Abstract
The use of enzyme immunoassays (EIA) for the non-invasive measurement of glucocorticoids provides a valuable tool for monitoring health and welfare in sensitive species. We validated methods for measuring fecal glucocorticoid metabolites (FGM) using the response to veterinary exams for four species of callitrichine monkeys: golden lion tamarin (Leontopithecus rosalia, n = 7), callimico (Callimico goeldii, n = 2), pied tamarin (Saguinus bicolor, n = 2), and white-fronted marmoset (Callithrix geoffroyi, n = 2). Routine veterinary exams were performed for the golden lion tamarins and callimicos, but exams for the pied tamarins and white-fronted marmosets were prompted by the death of a social partner. Prior to veterinary exams, fecal markers were evaluated to allow collection of individual samples and estimate approximate gut transit times. Based on this assessment, individual markers were fed in the afternoon, and fresh morning fecal samples were collected throughout this study. Following a veterinary exam, FGM increased roughly 3- to 28-fold above baseline in all species. Although FGM for most species returned to baseline concentrations within 24–48 h, the marmosets exhibited a progressive increase in FGM after an exam in response to the death of a breeding female and subsequent hand-rearing of a neonate. Individual differences were noted in the callimicos and tamarins, with higher baseline FGM levels in females vs. males, although small sample size precluded a clear determination of sex differences. To our knowledge, this is the first study to measure FGM in callimicos and white-fronted marmosets and the first to compare FGM across callitrichine species. These findings highlight the broad applicability of this EIA to measure the stress response of callitrichine monkeys. The progressive increase in FGM in the marmosets during hand-rearing of a neonate suggests that care should be taken to minimize this disturbance as much as possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurement of glucocorticoids can provide valuable insights into the health and welfare of animals. Metabolites of these hormones, typically cortisol or corticosterone, can be measured non-invasively through feces using enzyme immunoassay (EIA), providing a valuable tool for monitoring sensitive species, with minimal disruption (Keay et al. 2006; Schwarzenberger 2007). However, as significant variation exists in the metabolism and excretion time of glucocorticoids between species, as well as possible individual differences in hormone production within a species, it is necessary to validate the EIA using an event known to activate the hypothalamic–pituitary–adrenal (HPA) axis (Touma and Palme 2005).
One common method involves the administration of adrenocorticotropic hormone (ACTH), a precursor in the HPA axis known to stimulate glucocorticoid production (Heistermann et al. 2006). Although this represents a gold standard, ACTH challenges are not always possible or may not be ideal in some situations. Alternatively, assessing glucocorticoids before and after stressful events, such as capture, restraint, and anesthesia during veterinary exams (Armstrong and Santymire 2013) or translocation between institutions (Heistermann et al. 2006), can indicate the biological validity of an assay. The demonstration of circadian variations in glucocorticoids (Sousa and Ziegler 1998) or increases in relation to parturition (Murray et al. 2013) may provide an additional means for validating an assay.
Fecal glucocorticoid metabolites (FGM) have been previously assessed in some species of the family Callitrichinae, a group of arboreal, small-bodied New World monkeys (common marmoset, Callithrix jacchus: Sousa and Ziegler 1998; Raminelli et al. 2001; Heistermann et al. 2006; golden lion tamarin, Leontopithecus rosalia: Bales et al. 2005, 2006; pied tamarin, Saguinus bicolor: Armstrong and Santymire 2013; cotton-top tamarin, Saguinus oedipus: Ziegler and Sousa 2002; Fontani et al. 2014; mustached tamarin, Saguinus mystax: Huck et al. 2005). Although previous reports describe largely similar patterns of cortisol metabolite excretion, some discrepancies have been noted. For example, in a study of female common marmosets, Sousa and Ziegler (1998) demonstrated diurnal variation in cortisol, with higher concentrations detected in feces during the afternoon than the morning. Previous studies on marmosets have documented diurnal variation in both urine (Wied’s tufted-ear marmoset; Smith and French 1997) and saliva (common marmoset; Cross and Rogers 2004). Raminelli et al. (2001), however, identified elevated FGM during the afternoon in female common marmosets but not in males, and Huck et al. (2005) observed no diurnal variation in mustached tamarins. Similarly, elevated FGM concentrations during late pregnancy have been reported in golden lion tamarins (Bales et al. 2005) and common marmosets (Ziegler and Sousa 2002), but not in mustached tamarins (Huck et al. 2005). These differences highlight the need for careful validation of FGM measures for each species, even when dealing with closely related species.
