Abstract
Yellow vein clearing disease of citrus, associated with citrus yellow vein clearing virus (CYVCV), is transmitted by Aphis spiraecola, A. crassivora, and Dialeurodes citri and spreading quickly in Asia and the Near East. Because A. gossypii is common in affected orchards, adults were tested as vectors with either a 24-h acquisition access period (AAP) on infected sour orange then a 24-h inoculation access period (IAP) on virus-free seedlings, or a 48-h AAP/48-h IAP. After 3 months, 63.3% of the seedlings were infected after the 24-h/24-h IAP/AAP, 66.7% after the 48-h/48-h IAP/AAP. This is the first report of CYVCV transmission by Aphis gossypii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First reported in 1988 from Pakistan (Catara et al. 1988), yellow vein clearing disease of citrus was thereafter reported in India, Turkey, China, Iran, and more recently in Cyprus (Ahlawat 1997; Alas et al. 2019; Hashmian and Aghajanzadeh 2017; Önelge 2003; Zhou and Li 2010). Some infected citrus species and cultivars quickly exhibit symptoms, while others are latently infected (Alshami et al. 2003; Catara et al. 1988; Önelge 2003). On lemon (Citrus limon) and sour orange (C. aurantium), the symptoms include yellow vein clearing, chlorotic spot, water soaking, leaf flecking, leaf curling, mottling, and veinal necrosis (Alshami et al. 2003; Önelge 2003). In China, yield losses can range from 50 to 80%, and fruit quality is reduced (Li et al. 2017; Zhang et al. 2018).
The pathogen associated with the disease, citrus yellow vein clearing virus (CYVCV) is a flexuous particle, 13–14 nm in diameter, with a modal length of 685 nm and encapsidating single-stranded positive-sense RNA of 7.5 kb (Grimaldi and Catara 1996; Loconsole et al. 2012; Önelge et al. 2011). It has been classified in the genus Mandarivirus within the Alphaflexiviridae family, order Tymovirales (Adams et al. 2014).
CYVCV can be transmitted by grafting to 16 citrus cultivars including lemon and sour orange and mechanically to quinoa (Chenopodium quinoa), lambs quarters (Chenopodium amaranticolor), common bean (Phaseolus vulgaris), and cowpea (Vigna unguiculata) (Alshami et al. 2003; Önelge et al. 2011). Aphis spiraecola and Aphis craccivora transmit the virus from bean to bean and from lemon to bean, while A. spiraecola and Dialeurodes citri transmit it from citrus to citrus (Önelge et al. 2011; Zhang et al. 2018, 2019).
Because CYVCV is rapidly spreading in citrus orchards in Çukurova region (Turkey) and the cotton aphid, A. gossypii, is widely distributed in the area, here we tested whether this aphid can acquire CYVCV from infected sour orange and transmit it to virus-free sour orange.
Materials and methods
Plant materials
About 500 sour orange seeds were treated with a solution of 1% (w/v) 8-hydroxyquinoline hemisulfate, sown in sterilized potting soil (2/3 peat and 1/3 sand) and watered twice a week in an isolated insect-proof greenhouse at 20–25 °C. Ten-week-old seedlings were transferred to individual 5-l grow bags, labelled, and kept in a greenhouse for further use. Two years later, total nucleic acid (TNA) was extracted from new flushes with CTAB buffer according to a modified protocol proposed by Li et al. (2008). Four (4) µl of the extracted TNA, 1 µl of dNTP, 1 µl of random hexamer primer and 6 µl of RNase-free water were denaturated at 95 °C for 3 min and immediately chilled on ice for 10 min. Four microliters of M-MLV-RT buffer 1 × (Promega, Madison, WI, USA), 2 µl of DTT (0.1 M), 0.2 µl of M-MLV-RT (200 U/µl), and 1.8 µl of RNase-free water were added to the chilled product and the mixture incubated at 42 °C for 40 min and 70 °C for 15 min. The 25 µl reaction mixture for conventional PCR contained 2 µl of cDNA, 12.5 µl of 2 × DreamTaq Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), 8.5 µl of RNase-free water, and 1 µl of each of the primers (5′-TACCGCAGCTATCCATTTCC-3′ and 5′-GCAGAAATCCCGAACCACTA-3′) designed by Chen et al. (2014). The PCR program consisted of one cycle of 94 °C for 3 min; 35 cycles at 94 °C for 1 min, 59 °C for 1 min, 72 °C for 1 min 30 s; and a final extension at 72 °C for 7 min. The amplicons were electrophoresed in 1.5% (w/v) agarose gel and stained with ethidium bromide. Products were visualized under UV light, revealing that the seedlings were virus-free and could be used for the transmission tests.
In addition, one symptomatic sour orange tree that tested positive for CYVCV was used as a virus source plant at Çukurova University’s trial fields.
