Abstract
Trichoderma harzianum, a biocontrol agent for various plant pathogens, is known to degrade fungal cell walls; this mycoparasitism is believed to require secretion of cell-wall-degrading enzymes against host pathogens. In this study, we identified a homologue of yeast SNF1 (sucrose nonfermenting 1) encoding protein kinase in T. harzianum (ThSNF1) by draft genome sequencing of strain T36. Targeted gene disruption of ThSNF1 was performed using the PEG method with fusion PCR products. Growth of mutant ΔThSNF1 was markedly less than for the wild-type strain on minimal medium with chitin as a carbon source. The mutant exhibited reduced expression of the genes encoding chitinase and polygalacturonase and markedly reduced spore production. Mycoparasitism against plant pathogens such as Fusarium oxysporum f. sp. cubense (Panama disease) and Fusarium graminearum (Fusarium head blight) was clearly impaired in the mutant. The results suggest that ThSNF1 is critical for asexual development, utilization of certain carbon sources and virulence on fungi, and is therefore important for the biocontrol ability of T. harzianum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the genus Trichoderma are known as biocontrol agents because of their mycoparasitism against many plant pathogenic fungi. Some Trichoderma spp. can penetrate hyphae and kill the host fungus (Abdullah et al. 2007). Trichoderma spp. have been reported to control several pathogens of diverse crops via various mechanisms, such as the production of antifungal metabolites, competition for nutrients and space, mycoparasitism and efficiency in promoting plant defense mechanisms (Hoyos-Carvajal et al. 2008; Woo and Lorito 2007). The mycoparasitism of Trichoderma is characterized by hyphae that coil around host hyphae and penetrate host cells (Abdullah et al. 2007). Release of a range of enzymes, such as β-1,3-glucanase, pectinase, xylanase and chitinases, is thought to be important for the biocontrol activity because they enable Trichoderma to degrade the host’s cell walls (Hjeljord and Tronsmo 1998). Involvement of specific chitinase genes in the biocontrol properties of Trichoderma reesei was investigated using genome-wide analysis of chitinase genes (Seidl et al. 2005).
Serine/threonine protein kinase is an important mediator of fungal proliferation and development, signal transduction and infection-related morphogenesis in filamentous fungi (Dickman and Yarden 1999). Carbon catabolite repression is a universal regulatory principle that leads to the inhibition of expression of genes encoding enzymes needed to utilize complex carbon sources such as glucose and other simple sugars. In yeast, release from catabolite repression requires expression of the Snf1p protein kinase (Celenza and Carlson 1984; Ruijter and Visser 1997). Treitel et al. (1998) described Snf1p (encoded by SNF1) as a protein kinase that phosphorylates the DNA-binding transcriptional repressor Mig1p (called creA in filamentous fungi) (Ronne 1995).
The SNF1-mediated process controls expression of multiple cell-wall-degrading enzyme genes (Tonukari et al. 2000). Therefore, modification of this process through disruption of SNF1 homologues in fungi could lead to loss of production of multiple cell-wall-degrading enzymes and hence be useful for investigations into the role of these enzymes in regulating the expression of virulence genes in plant pathogens (Tonukari et al. 2000). In Cochliobolus carbonum, SNF1 homologue ccSNF1 controls expression of genes for several cell-wall-degrading enzymes and is also important for virulence against the maize host (Tonukari et al. 2000). Disruption of Fusarium oxysporum SNF1 (FoSNF1) reduces virulence on cabbage and Arabidopsis (Ospina-Giraldo et al. 2003). In Gibberella zeae, GzSNF1 is reported to be required for normal sexual and asexual development (Lee et al. 2009).
These investigations have demonstrated that SNF1 is involved in development, production of cell-wall-degrading enzymes and virulence of plant pathogens. However, the role and importance of the gene in mycoparasitism in Trichoderma and other biocontrol agents is currently unknown. To address this question, we analyzed the draft genome sequences of the fungus and identified the T. harzianum SNF1 homologue (ThSNF1) and functionally characterized the gene. This is the first report of a functional analysis of an SNF1 ortholog in the biocontrol fungus T. harzianum.
