close approach of rival howler groups, it is possible that our analyses failed to capture the social context(s) in which the importance of kinship is most evident.
More generally, the role of kinship in shaping social behavior remains unclear, with a growing body of evidence indicating that tolerance and cooperation can occur among primates in the absence of close kinship (Silk 1994; Langergraber et al. 2007, 2009). Indeed, although kinship has been linked to social affiliation and cooperative behavior in multiple species and adaptive contexts, such interactions can also occur among unrelated individuals (West et al. 2002; Clutton-Brock 2009; Langergraber et al. 2007, 2009). While our data suggest that within social groups, adult male howler monkeys are more closely related to each other than are adult females, neither the processes contributing to this pattern nor the adaptive significance of this finding are yet understood. In particular, further study is required to assess the generality of this outcome and to elucidate the importance of kinship among male group-mates in shaping the social structure of this species.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Bezanson M, Garber PA, Murphy JT, Primo LS (2008) Patterns of subgrouping and spatial affiliation in a community of mantled howling monkeys (Alouatta palliata). Am J Primatol 70:282–293
Brockett RC, Horwich R, Jones CB (2000) Female dispersal of the Belizean black howling monkey (Alouatta pigra). Neotrop Primates 8:32–34
Butts CT (2010) sna: Tools for social network analysis. R package version 2.2-0
Bygott JD, Bertram BCR, Hanby JP (1979) Male lions in large coalitions gain reproductive advantages. Nature 282:839–841
Calegaro-Marques C, Bicca-Marques JC (1996) Emigration in a black howling monkey group. Int J Primatol 17:229–237
Carpenter CR (1934) A field study of the behavior and social relations of howling monkeys (Alouatta palliata). Compar Psychol Monogr 10:1–168
Carpenter CR (1964) Grouping behavior of howling monkeys. In: Carpenter CR (ed) Naturalistic behavior of nonhuman primates. Pennsylvania State University Press, University Park, pp 386–391
Chapais B, Berman CM (2004) Introduction: the kinship black box. In: Chapais B, Berman CM (eds) Kinship and behaviour in primates. Oxford University Press, Oxford, pp 3–11
Chapman CA, Balcomb SR (1998) Population characteristics of howlers: ecological conditions or group history. Int J Primatol 19:385–403
Cheney DL, Seyfarth R (1983) Non-random dispersal in free-ranging vervet monkeys: social and genetic consequences. Am Nat 122:392–412
Clarke MR, Glander KE (2004) Adult migration patterns of the mantled howlers of La Pacifica. Am J Primatol 62:87
Clarke MR, Glander KE (2008) Natal emigration by both sexes in the La Pacifica population of mantled howlers: when do some stay? Am J Primatol 70:195–200
Clutton-Brock TH (1989) Female transfer and inbreeding avoidance in social mammals. Nature 337:70–72
Clutton-Brock TH (2009) Cooperation between non-kin in animal societies. Nature 462:51–57
Crockett CM (1984) Emigration by female red howler monkeys and the case for female competition. In: Small MF (ed) Female primates: studies by women primatologists. A.R. Liss, New York, pp 159–173
Crockett CM, Eisenberg JF (1987) Howlers: variations in group size and demography. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 54–68
Crockett CM, Pope TR (1993) Consequences of sex differences in dispersal for juvenile red howler monkeys. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development and behavior. Oxford University Press, Oxford, pp 104–118
Dekker D, Krackhardt D, Snijders TAB (2003) Mulicollinearity robust QAP for multiple regression. CASOS Working Paper, Carnegie Mellon University
