Abstract
Knowledge of endocrine stress responses can be advantageous for understanding how animals respond to their environment. One tool in wildlife endocrinology is to measure the adrenocortical activity as a parameter of disturbance of animals. Fecal glucocorticoid metabolites (GCMs) provide a noninvasive assessment of adrenocortical activity. Using an adrenocorticotropic hormone (ACTH) challenge administered to 28 captive coyotes (Canis latrans), we measured the levels of plasma cortisol, and fecal cortisol and corticosterone metabolites (i.e., GCMs). Our goal was to determine the dose-response in the plasma and fecal samples following the injection and determine if there were effects of sex, age, and time of day. Specifically, animals were anesthetized for ~ 90 min with treatment animals intravenously injected with exogenous ACTH and control animals receiving saline. We collected blood samples prior to injection and at 4 different time points post-injection. We also collected fecal samples 2 days pre- and 2 days post-injection to measure fecal GCMs and determine if an endocrine stress response could be detected in fecal samples. We found a definite response in cortisol levels in the plasma for coyotes to the ACTH challenge. There was a response in fecal corticosterone 1 day post-injection, but the control males showed a similar response indicating a handling effect. Fecal cortisol levels did not indicate a response to the ACTH challenge, and were significantly lower than corticosterone concentrations. We also found significant sex, but not age or diurnal, differences in fecal GCMs. Radioimmunoassays for fecal corticosterone levels appeared to be a reliable indicator of physiological stress in coyotes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endocrine system plays a vital role in the body’s ability to acclimatize to threatening situations (Boonstra 2004; Sheriff et al. 2011) by altering physiological and behavioral responses of the organism. When the endocrine system reacts to a threatening situation or stressor, it mobilizes the energy required to maintain homeostasis and survive (Aguilera 2011; Boonstra 2004; French et al. 2009). This energy mobilization is vital for an immediate stress response, and, depending on whether the stressor is acute or chronic, changes the energy cost of maintaining homeostasis necessary for survival (French et al. 2009; Keay et al. 2006; Möstl and Palme 2002; Touma and Palme 2005; Young et al. 2004). An acute stressor quickly mobilizes energy for the flight or fight stress response, increases catecholamines, coagulation, glucagon stimulation, and breathing, and increases the chance of survival (Arnemo and Caulkett 2007; Boonstra 2004).
Glucocorticoid (GC) concentrations give insight into adrenocortical activation (Arnemo and Caulkett 2007; Barja et al. 2008; Keay et al. 2006; Monfort et al. 1998). GCs (i.e., cortisol and corticosterone) are steroid hormones that are elevated in response to stressors (Dalmau et al. 2007; Millspaugh and Washburn 2004; Möstl et al. 1999; von der Ohe and Servheen 2002) and can provide a quantitative means for evaluating physiological stress in animals (von der Ohe and Servheen 2002). There are several different means for measuring GC concentrations: hair, feathers, saliva, plasma, urine and fecal samples (Sheriff et al. 2011). Some of these methods are more intrusive than others, with the noninvasive methods being more desirable in many cases. There have been a number of studies conducted using noninvasive methods for testing GC levels in animals (e.g., Creel 2005; Keay et al. 2006; McLeod et al. 1996; Touma et al. 2003; Wasser et al. 2000). When animals are handled they have a natural stress reaction, therefore, a noninvasive means of measurement (e.g., fecal or urine sample) may provide more valid information on the physiological state of the animal (Millspaugh and Washburn 2004; Möstl and Palme 2002; Touma and Palme 2005; Viljoen et al. 2008). Concentrations measured in fecal and urine samples are from metabolized GCs, meaning the levels produced in excrete are from a previous time period, such as the day before in feces (Hulsman et al. 2011; Touma and Palme 2005; Young et al. 2004) or hours before in urine (Galeandro et al. 2014; Zeugswetter et al. 2013). In contrast, plasma measurements indicate an immediate response to a stressor (Romero 2002).
Use of fecal cortisol and corticosterone metabolites (from this point forward will be grouped together as glucocorticoid metabolites or GCMs) should be validated to ensure the results are biologically meaningful (Keay et al. 2006; Touma and Palme 2005). There is a precedent in the literature to conduct validation procedures using radioactive tracers to determine the excretive fate of GCMs. For example, an experiment using radioactive tracers in domestic dogs (Canis familiaris), demonstrated that only a small amount of radioactive cortisol is excreted via the feces ~ 23%, whereas some is also excreted via the urine ~ 77% (Schatz and Palme 2001). Another prominent procedure for validating the use of fecal GCMs is to conduct an adrenocorticotropic hormone (ACTH) challenge. The injection of ACTH causes the activation of the hypothalamic–pituitary–adrenal axis (HPA-axis), and therefore, an increase in the release of GCs. Inducing the HPA-axis in this form provides the researcher with the knowledge that there should be an increase in fecal GCMs if they are a valid method for determining GC concentrations (Keay et al. 2006; Touma and Palme 2005). It is also important to consider age, sex, and circadian differences in GC release (Hoon Son et al. 2011; Keay et al. 2006; Touma et al. 2003; Touma and Palme 2005).
