Abstract
In 2017, premature abscission of leaves and dry rot with discoloration on the epidermis of the stem were observed in the field of reproductive-stage soybean in southern Shan State and the Nay Pyi Taw region in Myanmar. On the basis of morphological characteristics, two fungal genera, Colletotrichum and Diaporthe, were identified. Multilocus phylogenetic analyses based on the ITS, TUB and EF1-α genes for Diaporthe and the ITS, ACT, GAPDH, and CHS-1 genes for Colletotrichum were used to identify the species as Colletotrichum plurivorum, C. truncatum, Diaporthe endophytica and D. melonis. The pathogenicity of the collected isolates was confirmed by inoculation of soybean cultivar (Yezin-10), and all species except for C. plurivorum were virulent. The isolates of C. plurivorum were less aggressive than the other isolates. Koch’s postulate was fulfilled by reisolation of the original inoculated fungal isolates from the symptomatic tissue. In two locations in Myanmar, C. plurivorum, D. endophytica and D. melonis occurred in southern Shan State, whereas only one C. truncatum was found in the Nay Pyi Taw region. This is the first report of Colletotrichum and Diaporthe species associated with soybean stem diseases in Myanmar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max) is a major crop in Myanmar, where it is cultivated on 0.15 million hectares and yield averaged 1.07 tons per ha in 2016 (CSO 2017); however, the yield is approximately one-third of the world average yield (USDA 2018) because of low agricultural inputs and problems with pests and diseases. The yield and quality of the product are critical to soybean farmers. Unfortunately, plant diseases are a significant constraint on soybean production and caused losses of approximately 59.9 million metric tons in the top eight producing countries in 2006 (Wrather et al. 2010). Commonly in Myanmar, soybean farmers do not adopt any control measures against plant diseases, so they face losses when weather conditions favour disease development.
Fungal pathogens were the causal agents of the majority of the 24 reported soybean diseases in the top 10 soybean-producing countries in 1994 (Wrather et al. 1997). Among these diseases, stem blight, pod rot and seed decay are caused by Diaporthe spp. (and their Phomopsis anamorphs) and result in yield and quality losses (Santos et al. 2011). Moreover, a Diaporthe species complex (including D. endophytica, D. longicolla, D. phaseoli, D. phaseolorum and D. sojae) is associated with soybean (Gomes et al. 2013; Udayanga et al. 2015; Zhang et al. 1998).
Soybean anthracnose is mainly caused by Colletotrichum truncatum (Schwein.) Andrus and W. D. Moore and is an economically important disease in soybean production. The estimated yield reductions caused by anthracnose in China and India in 2006 were 1.66 million tons and 0.18 million tons, respectively (Wrather et al. 2010). Other species such as C. gloeosporioides (Penz) Penz & Sacc. (teleomorph Glomerella cingulata), C. coccodes (Wallr.) Hughes, and C. destructivum O’Gara (teleomorph G. glycine F.Lehm. and F.A.Wolf) are also related to anthracnose diseases in soybean (Chen et al. 2006; Manandhar 1986; Riccioni et al. 1998). However, some of the strains that were previously identified as C. gloeosporioides and C. truncatum could in fact be any of the newly described species in the Colletotrichum orchidacearum species complex (Damm et al. 2019). One species that belongs to this species complex, C. plurivorum (formerly C. cliviae), was recently reported in Brazil as a causal agent of soybean disease (Barbieri et al. 2017).
As described above, soybean pod and stem blight are caused by several Diaporthe spp., and soybean anthracnose is caused by the Colletotrichum orchidacearum species complex. Identification of causal pathogens is important for disease management. Traditional techniques of identification based on morphological characteristics present limitations because of phenotypic variation in the different geographical locations and environmental conditions in which Colletotrichum species occur (Bailey and Jeger 1992). Moreover, the identification of Diaporthe species is complicated due to inter- and intraspecific variability and a wide host range (Mostert et al. 2001; Rehner and Uecker 1994). Therefore, identification at the species level based on morphological characteristics alone is impossible for these species. Currently, species delimitation via molecular identification based on internal transcribed spacer (ITS) sequences of ribosomal DNA (rDNA) and other loci such as actin (ACT), beta-tubulin (TUB), chitin synthase (CHS-1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and translation elongation factor 1-alpha (EF1-α) has become the standard (Santos et al. 2011; Udayanga et al. 2015; Weir et al. 2012).