Callitrichine species are notable for the occurrence of monogamous pair bonds and communal support in raising offspring. In laboratory settings, disruption of stable social groups resulting from the removal and placement of individuals with new pair mates or peer groups has been shown to increase cortisol levels (urine: Ziegler et al. 1995; Smith et al. 2011; plasma: Johnson et al. 1996). As small-bodied prey species with rapid reproduction, periods of social instability, such as after the death of group members or during conflict between breeding animals and mature offspring, occur regularly and, for groups living in the wild, are typically brief and resolved through immigration and emigration between groups (Lazaro-Perea et al. 2000). In zoo and lab settings, however, natural immigration and emigration between groups to resolve social instability is not always immediately possible. Therefore, understanding the impact of social change is important for animal managers, as social instability may impact welfare (Johnson et al. 1996; Smith et al. 2011).
The aim of this investigation was twofold. The first objective was to develop methods of individual fecal identification by conducting a fecal marker assessment and to validate the measurement of FGM concentrations in four species of callitrichine monkeys: golden lion tamarin (L. rosalia), callimico (Callimico goeldii), pied tamarin (S. bicolor), and white-fronted marmoset (Callithrix geoffroyi). This is the first study to measure FGM concentrations in white-fronted marmosets and callimicos, and the first to compare FGM responses among callitrichine species. Biological validation was conducted using the stress response to veterinary exams. Second, we evaluated the impact of deaths of group members on FGM levels in the pied tamarin and white-fronted marmoset groups. We hypothesized that the death of breeding animals would lead to social instability and would thus alter FGM levels.
Methods
Subjects and housing
This study involved 17 monkeys from four species: golden lion tamarin (n = 7), callimico (n = 2), pied tamarin (n = 3), and white-fronted marmoset (n = 5; Table 1). Animals were housed in similar single-species exhibits in the RainForest building of Cleveland Metroparks Zoo. The monkeys were fed in the morning (10:00 AM) and late afternoon (4:30 PM) a diet consisting of canned marmoset diet (Mazuri; PMI Nutrition International LLC, St. Louis, MO, USA), New World primate biscuit (Mazuri), vegetables, fruit, and mealworms. The pied tamarins also received a calcium supplement, and the white-fronted marmoset diet included gum arabic. Water was available ad libitum.
Fecal marker assessment
Four indigestible items were qualitatively evaluated as fecal markers (see Online Resource 1): food dye (McCormick & Company, Inc., Sparks, MD, USA), food coloring paste (Wilton Industries, Woodridge, IL, USA), 2-mm glass jewelry beads (Darice, Inc., Strongsville, OH, USA), and non-toxic glitter (Advantus Corporation, Jacksonville, FL, USA). Fecal markers were hand-fed in banana pieces on Monday and Wednesday afternoons (1:00 to 3:00 PM), and morning fecal samples were evaluated for evidence of the marker for 48 h. During fecal marker evaluation, one marker type was fed per day for all species, and all individuals in a group received markers of the same color.
Fecal collection and hormone analysis
Fresh, uncontaminated fecal samples were collected in the morning (7:00 AM to 12:00 PM) before and after veterinary exams. For the white-fronted marmosets, fecal collection following the exam was extended to the afternoon because of small fecal size and inconsistent morning voids. During this time, all fresh marmoset fecal samples observed during 2-h periodic checks were collected. Fecal samples were individually identified using glitter for the golden lion tamarins, pied tamarins, and white-fronted marmosets. No fecal marker was used with the callimicos, and individual samples were visually identified by an observer as the animals defecated in the morning. On the morning after the veterinary exam, fecal samples from the callimicos were present before the lights were turned on, and these individually unidentifiable samples were collected and shown for reference. Complete and separate fecal voids were stored in Whirl-Pak fecal bags (Nasco, Fort Atkinson, WI, USA) at −20 °C. Feces were lyophilized (FreeZone 4.5-L freeze-dry system, model #7751020; Labconco Corporation, Kansas City, MO, USA), pulverized, homogenized, and sifted to remove food particles and glitter. Hormone metabolites were extracted using a method adapted from Brown (2008): 5 ml of methanol was added to 0.2 g dry feces, briefly hand-vortexed, mixed for 1 h (large-capacity mixer, model #099A LC1012; Glas-Col, LLC, Terre Haute, IN, USA), and centrifuged at 2500 RPM for 20 min (Sorvall Legend RT Plus; Thermo Scientific, Waltham, MA, USA). The supernatant was poured off and stored in a freezer at −20 °C prior to analysis, and was assayed within 6 months.