Sex differences in excretory GCMs have been observed in European stonechats (Saxicola torquata rubicola), and mice (Mus musculus f. domesticus), but not in coyotes or red deer (Cervus elaphus) (Goymann 2005; Huber et al. 2003; Schell et al. 2013; Touma et al. 2003). A study examining Steller sea lions (Eumetopias jubatus) found both sex and age class differences (Mashburn and Atkinson 2004), however, a study examining brown bears (Ursus arctos horibilis) did not (von der Ohe et al. 2004). There have also been a number of studies that have found circadian, or diurnal, effects on GCMs, which can influence ability to detect stress-related changes in GCMs (Brown et al. 2010; Dickmeis 2009; Smith et al. 2012; Touma et al. 2003). Also, when validating the use of fecal GCM concentrations as a tool to measure physiological stress, the method used to determine fecal GCM concentrations [i.e., radioimmunoassay (RIA) versus enzyme immunoassays (EIA)] may have an effect on which GC, cortisol or corticosterone, should be examined. Young et al. (2004) not only found that there was a difference depending upon which immunoassay was run, but as well as the species of the animal in question. In elephants, Brown et al. (2010) found that the different immunoassays produced similar results, but the type of urine samples (un-extracted, extracted) combined with the assay used impacted the GCM concentration. All of these variables, that may have an effect on the concentration outcomes, are important to consider when validating the use of fecal GCM concentration calculations for different species of interest.
Validations of fecal GCMs have been conducted in a number of species from many different taxon (Dloniak et al. 2004; Hulsman et al. 2011; Hunt et al. 2004; Hunt and Wasser 2003; Santymire et al. 2012; Schatz and Palme 2001; Wasser et al. 2000; Young et al. 2004). In the Canidae family, ACTH challenges have been conducted in domestic dogs, crab-eating foxes (Cerdocyoun thous), African wild dogs (Lycaon pictus), coyotes (Canis latrans), red wolves (Canis rufus), maned wolves (Chrysocyon brachyurus) and gray wolves (Canis lupus) providing information about the physiological stress response (de Villiers et al. 1997; Monfort et al. 1998; Rodrigues da Paz et al. 2014; Sands and Creel 2004; Schatz and Palme 2001; Schell et al. 2013; Vasconcellos et al. 2011; Young et al. 2004). However, none of these studies evaluated whether the pattern of the response of fecal GCMs were similar to the response found in the blood GCs (i.e., peaks in GCMs following the ACTH injection).
For the present study, the primary objectives were to (1) determine the glucocorticoid response in plasma (cortisol) and fecal (corticosterone and cortisol) samples collected following an ACTH injection, and (2) determine if there are effects of sex, age, and time of day of sample collection on fecal GCM concentrations. We tested the hypothesis that plasma cortisol and fecal GCMs would show a significant response to a controlled stimulation of the HPA-axis via ACTH. We predicted that the response in fecal GCMs would resemble the response in plasma cortisol, albeit the timescales (days versus minutes, respectively) would vary. We also predicted that the GCM response would vary between the sexes, according to age class, and the time of day. From the results of these main objectives, we also evaluated if the response in fecal GCMs resembled the response in plasma cortisol as a means of validating the use of fecal GCMs through radioimmunoassay. We were also interested in determining which GC antibody is better suited for measuring fecal GCMs in coyotes using RIA.
Materials and methods
Study location
The experiment was conducted at the U.S. Department of Agriculture, Wildlife Services, National Wildlife Research Center, Predator Research Facility, Millville, Utah, USA. During testing, coyotes were individually housed in outdoor kennels. The coyotes were housed in either a raised or floored kennel (raised floor 2.4 × 1.2 × 1.8 m; small floored 3.7 × 0.9 × 2.0 m; large floored 3.7 × 1.8 × 2.0 m). Each kennel type was equipped with a den box. Coyotes were moved into kennels one week prior to testing to allow for acclimation. Animals were fasted 24 h prior to injection to prevent complications while under anesthesia; all other days they were provided with their normal ration (650 g) of commercial mink food. Water was provided ad libitum. Kennels were checked every day and cleaned once per day except on the fast day. The experiment ran from 6 November to 18 December 2010. Animal care, anesthesia, and handling procedures were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC-QA 1809) at the USDA-National Wildlife Research Center and Utah State University.
Study animals
We initially used 32 coyotes (16 males and 16 females) for this experiment, ranging in age from 1.5 to 5 years. However, it should be noted that four animals (one male and three females) were later removed from the study for experimental reasons (see “sample collections” for details). Each individual was randomly assigned as either a treatment or a control animal (8 males and 8 females per group). On the day of the challenge, the coyotes were coaxed into their den boxes and manually restrained with pin sticks for an intramuscular injection of a 5:1 ketamine to xylazine anesthesia solution (two animals were anesthetized at a time); this type of restraint was a routine activity and a standard operating procedure for handling animals at the research facility. It should be noted that ketamine can affect cortisol concentrations, however, all animals including controls were treated the same (Hergovich et al. 2001; Khalili-Mahani et al. 2015). The amount of anesthesia drug administered varied between coyotes, but was based on an individual’s body size (range 10.71–16.67 mg/kg ketamine and 1.7–2.77 mg/kg xylazine). Once the animals were anesthetized, we weighed them, and initiated measurements of temperature, respiration, and pulse. The treatment group was given an injection of ACTH (4 IU/kg) (Porcine pituitary; Sigma-Aldrich, Co., St. Louis, MO) and the control group was given an injection of sterile saline solution (4 IU/kg); both were administered intravenously.