Maintenance of A. gossypii colonies
Colonies of A. gossypii were collected from asymptomatic citrus trees that previously tested negative to the virus; a subset of 25 aphids tested negative for the virus by RT-PCR. So the remaining aphids were reared for 1 week on the 2-year-old virus-free sour orange seedlings that were grown from seeds and kept in the insect-proof greenhouse as described earlier. The aphids were transferred to new virus-free sour orange seedlings every day; a subset of 25 aphids was collected and shown by RT-PCR to be negative for the virus, so these colonies were then assessed for virus transmission.
Transmission
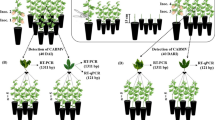
Spring flushes were collected from the sour orange tree identified as a virus source and used to feed the virus-free A. gossypii in a laboratory at 20–25 °C for an acquisition access period (AAP) of 24 h or 48 h. From each AAP group, 25 aphids were pooled and confirmed by RT-PCR as described above to be virus-positive (Fig. 1). The 24-h AAP aphids were used in expt. 1; the 48-h AAP aphids were used in expt. 2. In each experiment, 100 virus-positive aphids were placed on young flushes of each of 10 2-year-old virus-free sour orange seedlings for an inoculation access period (IAP) of 24 h in expt. 1 and 48 h in expt. 2. The receptor plants were then treated with insecticide and placed in insect cages. Every experiment was done three times. Three months later, the receptor plants were analyzed by RT-PCR for CYVCV. The total numbers of infected and noninfected seedlings were compared between the two AAP/IAP groups for significant differences using Fisher's exact test in SPSS V21 (IBM, Armonk, NY, USA).
Results
A. gossypii tested positive for CYVCV after the 24-h and 48-h AAP, suggesting that this aphid is a candidate for the transmission of the CYVCVV. In addition, some receptor plants had characteristic symptoms of CYVCV such as water soaking, leaf flecking, and leaf curling by 3 months after the IAP. The RT-PCR test of receptor plants (Fig. 2) revealed CYVCV was present in 70%, 50%, and 50% of the plants in the three respective tests, after the 24-h AAP/24-h IAP (mean 63.3%) and in 90%, 60%, and 50% after 48-h AAP/48-h IAP (mean 66.7%). Fisher’s exact test result (2-tail, P > 0.05) indicated that there was no significant difference in transmission between the two acquisition and inoculation periods (Table 1).
CYVCV extracted from A. gossypii was amplified by RT-PCR and sequenced with an ABI 3730XL Sanger sequencer (Applied Biosystems, Foster City, CA, USA) using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) at Macrogen Europe (Macrogen, Amsterdam, Netherlands). The sequence was analyzed and deposited in GenBank as accession MT395314. It shared 99.64% nucleotide sequence identity with reference isolate Y1.
Discussion
A. gossypii is polyphagous, affecting at least 700 plant species (Carletto et al. 2009; IRAC, 2020), and is an effective vector of more than 50 plant viruses including citrus tristeza virus (CTV) and citrus vein enation virus (CVEV) (Bar-Joseph and Loebenstein 1973; de Mendoza et al. 1993; IRAC 2020).
In previous studies on CYVCV transmission, the acquisition and transmission of the virus by A. spiraecola, A. crassivora, and D. citri were highlighted. Because A. spiraecola and D. citri belong to different families, Zhang et al. (2019) concluded that CYVCV can be transmitted by a broad range of insect vectors and that common insects in citrus orchards, including A. gossypii, should be tested as potential vectors. Our present study on the transmission of CVYVC by A. gossypii from infected sour orange to virus-free sour orange confirmed their hypothesis that other insects can acquire and transmit CVYVC.
The average rates of virus transmission after 3 months were 63.3% and 66.7% for AAP/IAP of 24 h/24 h and 48 h/48 h, respectively, much higher than the transmission by A. spiraecola (4.4% after 24 h/24 h AAP/IAP and 23.3% after 48 h/48 h; Zhang et al. 2018) and by Dialeurodes citri (31.9% after 48 h/48 h AAP/IAP; Zhang et al. 2019) after 3 months. This difference may be explained by the high efficiency of A. gossypii for transmitting plant viruses. Indeed, this aphid can acquire CTV in a minimum of 30 min and transmit it within 60 min (Campolo et al. 2014). Inoculation with one individual of A. gossypii can transmit papaya ringspot virus (PRSV) at, a rate of 53%, and a group of five can achieve 100% transmission (Kalleshwaraswamy and Kumar 2008).
Rate of transmission between the AAP/IAP of 24 h/24 h and 48 h/48 h did not differ significantly in contrast with the results of de Mendoza et al. (1993), who found that longer acquisition and inoculation access periods increase the transmission rate of CVEV by Myzus persicae and A. gossypii.
This first evidence of the transmission of CYVCV from citrus to citrus by A. gossypii contributes to a better understanding of the epidemiology of citrus yellow vein clearing. More attention needs to be given to this aphid and other insects as vectors and to designing integrated pest management strategies to control the vectors.