Materials and methods
Fungal strains and culture conditions
In this study, T. harzianum strain T36 was used as the wild-type strain. The isolate was obtained from stock collections at the Biotechnology Research Center of Ecuador (CIBE-ESPOL). The wild type and transformants were maintained on potato dextrose agar (PDA, Difco, Detroit, MI, USA) at 25 °C. Mycelial fragments were stored in 20 % glycerol at -80 °C. The strains were cultured in potato dextrose broth (PDB) and yeast peptone glucose (YPG) medium for DNA extraction. F. oxysporum f. sp. cubense (Fo-01) (Panama disease) was obtained from stock collection at the CIBE-ESPOL, and Fusarium graminearum (No. 201) (Fusarium head blight) was kindly provided by Dr. Suga (Gifu University, Japan). The isolate of F. oxysporum f. sp. cubense was used under the special permission from the Minister of Agriculture, Forestry and Fishery of Japanese Government. Culture media with different carbon sources were prepared with minimal medium (2 g/L KH2PO4, 1.4 g/L (NH4)2SO4, 0.3 g/L MgSO4·7H2O, 0.3 g/L CaCl2·2H2O, 0.005 g/L FeSO4·7H2O, 0.002 g/L ZnSO4·7H2O and 0.002 g/L MnSO4·H2O) by adding 1 % glucose or 1 % colloidal chitin (Wako Chemicals, Osaka, Japan).
Isolation and gene targeting of ThSNF1
The sequences of the PCR primers used in this study are shown in Table 1. The gene encoding SNF1 protein kinase homologue ThSNF1 (GenBank accession LC002817) in T. harzianum was determined by analyzing the draft sequence data of the T36 strain obtained with Illumina HiSeq 2000 using SNF1 genes from Saccharomyces cerevisiae (Celenza and Carlson 1984), F. oxysporum (Ospina-Giraldo et al. 2003) and C. carbonum (Tonukari et al. 2000) as queries. The size of the full-length ThSNF1 gene is 2361 bp, and it encodes a protein containing 710 amino acids.
An outline of the PCR approach (Kuwayama et al. 2002; Nayak et al. 2006; Ninomiya et al. 2004) for constructing the gene disruption vectors is shown in Fig. 1a. Genomic DNA of T. harzianum T36 was used to amplify a 994-bp fragment (left-side arm of the vector) and a 603-bp fragment (right-side of the vector) from ThSNF1 with PCR primers Thsnf1AF/Thsnf1AR and Thsnf1BF/Thsnf1BR, respectively. The primer sets were designed for the deletion of the ThSNF1 internal sequence, which encodes a serine/threonine protein kinase (SNF1) homologue. The hph marker cassette was amplified by PCR from p71sfi plasmid, which contains a hygromycin B phosphotransferase gene, with primers fushphF/fushphR. The final fusion products were amplified using primers Thsnf1AF/Thsnf1BR. The PCR was performed using a Thermal Cycler Dice TP650 (Takara Bio, Ohtsu, Japan) with an initial denaturing step of 5 min at 95 °C; followed by 30 cycles of 15 s at 95 °C, 15 s at 59 °C, and 30 s at 72 °C; and a final extension of 5 min at 72 °C. The final fused products were purified with a QIAquick Kit (Qiagen, Tokyo, Japan) before transformation of the T. harzianum T36 strain.