Sample collections
Fecal sample collections were initiated 2 days prior to injection and then continued for 2 days post-injection: scats were collected three times per day as available (morning, mid-day, evening) with each sample collected by the same person. This collection schedule allowed for a baseline measurement of fecal GCMs while also considering the diurnal fluctuations of the cortisol and corticosterone metabolites. To ensure the freshest sample was collected each time, any remaining scat was removed from the kennel. Fecal samples were frozen in a − 20 °C freezer until extraction.
Blood sampling occurred at five different time intervals: pre-injection (first blood draw after anesthetizing), and then at four times post-injection (10, 30, 60 and 90 min post-injection). The amount of blood collected at each interval was approximately 2 mL. The coyotes were kept under anesthesia for approximately 90 min and allowed to recover without any drug reversal. Prior to centrifuging, blood clots were removed from the blood tubes. The blood was centrifuged for 20 min at room temperature; the plasma was then collected into a cryovial and frozen at − 20 °C until extraction.
During anesthesia we removed four animals from the study (three females and one male) due to procedural problems that would influence the accurate and unbiased measurement of cortisol and corticosterone metabolites. The male was removed because he recovered from the anesthesia too early and consequently received an additional injection of Telazol, to which he reacted poorly. One female was removed due to her becoming hypothermic during anesthesia. The other two females were removed due to human error during the ACTH injections (i.e., the animals did not receive the proper ACTH dosage). The first two instances may have caused physiological stress, while the latter two instances would not have achieved a valid dose–response.
Hormone analyses
Fecal cortisol and corticosterone were extracted using a phosphate–methanol buffer solution following the methods of Shideler et al. (1994) and Bauman and Hardin (1998). The methods for extracting cortisol from the plasma followed the protocol in Neuman-Lee and French (2014). Plasma cortisol and fecal cortisol metabolite concentrations were determined using a radioimmunoassay kit (Siemens Coat-a-count cortisol RIA kit, Siemens Healthcare Diagnostics Inc., USA; cross reactivity with cortisol and cortisone is < 1%) and fecal corticosterone metabolite concentrations were determined using a double antibody radioimmunoassay kit (ImmuChemTM Double Antibody RIA kit, MP Biomedicals, Orangeburg, NY; cross reactivity with other metabolites is < 1%). Although, it should be noted that cross reactivity for fecal GCM may be higher than that reported by the manufacturer (Schatz and Palme 2001; Wasser et al. 2000). For both kits, we followed the assay procedure provided with the RIA kits. For the cortisol RIA kits, we added 10 µL of the fecal sample supernatant and 25 µL of the zero calibrator diluent, and for plasma we added 50 µL of plasma and 25 µL of the zero calibrator diluent. Some samples fell below the level of detectability and thus we re-ran these samples and doubled the amount of sample and only added 15 µL of the zero calibrator. We only examined the cortisol concentrations in the plasma because a preliminary examination determined the plasma corticosterone levels were too low to be accurately measured. For the corticosterone RIA kits, we added 10 µL of the fecal sample supernatant and 15 µL of the steroid diluent. Some samples fell outside of the standard curve and thus we re-ran these samples at a different dilution such that the re-run values fell within the range of detectability of the assay; even then, some extrapolated sample values were outside the standard curve. All samples, including the extrapolated values, were retained for statistical analyses.
For fecal cortisol and corticosterone, the method for calculating the concentrations of the metabolites was the same, following the procedures provided with radioimmunoassay kits. All samples were run in duplicate. Final concentrations were corrected for dilution factor and for dry fecal mass resulting in a final concentration of hormone per gram of fecal matter. Plasma cortisol samples were also corrected via individual recovery values that were calculated by adding a small amount of radioactivity to all samples prior to extraction. A subsample of each assayed sample was then run through a liquid scintillation counter to correct for any sample loss that happened during the extraction process.
Statistical analyses
We performed statistical analyses on 15 males and 13 females that were not excluded from the study. For the plasma cortisol samples, the first statistical test performed was a multi-way ANOVA to determine the influence of sex (male, female), treatment type (ACTH, saline), and the time of the blood draw (0, 10, 30, 60, 90 min) on concentrations of cortisol. Next we separated the data for the treatment (ACTH) and control (saline) groups, due to treatment type being a significant factor. We then ran a one-way ANOVA with a post-hoc Tukey’s test for each of these groups (ACTH, saline) separately to determine which times of blood collection were significantly different. In addition, we performed a repeated measures ANOVA to determine whether the individual coyotes responded to treatment differently. We separated the subjects by sex and treatment type to examine if the levels of cortisol were influenced by individual variation (between subjects), versus the time of blood collection (within subjects).