Dekker D, Krackhardt D, Snijders TAB (2007) Sensitivity of MRQAP tests to collinearity and autocorrelation conditions. Psychometrika 72:563–581
Di Fiore A, Fleischer RC (2005) Social behavior, reproductive strategies and population genetic structure of Lagothrix poeppigii. Intern J Primatol 26:1137–1173
Di Fiore A, Link A, Campbell CJ (2011) The atelines: behavioral and socioecological diversity in a New World radiation. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford, pp 155–188
Dobson FS (1982) Competition for mates and predominant juvenile male dispersal in mammals. Anim Behav 30:1183–1192
Ellsworth JA (2000) Molecular evolution, social structure and phylogeography of the mantled howler monkey (Alouatta palliata). Ph.D. thesis, University of Nevada
Glander KE (1980) Reproduction and population growth in free-ranging mantled howling monkeys. Am J Phys Anthropol 53:25–36
Glander KE (1992) Dispersal patterns in Costa Rican mantled howling monkeys. Int J Primatol 13:415–436
Glander KE, Fedigan LM, Fedigan L, Chapman C (1991) Field methods for capture and measurement of three monkey species in Costa Rica. Folia Primatol 57:70–82
Gouzoules S, Gouzoules H (1987) Kinship. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 299–305
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Hamilton WD (1964) The genetical evolution of social behavior I and II. J Theor Biol 7:1–52
Hinde RA (1983) A conceptual framework. In: Hinde RA (ed) Primate social relationships. Blackwell, Oxford, pp 1–7
Hladik CM (1978) Adaptive strategies of primates in relation to leaf-eating. In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Press, Washington D.C., pp 373–396
Hopkins ME (2013) Relative dominance and resource availability mediate mantled howler (Alouatta palliata) spatial response to neighbors’ loud calls. Int J Primatol 34:1032–1054
Jack KM, Fedigan LM (2004) Male dispersal patterns in white-faced capuchins (Cebus capucinus). Part I: patterns and causes of natal emigration. Anim Behav 67:761–769
Jones CB (1980) The function of status in the mantled howler monkey, Alouatta palliata Gray: intraspecific competition for group membership in a Neotropical folivorous primate. Primates 21:389–405
Jones CB (1999) Why both sexes leave: effects of habitat fragmentation on dispersal behavior. Endanger Species Update 16:70–73
Kalinowski ST, Wagner AP, Taper ML (2006) ML-Relate: a computer program for maximum likelihood estimation of relatedness and relationship. Mol Ecol Notes 6:576–579
Kapsalis E (2004) Maternal kinship and primate behavior. In: Chapais B, Berman CM (eds) Kinship and behavior in primates. Oxford University Press, Oxford, pp 153–176
Kitchen DM (2004) Alpha male black howler monkey responses to loud calls: effect of numeric odds, male companion behaviour and reproductive investment. Anim Behav 67:125–139
Koenig A, Beise J, Chalise MK, Ganzhorn JU (1998) When females should contest for food—testing hypotheses about resource density, distribution, size and quality with Hanuman langurs (Presbytis entellus). Behav Ecol Sociobiol 42:225–237
Kowalewski MM, Garber PA (2010) Mating promiscuity and reproductive tactics in female black and gold howler monkeys (Alouatta caraya) inhabiting an island on the Parana River, Argentina. Amer J Primatol 72:734–748
Krackhardt D (1987) QAP partialling as a test of spuriousness. Soc Networks 9:171–186
Krackhardt D (1988) Predicting with networks—nonparametric multiple-regression analysis of dyadic data. Soc Networks 10:359–381
Langergraber KE, Mitani JC, Vigilant L (2007) The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci 104:7786–7790
Langergraber KE, Mitani JC, Vigilant L (2009) Kinship and social bonds in female chimpanzees (Pan troglodytes). Am J Primatol 71:840–851
Leigh EG, Rand AS, Windsor DW (1982) Ecology of a tropical forest. Smithsonian Press, Washington, D.C.