Deletion strategies for ThSNF1 in the genome of the Trichoderma harzianum T36 strain. a A fusion PCR method was used to construct the ThSNF1 replacement vector. All PCR primers used are listed in Table 1. The 5′ region of SNF1 was amplified by PCR with primer pair ThSNF1AF/ThSNF1AR; the 3′ region was amplified with primer pair ThSNF1BF/ThSNF1BR. The hph gene was amplified with primer pair fushphF/fushphR. The three PCR products were then used as a template for fusion PCR using primer pair ThSNF1AF/ThSNF1BR; the resulting PCR product was used for transformation. White, black and gray indicate sequences of SNF1, hph and flanking regions of SNF1, respectively. b PCR analysis of gene replacement events in ThSNF1 in the wild-type strain (WT) and the ΔThSNF1 mutant using primer pairs hphF/hphR (b)-hph, Thsnf1inF/Thsnf1inR (b)-SNF1, Thsnf1homoF/HphhomoR (b)-Homologous 1, and HphhomoF/Thsnf1homoR (b)-Homologous 2. c Expression of ThSNF1 in the wild-type T36 (WT) and ΔThSNF1 strains of T. harzianum. RT-PCR primer sets listed in Table 1 were used to detect ThSNF1 and the β-tubulin gene of T. harzianum
Fungal protoplasts were prepared using the method previously described by Akamatsu et al. (1997) with modifications. Protoplasts at a concentration of 1.25 × 108 protoplasts/mL in a final volume of 80 μL were transformed with the disruption vector as previously described (Akamatsu et al. 1997). To identify ThSNF1 deletion mutants, we used three sets of primers for hygromycin B-resistant colonies (Table 1). Initially, a pair of primers for the hph cassette was used to verify the insertion of the vector. Next, primer set Thsnf1inF/Thsnf1inR was used to verify the insert. Primer pairs Thsnf1homoF/HphhomoR and HphhomoF/Thsnf1homoR were used to examine integration of the hph cassette by a double-crossover homologous recombination event at the ThSNF1 locus. Putative mutant strains determined by the expected diagnostic amplification fragments were purified by single spore isolation.
Gene expression analysis
For expression analysis, total RNA was extracted from fungal mycelia grown in minimal medium supplemented with glucose or the autoclaved F. oxysporum f. sp. cubense mycelium (0.5 % w/v) as the carbon source as previously described (Vieira et al. 2013). After 3 days of culture, the mycelia were harvested through filter paper and washed with distillated water. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. Total RNA was treated with DNaseI (Takara Bio) to remove traces of contaminating DNA. A total of 1 μg of the RNA sample was converted into cDNA using the PrimeScript RT-PCR Kit (Takara Bio) with random 6-mer primers according to the manufacturer’s instructions. The resulting cDNA was used as a template for RT-PCR with primer sets Thsnf1inF/Thsnf1inR, Chi33H3F/Chi33H3R, Thpgx1F/Thpgx1R and ThTUBF/ThTUBR for ThSNF1, Chi18-17 (chitinase), PGX1 (polygalacturonase) and the β-tubulin gene, respectively. The PCR primer sequences of those genes were designed by using the draft genome data of the T. harzianum strain T36.
Morphology and colony growth
Phenotypes of the mutant were examined on minimal media supplemented with different carbon sources or on PDA. Colony morphology and radial growth of the mutant and the wild-type strains were checked every day. Conidia were counted as previously described by López-Mondéjar et al. (2009).
In vitro mycoparasitism assay
The antagonism test was performed in triplicate on PDA by placing a mycelial disc (5 mm in diameter) of each pathogenic fungus (F. oxysporum f. sp. cubense or F. graminearum) on one side of a Petri dish; the wild type or ΔThSNF1 mutant of T. harzianum was placed on the other side. After 10 days, mycoparasitism by Trichoderma spp. was evaluated using the following scale (Ezziyyani et al. 2004) based on the percentage of the colony surface of the pathogen covered by Trichoderma and sporulation by Trichoderma: 0: 0 % coverage; 1: 25 % coverage; 2: 50 % coverage; 3: 100 % coverage; 4: 100 % coverage plus sporulation.