In the case of Myanmar, scientific information on soybean fungal diseases is scant, and it is very difficult to precisely diagnosis the species, which is essential for disease management. During a field survey in the rainy season of 2017, we found that soybean plants in farmers’ fields were affected by fungal diseases causing premature leaf fall and unfilled pods. Therefore, the objective of this study was to characterize the causal fungal species community in soybeans from two major soybean production areas to develop disease control strategies in Myanmar.
Materials and methods
Sample collection and isolation
Samples were collected in September 2017 in a survey of two major crop production locations in Myanmar. The first location, in central Myanmar in the Nay Pyi Taw area (19.8°N, 96.20°E), has a tropical climate with intermittent rainfall, and the second location, Lawksawk (21.78°N, 96.92°E), is a mountainous area and has a mild climate with high humidity in the growing season. During field collections, premature leaf fall, dry rot, discoloration on the epidermis of the stem and pods with shrivelled seeds were observed (Fig. 1). These typical disease symptoms were widespread and easily observed throughout the fields of both locations during the field survey. Twenty diseased stem samples with black specks and blotching were collected from each location. The epidermis of the collected stem samples was cut into 2–3 mm pieces and surface-sterilized with 2% (v/v) NaOCl solution, then incubated on water agar at 25 ± 1 °C for 24 h. The emerging mycelial tips were transferred to potato dextrose agar (PDA) plates, and single-spore cultures and single mycelial tips from the cultures for non-spore-forming isolates were prepared to obtain pure cultures for further studies.
Morphological characterization
Agar discs (5 mm diameter) of each fungal isolate were placed on PDA and incubated at 25 ± 1 °C with four replicates. Colony diameter was measured at 24-h intervals for 7 days, and colony morphology was examined. The isolates were placed on sterilized soybean stems on water agar to observe the morphology of the fruiting bodies. Images were obtained using a light microscope mounted with an Olympus DP70 camera (Olympus, Tokyo, Japan). Only Colletotrichum and Diaporthe species were isolated from the diseased stem samples, and representative isolates were selected from each morphologically similar group for the experiment.
Pathogenicity on soybean
The pathogenicity of the collected isolates was examined using a stem cutting inoculation technique (Li et al. 2010) with slight modifications. Soybean (Yezin-10 cultivar) seeds were surface-sterilized with a 2% (v/v) NaOCl solution and grown in autoclaved potted soil in a phytotron (Biotron Application Center, Kyushu University) at 25 ± 2 °C. Two-week-old seedling stems were cut into equal lengths at 15 cm above the soil line but below the first trifoliate leaf. Then, small mycelial discs were collected from the margins of 10-day-old cultures on PDA plates using the large end of 200 µl micropipette tips and immediately placed onto fresh-cut stems. Inoculation with PDA-only discs served as the control. A completely randomized design (CRD) was used with five replications, and each replicate included three plants. The micropipette tips were removed 2 days after inoculation, and stem and diseased lesion lengths were measured at 10 days after inoculation. Lesion length was measured and calculated as a percentage of total stem length using the following formula: Lesion length (as % of total stem length) = (Lesion length/Stem length) × 100 (Li et al. 2010). Analysis of variance with CRD was performed, and the mean values from each treatment were compared on the basis of the least significant difference at P ≤ 0.05 with Statistix version 8.0 (Analytical Software, Tallahassee, FL, USA). The inoculated fungal isolates were reisolated from the symptoms that developed after inoculation, and the morphological characteristics were checked to confirm their pathogenicity and whether Koch’s postulates were fulfilled.
DNA extraction, PCR amplification and sequencing analysis
Genomic DNA from 15 randomly selected isolates was extracted using the DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany). After the extraction of template DNA, the samples were stored at − 20 °C until use.