Longmire JL, Lewis AK, Brown NC, Buckingham LM, Clark KM, Jones MD, Meincke LJ, Mayne J, Ratliff RL, Ray FA, Wagner RP, Moyzis RK (1988) Isolation and molecular characterization of a highly polymorphic centromeric tandem repeat in the family Falconidae. Genomics 2:14–24
McCann CM, Rothman JM (1999) Changes in nearest-neighbor associations in a captive group of western lowland gorillas after the introduction of five hand-reared infants. Zoo Biol 18:261–278
McFadden D (1974) Conditional logit analysis of qualitative choice behavior. In: Zarembka P (ed) Frontiers in econometrics. Academic Press, New York, pp 105–142
Meikle DB, Vessey SH (1981) Nepotism among rhesus monkey brothers. Nature 294:160–161
Melnick DJ, Hoelzer GA (1996) Genetic consequences of macaque social organization and behavior. In: Fa JE, Lindburg DG (eds) Evolution and ecology of macaque societies. Cambridge University Press, Cambridge, pp 412–422
Melnick DJ, Pearl MC (1987) Cercopithecines in multi-male groups: genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 121–134
Milton K (1980) The foraging strategy of howler monkeys: a study in primate economics. Columbia University Press, New York
Milton K (1982) Dietary quality and population regulation in a howler monkey population. In: Leigh EG, Rand AS, Windsor DM (eds) The ecology of a tropical forest. Smithsonian Press, Washington, D.C., pp 273–290
Milton K (1996) Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. J Zool 239:39–63
Milton K, Lozier JD, Lacey EA (2009) Genetic structure of an isolated population of mantled howler monkeys (Alouatta palliata) on Barro Colorado Island, Panama. Conserv Genet 10:347–358
Miranda JMD, Passos FC (2005) Composicao e dinamica de grupos Alouatta guariba clamitans Cabrera (Primates, Atelidae) em Floresta Ombrofila Mista no Estado do Parana, Brazil. Rivista Brasilera de Zoologia 22:99–106
Nishimura A (2003) Reproductive parameters of wild female Lagothrix lagotricha. Int J Primatl 24:707–722
Oklander LI, Kowalewski MM, Corach D (2010) Genetic consequences of habitat fragmentation in black-and-gold howler (Alouatta caraya) populations from northern Argentina. Int J Primatol 31:813–832
Ostro LET, Silver SC, Koontz FW, Horwich RH, Brockett R (2001) Shifts in social structure of black howler (Alouatta pigra) groups associated with natural and experimental variation in population density. Int J Primatol 22:733–748
Packer C, Gilbert D, Pusey AE, O’Brian SJ (1991) A molecular genetic analysis of kinship and cooperation in African lions. Nature 351:562–565
Perry S (2012) The behavior of wild white-faced capuchins: demography, life history, social relationships, and communication. In: Brockmann HJ, Roper TJ, Naguib M, Mitani JC, Simmons LW (eds) Advances in the study of behavior, vol 44. Academic Press, Burlington, pp 135–181
Perry S, Manson JH, Muniz L, Gros-Louis J, Vigilant L (2008) Kin-biased social behavior in wild adult female white-faced capuchins, Cebus capucinus. Anim Behav 76:187–199
Pope TR (1990) The reproductive consequences of male cooperation in the red howler monkey. Behav Ecol Sociobiol 27:439–446
Pope TR (1992) The influence of dispersal patterns and mating system on genetic differentiation within and between populations of the red howler monkey (Alouatta seniculus). Evolution 46:1112–1128
Pope TR (1996) Socioecology, population fragmentation, and patterns of genetic loss in endangered primates. In: Avise JC, Hamrick JL (eds) Conservation genetics: case histories from nature. Chapman & Hall, New York, pp 119–159
Pope TR (1998) Effects of demographic change on group kin structure and gene dynamics of populations of red howling monkeys. J Mammal 79:692–712
Pope TR (2000) The evolution of male philopatry in Neotropical monkeys. In: Kappeler PM (ed) Primate males. Cambridge University Press, Cambridge, pp 219–235
Pusey AE, Packer C (1987) Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 250–266
R Development Core Team (2014) R: a language and environment for statistical computing, version 3.1.2. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Rumiz DI (1990) Alouatta caraya: population density and demography in northern Argentina. Am J Primatol 21:279–294
Ryan S, Starks PT, Milton K, Getz WM (2008) Intersexual conflict and group size in Alouatta palliata: a 23-year evaluation. Int J Primatol 29:405–420
Saavedra CJ (1984) Spatial and social relationships of males in two groups of red howler monkeys (Alouatta seniculus). M.S. thesis, University of Florida
Sekulic R (1981) Male relationships and infant deaths in red howler monkeys (Alouatta seniculus). Z Tierpsychol 61:185–202
Sekulic R (1982) The function of howling in red howler monkeys (Alouatta seniculus). Behaviour 81:38–54