Results
Cloning and targeted disruption of ThSNF1 in T. harzianum
The gene encoding the serine/threonine protein kinase SNF1 homologue from T. harzianum was identified by analyzing the draft sequence of the T36 strain and was designated ThSNF1 (GenBank accession number LC002817). The size of the full-length ThSNF1 gene was 2361 bp, encoding a protein of 710 amino acids. The deduced amino acid sequences of ThSNF1 showed homology to S. cerevisiae SNF1 (Celenza and Carlson 1984), C. carbonum ccSNF1 (Tonukari et al. 2000), F. oxysporum FoSNF1 (Ospina-Giraldo et al. 2003), G. zeae GzSNF1 (Lee et al. 2009) and other fungal SNF1 homologues. Alignment of the amino acid sequences of ThSNF1 with ascomycetes SNF1 orthologs FoSNF1 and ccSNF1 showed high homology, especially in the serine/threonine protein kinase catalytic domain (Suppl. Figure 1).
To examine the role of ThSNF1 in morphology, growth, development and mycoparasitism of T. harzianum, the gene was deleted from the fungus using transformation-mediated gene disruption. The targeting vector containing the 3′- and 5′-flanking sequences of ThSNF1 was constructed to disrupt the gene by homologous recombination (Fig. 1a). Transformation of T36 protoplasts with the ThSNF1-disruption vector resulted in hygromycin B-resistant colonies; homologous integration of the transformants was further examined by PCR screening. Primer set HphF/HphR produced the expected 0.4-kb band from the ΔThSNF1 mutant (Fig. 1b). Primer set Thsnf1inF/Thsnf1inR resulted in no amplified fragments from the ΔThSNF1 mutant (Fig. 1b), suggesting that ThSNF1 was deleted by homologous integration of the vector. To confirm ThSNF1 disruption, we used primer combinations Thsnf1homoF/HphhomoR and HphhomoF/Thsnf1homoR to detect the junctions between the recipient ThSNF1 region and the integrated vectors, respectively (Fig. 1b). With these primer combinations, the PCR failed to amplify any DNA fragments from the wild-type strain. By contrast, primer combinations Thsnf1homoF/HphhomoR and HphhomoF/Thsnf1homoR amplified the expected-sized bands in the mutant (Fig. 1b). The deletion strain was used for further work.
The expression of ThSNF1 in the wild-type strain and the ΔThSNF1 mutant was confirmed by RT-PCR analysis. ThSNF1 expression was not detected in the mutant strain (Fig. 1c).
Phenotypic characterization of the ThSNF1-targeted strain
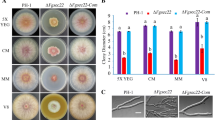
The effects of the ΔThSNF1 mutation on morphology, conidiation and vegetative growth were examined. Agar blocks from colonies grown on PDA plates were transferred onto minimal media supplemented with different nutritional sources. In the presence of glucose, the growth rate of the ΔThSNF1 mutant was not markedly reduced compared with that of the wild type (Fig. 2a). In contrast, growth was clearly reduced when chitin was added as the sole carbon source (Fig. 2a), indicating that the mutant had decreased ability to utilize chitin. Additionally, there was a significant difference in the conidial yield between the wild type and the mutant strain on PDA and on minimal medium supplemented with glucose (MMG) (Fig. 2b). However, conidial morphology and the germination rate of the mutant were the same as those of the wild type (data not shown).
Colony growth and sporulation of wild-type strain T36 (WT) and mutant ΔThSNF1 of Trichoderma harzianum. a Growth rates between the wild-type T36 (WT) and mutant ΔThSNF1 on minimal media supplemented with different carbon sources. Colony growth was measured daily for 7 days to calculate the mean growth rate (mm/day). b Mean number (± SD) of conidia produced by wild-type T36 (WT) and mutant ΔThSNF1 of T. harzianum. Conidia from 7-day-old cultures grown on PDA or minimal media supplemented with glucose (MMG) were harvested and counted. N = 3
The expression of genes encoding cell-wall-degrading enzymes in the ThSNF1-targeted strain
The wild-type and mutant strains were grown in liquid shake cultures for 3 days. The expression of a chitinase gene (Chi18-17) (Seidl et al. 2005) and a polygalacturonase gene (PGX1) of T. harzianum was examined by RT-PCR. Involvement of these enzyme genes in mycoparasitism has been previously reported (Seidl et al. 2005; Viterbo et al. 2001). Expression of the genes was undetectable in the ΔThSNF1 mutant under conditions that ordinarily would induce these genes (Fig. 3).