When we conducted the statistical tests for the fecal samples, the samples we used were from the first time period (morning) collection due to the lack of consistent collection during the other two time periods. For both the cortisol and corticosterone metabolite levels, we first performed a multi-way ANOVA to determine the influence of sex (male, female), treatment type (ACTH, saline) and the day of feces collection before or after injection (2 days before, 1 day before, 1 day after, 2 days after). Next, due to the sex of the animal being a highly significant factor, we separated the groups by males and females. Then we ran a one-way ANOVA and a post-hoc Tukey’s test to determine which days of fecal collection were significantly different from one another for the GCM concentrations. In addition, we also performed a repeated measures ANOVA, for both the cortisol and corticosterone metabolite levels, separated by sex and treatment type, to determine if there was significant individual variation (between subjects) versus significant differences between the fecal collection days (within subjects) on the levels of GCM metabolite concentrations in the fecal samples.
Results
Plasma cortisol
For the cortisol concentrations in the coyote plasma, for both treatment types combined, we found that 71% of the variation in cortisol levels was explained by the sex of the coyote, treatment type, and time of blood draws, and the interactions of these three variables. There was significant influence in the multi-way ANOVA of the treatment type, the time of the blood draw, the interaction of the treatment type, and the time of the blood draw (Table 1). Our results indicated that the treatment type (44.8% of the variance) of the coyote was a larger influence compared to the time of the blood draw (8.7% of the variance), and the interaction of the treatment type and the time of the blood draw (15.7% of the variance, Table 1; Fig. 1). The high influence of treatment type was expected as there should be a higher dose-response from the ACTH injection compared to the saline injection (control). According to the results of the one-way ANOVA and post-hoc Tukey’s test, we found that the time of the blood draw was a highly significant factor (R 2 = 0.312, F = 7.385, P < 0.001; Fig. 1).
Mean (± SD) cortisol concentrations (ng/mL) in coyote plasma for a control and ACTH males, and b control and ACTH females, at five different blood draw times (0, 10, 30, 60, 90 min) during an ACTH challenge, National Wildlife Research Center, Predator Research Facility, Millville, Utah, November–December 2010
Our results from the repeated measures ANOVA for plasma cortisol concentrations in coyotes showed significant differences for the influence of the time of the blood draw (Table 2). This indicated that we found dose-responses for the ACTH injections and handling responses for the control animals. The repeated measures ANOVA also found significant differences between control animals (Table 2) indicating that individual animals had different responses to handling, or rather individual variation. Interestingly, the repeated measures ANOVA model found no significant differences between the ACTH treatment individuals (Table 2) indicating that the ACTH injection had more of an influence than handling. The assay sensitivity was 2 ng/mL, with the intra-assay coefficient of variation < 5%.
Fecal GCMs
Fecal cortisol metabolites
For the cortisol metabolite concentrations in the coyote scats, we found that only 15% of the variation in the concentrations was explained by the sex of the coyote, the treatment type, the day of fecal collection, and the interactions of these three variables (Table 3). From the multi-way ANOVA we found the day of the fecal collection to be a significant factor, which implied the cortisol levels varied among the days of collection, but a dose–response from the ACTH injection was not indicated (Fig. 2) and there was likely a handling response among the control animals. The results from the one-way ANOVA and post-hoc Tukey’s test did not find a significant (at P = 0.05) influence of the day of collection for the fecal cortisol metabolite concentrations, however, it did appear to have some influence on male coyotes (males: R 2 = 0.128, F = 2.749, P = 0.051; females: R 2 = 0.082, F = 1.434, P = 0.244). We found no significant influences, in the one-way ANOVAs, for fecal cortisol metabolite concentrations (Table 4) indicating that there was no influence of the day of the fecal collection for fecal cortisol metabolite concentrations (Fig. 2). The assay sensitivity was 2 ng/mL, with the intra-assay coefficient of variation < 10% and the interassay coefficient of variation < 20%.
Mean (± SD) cortisol metabolite concentrations (ng/g) in coyote feces for a control and ACTH males, and b control and ACTH females, over four different fecal collection days before and after an ACTH challenge, National Wildlife Research Center, Predator Research Facility, Millville, Utah, November–December 2010
Fecal corticosterone metabolites
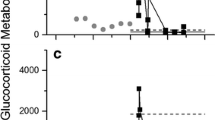
For the fecal corticosterone metabolite concentrations, we found 44% of the variation in concentrations was explained by the sex of the coyote, the treatment type, the day of fecal collection, and the interactions of these three variables, from the multi-way ANOVA (Table 3). Our results indicated that the sex of the coyote was a highly significant variable (Fig. 4). The time of the fecal collection was also a significant influence (Fig. 3). From the one-way ANOVAs, with post-hoc Tukey’s tests, we found the time of the fecal collection was a significant factor for males but not for females (males: R 2 = 0.564, F = 24.132, P < 0.001; females: R 2 = 0.138, F = 2.558, P = 0.066).