Fecal glucocorticoid metabolites were analyzed via the enzyme immunoassay (EIA) protocol described by Brown (2008) using polyclonal cortisol antiserum (R4866, 1:300,000 dilution) provided by Coralie Munro (University of California, Davis, CA, USA). The EIA was validated for each species by demonstrating (1) parallelism of the binding inhibition curve of pooled fecal extract dilutions (1:1 to 1:1024) with the cortisol standard (R2 >94 %), and (2) significant recovery (>92.0 %) of exogenous cortisol from diluted fecal samples spiked with the quality control high (200 pg/well) and low (5 pg/well). The mean inter-assay coefficient of variation (CV) of standards and high- and low-value quality controls was 7.2 %. Intra-assay CVs were less than 10 %. Microtiter plates were read using a spectrophotometer (Epoch; BioTek Instruments, Inc., Winooski, VT, USA) at 450-nm wavelength using Gen5 software (v2.03; BioTek Instruments, Inc.).
Biological validation
For biological validation, FGM levels were assessed before and after veterinary exams. Fecal samples for the golden lion tamarins and callimicos were collected before and after a routine veterinary exam. Samples were collected 6 days prior to and 10 days post-exam for the golden lion tamarins and 5 days prior to and 7 days post-exam for the callimicos. An average of 19 and 23 samples per individual were collected from the golden lion tamarin and callimico groups, respectively. Prior to the exam, the golden lion tamarins could not be reliably identified by researchers or staff, and a pooled fecal sample from the group was collected each morning. During the exam, the golden lion tamarins were marked (Flouro Tell Tail Animal Marker; Fil, Mount Maunganui, New Zealand), allowing for individual hand-feeding of fecal markers and identification of samples post-exam.
Fecal samples were collected from the pied tamarins and white-fronted marmosets opportunistically after veterinary exams prompted by the death of a social partner. For the pied tamarins, the entire group (TF1, TM1, and TM2) was captured and transported to the veterinary hospital for an exam on the breeding male (TM1). During this exam, the breeding male (TM1) was euthanized as a result of an age-related decline in health, and the female received an exam and injection of Depo-Provera (Pfizer Inc., New York, NY, USA) for contraception. The male offspring (TM2) did not receive an exam and remained in a transport box for the duration of the exam (≈1 h). Data were available from 27 days before the exam to 22 days after the exam, with an average of 39 samples collected per individual.
In the white-fronted marmoset group, the breeding female (MF1) died unexpectedly, and a 2-week-old neonate was temporarily removed from the group for hand-rearing. The breeding male (MM1) received a physical exam the day after this event; the male offspring (MM2) did not receive an exam and remained in the marmoset exhibit and holding area during MM1’s exam. Two days after this exam, the neonate was returned to the holding area for hand-rearing procedures. Baseline data were available from 113 to 50 days before this event. Fecal samples from the marmosets were collected for 10 days post-exam. During this time, the neonate was housed in an incubator, within audiovisual contact of the marmoset holding area. Staff entered the holding area between 6:00 AM and midnight to feed the neonate. Additional data collection began 19 days after this initial collection period, at which point the neonate was housed in a wire mesh cage attached to the holding cage, which allowed for physical contact between the neonate and adult marmosets (MM1 and MM2). Staff entered the holding area between 6 AM and 6 PM for hand-rearing procedures. An average of 55 fecal samples per individual were collected during this study.
Two additional deaths occurred in the white-fronted marmoset group from unrelated causes. An adult male offspring (MM3) died following exploratory surgery due to illness 89 days prior to the death of the breeding female. The breeding male marmoset (MM1) was euthanized 40 days after the death of the breeding female (MF1), following progressive weight decline despite treatment attempts.
Statistical analysis
The baseline FGM concentration was calculated for each individual using an iterative process whereby all baseline samples greater than the mean plus 2 standard deviations (SD) were removed, and this statistic was recalculated until no additional samples were greater than this value, at which point the mean was designated as the individual baseline (Brown et al. 1999). A sample’s FGM concentration was considered elevated if it exceeded 2 SD of the individual’s baseline value. For the female callimico (CF1), one elevated sample that occurred prior to the exam was excluded from baseline calculations to generate a representative baseline for this individual, but this value was retained in this individual’s validation graph. For presentation, data are shown from 200 h before and after the vet exam for the golden lion tamarin, callimico, and pied tamarin groups. As multiple deaths occurred in the marmoset group, all available data are shown.