For the identification of Diaporthe spp., the ITS, beta-tubulin (TUB) and translation elongation factor 1-α (EF1-α) genes were amplified using the ITS1 and ITS4, Bt-2a and Bt-2b, and EF1-728F and EF1-986R primers, respectively. For Colletotrichum spp., ITS, actin (ACT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and chitin synthase (CHS-1) were amplified by using primer pairs ITS1 and ITS4, ACT-512F and ACT-783R, and GDF and GDR, and CHS-79F and CHS-345R, respectively (Table 1).
PCR amplification was carried out in a 25 µl reaction volume containing 2.5 µl of 10 × reaction buffer, 2 µl of dNTPs, 1.0 µl of each primer, 0.25 µl of Taq polymerase (2.5 U/µl) (Toyobo, Osaka, Japan), 2 µl of template DNA and 16.25 µl of MilliQ water. PCR was performed in a thermal cycler (TProfessional Basic Gradient Thermocycler, Biometra, Göttingen, Germany). The PCR conditions for ITS were 95 °C for 2 min, 39 cycles 95 °C for 30 s, 55 °C for 50 s, and 72 °C for 1 min, and a final step at 72 °C for 5 min; the annealing temperature was different for other genes: 58 °C for TUB and EF1-α (Udayanga et al. 2014), 58 °C for ACT and CHS-1, and 60 °C for GAPDH (Weir et al. 2012).
The quality of the PCR products was checked by electrophoresis in 1.5% (w/v) agarose stained with ethidium bromide, and the products were purified with a QIAquick PCR kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Then the purified PCR products were sent to Fasmac Co. (Kanagawa, Japan) for sequencing.
Phylogenetic analysis
The accession numbers of all sequences that were newly generated in this study are listed in Table 2. The sequences of the ITS, TUB and EF1-α genes of Diaporthe species were separately aligned with verified sequences published by Udayanga et al. (2015). The reference sequences of the ITS, ACT, GAPDH and CHS-1 genes of Colletotrichum spp. were obtained from Damm et al. (2019) and Weir et al. (2012). The nucleotides of the DNA sequences were aligned via the ClustalW method (Thompson et al. 1994) using MEGA software version 7 (Kumar et al. 2016), and alignment gaps were treated as gap data. Multilocus phylogenetic analyses of the Colletotrichum (based on ITS, ACT, GAPDH, and CHS-1 genes) and Diaporthe (based on ITS, EF1-α and TUB genes) genera were performed separately by the maximum likelihood (ML) method with a distance matrix based on Kimura’s two-parameter correlation for multiple hits (Kimura 1980) using MEGA 7. The confidence estimate for tree topologies was determined by bootstrap analysis with 1000 replicates. Additionally, phylogenetic trees for the two genera with Bayesian probabilities were also constructed using the Markov chain Monte Carlo (MCMC) algorithm with MrBayes (version 3.1.2) (Ronquist et al. 2012). Nucleotide substitution models for each gene were selected by using PartitionFinder (version 1.1.1) (Lanfear et al. 2012). The analysis of MCMC chains was run for 1,000,000 generations, and trees were sampled every 1000th generation. The first 25% of the trees were discarded, and the posterior probabilities were calculated using the remaining trees. The resulting tree was viewed by using MEGA 7.
Results
Morphological characteristics of the collected fungal isolates
From the diseased soybean samples from the two locations in Myanmar, 12 representative fungal isolates were obtained from the Lawksawk and three from Nay Pyi Taw areas (Table 2). The observed morphological characteristics indicated that the isolates were species of Colletotrichum and Diaporthe. Species identification was completed based on the molecular phylogenetic analysis. The morphological characteristics of the four pathogenic fungal species identified in this experiment were as follows.
Diaporthe melonis: Colony colour of the isolates was whitish to grey, and no colour staining was observed on PDA. The growth rate of the isolates was 6.1 ± 0.2 mm/day (n = 12) in the dark at 25 °C. Pycnidia on sterilized soybean stems were globose and 189.9 ± 28.2 µm (n = 50) in diameter containing abundant α-conidia. The alpha conidia were hyaline, smooth, ovoid to ellipsoid, biguttulate, and 6.9 ± 0.5 µm × 2.4 ± 0.2 µm (n = 50) in size. Beta conidia were not observed (Fig. 2a-1, a-4). The morphology of D. melonis in this experiment was the same as the taxonomic description of Udayanga et al. (2015).