Sikes RS, Gannon WL, Animal Care and Use Committee of the American Society of Mammalogists (2011) Guidelines of the American Society of Mammalogists for the use of wild animals in research. J Mammal 92:235–253
Silk JB (1987) Social behavior in evolutionary perspective. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 318–329
Silk JB (1994) Social relationships of male bonnet macaques: male bonding in a matrilineal society. Behaviour 130:271–291
Silk JB, Alberts SC, Altmann J (2004) Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim Behav 67:573–582
Smith CC (1977) Feeding behaviour and social organization in howling monkeys. In: Clutton-Brock TH (ed) Primate ecology. Academic Press, London, pp 97–126
Smuts BB (1987) Gender, aggression, and influence. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 400–412
Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (1987) Primate societies. University of Chicago Press, Chicago
Strier KB, Dib LT, Figueira JEC (2002) Social dynamics of male muriquis (Brachyteles arachnoides hypoxanthus). Behav 139:315–342
Trivers R (1985) Social evolution. Benjamin/Cummings Publishing Co, Menlo Park
Van Belle S, Estrada A (2008) Group size and composition influence male and female reproductive success in black howler monkeys (Alouatta pigra). Am J Primatol 70:613–619
Van Belle S, Estrada A, Strier KB (2008) Social relationships among male Alouatta pigra. Int J Primatol 29:1481–1498
Van Belle S, Estrada A, Ziegler TI, Strier KB (2009) Sexual behavior across ovarian cycles in wild black howler monkeys (Alouatta pigra): male mate guarding and female mate choice. Intern J Primatol 71:153–164
Van Belle S, Estrada A, Di Fiore A (2014a) Kin-biased spatial associations and social interactions in male and female black howler monkeys (Alouatta pigra). Behav 151:2029–2057
Van Belle S, Garber PA, Estrada A, Di Fiore A (2014b) Social and genetic factors mediating male participation in collective group defense in black howler monkeys. Anim Behav 98:7–17
Van Horn RC, Altmann J, Alberts SC (2008) Can’t get there from here: inferring kinship from pairwise genetic relatedness. Anim Behav 75:1173–1180
Wagner AP, Creel S, Kalinowski ST (2006) Estimating relatedness and relationship using microsatellite loci with null alleles. Heredity 97:336–345
Wang E, Milton K (2003) Intragroup social relationships of male Alouatta palliata on Barro Colorado Island, Republic of Panama. Int J Primatol 24:1227–1244
West SA, Pen I, Griffin AS (2002) Cooperation and competition between relatives. Science 292:72–75
White FJ, Chapman CA (1994) Contrasting chimpanzees and bonobos: nearest neighbor distances and choices. Folia Primatol 63:181–191
Widdig A, Nurnberg P, Krawezak M, Streich WJ, Bercovich F (2001) Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc Natl Acad Sci USA 98:13769–13773
Xia D, Li J, Garber PA, Sun L, Zhu Y, Sun B (2012) Grooming reciprocity in female Tibetan macaques Macaca thibetana. Am J Primatol 74:569–579
Zucker EL, Clarke MR (1998) Agonistic and affiliative relationships of adult female howlers (Alouatta palliata) in Costa Rica over a 4-year period. Int J Primatol 19:433–449
Acknowledgments
We thank the Smithsonian Tropical Research Institute and the Autoridad Nacional del Ambiente of Panama (ANAM) for their help and facilitation of this research. Special thanks to Vicente Jaramillo and Frederico Rodrigues for field assistance, to Dana Bardolf for assistance with data collection, to Paul Elsen for organizing the spatial and behavioral data for analysis, to Steve Selvin for statistical advice and to Tony Di Fiore for assistance with relatedness permutation analyses. For assistance with microsatellite analyses we thank Aimee Ellison. For insightful comments and suggestions, we thank the reviewers of this manuscript. Research funding was provided by the California Agricultural Experimental Station (KM and KE) and by the Museum of Vertebrate Zoology (EAL) at UC Berkeley. All work was conducted in compliance with national and institutional regulations and adhered to the legal requirements of the Republic of Panama, the American Society of Mammalogists’ guidelines for research involving live mammals (Sikes and Gannon 2011) and ASP principles for the ethical treatment of nonhuman primates.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Milton, K., Nolin, D.A., Ellis, K. et al. Genetic, spatial, and social relationships among adults in a group of howler monkeys (Alouatta palliata) from Barro Colorado Island, Panama. Primates 57, 253–265 (2016). https://doi.org/10.1007/s10329-016-0523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-016-0523-5