Expression of genes encoding cell-wall-degrading enzymes in wild-type T36 (WT) and mutant ΔThSNF1 of Trichoderma harzianum. Total RNA was extracted from fungal mycelia grown in minimal medium supplemented with glucose as the only carbon source. RT-PCR primer sets listed in Table 1 were used for detection of chitinase gene Chi18-17 (lane 1), polygalacturonase gene PGX1 (lane 2) and the β-tubulin gene (lane 3) of T. harzianum
Mycoparasitic ability of the ThSNF1-targeted strain
Loss of mycoparasitic ability of the ΔThSNF1 mutant against two pathogens (F. oxysporum f. sp. cubense and F. graminearum) on dual culture plates was evident 10 days after inoculation. Mycelia of T. harzianum came into contact with the pathogen colonies. Following contact, mycelia of the wild-type strain covered the pathogen colonies and sporulated, indicating strong mycoparasitism of the pathogens (Fig. 4). The colonies of the pathogens were obscured compared with the colonies cultured with the ΔThSNF1 mutant. In contrast, the ΔThSNF1 mutant did not overgrow the pathogen or sporulate on the plates, and the pathogen colonies continuously expanded after contact (Fig. 4a, b).
Dual-culture antagonism test of wild-type (WT) and mutant ΔThSNF1 Trichoderma harzianum against either Fusarium oxysporum f. sp. cubense (Foc) or Fusarium graminearum (Fg) A 5-mm-diameter mycelial disk of each fungus was placed on PDA; pathogen is on lower side, Trichoderma on upper side. a The antagonism test was performed on PDA by placing a mycelium disc (5 mm in diameter) of each pathogenic fungus) on one side of a Petri dish; the opposite side of each dish was inoculated with the Trichoderma strains. The plates were incubated at 25 °C for 10 days. b Mycoparasitism of the Trichoderma strains against the pathogens as determined in triplicate using the scale described in the “Materials and methods”. Each value is the mean of three replicates per treatment
Discussion
Trichoderma spp. are useful in agriculture, and T. harzianum is a well-known, effective biological control agent for alternative pathogen control (Chet 1987). Among the various mechanisms used by antagonistic Trichoderma spp. (Hoyos-Carvajal et al. 2008; Woo and Lorito 2007), cell wall degradation of host plant pathogenic fungi is an important strategy for mycoparasitism (Benítez et al. 2004). Because chitin is the major cell wall component of many plant pathogenic fungi, the role of chitinase and its gene in mycoparasitism and biocontrol activity has been investigated (Seidl et al. 2005; Viterbo et al. 2001). A comprehensive survey of Trichoderma chitinase genes using genome-wide analysis revealed multiple chitinase gene homologues in the Trichoderma genome (Seidl et al. 2005).
Functional analysis of genes encoding these cell-wall-degrading enzymes is difficult due to the multiple copies of the genes in the fungal genome, as well as for the genes encoding enzymes that degrade the cell walls of plant pathogens (Tonukari et al. 2000; Walton 1994). The major obstacle in examining the role of such genes and enzymes is redundancy. Plant pathogens have multiple genes for multiple cell-wall-degrading enzymes, including chitinase, glucanase and pectinase (Walton 1994). Therefore, mutation of these genes using molecular techniques results in retention of at least some residual enzyme activity. This technical obstacle has been resolved through loss of function of the SNF1 homologue in the plant pathogenic fungus C. carbonum (Tonukari et al. 2000). Because the yeast SNF1 ortholog in C. carbonum (ccSNF1) is required for derepression of catabolite-repressed genes, mutation of the gene in the pathogen caused downregulation of catabolite-repressed cell-wall-degrading enzymes. Therefore, SNF1-disrupted mutants were useful for determining whether the cell-wall-degrading enzyme complex is important for fungal pathogenicity in hosts.