Baseline FGM levels were compared between the male and female callimicos and pied tamarins using distribution-free confidence intervals (95 % CIdf) around the median. As the male pied tamarin’s (TM2) FGM levels appeared to increase following the death of his father (TM1), the male and female’s FGM levels were again compared using baseline data from a research project that began 98 days after the death of the breeding male. We considered a difference significant if confidence intervals did not overlap. Data were analyzed using SAS University Edition software (SAS Institute Inc., Cary, NC, USA).
Results
Assessment of fecal markers
Fecal markers were fed in the afternoon (1:00 to 3:00 PM), and all were observable by the following morning voids (see Online Resource 1). Although this time frame was successful for most species, when the pied tamarins were fed in early afternoon (1:00 PM), evidence of the food coloring marker was observed prior to first morning voids, indicating that the marker was excreted before the tamarins entered their nest box for the evening (5:00 to 6:00 PM). In addition, when the tamarins were fed glitter, fecal samples would occasionally contain glitter colors fed to different individuals, even though food sharing did not occur during hand feeding sessions. Although not witnessed during this project, coprophagy has been observed in this group. For the pied tamarins, feeding fecal markers later in the afternoon (3:00 PM) was found to be more successful.
Consumption of the fecal markers differed drastically between species. The golden lion tamarins and pied tamarins readily consumed all fecal markers, whereas the callimicos and white-fronted marmosets reacted aversely to the food coloring and food paste, often dropping the banana pieces, and would eject the beads out of the side of their mouths. As glitter was the only fecal marker readily consumed by all species, this marker was chosen and subsequently used for the remainder of the study.
Changes in FGM levels in relation to veterinary exams and animal deaths
In all four species, we observed a significant increase in FGM concentrations 24–48 h after a veterinary exam (Table 2). As the magnitude and pattern of the increase differed across species, these results are presented separately for each group.
Golden lion tamarin
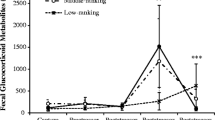
After capture and anesthesia during a routine veterinary exam, all golden lion tamarins demonstrated elevated FGM levels within 49 h post-exam (Fig. 1; representative individuals). In addition, all individually unidentifiable samples collected the afternoon of the exam were elevated above all individuals’ baseline FGM values. Although most individuals displayed a single FGM elevation following the exam (LF1, LF3, LM1, and LM4; see Fig. 1a, c for representative profiles), a secondary elevation in FGM levels, occurring at roughly 48–72 h post-exam, was observed in some individuals (LF2, LM2 and LM3; see Fig. 1b, d for representative profiles). The magnitude of the primary FGM increases varied considerably across individuals (~3- to 28-fold increase compared to baseline), with a mean 12-fold increase above baseline for the group. Individual FGM had returned to baseline levels by 24–48 h.
Fecal glucocorticoid levels of four golden lion tamarins (a LF1, b LF2, c LM1, d LM2) after capture and anesthesia during a routine veterinary exam (time 0:00 h). Pooled samples from the group collected before the exam (grey circles) are shown for reference but were not used in individual baseline calculations. Baseline and baseline +2SD are indicated by solid and dashed lines, respectively
Callimico
Both the male and female callimico showed a peak FGM level roughly 20 h post-exam (Fig. 2). The magnitude of this peak was similar for the two animals: 3.3 and 2.5 times greater than baseline for the male and female, respectively. By 24 h post-exam, FGM had temporarily returned to baseline concentrations for both individuals. Secondary peaks comparable in magnitude to the primary peaks were also observed (male, 43 and 68 h post-exam; female, 68 h post-exam).
Fecal glucocorticoid metabolite concentrations in a female (a) and male (b) callimico before and after a routine veterinary exam (time 0:00 h). Baseline and baseline +2 SD are indicated by solid and dashed lines, respectively. Individually unidentifiable samples collected the morning after the exam are shown for reference and denoted with an X (see “Methods”)
Pied tamarin
After a veterinary exam on the breeding male (TM1) and female (TF1) pied tamarins, during which the breeding male was euthanized due to age-related decline in health, the breeding female pied tamarin and her adult male offspring (TM2) demonstrated elevated FGM concentrations post-exam, with peak concentrations occurring at 20 h for the breeding female and her male offspring (Fig. 3). The breeding female’s FGM peak after the exam was 21.3 times greater than at baseline. The male offspring, who was transported to the veterinary hospital but not examined, exhibited a lower-magnitude FGM increase 4.3 times greater than baseline. Both individuals demonstrated a return to baseline FGM levels within 48 h.