Mean (± SD) corticosterone metabolite concentrations (ng/g) in coyote feces for a control and ACTH males, and b control and ACTH females, over four different fecal collection days before and after an ACTH challenge, National Wildlife Research Center, Predator Research Facility, Millville, Utah, November–December 2010
Mean (± SD) corticosterone metabolite concentrations (ng/g) in feces comparing a control males and females, and b ACTH males and females, over four different fecal collection days before and after an ACTH challenge, National Wildlife Research Center, Predator Research Facility, Millville, Utah, November–December 2010
Our results from the repeated measures ANOVAs indicated that the day of the fecal collection influenced control males, ACTH males, and ACTH females (Table 4). However, there was not a significant influence of the day of fecal collection for control females. We also found that there was no significant variation between subjects for the fecal corticosterone metabolite concentrations (Table 4). These results suggested that there was a dose–response of the ACTH injections for both males and females, but also a handling response among the control males which confounded the treatment response among the males. Also, there was an indication that individual variation was not a factor that influenced fecal corticosterone metabolite concentrations. The assay sensitivity was 7.7 ng/mL, with the intra-assay coefficient of variation < 10% and the interassay coefficient of variation < 20%.
Other possible factors on GCM levels
We also examined the fecal GCM diurnal fluctuation for both cortisol and corticosterone metabolites (Fig. 5) on two different days before the ACTH challenge. We only separated the data by sex for the corticosterone metabolite samples due to no sex differences between males and females in the cortisol metabolite samples. Though there does appear to be some influence of diurnal fluctuation, the individual variation was a larger influence. The standard deviation error bars overlap for the three different time periods on both days indicating no influence of the time of collection on cortisol and corticosterone metabolite levels in the fecal samples.
Mean (± SD) fecal a cortisol metabolite concentrations (ng/g) for males and females, and corticosterone (cort.) metabolite concentrations (ng/g) for b males, and c females, at three (1—morning, 2—mid-day, 3—evening) different time periods on two different days, to examine diurnal fluctuation, National Wildlife Research Center, Predator Research Facility, Millville, Utah, November–December 2010
We were also interested in determining if the time of handling on the test day was an influence for the baseline plasma cortisol concentrations. The animals were classified based on the time of handling with the morning group consisting of animals handled before 12 p.m. and the afternoon group was handled after that time. Mean plasma cortisol concentrations for animals handled in the morning [2.864 ng/mL ± 1.735 (SD)] was not different than values from animals handled in the afternoon (3.235 ng/mL ± 2.588 L t = 0.485 m, P = 0.31).
Another variable we examined was the age of the coyote at the time of the ACTH trial. The data was separated by age class, and the baseline measurements for each class were calculated. Mean plasma cortisol concentrations across the five age classes (Table 5) did not significantly differ (F = 0.03, df = 4, 27, P = 0.998). Similarly, mean fecal cortisol metabolite concentrations did not significantly differ among the five age classes (F = 1.61, df = 4, 27, P = 0.20; Table 5). We also examined the influence of age on corticosterone metabolites in the fecal samples collected 1 day before the ACTH trial. Mean corticosterone metabolite concentrations in the fecal samples collected 1 day prior to the trial did not significantly differ among the five age classes for males (Table 5) or four age classes for females (Table 5).
Discussion
Plasma versus fecal responses to ACTH
The present study was the first to comprehensively compare the results of plasma cortisol and fecal GCMs in response to an ACTH challenge for a species in the family Canidae. Overall our results supported our hypothesis and demonstrated a dose–response in both the plasma and fecal samples following an ACTH injection, and that fecal GCM concentration (mainly the assay for corticosterone) showed a dose–response pattern similar to the plasma cortisol concentrations. In the plasma samples, we found our exogenous ACTH induced peaks ~ 30–60 min post ACTH injection. Fecal GCMs showed clear peaks with the corticosterone metabolites peaking ~ 1 day (~ 12 h) post-injection for both females and males, but the control males also showed a similar peak, indicating a handling effect. We found no significant effect of ACTH treatment on fecal cortisol metabolites for either sex. It is important to note that fecal GCM peaks may have occurred sooner, however the coyotes were fasted the day before the ACTH challenge. Other ACTH challenge studies comparing blood GC to fecal GCM concentrations also found fecal GCMs to show similar results in different species including snowshoe hares (Lepus americanus; Sheriff et al. 2010), Belding’s ground squirrels (Spermophilus beldingi; Mateo and Cavigelli 2005), and female ring-tailed lemurs (Lemur catta; Cavigelli 1999).
Sex dependent effects
Coyotes in the current study also showed significant sex-dependent differences in plasma GC and in fecal GCM response to handling stress. Specifically, we found that our control animals given saline injections rather than ACTH still had a stress response from the handling and anesthesia, especially in plasma cortisol concentrations; although the peaks for the control animals were relatively lower than ACTH animals. This handling stress response was significant in fecal GCMs for the control males but not the control females, suggesting a sex-dependent difference in response to handling. Male coyotes are the main defender of territorial boundaries against intruders (Gese 2001) and may have responded more strongly to our intrusion during handling than the females. Interestingly, Laver et al. (2012) found similar results, where the control male banded mongooses (Mongos mongo) had elevated GCM concentrations post-handling. Our results also indicated sex differences in corticosterone fecal metabolite levels, with the females having higher concentrations pre- and post-injection. This is not surprising given that many different species show significant sex differences in circulating steroid concentrations (Handa et al. 1994; Neuman-Lee and French 2017; Neuman-Lee et al. 2017). However, we found no differences between the sexes for plasma cortisol and fecal cortisol metabolite concentrations, which are present at relatively lower concentrations than fecal corticosterone metabolites. Moreover, Schell et al. (2013) were unable to detect sex differences in fecal cortisol metabolites using an EIA. The ability of the current study to detect sex differences with a corticosterone RIA, suggests that utilizing the correct antibody is essential for detecting biological differences. These contradictory findings correspond to the varied patterns in sex-specific response in that studies have shown no consistent pattern (reviewed in Touma and Palme 2005). The lack of a predictable pattern in both sex-specific responses to stressors and metabolism of GC products further impresses the importance of using both sexes when assessing physiological responses to stress (reviewed in Touma and Palme 2005).