An SNF1 ortholog in Trichoderma has been identified and analyzed in the cellulolytic industrial species T. reesei (Cziferszky et al. 2003). The Snf1 kinase of the fungus phosphorylates regulation-relevant serine residues in the yeast carbon catabolite repressor Mig1, but not in the filamentous fungal counterpart Cre1 (Cziferszky et al. 2003). However, the role of the SNF1 ortholog in Trichoderma spp. in mycoparasitism during biocontrol has not yet been elucidated.
The SNF1 ortholog (ThSNF1) in biocontrol strain T36 of T. harzianum was identified in this study using draft genome data of the strain. Involvement of the gene in mycoparasitism activity was examined using a ΔThSNF1 mutant. The ThSNF1 gene is structurally and functionally related to the SNF1 orthologs in F. oxysporum (FoSNF1) (Ospina-Giraldo et al. 2003), C. carbonum (ccSNF1) (Tonukari et al. 2000) and others. These proteins showed high similarity, particularly in the serine/threonine protein kinase catalytic domain. Previous studies indicated that SNF1 homologues in C. carbonum and F. oxysporum were involved in the utilization of certain sugars as carbon sources (Ospina-Giraldo et al. 2003; Tonukari et al. 2000). Moreover, these SNF1 homologues control expression of genes for several cell-wall-degrading enzymes and, hence, contribute to virulence against host plants.
Deletion of ThSNF1 in T. harzianum resulted in a phenotype similar to those pathogens, including impaired ability to utilize certain carbon sources such as chitin, reduced expression of genes encoding cell-wall-degrading enzyme and reduced virulence/mycoparasitism against Fusarium pathogens. The production of cell-wall-degrading enzymes such as chitinase is an important factor for mycoparasitism by Trichoderma spp. (Seidl et al. 2005). Transgenic plants that expressed the T. harzianum chitinase gene became resistant to several plant pathogenic fungi, indicating the involvement of the genes in the antagonistic and antifungal activities of the biocontrol fungus T. harzianum (Lorito et al. 1998). Thus, the impaired production of such enzymes as a result of defects in ThSNF1 most likely disturbed mycoparasitic invasion of host fungi, the most common mechanism in the biocontrol activity of Trichoderma spp.
The results of the SNF1 mutation do not differentiate the role of each individual cell-wall-degrading enzyme during mycoparasitism because all of the enzymes might be downregulated. However, the SNF1 modification is a valuable strategy to examine the contribution of genes for the cell-wall-degrading enzyme complex, including the chitinase, polygalacturonase and glucanase genes, in virulence against host plants or fungi by plant pathogenic or mycoparasitic fungi.
References
Abdullah F, Ilias GNM, Nelson M (2007) Hyperparasitic mechanisms employed by the fungal biocontrol agent in a Trichoderma–Ganoderma interaction. In: Exploring life as a catalyst for technological advancement. Proceedings of the 9th symposium of the Malaysian Society of Applied Biology, Universiti Sains Malaysia. Malaysian Society of Applied Biology, Kuala Lumpur, pp 107–130
Akamatsu H, Itoh Y, Kodama M, Otani H, Kohmoto K (1997) AAL-toxin-deficient mutants of Alternaria alternata tomato pathotype by restriction enzyme-mediated integration. Phytopathology 87:967–972
Benítez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Celenza JL, Carlson M (1984) Cloning and genetic mapping of SNF1, a gene required for expression of glucose-repressible genes in Saccharomyces cerevisiae. Mol Cell Biol 4:49–53
Chet I (1987) Trichoderma: application, mode of action and potential as a biocontrol agent of soilborne plant pathogenic fungi. In: Chet I (ed) Innovative approaches to plant disease control. Wiley, New York, pp 137–160
Cziferszky A, Seiboth B, Kubicek CP (2003) The Snf1 kinase of the filamentous fungus Hypocrea jecorina phosphorylates regulation-relevant serine residues in the yeast carbon catabolite repressor Mig1 but not in the filamentous fungal counterpart Cre1. Fungal Genet Biol 40:166–175
