Abstract
Microplastics have been recently detected in many environmental media and living organisms, yet their transfer and toxicity to humans are poorly known. Here, we review microplastic transfer in the food chain with focus on microplastic pollution sources, methods to analyze microplastics in food, health impact of food-related microplastic exposure, and remediation of microplastic pollution. Microplastic pollution sources include seafood, food additives, packaging materials, and agricultural and industrial products. Remediation techniques comprise the use of microbial enzymes and biofilms. Microplastic detection methods in food rely on separation and quantification by optical detection, scanning electron micrography, and Fourier-transform infrared spectroscopy. Human health impact following microplastic ingestion include cancers, organ and respiration damage, and reproductive impairments. Overall, microplastic toxicity is mainly due to their ability to enter the metabolism, adsorption into the circulatory system for translocation, and difficulty, if not impossibility, of excretion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The plastic industries have recorded enormous growth in the last few decades, with global production of plastic above 367 million tons as of 2021. Consequently, plastic waste released into various environmental compartments has increased due to usage, and disposal (Huang et al. 2022; Anand et al. 2023; Osman et al. 2023; Ali et al. 2023; Sharma et al. 2023; Chia et al. 2023). Microplastics are generated when discarded plastic materials are exposed to external factors such as physical and chemical changes, mechanical force, and biological transformations (Novotna et al. 2019). Microplastics are plastic fragments less than 5 mm in size (Osman et al. 2023). Secondary microplastics are produced when raw plastic particles are degraded by chemical, biological, and physical processes in the environment, while primary microplastics are basic materials utilized in household and personal care goods (Padervand 2017; Padervand et al. 2020). Microplastics are of great concern and a challenge to environmentalists due to their long-term durability, their polymeric structure and ease of transfer between different environments (Chia et al. 2021). Microplastics are quite prevalent as a result of poor plastic waste management (Akan et al. 2021; Enechi et al. 2021; Deme et al. 2022; Okeke et al. , 2022a, b, 2023a; Okoye et al. 2022a). Microplastics' degradative mechanisms are not completely understood (Othman et al. 2021; Huang et al. 2022). For 3 years, under simulated realistic weather circumstances, the deteriorating changes of the chemical structure of two types of microplastics, including polypropylene and polyethylene, were evaluated (Brandon et al. 2016). They discovered some minor nonlinear variations in time in moieties like carbonyl, hydroxyl, and carbon–oxygen bonds using Fourier-transform infrared (FTIR) spectroscopy analysis (Huang et al. 2022; Chia et al. 2023), which suggests that microplastics degrade slowly (Brandon et al. 2016). Several methods have been employed in the removal of microplastics from different media including sorption and filtration methods (Adsorption on green algae, removal using membrane technology), advanced filtration technologies, chemical methods, biological removal and ingestion (using marine organisms, using bacteria, via ingestion (Padervand et al. 2020)).
A plethora of evidence from previous studies has demonstrated the retention of microplastics in various ecosystems, including those of the ocean, soil, freshwater, and the atmosphere (Akan et al. 2021; Deme et al. 2022; Okeke et al. 2022a, b, c, 2023a, b; Okoye, et al. 2022a, b). As a result, it is reasonable to assume that Microplastics will find their way into and accumulate in the food chain, which is based on above-ground ecosystems, before being consumed by man via diet. There are numerous studies on inner aquatic products at the moment because the majority of Microplastics detection and identification assessments are conducted on aquatic ecosystems (Zhao et al. 2022). However, foodborne Microplastics are not just found in aquatic foods; they are also found in some commonly ingested processed foods and terrestrial crops (Piyawardhana et al. 2022). Additional sources of Microplastics include fast food deliveries, foodstuffs packed and stored in plastic containers (Li et al. 2020b) and even indoor air (Zhang et al. 2022). Therefore, more effort is needed by researchers into both quantitative and qualitative research on different categories of food items, as this will serve as the foundation for determining the health risks associated with microplastics in humans. There are numerous documented detrimental consequences resulting from microplastics including reproductive toxicity (Liu et al. 2022b), inhibition of growth (Vickers 2017), impairment of nutrient absorption in plants as well as nervous system disorders (Lee et al. 2022). Results from previous study show that microplastics ingested through diet resulted in intestinal disorder in humans (Lu et al. 2019). The altered intestinal integrity could result to a concomitant anomaly in digestion and absorption of nutrients as well as altered mammalian homeostasis (Luissint et al. 2016), with resultants immune system dysfunction, metabolic disorders and pathogenesis of various diseases (Ganal-Vonarburg et al. 2020). In summary, it can be seen from these previous studies that microplastics have detrimental impacts on human health and the environment and could get into the human body via contaminated food or food packaging materials. Here, we review microplastics in food, effects on food security, and potential health risks.