Age, diurnal fluctuations, and other factors
While we found expected differences in responses between the sexes, we did not observe any age-related differences in coyote plasma cortisol or fecal GCM concentrations. Our results are similar to von der Ohe et al. (2004) who also did not find differences in age class for brown bears (U. arctos horribilis), and Nováková et al. (2008) who found no significant differences between adult and sub-adult spiny mice (Acomys cahirinus). We also found the time of handling did not influence the initial plasma cortisol concentrations. There did appear to be diurnal fluctuation in fecal GCMs, but they were not statistically significant. Sampling was dependent on the individual coyote’s defecation time (i.e., fecal swabs would impose additional stress and so were not used), and thus we were unable to collect fecal samples from all of the animals during all three time periods. Another possible explanation for the lack of statistically significant diurnal fluctuation in our study could be due to the season of fecal collection. Our study was conducted during the fall; however, a different study in coyotes by Schell et al. (2013) found evidence of diurnal fluctuations of fecal GCM concentrations for coyotes that were sampled during the summer. Owen et al. (2005) also found season to be an influence over diurnal fluctuations of GCMs for giant pandas (Ailuropoda melanoleuca). They found significant differences in winter and spring, but no significant differences during the other seasons.
Finally, we also found high individual variation of plasma cortisol and fecal GCM concentrations and responses to handling and ACTH challenges. Carnes et al. (1988) found individual variation in the rhythm of cortisol and ACTH micropulse secretions for Rhesus monkeys (Mucaca mulatta). Research has demonstrated that many different factors may lead to variation in stress reactivity (Meaney 2001), such as varying personalities in birds (Cockrem 2013). Even though there was individual variation in responses, we still found dose-responses in the treatment animals and handling responses in the control animals. A majority of previous ACTH challenge studies had sample sizes between 1 and 4 animals, and therefore, due to the smaller sample sizes, variation reported in those studies may be misleading.
Antibody and assay considerations
We found orders of magnitude differences in the concentrations of fecal GCMs with two commonly used antibodies. The ImmuChemTM double antibody RIA kit detected higher levels of GCMs than the Siemens Coat-a-count cortisol RIA kit (Fig. 6). Most importantly, the corticosterone antibody detected a significant response to ACTH and handling stress, whereas the cortisol antibody did not. Our findings also differ from those reported by Schell et al. (2013), who found higher cortisol metabolite concentrations in the feces in coyotes, but this discrepancy is likely explained by different cross-reactivity of the antibodies used in the different assay and extraction methods. In support of this idea, Wasser et al. (2010) demonstrated high cross reactivity of the ICN antibody with other GCMs even though the manufacturer reported cross reactivity is < 1% for cortisol. Young et al. (2004) found similar differences between radioimmunoassay and enzyme immunoassays for carnivores as well.
These differences in antibodies and assay approaches are highly relevant as the noninvasive fecal GCM technique has become an important method for monitoring the welfare of a vast number of species (Dloniak et al. 2004; Hulsman et al. 2011; Hunt et al. 2004; Santymire et al. 2012; Schatz and Palme 2001; Wasser et al. 2000; Young et al. 2004), including those from the Canidae family (de Villiers et al. 1997; Monfort et al. 1998; Rodrigues da Paz et al. 2014; Sands and Creel 2004; Schatz and Palme 2001; Schell et al. 2013; Vasconcellos et al. 2011; Young et al. 2004).
Conclusions
In conclusion, we found the corticosterone radioimmunoassay of fecal GCMs for coyotes showed a dose-response to the ACTH challenge similar to the levels of plasma cortisol, albeit on different timescales. We also observed significant sex, but not age related, differences in concentrations of fecal GCMs with females having higher concentrations. The sexes also differed in their response to handling stress, where control males mounted a significant fecal GCM response to handling stress, but females did not. While we found diurnal variations in GCMs, they were not statistically significant, and this was likely due in part to high inter-individual variation in fecal GCMs and GCM responses to stress. Overall, these results demonstrate the effectiveness of using fecal GCMs (namely the radioimmunoassay for corticosterone) for assessing physiological stress responses and sex-related response differences among coyotes.