White-fronted marmoset
Fecal samples from both male marmosets (MM1 and MM2) displayed a progressive increase in FGM levels following a veterinary exam on the breeding male (MM1) prompted by the death of the breeding female (MF1) and temporary removal of a neonate (Fig. 4). The neonate was returned to the marmoset holding area 2 days after the exam and housed in an incubator for hand-rearing procedures. The progressive increase in FGM reached a peak magnitude 143 times greater than baseline for individual MM1 (11 days post-exam) and 19.6 times greater than baseline for individual MM2 (8 days post-exam). Fecal samples collected during the afternoon were elevated at 24 h post-exam for the breeding male and 48 h for the male offspring, who did not receive an exam but did experience a capture event. Morning fecal samples from the breeding male were not available until 69 h post-exam, and were elevated at this time. Morning fecal samples were available for MM2 following the exam, but morning FGM levels were not elevated until 69 h post-exam. Fecal samples 27 days post-exam showed a return to near baseline FGM levels, although a consistent return to baseline levels was not observed for either individual. No FGM increase was observed after the death of an adult male offspring (MM3) or after the death of the breeding male (MM1).
Fecal glucocorticoid concentrations of two male white-fronted marmosets (a MM1, b MM2) before and after the death of the breeding female, temporary removal of a neonate, and subsequent veterinary exam (arrow at time 0:00 h). The neonate was returned to the off-exhibit holding area after the veterinary exam (arrow at time 48 h). An adult offspring (MM3) died before the veterinary exam validation and is shown for reference (arrow at time −2124 h). Individual MM1 died during data collection, and this event is noted for MM2 (b arrow at time 924 h). Afternoon fecal samples were collected following the exam and are indicated as “PM”. Baseline and baseline +2 SD are indicated by solid and dashed lines, respectively
Individual differences in baseline FGM concentration
Although the small sample size precluded a comparison between sexes, several differences between individuals were observed. FGM values were significantly higher in the female callimico (Mdn = 402.5 ng/g, 95 % CIdf = 281.3–762.1) than the male (Mdn = 156.5 ng/g, 95 % CIdf = 125.8–264.8). In the pied tamarin group, the breeding female exhibited higher FGM levels (Mdn = 120.0 ng/g, 95 % CIdf = 78.9–170.1) than both the breeding male (TM1 Mdn = 43.6 ng/g, 95 % CIdf = 31.0–48.9) and their male offspring (TM2 Mdn = 58.7 ng/g, 95 % CIdf = 51.4–76.4). However, during a subsequent study conducted 98 days after the death of the breeding male, we observed no significant difference between the female (Mdn = 148.7 ng/g, 95 % CIdf = 104.5–189.7) and her male offspring (Mdn = 129.6 ng/g, 95 % CIdf = 104.4–182.3).
Discussion
To our knowledge, this is the first study to provide information on the fecal glucocorticoid response of multiple callitrichine species. All animals assessed in this study (n = 13) demonstrated elevated FGM following a veterinary exam, highlighting the broad applicability of this cortisol EIA to measure the stress response of callitrichine species. For most individuals, this response occurred within 24–48 h of the exam. Contrary to expectations, we did not observe clear evidence that the death of group mates led to social instability and elevated FGM levels. Elevated FGM levels were observed in the marmoset group following the death of the breeding female and subsequent veterinary exam, but this response was confounded by the hand-rearing of a neonate in the marmoset holding area. The prolonged FGM response of the marmosets does suggest that FGM measures are sensitive to both acute and chronic stressors. Differences in baseline FGM concentrations between males and females were observed in some groups, although small sample size and social changes prevented a clear determination of sex differences for these species.
Assessment of fecal markers for use in callitrichine monkeys
As fecal samples were collected from multiple animals within groups each day, identification of a reliable fecal marker was necessary. We observed strong differences across species in their reaction to fecal markers, with glitter being the only marker that was readily consumed by all species. Although all animals within a group reacted similarly, the observed preferences may be group-specific, as other studies have described different preferences (Fuller et al. 2011).