Sources of microplastics in the food chain
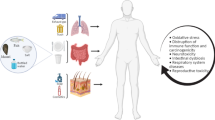
Increasing incidence and consequent health risks of microplastics continue to raise concerns over entry into humans, by inhalation, ingestion and dermal contact (Asif et al. 2017). Microplastics incidence in the food chain is from the aquatic (Panel and Chain 2016; Du et al. 2020; Benson et al. 2022) and terrestrial (He et al. 2021a) environments (Fig. 1). Thus, both the aquatic and terrestrial ecosystems act as microplastics reservoirs. Extensive exposure via the food web involves seafood, drinking water, salt, honey, milk, sugar and meat (Panel and Chain 2016; He et al. 2021a; Jadhav et al. 2021; Benson et al. 2022; Alberghini et al. 2022; Vitali et al. 2023). We summarize some entry routes of microplastics to humans via the food web in the following sections and in Fig. 1 below. Overall, water sources and packing materials constitute the highest microplastics entrance route to the human food chain. For instance, salt retains and transmits microplastics through intake but terrestrial foods like cattle have a method for their elimination. We discuss below several pathways through which microplastics may migrate into food samples.
Microplastics can enter different environmental compartments and human food chain through various pathways. These include industrial operations, wastewater discharge, and the decomposition of larger plastic trash. Once in the environment, microplastics can spread through air, water, and soil, accumulating in different ecosystems, both aquatic and terrestrial. Animals residing in these habitats, such as fish, plankton, and bivalves, can ingest microplastics. Microplastics can enter the human body through the intake of tainted seafood, meat, or vegetables. They can also enter the body through drinking water and airborne particles
Water
Polyamide, polyethylene, polyester, polyvinyl chloride, polytetrafluoroethylene, etc. from food packaging, toys, personal care products and sewage have been reported in water (Muhib et al. 2023; Sewwandi et al. 2023). Microplastics including acrylonitrile, butadiene, polyethylene terephthalate, poly(ester amide) and styrene have been traced to production water sources including underground water, public water sources, surface water and rainwater (Schymanski et al. 2018; Makhdoumi et al. 2021; Samandra et al. 2022; Muhib et al. 2023; Sewwandi et al. 2023) with eventual incidence in the human food chain. For example, a study on comprehensive water chain reported epoxy resin, polyethylene, polyamide, polystyrene, and polyvinyl chloride at an average concentration of 700 particles/L (Pironti et al. 2021). Enormous microplastics are available to humans via treated water, with the World Health Organization reporting a concentration of 5 particles/L of tap water and worse for individuals drinking only bottled water (Pironti et al. 2021). Introduction of microplastics from water and drinks represents about the most important entry route considering the integral place of water in human nutrition (Miller and Young 2023) and the place of water as an important reservoir of microplastics (Sewwandi et al. 2023). In summary, water is critical to human nutrition and an important route of microplastics entry into humans.
Seafood
Seafood represents a preferred protein source in human diets (De-la-torre and De-la-torre 2020), but an important route of microplastics entry into the human food chain (Table 1) (Schymanski et al. 2018; Cverenkárová et al. 2021; Benson et al. 2022; Alberghini et al. 2022; Sewwandi et al. 2023). Microplastics incidence in fish is either direct via ingestion or passive via gill respiration and ingesting of contaminated prey (De-la-torre and De-la-torre 2020). Studies report the presence of microplastics in seafood including shellfish and fish (Cverenkárová et al. 2021; Alberghini et al. 2022; Sewwandi et al. 2023). The localization of microplastics in the usually discarded seafood gastrointestinal tract (Alberghini et al. 2022; Sewwandi et al. 2023) offers some respite, but they have been reported in some edible parts (Sridhar et al. 2022). Further, the presence of microplastics on the external intestinal linings of lower marine species suggests bioavailability to humans (Pironti et al. 2021; Benson et al. 2022). Similarly, shell fishes are sometimes eaten with the gastrointestinal tract, increasing entry to the food chain (De-la-torre and De-la-torre 2020; Cverenkárová et al. 2021). For example, Teng et al. (2019) reported microplastics present in 84% of the oyster samples studied in China (Table 1) (Teng et al. 2019).