Morphological characteristics of fungal pathogens isolated in this study. a-1 to a-4Diaporthe melonis. a-1 Colony on PDA after 10 days, a-2 pycnidia on inoculated sterilized soybean stems on water agar, a-3 conidiophores, a-4 alpha conidia (bars: a-2 = 3 mm, a-3 and a-4 = 10 µm). b-1 to b-4Diaporthe endophytica. b-1 Colony on PDA after 10 days, b-2 lesions after inoculation of soybean stem, b-3 pycnidia on epidermis of inoculated soybean stem, b-4 alpha conidia (bar: b-2 = 2 mm, b-3 = 50 µm, and b-4 = 10 µm). c-1 to c-3Colletotrichum truncatum. c-1 Colony on PDA after 7 days, c-2 acervulus on sterilized soybean stem, c-3 conidia (bar: c-2 = 50 µm and c-3 = 20 µm). d-1 to d-4Colletotrichum plurivorum. d-1 Colony on PDA after 10 days, d-2 ascocarp on inoculated soybean stem, d-3 ascus and ascospores, and d-4 conidia (bar: d-2 = 50 µm, d-3 = 10 µm, d-4 = 20 µm)
Diaporthe endophytica: The colony morphology of the fungus was similar to that of D. melonis. The growth rate was 5.6 ± 0.3 mm/day on PDA in the dark at 25 °C. There was no sporulation on PDA. However, pycnidia and alpha conidia were observed on lesions of the inoculated soybean plant stems. Pycnidia were round to ovoid with ostioles, 135 ± 28.2 µm in diameter, and the morphology of the alpha conidia was similar to those of D. melonis, with a size of 6.8 ± 0.5 µm × 2.6 ± 0.3 µm (n = 50). Beta conidia were not observed (Fig. 2b-1, b-4). However, sporulation of D. endophytica was not reported on either media or sterilized host plant tissue by Gomes et al. (2013). In our experiment, the morphology of D. endophytica was almost the same as that of D. melonis.
Colletotrichum truncatum: The colony on PDA was grey to dark grey with white to grey aerial mycelia, and the PDA was stained black. The colony was thick, and the growth rate was 4.6 ± 0.7 mm/day in the dark at 25 °C. Acervuli formed on the inoculated stems, and black setae were dominant, with a width of 208.8 ± 62.1 µm. The conidia were falcate and tapered at each end, 26.2 ± 1.5 × 4.5 ± 0.4 µm (n = 50) (Fig. 2c-1, c-3). The conidial dimensions were similar to those of C. truncatum isolate CBS112998 (Damm et al. 2009).
Colletotrichum plurivorum: The colony was whitish grey initially and later became darker grey; the aerial mycelia were sparse, and the ascomata (sexual morph) formed clusters at 2 weeks after incubation on PDA at 25 °C. Conidia (asexual morph) were rarely found on PDA and were mainly observed on inoculated soybean stems; they were smooth-walled, one-celled, hyaline, and cylindrical to oblong with rounded ends, 15.5 ± 0.9 µm × 5.1 ± 0.3 µm (n = 50). The ascostroma were dark and globose to subglobose, and they contained asci with thin walls; in addition, they were unitunicate and clavated, with dimensions of 45.2 ± 9.8 µm × 8.9 ± 2.1 µm (n = 50). Eight ascospores were arranged in each ascus and slightly curved, and they were narrow at each rounded end, with dimensions of 16.5 ± 3.2 µm × 5.5 ± 0.9 µm (n = 50) (Fig. 2d-1, d-4). The morphological characteristics of the isolates (SoyYS1707, SoyYS1709, SoyYS1726, SoyYS1730, and SoyYS1731) matched those of C. plurivorum (CBS 125,474) (Damm et al. 2019).