References
Adams MJ, Lefkowitz EJ, King AMQ, Carstens EB (2014) Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2014). Arch Virol 159:2831–2841
Ahlawat YS (1997) Viruses, greening, bacterium, and viroids associated with citrus (Citrus species) decline in India. Indian J Agr Sci 67:51–57
Alas T, Baloglu S, Caglar BK, Gunes A (2019) Detection and characterization of citrus tatter leaf virus (CTLV) and citrus yellow vein clearing virus (CYVCV) in citrus trees from Cyprus. Saudi J Biol Sci 26:995–998
Alshami AAA, Ahlawat YS, Pant RP (2003) A hitherto unreported yellow vein clearing disease of citrus in India and its viral aetiology. Indian Phytopathol 56:422–427
Bar-Joseph M, Loebenstein G (1973) Effects of strain, source plant, and temperature on the transmissibility of citrus tristeza virus by the melon aphid. Phytopathology 63:716–720
Campolo O, Chiera E, Malacrinò A, Laudani F, Fontana A, Albanese GR, Palmeri V (2014) Acquisition and transmission of selected CTV isolates by Aphis gossypii. J Asia-Pac Entomol 17:493–498
Carletto J, Lombaert E, Chavigny P, Brevault T, Lapchin L, Vanlerberghe-Masutti F (2009) Ecological specialization of the aphid Aphis gossypii Glover on cultivated host plants. Mol Ecol 18:2198–2212
Catara A, Azzaro A, Moghal S, Khan D (1988) Virus, viroid, and prokaryotic diseases of citrus in Pakistan. Proceeding of the 6th international citrus congress, Israel Balaban publisher, Rehovot, Israel. 957–962
Chen HM, Li ZA, Wang XF, Zhou Y, Tang KZ, Zhou CY, Zhao XY, Yue JQ (2014) First report of citrus yellow vein clearing virus on lemon in Yunnan, China. Plant Dis 98:1747–1747
de Mendoza AH, Pina JA, Ballester-Olmos JF, Navarro L (1993) Persistent transmission of citrus vein enation virus by Aphis gossypii and Myzus persicae. In: international organization of citrus virologists conference proceedings (1957–2010), 12(12). Retrieved from https://escholarship.org/uc/item/4rw738cf. Cited 21 Jul 2020
Grimaldi V, Catara A (1996) Association of a filamentous virus with yellow vein clearing of lemon. In: proceeding of the 13th conference of international organization of citrus, UC Riverside, CA, USA. 343–345
Hashmian B, Aghajanzadeh S (2017) Occurrence of citrus yellow vein clearing virus in Citrus species in Iran. J Plant Pathol 99:290
IRAC [Insecticide Resistance Action Committee] (2020) Mellon & cotton aphid. Available via https://irac-online.org/pests/aphis-gossypii/. Cited 08 Jun 2020
Kalleshwaraswamy CM, Kumar NKK (2008) Transmission efficiency of Papaya ringspot virus by three aphid species. Phytopathology 98:541–546
Li R, Mock R, Huang Q, Abad J, Hartung J, Kinard G (2008) A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J Virol Methods 154:48–55
Li XT, Su HN, Tan KG, Tong X-N, Zhong BL (2017) Fruit malformation of satsuma mandarin (Citrus unshiu Marc.) infected by Citrus yellow vein clearing virus. J Phytopathol 165:283–288
Loconsole G, Önelge N, Potere O, Giampetruzzi A, Bozan O, Satar S, De Stradis A, Savino V, Yokomi RK, Saponari M (2012) Identification and characterization of Citrus yellow vein clearing virus, a putative new member of the genus Mandarivirus. Phytopathology 102:1168–1175
Önelge N (2003) First report of yellow vein clearing of lemons in Turkey. J Turk Phytopathol 32:53–55
Önelge N, Satar S, Elibüyük Ö, Bozan O, Kamberoğlu M (2011) Transmission studies on citrus yellow vein clearing virus. In: international organization of citrus virologists conference proceedings (1957–2010), 18(18). Retrieved from https://escholarship.org/uc/item/4134f1xr/. Cited 21 Jul 2020
Zhang Y, Wang Y, Wang Q, Cao M, Zhou C, Zhou Y (2018) Identification of Aphis spiraecola as a vector of citrus yellow vein clearing virus. Eur J Plant Pathol 152:841–844
Zhang YH, Wang YL, Zhou CY, Zhou Y, Liu CH, Wang Q (2019) Identification of Dialeurodes citri as a vector of citrus yellow vein clearing virus in China. Plant Dis 103:65–68
Zhou C, Li Z (2010) Current occurrence and research status of graft-transmissible citrus diseases in P. R. China. In: proceeding of the 18th conference of international organization of citrus virologists, UC Riverside, Sao Paulo, Brazil. Pp. 44
Acknowledgements
This work was supported by Çukurova University under the research grant FDK-2018-11365.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afloukou, F.M., Çalişkan, F. & Önelge, N. Aphis gossypii Glover is a vector of citrus yellow vein clearing virus. J Gen Plant Pathol 87, 83–86 (2021). https://doi.org/10.1007/s10327-020-00976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-020-00976-6