Food packaging
With 40% of the total plastic production, food packaging (cans, bottles, wrappings, cups) uses the highest volume of plastics (Sridhar et al. 2022; Muhib et al. 2023), accounting for appreciable levels of microplastics migration into the human food chain (Al Mamun et al. 2023). In fact, food packaging and water may represent the most important route of microplastics in food explained by atmospheric fallout, surface leaching due to loose/rough structure, mechanical degradation and stress (opening methods) (Mason et al. 2018; Winkler et al. 2019; Du et al. 2020; Sobhani et al. 2020; Muhib et al. 2023). These mechanisms account for ingestion of about 2.98 × 103 microplastics person−1 year−1 and may increase based on ingestion frequency. For example, bottled water and drinks are severally reported with high incidence of microplastics that end up in human food (Table 2) (Panel and Chain 2016; Jadhav et al. 2021; Cverenkárová et al. 2021; Sridhar et al. 2022; Sewwandi et al. 2023; Vitali et al. 2023). The global bottle water consumption is above 22 billion liters annually (Luo et al. 2018), with microplastics in approximately 93% of bottle water samples studied in different countries (Pironti et al. 2021). Microplastics in bottled water are about twice the quantity reported in tap water (Table 2) (Mason et al. 2018; Jadhav et al. 2021). Other studies also report the presence of microplastics in bottled water examining, single use, beverage cartons (Table 2) (Schymanski et al. 2018; Muhib et al. 2023).
Further, the replacement of traditional paper wrapper to plastic materials for teabags also presents an important route of microplastics entry into human food chain. Irrespective of quality, plastic materials degrade from 40 °C over time. Therefore, tea bags that may be brewed almost 100 ° release billions of microplastics in a single teacup (Jadhav et al. 2021; Carr Kinnear et al. 2021; Sewwandi et al. 2023), with high toxicity index. Further disposable paper cups with hydrophobic inner linings release substantial microplastics when used to serve hot drinks (Liu et al. 2022a). This presents another synergy for high microplastics contamination from water and packing material, in addition to the high microplastics contaminations present in water sources, including treated tap water.
Microplastics incidence in milk and dairy products due to hygiene and corrosion from the milk processing and packaging materials have been reported. Thermoplastic sulfone polymers used in the ultrafiltration of milk have been reported due to high pressure-induced peel-off and chemical stress on the membranes (Benson et al. 2022; Vitali et al. 2023). Further, about 16 million microplastics/L are released due to shaking and preparation temperature in feeding bottles, representing a worrisome position as milk is mostly consumed by infants (Li et al. 2021b; Sewwandi et al. 2023). Microplastic intake from feeding bottles is 3000 times adults exposure from air, food and water at 1,580,000 per child (Li et al. 2020c; Jadhav et al. 2021). Obviously, the recent preference of plastic materials for food package is a critical route of entry for microplastics into the human food chain, form plastic tea bags, to feeding bottles and plastic bottles for water and other drinks, all account for appreciable levels of microplastics in human food.
Agricultural products
Soil is an important sink for microplastics, with 4–23 times the volume of microplastics in water, distributed in agricultural soils, industrial and remote areas (He et al. 2021a). Agriculture uses 38% of all global land (Zabel et al. 2019) and soil microplastics contamination results from anthropogenic activities and degradation (He et al. 2021a). The soil–plant pathway contributes 99% of human calories but represents an important microplastics entry route to the human food chain (Abbasi et al. 2020). Studies have shown the size-dependent root intake of microplastics from contaminated soils related to a crack-entry route in the root and subsequent translocation to the stem and then to other edible plant parts (He et al. 2021a; Wen et al. 2022). Also, another study has described the vertical migration of microplastics entry into plants aided by infiltration, fauna activities and root growth (Li et al. 2021b).