As callitrichines have demonstrated rapid gut passage times (2.5–4.5 h; Power and Oftedal 1996; Heymann and Smith 1999), fecal markers were fed in the early afternoon for identification of morning voids the following day. The pied tamarins appeared to have a more rapid excretion time than other species, as fecal markers fed in the early afternoon were identifiable in the late afternoon of the same day. Differences in gut transit times among callitrichines have been noted and linked to dietary differences, with shorter retention times for more frugivorous species, such as tamarins, compared to marmosets, which specialize on gum exudativory (Power and Oftedal 1996).
Effect of veterinary exams and social changes on FGM concentration
Veterinary exams produced a consistent FGM increase across species that was visible for most individuals within 24 h of the exam, with peak concentrations often observed within 48 h, similar to the time course reported by Armstrong and Santymire (2013) for pied tamarins following a veterinary exam. Following the initial peak, secondary increases were noted in several species before FGM concentrations returned to baseline levels. This pattern has been observed in other species (Steller sea lion: Hunt et al. 2004; greater sage grouse: Jankowski et al. 2009), including primates (yellow baboon: Wasser et al. 2000), and these authors have suggested that a secondary increase may be a byproduct of enterohepatic recirculation of hormones (Roberts et al. 2002) or negative feedback regulation of HPA activity (Jankowski et al. 2009). Although the presence of individually unidentified samples post-exam prevents a clear understanding of the absolute magnitude increase in FGM levels for each individual, the callimicos appeared to exhibit a reduced and variable FGM response compared to the golden lion tamarins and pied tamarins. Differences in the excretion or metabolism of cortisol may exist between this species and other callitrichines.
The response of the two male marmosets differed from the overall pattern observed in the other study species, with a consistent increase in FGM concentrations observed for 10 days after an exam in response to the death of the breeding female, which for one individual reached a peak FGM concentration 143 times greater than baseline. The increase in this male was considerably larger than those of other individuals in this study and those reported by other studies, such as the 10.8-fold increase over baseline described by Heistermann et al. (2006) in two common marmosets after translocation to a new institution. Following the exam, afternoon fecal samples were collected from the marmosets in addition to morning samples, because of small fecal size and inconsistent morning voids (e.g., no morning fecal sample was observed for the first 48 h post-exam for one marmoset). Although diurnal variation in FGM levels has been reported in other callitrichine species, and may have influenced FGM concentrations (common marmoset: Sousa and Ziegler 1998; Raminelli et al. 2001), elevated morning FGM levels were consistently observed in both individuals after 69 h post-exam, including the peak FGM concentrations measured.
Two days after the marmoset’s veterinary exam, a neonate that was born 9 days prior to the death of the breeding female was returned to the marmoset off-exhibit holding area for hand-rearing in an incubator. The presence of the neonate and its frequent vocalizations may have contributed to this progressive increase in FGM, as several studies have found that male marmosets are highly motivated by infant vocal cues (Zahed et al. 2008). The playback of infant distress calls to unrelated male common marmosets was found to increase serum cortisol, with no evidence of habituation after repeated exposure (Barbosa and Mota 2014). As these authors point out, marmosets exhibit a high degree of male parental investment, and increased cortisol in response to neonate cues may be an important mechanism in promoting care-giving behavior. In addition to the presence of the neonate, increased human activity in the holding area during the hand-rearing process may have negatively influenced the marmosets. Previous studies on laboratory-housed common marmosets have reported increased fecal glucocorticoids (Barbosa and Mota 2009) and salivary cortisol (Cross et al. 2004) in response to increased human activity.
As these are monogamous pair-bonded primates, the death of the breeding female marmoset may have contributed to this prolonged stress response. Partner-directed affiliative and protective behaviors have been reported in a wild male common marmoset toward a dying female mate (Bezerra et al. 2014). However, it is unlikely that the death of the female directly contributed to the dramatic increase in FGM concentrations observed, for two reasons. First, FGM levels were not consistently elevated until 48 h after the exam, which corresponds to 72 h after the death of the breeding female and removal of the neonate. Based on the time lag to elevated FGM concentrations observed in other animals in this study (24–48 h), this initial increase was more likely in response to the exam than to the female’s death. Second, this dramatic increase in FGM concentrations that occurred in both the breeding male (MM1) and adult offspring (MM2) was not observed after the death of an adult male offspring (MM3) or after the death of the breeding male. Similarly, the pied tamarins demonstrated a rapid return to baseline FGM levels after a veterinary exam and death of the breeding male, with no prolonged response to the male’s absence. Huck et al. (2005) also reported no increase in FGM in a group of wild mustached tamarins after the death of a breeding female.