Pathogenicity test
Twelve representative isolates consisting of four isolates of D. melonis, two isolates of D. endophytica, three isolates of C. truncatum and three isolates of C. plurivorum were used for the pathogenicity tests. Pathogenicity was determined by measuring the percentage lesion length on the inoculated soybean stems. The inoculated symptoms of the representative fungal isolates of Colletotrichum spp. and Diaporthe spp. are shown in Fig. 3. Lesion lengths were 19.05–53.62% of the respective stem length, and there were significant differences in percentage lesion length among the species (Table 3). D. melonis had the longest lesions at 45.16%, significantly larger than C. plurivorum, C. truncatum and D. endophytica. Lesion lengths of C. truncatum and D. endophytica did not differ significantly. However, C. plurivorum caused the shortest lesions (19.48% of the stem length).
Phylogenetic analysis
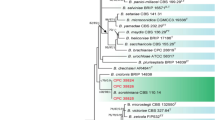
Phylogenetic analysis of Diaporthe spp. based on ITS, EF1-α and TUB sequences of 64 strains, including closely related reference Diaporthe spp. strains. Gene boundaries were as follows: ITS, 1–492; EF1-α, 493–871; TUB, 872–1318. D. vaccinia (DP5032) was used as an outgroup. For the Bayesian analysis, the substitution models used were as follows: GTR + I + G for the ITS and HKY + G for the EF1-α and TUB genes. The topologies of the phylogenetic trees obtained via the maximum likelihood method using MEGA 7 software and Bayesian analysis were similar, and parsimony bootstrap (≥ 70%) and Bayesian posterior probability (≥ 0.90) values are shown on the branches in Fig. 4. The isolates obtained in the present study (SoyYS1701, SoyYS1703, SoyYS1704, SoyYS1706, SoyYS1711, SoyYS1712 and SoyYS1713) formed a single clade and were very closely related to D. melonis (CBS435.87). Two isolates (SoyYS1732 and SoyYS1733) fell into a single clade with the ex-type isolates of D. endophytica (LGMF948 and CBS133811).
Phylogenetic trees inferred from the ITS, EF1-α, and TUB genes of Diaporthe species using the maximum likelihood method. Bootstrap support values (≥ 70%)/Bayesian posterior probabilities (≥ 0.90) are displayed at each branch, and black squares (filled rectangle) indicate isolates from the present study
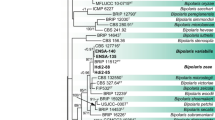
For phylogenetic analysis of Colletotrichum spp., the ITS, GAPDH, CHS-1 and ACT sequences of the three representative isolates (SoyYZ1715, SoyYZ1716 and SoyYZ1725) from Nay Pyi Taw and three representative isolates (SoyYS1707, SoyYS1709, and SoyYS1726) from Lawksawk were aligned with closely related verified sequences for phylogenetic analysis. The alignment contained 37 taxa, and Monilochaetes infuscans (CBS 869.96) served as the outgroup. The gene boundaries, including gaps, were as follows: ITS, 1–519; GAPDH, 520–826; CHS-1, 827–1116; and ACT, 1117–1364. The substitution models selected for Bayesian analysis were GTR + G for ITS, HKY + I for GAPDH and HKY + G for the CHS-1 and ACT genes. The results showed that the SoyYZ1715 and SoyYZ1716 isolates were identical to the CBS195.32 and CMES 1036 sequences, and the three isolates obtained in this study were also closely related to the C. truncatum type strain (CBS151.35). The other three isolates (Soy YS1707, SoyYS1709 and SoyYS1726) were in the same clade as C. plurivorum (CBS125474 and LFN0008) (Fig. 5).
Phylogenetic trees inferred from the ITS, ACT, GAPDH and CHS-1 genes of Colletotrichum species using the maximum likelihood method. Bootstrap support values (≥ 70%)/Bayesian posterior probabilities (≥ 0.90) are displayed at each branch, including detailed descriptions of the symbol as per Fig. 4
Discussion
The symptoms of soybean stem diseases caused by the Diaporthe species complex are described as black blotching with or without pycnidia on the stem (Kmetz et al. 1978). Moreover, groups of Colletotrichum species are also associated with the stem and cause anthracnose on soybeans (Yang et al. 2014), and the morphological characteristics among the Colletotrichum species were also similar. Consequently, we differentiated the collected isolates only to the genus level as Colletotrichum spp. and Diaporthe spp. based on morphology.