Important food plants and vegetable including apple, broccoli, carrot, corn, cucumber, lettuce, onions, pears, wheat crops are able to take up microplastics of between 50 and 700 nm from the soil via the root (Table 3) (Sewwandi et al. 2023; Wen et al. 2022; Li et al. 2021a, b, c; Oliveri Conti et al. 2020). These contaminants are incident in livestock (cow, pig and poultry), but don’t have a direct entry into the food chain, due to an elimination mechanism in animals (Wu et al. 2021; Wen et al. 2022). Ingested microplastics do not get absorbed from the gastrointestinal tract and are not present in edible parts (Wen et al. 2022). However, consumption of the animal intestine is a relished cuisine in parts of Africa, such as eastern Nigeria. Further, chopping boards and packaging materials such as trays and films have been implicated for microplastics in meat products (Wu et al. 2021; Habib et al. 2022). Seafood is also a source of introduction of microplastics into the poultry and livestock industry with eventual transfer to human food chain explained by trophic level food transfer mechanism (Panel and Chain 2016). Incidence of microplastics in poultry has been demonstrated to pose significant risk of introduction to the human food web (Pironti et al. 2021; Sewwandi et al. 2023). Studies showed route of entry into chicken and increasing concentration traced from soil to chicken feces to gizzard (Habib et al. 2022) and more recently polyvinyl chloride and polystyrene have been reported in chicken flesh (Pironti et al. 2021). Agriculture is at the very bottom of human food chain, supplying all needed nutrients from both plant and animal produce. As expected, agriculture is an important source of microplastics in human food, worse from plants but also from animal sources.
Food additives
Salt is a carrier of microplastics, with a maximum human exposure of 6110 microplastic/year, presenting an important ingestion route based on the World Health Organization recommended daily consumption of 5 g daily (Pironti et al. 2021; Benson et al. 2022; Sewwandi et al. 2023). Global incidence of microplastics has been reported in edible salt, with least contamination in China and highest in Croatia at 2000 particles/kg of salt (Pironti et al. 2021; Kuttykattil et al. 2023). Mostly, the plastic particles found in salt consist of cellophane, cellulose, paraffin wax, polyvinyl chloride, etc. The highest Microplastics contamination is reported in sea salt, suggesting industrial effluents or the degradation of larger plastics as main sources of microplastics (Kim et al. 2018). Specifically, Kim and others reported microplastic contents of 0–1674 n/kg in sea salt, 0–148 n/kg in rock salt and 28–462 n/kg in lake salt showing salt as an important source of microplastics in food. The high incidence of microplastics in salt indicates that evaporation is unable to remove contaminant, exposure humans to microplastics. Further, the primary source of salt contributes to microplastics load in salt such that sea salt was most contaminated, explain by the disposal of contaminated industrial wastes into the sea. Microplastics are present in other food additives (Table 3) (Sewwandi et al. 2023). For example, the presence of microplastics in honey is traced beekeepers and atmospheric contamination during production (Sewwandi et al. 2023). Similarly, microplastics contamination has been reported in sugar, though likely below acceptable safe limits (Vitali et al. 2023). Appreciable levels of microplastics end up in food from salt, honey, sugar and other additives traced to production sources and staff.
Industrial products
Many industries are implicated for the incidence of microplastics in food and eventual entry into the food chain. Earlier in this section, we highlighted the routes of microplastics incidence to include air, water and soil environments and here, we elucidate the role of industries in the introduction of microplastics. Globally, personal care products from wastewater treatment plants contribute about 1500 tons/year of microplastics to the aquatic environment alone with a global emission of 1.2 × 104 tons/year and an average microplastics of 2162 particles/g (Sun et al. 2020a). These microplastics in the aquatic environment could contaminate seafood and public water sources and end up in the human food chain as already discussed in this review. Further, the textile industry is reputed for the release of fiber from industries into the aquatic environment (Periyasamy and Tehrani-Bagha 2022). These fiber materials have been traced to seafood and eventually the human food chain (Table 3). Research has demonstrated the incidence of Microplastics resulting from industrial emissions via ingestion, inhalation and dermal absorption with enormous health implication (Auguet et al. 2022). Industrial release of microplastics into the atmosphere with subsequent settling on plants, soils and food materials is also implicated for the incidence of microplastics in the human food chain (Pironti et al. 2021). These industrial releases actually represent a primary source of microplastics for the various routes of entry into the human food chain.
Analysis of microplastics in food
The process of microplastic analysis in food generally involves sample digestion (food matrix destruction), sample extraction/separation, and sample identification, measurement, and characterization. The pretreatment of the sample is essential for a successful spectroscopic analysis of microplastics in food because it maximizes extraction efficiency, protects polymers, and yields reliable results. The kind of food matrices and targeted microplastics determines the suitable sample pretreatment to use. Because there are many different types of microplastics in samples, it is frequently challenging to recover all of them using a single technique. Understanding the advantages and disadvantages of each sample pretreatment technique are crucial.