Dickman MB, Yarden O (1999) Serine/threonine protein kinase and phosphatases in filamentous fungi. Fungal Genet Biol 26:99–117
Ezziyyani M, Pérez SC, Ahmed AS, Requema ME, Candela ME (2004) Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L) (in Spanish). Anal Biol 26:35–45
Hjeljord L, Tronsmo A (1998) Trichoderma and Gliocladium in biological control: an overview. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium, vol 2., Enzymes, biological control and commercial applicationsCRC Press, London, pp 129–151
Hoyos-Carvajal L, Duque G, Orduz PS (2008) Antagonismo in vitro de Trichoderma spp. sobre aislamientos de Sclerotinia spp. y Rhizoctonia spp. (in Spanish). Rev Colomb Cienc Hortic 2:76–86
Kuwayama H, Obara S, Morio T, Katoh M, Urushihara H, Tanaka Y (2002) PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res 30:e2. doi:10.1093/nar/30.2.e2
Lee SH, Lee J, Lee S, Park EH, Kim KW, Kim MD, Yun SH, Lee YW (2009) GzSNF1 is required for normal sexual and asexual development in the ascomycete Gibberella zeae. Eukaryot Cell 8:116–127
López-Mondéjar R, Catalano V, Kubicek CP, Seidl V (2009) The β-N-acetylglucosaminidases NAG1 and NAG2 are essential for growth of Trichoderma atroviride on chitin. FEBS J 276:5137–5148
Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zoina A, Tuzun S, Scala F (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95:7860–7865
Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR (2006) A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566
Ninomiya Y, Suzuki K, Ishii C, Inoue H (2004) Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc Natl Acad Sci USA 101:12248–12253
Ospina-Giraldo MD, Mullins E, Kang S (2003) Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr Genet 44:49–57
Ronne H (1995) Glucose repression in fungi. Trends Genet 11:12–17
Ruijter GJG, Visser J (1997) Carbon repression in Aspergilli. FEMS Microbiol Lett 151:103–114
Seidl V, Huemer B, Seiboth B, Kubicek CP (2005) A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS J 272:5923–5939
Tonukari NJ, Scoot-Craig JS, Walton JD (2000) The Cochliobolus carbonum SNF1 gene is required for cell wall-degrading enzyme expression and virulence on maize. Plant Cell 12:237–247
Treitel MA, Kuchin S, Carlson M (1998) Snf1 protein kinase regulates phosphorylation of the Mig1 repressor in Saccharomyces cerevisiae. Mol Cell Biol 18:6273–6280
Vieira PM, Coelho ASG, Steindorff AS, de Siqueira LSJ, Silva RN, Ulhoa CJ (2013) Identification of differentially expressed genes from Trichoderma harzianum during growth on cell wall of Fusarium solani as a tool for biotechnological application. BMC Genom 14:177
Viterbo A, Haran S, Friesem D, Ramot O, Chet I (2001) Antifungal activity of a novel endochitinase gene (chit36) from Trichoderma harzianum Rifai TM. FEMS Microbiol Lett 200:169–174
Walton JD (1994) Deconstructing the cell wall. Plant Physiol 104:1113–1118
Woo SL, Lorito M (2007) Exploiting the interactions between fungal antagonists, pathogens and the plant for biocontrol. In: Vurro M, Gressel J (eds) Novel biotechnologies for biocontrol agent enhancement and management. Springer, Netherlands, Amsterdam, pp 107–130
Acknowledgments
We thank H. Suga for providing the fungal strains and R. P. Oliver for providing the transformation vector. This work was supported by the Global COE Program “Advanced Utilization of Fungus/Mushroom Resources for Sustainable Society in Harmony with Nature,” MEXT, Japan. We thank the National Secretary of Higher Education, Science, Technology and Education of Ecuador and the Biotechnology Research Center of Ecuador, Higher Polytechnic College of the Littoral CIBE-ESPOL for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galarza, L., Akagi, Y., Takao, K. et al. Involvement of ThSNF1 in the development and virulence of biocontrol agent Trichoderma harzianum . J Gen Plant Pathol 81, 211–217 (2015). https://doi.org/10.1007/s10327-015-0590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-015-0590-2