The pathogenicity test using the stem cutting inoculation method reproduced necrotic lesions with fruiting bodies on the cut stems of soybean. This method is mainly used for pathogenicity testing and varietal resistance screening experiments for Phomopsis diseases, Sclerotinia stem rot, and charcoal rot disease in soybean (Kull et al. 2007; Li et al. 2001; Shan et al. 2013; Twizeyimana et al. 2012). However, the inoculation test with the isolates of Colletotrichum spp. also reproduced necrotic lesions on cut stems, and we assumed that these species were fungal pathogens of soybean. Therefore, isolates of these two genera were identified separately by molecular phylogenetic analysis.
Currently, molecular tools such as the sequencing of different genes or intergenic regions have been shown to be applicable and more accurate for the identification of fungal species (Ash et al. 2010; Gomes et al. 2013; Yang et al. 2014). In strategies involving the use of genetic barcodes of different genes for species delimitation, the ITS region of r-DNA has been widely applied (Gardes and Bruns 1993; Schoch et al. 2012; Shishido et al. 2006). However, fungal species identification on the basis of ITS alone has limitations (Nilsson et al. 2008), and the ITS, HIS or TUB genes should be analysed for the species description of Diaporthe spp. (Gomes et al. 2013). Similarly, many Colletotrichum species cannot be identified using only the ITS (Weir et al. 2012). Therefore, other fungal barcoding genes were aligned with other sequences of verified reference isolates, and a phylogenetic study was carried out. According to the multilocus phylogenetic analysis, the isolates of Diaporthe from the Lawksawk area were similar to D. endophytica and D. melonis. The Colletotrichum isolates from Lawksawk and Nay Pyi Taw were identified as C. plurivorum and C. truncatum, respectively.
Known hosts of Diaporthe melonis include Annona squamosa, Carapa guianensis, Cucumis melo and Glycine max (Dissanayake et al. 2017), and this species is phylogenetically closely related to D. longicolla and D. sojae (Udayanga et al. 2015). D. endophytica has been isolated from seeds of Glycine max, as an endophyte on the leaves of Schinus terebinthifolius and on the petioles of Maytenus ilicifolia in Brazil (Gomes et al. 2013). In our pathogenicity tests, D. endophytica and D. melonis were pathogenic on soybean plants. On the other hand, C. truncatum is a common cause of anthracnose disease in major soybean-growing countries (Wrather et al. 1997, 2010), including Myanmar (CAB-International 2001). However, C. plurivorum has been reported on soybean in Brazil and Japan (Barbieri et al. 2017; Damm et al. 2019) and belongs to the C. orchidearum species complex (Damm et al. 2019). This species was recently reported as a pathogen that is associated with anthracnose disease on chili in China and the Andaman and Nicobar Islands (Liu et al. 2016; Sakthivel et al. 2018), on papaya in Taiwan (Sun et al. 2019), and on Pyrus spp. in China (Fu et al. 2018). In the present study, we revealed that C. plurivorum was weakly virulent, whereas C. truncatum was strongly virulent on soybean.
We identified isolates of C. plurivorum, D. endophytica and D. melonis in Lawksawk Township, southern Shan State, but only one species, C. truncatum, was isolated from the diseased samples from the Nay Pyi Taw area. More diverse fungal plant pathogen species were found in southern Shan State, which seems to be facilitated by mild temperatures (not very hot or cold) and humid weather. TeKrony et al. (1983) reported that disease incidence caused by Diaporthe species was significantly related to relative humidity and air temperature and that seed infection by these fungal pathogens is more dependent on moisture. The maximum disease incidence of soybean anthracnose caused by C. truncatum occurred at 28.4 °C and 76% relative humidity in September 1995 in India (Singh et al. 2001).
In Myanmar, 15 other species of Diaporthe and 16 species of Colletotrichum have been reported on different host plants (Thaung 2008). Additionally, infection of soybean by C. plurivorum, C. truncatum, D. endophytica and D. melonis in Myanmar was never checked before this study. During the field survey of two major soybean-growing areas, fungal diseases were prominent and severely affected the yield and income of the farmers. To our knowledge, this is the first report of the characterization of fungal pathogens on soybean in Myanmar, and effective control measures are urgently needed by farmers. We hope that the results of this investigation will support further experiments on the development of disease control and management strategies for soybean production.