References
Aguilera G (2011) HPA axis responsiveness to stress: implications for healthy aging. Exp Gerontol 46:90–95
Arnemo JM, Caulkett N (2007) Stress. In: West G, Heard D, Caulkett N (eds) Zoo animal and wildlife immobilization and anesthesia. Blackwell Publishing Professional, Iowa, pp 102–110
Barja I, Silván G, Illera JC (2008) Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J Chem Ecol 34:697–701
Bauman JE, Hardin A (1998) Measurement of steroids in animal feces with commercially available RIA kits intended for use in human serum. J Clin Ligand Assay 21:83
Boonstra R (2004) Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr Comp Biol 44:95–108
Brown JL, Kersey DC, Freeman EW, Wagener T (2010) Assessment of diurnal urinary cortisol excretion in Asian and African elephants using different endocrine methods. Zoo Biol 29:274–283
Carnes M, Kalin NH, Lent SJ, Barksdale CM, Brownfield MS (1988) Pulsatile ACTH secretion: variation with time of day and relationship to cortisol. Peptides 9:325–331
Cavigelli SA (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav 57:935–944
Cockrem JF (2013) Corticosterone responses and personality in birds: Individual variation and the ability to cope with environmental changes due to climate change. Gen Comp Endocrinol 190:156–163
Creel S (2005) Dominance, aggression, and glucocorticoid levels in social carnivores. J Mammal 86:255–264
Dalmau A, Ferret A, Chacon G, Manteca X (2007) Seasonal changes in fecal cortisol metabolites in Pyrean Chamois. J Wildl Manage 71:190–194
de Villiers MA, van Jaarsveld S, Meltzer DGA, Richardson PRK (1997) Social dynamics and the cortisol response to immobilization stress of the African wild dog, Lycaon pictus. Horm Behav 31:3–14
Dickmeis T (2009) Glucocorticoids and the circadian clock. J Endocrinol 200:3–22
Dloniak SM, French JA, Place NJ, Weldele ML, Glickman SE, Holekamp KE (2004) Non-invasive monitoring of fecal androgens in spotted hyenas (Crocuta crocuta). Gen Comp Endocrinol 135:51–61
French SS, Moore MC, Demas GE (2009) Ecological immunology: the organism in context. Integr Comp Biol 49:246–253
Galeandro L, Sieber-Ruckstuhl NS, Riond B, Hartnack S, Hofmann-Lehmann R, Reusch CE, Boretti FS (2014) Urinary corticoid concentrations measured by 5 different immunoassays and gas chromatography-mass spectrometry in healthy dogs and dogs with hypercortisolism at home and in the hospital. J Vet Internal Med 28:1433–1441
Gese EM (2001) Territorial defense by coyotes (Canis latrans) in Yellowstone National Park, Wyoming: who, how, where, when, and why. Can J Zool 79:980–987
Goymann W (2005) Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann NY Acad Sci 1046:35–53
Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamic-pituitary-adrenal axis. Horm Behav 28:464–476
Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, Jilma B (2001) Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men a randomized, double-blind, placebo- controlled trial. Neuropsychopharmacology 24:590–593
Hoon Son G, Chung S, Kim K (2011) The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front Neuroendocrinol 32:451–465
Huber S, Palme R, Arnold W (2003) Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus). Gen Comp Endocrinol 130:48–54
Hulsman A, Dalerum F, Ganswindt A, Muenscher S, Bertschinger HJ, Paris M (2011) Non-invasive monitoring of glucocorticoid metabolites in Brown Hyaena (Hyaena brunnea) feces. Zoo Biol 30:451–458
Hunt KE, Wasser SK (2003) Effect of long-term preservation methods on fecal glucocorticoid concentrations of Grizzly bear and African elephant. Physiol Biochem Zool 76:918–928
Hunt KE, Trites AW, Wasser SK (2004) Validation of a fecal glucocorticoid assay for Stellar sea lions (Eumetopias jubatus). Physiol Behav 80:595–601
Keay JM, Singh J, Gaunt MC, Kaur T (2006) Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. J Zoo Wildl Med 37:234–244
Khalili-Mahani N, Martini CH, Olofsen E, Dahan A, Niesters M (2015) Effect of subanaesthetic ketamine on plasma and saliva cortisol secretion. Br J Anaesth 115:68–75
Laver PN, Ganswindt A, Ganswindt SB, Alexander KA (2012) Non-invasive monitoring of glucocorticoid metabolites in banded mongooses (Mungos mungo) in response to physiological and biological challenges. Gen Comp Endocrinol 179:178–183
Mashburn KL, Atkinson S (2004) Evaluation of adrenal function in serum and feces of Steller sea lions (Eumetopias jubatus): influences of molt, gender, sample storage, and age on glucocorticoid metabolism. Gen Comp Endocrinol. 136:371–381
Mateo JM, Cavigelli SA (2005) A validation of extraction methods for non-invasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084
McLeod PJ, Moger WH, Ryon J, Gadbois S, Fentress JC (1996) The relation between urinary cortisol levels and social behaviour in captive timber wolves. Can J Zool 74:209–216
Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–1192
Millspaugh JJ, Washburn BE (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138:189–199
Möstl E, Palme R (2002) Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74
Möstl E, Messmann S, Bagu E, Robia C, Palme R (1999) Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J Vet Med A Physiol Pathol Clin Med 46:621–631