Both marmosets in the present study showed a reduction to near baseline levels 27 days after the exam, but neither demonstrated a complete return to baseline. During this subsequent collection period, the neonate had been moved to a temporary cage attached to the marmoset holding cages, which allowed for physical contact between the neonate and adult marmosets. Human activity from the hand-rearing procedure had also decreased by this point, but was still more frequent than during baseline periods. Taken together, it is likely that factors related to the nearby hand-rearing of a neonate contributed to the continual FGM response in the marmosets. Although audiovisual contact with conspecifics during the initial hand-rearing may benefit the neonate and is a recommended practice (Bairrão Ruivo 2010), the potential adverse effects on adults should be considered, and efforts should be made to minimize this disturbance as much as possible.
Demographic factors influencing FGM levels
Individual differences in FGM concentrations were observed in the callimicos and pied tamarins, with higher concentrations present in females than in males, although it is unknown whether these are true sex differences, given the limited sample size. Higher cortisol levels in females compared to males have been reported for some callitrichine species: pied tamarin (Armstrong and Santymire 2013), common marmoset (Johnson et al. 1996; Raminelli et al. 2001), and Wied’s black tufted-ear marmoset (Callithrix kuhlii, Smith and French 1997). In the pied tamarin group, the adult male offspring’s (TM2) baseline FGM level doubled following the death of the breeding male (TM1), and did not differ from the female’s baseline FGM level. Although some studies have found male social status to influence cortisol (Ziegler et al. 1996), others have not identified a relationship (Bales et al. 2006). Further research investigating sex differences in FGM and the influence of social changes is needed before drawing conclusions in these species.
Conclusion
This study demonstrated the broad applicability of a fecal cortisol EIA to measure the stress response of callitrichine monkeys through noninvasive methods. Although some differences between groups were noted, all individuals displayed an increase in FGM ranging from roughly 3- to 28-fold above baseline within 24–49 h after veterinary exams. The death of conspecifics in the pied tamarin and white-fronted marmoset groups did not appear to have a lasting impact on FGM levels. The progressive increase in FGM levels observed in two male marmosets following an exam in response to the death of the breeding female appeared to reflect the separation and hand-rearing of a neonate, highlighting the importance of considering the potential impact of hand-rearing on group members. Future research is needed to explore species-specific differences in HPA activity and to uncover the factors that may have contributed to these changes.
References
Armstrong DM, Santymire RM (2013) Hormonal and behavioral variation in pied tamarins housed in different management conditions. Zoo Biol 32:299–306
Bairrão Ruivo E (2010) EAZA husbandry guidelines for the Callitrichidae. ZooParc de Beauval, Beauval
Bales KL, French JA, Hostetler CM, Dietz JM (2005) Social and reproductive factors affecting cortisol levels in wild female golden lion tamarins (Leontopithecus rosalia). Am J Primatol 67:25–35
Bales KL, French JA, McWilliams J, Lake RA, Dietz JM (2006) Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia). Horm Behav 49:88–95
Barbosa MN, Mota MTS (2009) Behavioral and hormonal response of common marmosets, Callithrix jacchus, to two environmental conditions. Primates 50:253–260
Barbosa MN, Mota MTS (2014) Do newborn vocalizations affect the behavioral and hormonal responses of nonreproductive male common marmosets (Callithrix jacchus)? Primates 55:293–302
Bezerra BM, Keasey MP, Schiel N, Souto AS (2014) Responses towards a dying adult group member in a wild New World monkey. Primates 55:185–188
Brown JL (2008) Wildlife Endocrinology Manual. National Zoological Park, p. 75
Brown JL, Schmitt DL, Bellem A, Graham LH, Lehnhardt J (1999) Hormone secretion in the Asian elephant (Elephas maximus): characterization of ovulatory and anovulatory luteinizing hormone surges. Biol Reprod 61:1294–1299
Cross N, Rogers LJ (2004) Diurnal cycle in salivary cortisol levels in common marmosets. Dev Psychobiol 45:134–139
Cross N, Pines MK, Rogers LJ (2004) Saliva sampling to assess cortisol levels in unrestrained common marmosets and the effect of behavioral stress. Am J Primat 62:107–114