References
Ash GJ, Stodart B, Sakuanrungsirikul S, Anschaw E, Crump N, Hailstones D, Harper JDI (2010) Genetic characterization of a novel Phomopsis sp., a putative biocontrol agent for Carthamus lanatus. Mycologia 102:54–61
Bailey JA, Jeger MJ (eds) (1992) Colletotrichum: biology, pathology and control. CABI, Wallingford
Barbieri MCG, Ciampi-Guillardi M, Moraes SRG, Bonaldo SM, Rogerio F, Linhares RR, Massola NS (2017) First report of Colletotrichum cliviae causing anthracnose on soybean in Brazil. Plant Dis 101:1677
CAB International (2001) Colletotrichum truncatum [distribution map]. In: Distribution maps of plant diseases 1st edn, map no. 835. https://www.cabi.org/ISC/abstract/20066500835. CABI, Wallingford, UK.
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Chen LS, Chu C, Liu CD, Chen RS, Tsay JG (2006) PCR-based detection and differentiation of anthracnose pathogens, Colletotrichum gloeosporioides and C. truncatum, from vegetable soybean in Taiwan. J Phytopathol 154:654–662
CSO (2017) 2017 Myanmar statistical yearbook. Central Statistical Organization, Ministry of National Planning and Economics Development, Nay Pyi Taw
Damm U, Sato T, Alizadeh A, Groenewald JZ, Crous PW (2019) The Colletotrichum dracaenophilum, C. magnum and C. orchidacearum species complexes. Stud Mycol 92:1–46
Damm U, Woudenberg JHC, Cannon PF, Crous PW (2009) Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers 39:45–87
Dissanayake AJ, Phillips AJL, Hyde KD, Yan JY, Li XH (2017) The current status of species in Diaporthe. Mycosphere 8:1106–1156
Fu M, Crous PW, Bai Q, Zhang PF, Xaing J, Guo YS, Zhao FF, Yang MM, Hong N, Xu WX, Wang GP (2018) Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 42:1–35
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rust. Mol Ecol 2:113–118
Glass LN, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Gomes RR, Glienke C, Videira SIR, Lombard L, Groenewald JZ, Crous PW (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1–41
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kmetz KT, Schmitthenner AF, Ellette CW (1978) Soybean seed decay: prevalence of infection and symptom expression caused by Phomopsis sp., Diaporthe phaseolorum var. sojae, and D. phaseolorum var. caulivora. Phytopathology 68:836–840
Kull LS, Vuong TD, Powers KS, Eskridge KM, Steadman JR, Hartman GL (2007) Evaluation of resistance screening methods for Sclerotinia stem rot of soybean and dry bean. Plant Dis 87:1471–1476
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701
Li S, Bradley CA, Hartman GL, Pedersen WL (2001) First report of Phomopsis longicolla from velvetleaf causing stem lesions on inoculated soybean and velvetleaf plants. Plant Dis 85:1031
Li S, Hartman GL, Boykin DL (2010) Aggressiveness of Phomopsis longicolla and other Phomopsis spp. on soybean. Plant Dis 94:1035–1040
Liu F, Tang G, Zheng X, Li Y, Sun X, Qi X, Zhou Y, Xu J, Chen H, Chang X, Zhang S, Gong G (2016) Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci Rep 6:1–17
Manandhar JB, Hartman GL, Sinclair JB (1986) Colletotrichum destructivum, the anamorph of Glomerella glycines. Phytopathology 76:282–285
Mostert L, Crous PW, Kang J-C, Phillips AJL (2001) Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93:146–167
Nilsson RH, Kristiansson E, Ryberg M, Hallenberg N (2008) Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinform 4:193–201
Rehner SA, Uecker FA (1994) Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Can J Bot 72:1666–1674
Riccioni L, Conca G, Hartman GL (1998) First report of Colletotrichum coccodes on soybean in the United States. Plant Dis 82:959
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Sakthivel K, Manigundan K, Sneha S, Patel A, Charishma K, Neelam S, Gautam RK, Kumar A (2018) First report of Colletotrichum plurivorum from the Andaman and Nicobar Islands causing anthracnose in chilli (Capsicum annuum). New Dis Rep 38:26