Monfort SL, Mashburn KL, Brewer BA, Creel SR (1998) Evaluating adrenal activity in African Wild Dogs (Lycaon pictus) by fecal corticosteroid analysis. J Zoo Wildl Med 29:129–133
Neuman-Lee LA, Brodie ED Jr, Hansen T, Brodie ED III, French SS (2017) To stress or not to stress: physiological responses to tetrodotoxin in resistant gartersnakes vary by sex. Comp Biochem Physiol, Part A 209:34–40
Neuman-Lee LA, French SS (2014) Wound healing reduces stress-induced immune changes: evidence for immune prioritization in the side-blotched lizard. J Comp Physiol B 184:623–629
Neuman-Lee LA, French SS (2017) Endocrine-reproductive-immune interactions in female and male Galápagos marine iguanas. Hormones Behav 88:60–69
Nováková M, Palme R, Kutalová H, Janský L, Frynta D (2008) The effects of sex, age and commensal way of life on levels of fecal glucocorticoid metabolites in spiny mice (Acomys cahirinus). Phys Behav 95:187–193
Owen MA, Czekala NM, Swaisgood RR, Steinman K, Lindburg DG (2005) Seasonal and diurnal dynamics of glucocorticoids and behavior in giant pandas. Ursus 16:208–221
Rodrigues da Paz RC, Souza NP, Brown JL (2014) Evaluation of glucocorticoid faecal monitoring as a non-invasive assessment of stress in captive crab-eating fox (Cerdocyoun thous) after ACTH stimulation. J Steroids Horm Sci S12:008
Romero LM (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24
Sands J, Creel S (2004) Social dominance, aggression and faecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim Behav 67:387–396
Santymire RM, Freeman EW, Lonsdorf EV, Heintz MR, Armstrong DM (2012) Using ACTH challenges to validate techniques for adrenocortical activity analysis in various African wildlife species. Int J Anim Vet Adv 4:99–108
Schatz S, Palme R (2001) Measurement of faecal cortisol metabolites in cats and dogs: a non-invasive method for evaluating adrenocortical function. Vet Res Commun 25:271–287
Schell CJ, Young JK, Lonsdorf EV, Santymire RM (2013) Anthropogenic and physiologically induced stress responses in captive coyotes. J Mammal 94:1131–1140
Sheriff MJ, Krebs CJ, Boonstra R (2010) Assessing stress in animal populations: Do fecal and plasma glucocorticoids tell the same story? Gen. Comp Endocrinol 166:614–619
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887
Shideler SE, Savage A, Ortuno AM, Mooman EA, Lasley BL (1994) Monitoring female reproductive function by measurement of fecal estrogen and progesterone metabolites in the white–faced saki (Pithecia pithecia). Am J Primatol 32:95–108
Smith JE, Monclús R, Wantuck D, Florant GL, Blumstein DT (2012) Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen Comp Endocrinol 178:417–426
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74
Touma C, Sachser N, Möstl E, Palme R (2003) Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278
Vasconcellos AS, Chelini MM, Palme R, Guimarães MABV, Oliveira CA, Ades C (2011) Comparison of two methods for glucocorticoid evaluation in maned wolves. Pesqui Vet Bras 31:79–83
Viljoen JJ, Ganswindt A, Palme R, Reynecke HC, Du Toit JT, Langbauer WR Jr (2008) Measurement of concentrations of faecal glucocorticoid metabolites in free-ranging African elephants within the Kruger National Park. Koedoe 50:18–21
von der Ohe CG, Servheen C (2002) Measuring stress in mammals using fecal glucocorticoids: opportunities and challenges. Wildl Soc Bull 30:1215–1225
von der Ohe CG, Wasser SK, Hunt KE, Servheen C (2004) Factors associated with fecal glucocorticoids in Alaskan brown bears (Ursus arctos horribilis). Physiol Biochem Zool 77:313–320
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Wasser SK, Azkarate JC, Booth RK, Hayward L, Hunt K, Ayres K, Vynne C, Gobush K, Canales-Espinosa D, Rodríguez-Luna E (2010) Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species. Gen Comp Endocrinol 168:1–7
Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137:148–165
Zeugswetter FK, Neffe F, Schwendenwein I, Tichy A, Möstl E (2013) Configuration of antibodies for assay of urinary cortisol in dogs influences analytic specificity. Domest Anim Endocrinol 45:98–104
Acknowledgements
We thank the USDA/APHIS/WS/National Wildlife Research Center, Predator Research facility for funding and use of the captive coyote colony. We also thank the staff at the Predator Research facility, especially S. Brummer, J. Schultz, and N. Floyd for helping with the care of the coyotes. We also thank B. Roberts for teaching us the extraction method, M. Stevenson for the help extracting the hormones from fecal samples, and J. Young for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed. Animal care, anesthesia, and handling procedures were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC-QA 1809) at the USDA-National Wildlife Research Center and Utah State University.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Stevenson, E.T., Gese, E.M., Neuman-Lee, L.A. et al. Levels of plasma and fecal glucocorticoid metabolites following an ACTH challenge in male and female coyotes (Canis latrans). J Comp Physiol B 188, 345–358 (2018). https://doi.org/10.1007/s00360-017-1125-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1125-2