Fontani S, Vaglio S, Beghelli V, Mattioli M, Bacci S, Accorsi PA (2014) Fecal concentrations of cortisol, testosterone, and progesterone in cotton-top tamarins housed in different zoological parks: relationships among physiological data, environmental conditions, and behavioral patterns. J Appl Anim Welf Sci 17:228–252
Fuller G, Margulis SW, Santymire R (2011) The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol 30:379–398
Heistermann M, Palme R, Ganswindt A (2006) Comparison of different enzyme immunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primat 68:257–273
Heymann EW, Smith AC (1999) When to feed on gums: temporal patterns of gummivory in wild tamarins, Saguinus mystax and Saguinus fuscicollis (Callitrichinae). Zoo Biol 18:459–471
Huck M, Löttker P, Heymann E, Heistermann M (2005) Characterization and social correlates of fecal testosterone and cortisol excretion in wild male Saguinus mystax. Int J Primatol 26:159–179
Hunt KE, Trites AW, Wasser SK (2004) Validation of a fecal glucocorticoid assay for Steller sea lions (Eumetopias jubatus). Physiol Behav 80:595–601
Jankowski M, Wittwer D, Heisey D, Franson J, Hofmeister E (2009) The adrenocortical response of Greater Sage Grouse (Centrocercus urophasianus) to capture, ACTH injection, and confinement, as measured in fecal samples. Physiol Biochem Zool 82:190
Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP (1996) The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus). Biol Psychiatry 40:317–337
Keay JM, Singh J, Gaunt MC, Kaur T (2006) Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. J Zoo Wildl Med 37:234–244
Lazaro-Perea C, Castro CSS, Harrison R, Araujo A, Arruda MF, Snowdon CT (2000) Behavioral and demographic changes following the loss of the breeding female in cooperatively breeding marmosets. Behav Ecol Sociobiol 48:137–146
Murray CM, Heintz MR, Lonsdorf EV, Parr LA, Santymire RM (2013) Validation of a field technique and characterization of fecal glucocorticoid metabolite analysis in wild chimpanzees (Pan troglodytes). Am J Primatol 75:57–64
Power ML, Oftedal OT (1996) Differences among captive callitrichids in the digestive responses to dietary gum. Am J Primatol 40:131–144
Raminelli JLF, Sousa MBC, Cunha MS, Barbosa MFV (2001) Morning and afternoon patterns of fecal cortisol excretion among reproductive and non-reproductive male and female common marmosets, Callithrix jacchus. Biol Rhythm Res 32:159–167
Roberts M, Magnusson B, Burczynski F, Weiss M (2002) Enterohepatic circulation: physiological, pharmacokinetic, and clinical implications. Clin Pharmacokinet 41:751–790
Schwarzenberger F (2007) The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int Zoo Yearb 41:52–74
Smith TE, French JA (1997) Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiol Behav 62:225–232
Smith AS, Birnie AK, French JA (2011) Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol Behav 104:955–961
Sousa MBC, Ziegler TE (1998) Diurnal variation on the excretion patterns of fecal steroids in common marmoset (Callithrix jacchus) females. Am J Primatol 46:105–117
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Zahed SR, Prudom SL, Snowdon CT, Ziegler TE (2008) Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus). Am J Primatol 70:84–92
Ziegler TE, Sousa MBC (2002) Parent–daughter relationships and social controls on fertility in female common marmosets, Callithrix jacchus. Horm Behav 42:356–367
Ziegler TE, Scheffler G, Snowdon CT (1995) The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav 29:407–424
Ziegler TE, Wegner FH, Snowdon CT (1996) Hormonal responses to parental and non-parental conditions in male cotton-top tamarins, Saguinus oedipus, a New World primate. Horm Behav 30:287–297
Acknowledgments
We would like to thank Joe Ropelewski, Scott Parish, Terri Rhyner, Chris Gertiser, and Lynn Koscielny for their coordination and support of this project. In addition, we thank Jen Avondet, Suzy Peoples, Joan Rog, Ingrid Rinker, Elaine Leickly, and Christine Olle for their assistance in processing fecal samples. This research was funded by Cleveland Metroparks Zoo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed were approved by the appropriate institutional animal care and use committee (IACUC).
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Wark, J.D., Amendolagine, L., Lukas, K.E. et al. Fecal glucocorticoid metabolite responses to management stressors and social change in four species of callitrichine monkeys. Primates 57, 267–277 (2016). https://doi.org/10.1007/s10329-016-0514-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-016-0514-6