Santos JM, Vrandečić K, Ćosić J, Duvnjak T, Phillip AJL (2011) Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 27:9–19
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Consortium FB (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246
Shan Z, Li S, Liu Y, Yang Z, Yang C, Sha A, Chen H, Chen S, Zhou XA (2013) First report of Phomopsis seed decay of soybean caused by Phomopsis longicolla in South China. Plant Dis 96:1693
Shishido M, Yoshida N, Usami T, Shinozaki T, Kobayashi M, Takeuchi T (2006) Black root rot of cucurbits caused by Phomopsis sclerotioides in Japan and phylogenetic grouping of the pathogen. J Gen Plant Pathol 72:220–227
Singh R, Singh SB, Singh PN (2001) Effect of environmental conditions on development of anthracnose of soybean. Ann Plant Protein Sci 9:146–147
Sun YC, Damm U, Huang CJ (2019) Colletotrichum plurivorum, the causal agent of anthracnose fruit rot of papaya in Taiwan. Plant Dis 11:1040
TeKrony D, Egli D, Stuckey R (1983) Relationship between weather and soybean seed infection by Phomopsis sp. Phytopathology 73:914–918
Templeton MD, Rikkerink EHA, Solon SL, Crowhurst RN (1992) Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 122:225–230
Thaung MM (2008) Biodiversity survey of coelomycetes in Burma. Australas Mycol 27:74–110
Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Twizeyimana M, Hill CB, Pawlowski M, Paul C, Hartman GL (2012) A cut-stem inoculation technique to evaluate soybean for resistance to Macrophomina phaseolina. Plant Dis 96:1210–1215
Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD (2015) The Diaporthe sojae species complex: phylogenetic re-assessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol 119:383–407
Udayanga D, Castlebury LA, Rossman AY, Hyde KD (2014) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporellaD. foeniculina and D. rudis. Persoonia 32:83–101
USDA (2018) World agricultural production: crop production tables. Production, Supply and Distribution, Office of Global Analysis, Foreign Agricultural Service/USDA, Washington.
Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and amplification. Academic Press, San Diego, pp 315–322
Wrather JA, Anderson TR, Arsyad DM, Gai J, Ploper LD, Porta Puglia A, Ram HH, Yorinori JT (1997) Soybean disease loss estimates for the top 10 soybean producing countries in 1994. Plant Dis 81:107–110
Wrather JA, Shannon G, Balardin R, Carregal L, Escobar R, Gupta GK, Ma Z, Morel W, Ploper D, Tenuta A (2010) Effect of diseases on soybean yield in the top eight producing countries in 2006. Plant Health Prog. https://doi.org/10.1094/PHP-2010-0125-01-RS
Yang H-C, Haudenshield JS, Hartman GL (2014) Colletotrichum incanum sp. nov., a curved-conidial species causing soybean anthracnose in USA. Mycologia 106:32–42
Zhang AW, Riccioni L, Pedersen WL, Kollipara KP, Hartman GL (1998) Molecular identification and phylogenetic grouping of Diaporthe phaseolorum and Phomopsis longicolla isolates from soybean. Phytopathology 88:1306–1314
Acknowledgements
This research was supported by the Japanese Government (Monbukagakusho: MEXT) scholarship programme and a Grand-in-Aid for Scientific Research (no. 18K05652) from the Japan Society for the Promotion of Science. We thank Mr. Thet Tun Aung, Programme officer, and Mr. Aye Kyaw, Soybean/oil Seed Programme Officer, Value Chains and Rural Development, Taunggyi Field Office, Winrock International, Myanmar for their support during field sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaw, M., Aye, S.S. & Matsumoto, M. Colletotrichum and Diaporthe species associated with soybean stem diseases in Myanmar. J Gen Plant Pathol 86, 114–123 (2020). https://doi.org/10.1007/s10327-019-00902-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-019-00902-5