Separation techniques
Physical treatments
Membrane-based filtration
One of the effective techniques for isolating microplastics from food matrices is membrane-based separation. To recover microplastics and conduct additional analysis, various liquids, such as drinking water samples and certain beverages, can be filtered onto filter membranes. To retain the microplastics in other liquid or solid samples, filtering digestants are typically used after chemical or enzymatic digestion. The simple method uses a pressure difference to cause the liquid to pass through the membrane and does not require a complicated setup. Applying pressure enables the solution to keep the microplastics on the surface of various membrane filters with a distinct size separation. The membrane filtration technique for microplastic separation after treatment is depicted in Fig. 2. The potential to replace traditional methods that are energy-intensive and need sophisticated maintenance and operation has attracted interest in the technology (Sridhar et al. 2021). The size of the filtered particles can also be used to categorize the separation process; these include nanofiltration, ultrafiltration, reverse osmosis, and microfiltration.
Microplastic separation using membrane filtration for liquid and solid foods. The microplastic in solid food samples undergoes chemical digestion using the appropriate chemical, and the solvent is subsequently extracted. Similarly, microplastic-contaminated liquid food samples undergo microfiltration to extract the media containing microplastics. The solvent extracts are then passed through the membrane filtration apparatus with the appropriate membrane filters. The extracted microplastics are then characterized, and the digestion media is removed
Microplastics on filters could either be transferred to slides or used directly for spectroscopic analysis. Mesh, glass fiber, cellulose acetate membranes, cellulose nitrate, and metal-coated filters are a few of the frequently recommended filtering materials. Aluminum/gold-coated filters typically have a clear background, simplifying spectroscopic identification (Oßmann et al. 2018). Metal surfaces are more expensive than other filters, and they are also more prone to distortion. Concentrating microplastics within a particular size range is possible by using a number of filters with various pore sizes. In addition, the chemical compatibility of various filters varies. As a result, another crucial factor to take into account is the compatibility of filter materials with digesting solvents. The potential of membrane separation technology in the water sector has been thoroughly investigated. For instance, a study done in drinking water treatment plants to separate soluble microbial products (a mixture containing proteins, fulvic acids, polymers, organic matter, and polysaccharides) using ultrafiltration revealed a 20% enhancement in removal efficiency after biodegradation (Wu et al. 2019). The method has been explored for the separation of microplastics as a result of these encouraging results in the removal of particles, suspended solids and particular ions. A South Korean-based study examined the use of a membrane disk filter in conjunction with sand filtration and ozone treatment to separate microplastics (Hidayaturrahman and Lee 2019). Filtration technique was used for the isolation of microplastics from organic matter in tap water samples (Li et al. 2022). According to the results, wastewater removal effectiveness ranged from 75 to 91.9%. Another independent study carried out in the United States of America examined the impact of sewage released into the San Francisco Bay and other broad geographic regions (Mason et al. 2016). According to the study, 0.05 to 0.024 particles/L of wastewater were discovered in the 90 samples taken from 17 different facilities using a mix of chemical and microfiltration techniques with a mesh size of 125 m. When compared to industrial effluents, the microplastic observed in these WWTPs was lower between 50 thousand to 15 million particles/L (Mason et al. 2016). Conclusively, these studies using filtration based on membrane technology recorded promising results in separation of microplastics.
Recently, microplastics in food have also been detected using membrane separation technology. One intriguing study, for instance, looked at the presence of microplastics in branded milk (23 samples) from both foreign and local brands (Kutralam-Muniasamy et al. 2020). The samples were handled at 0.5 bar pressure and slightly warmed before filtering because casein and fat could clog the filters. Microfilters with pore diameters of 0.22, 0.45, 11, and 5 μm were selected, with 11 μm being the ideal size for separation. The findings revealed a total of 2.5% granules and 97.5% fibers, with polyethersulfone and polysulfone polymers making up the majority. The lowest concentration was 32 particles/L while the highest was recorded as 11 ± 3.54 particles/L. The average microplastic count was 6.5 ± 2.3 particles/L. One of the intriguing conclusions was that none of the milk samples included any of the typical polymers like polyester, polypropylene, polyethylene, or PET. Furthermore, it was found that thermoplastic sulfone polymers could be readily extracted from milk samples using membrane filters. Similar investigations were carried out to find microplastic in takeaway food packages. The microplastics were extracted using membrane filters with a 5 μm pore size. According to the findings, microplastics ranged from 3 to 29 particles per container across all of the containers, with polystyrene flakes making up roughly 77% of those particles. Further analysis revealed that those who order meals 4–7 times per week may consume 12–203 microplastics via food packaging material (Du et al. 2020). Based on the positive results recorded in the above studies, it can be concluded that membrane technology is a technique for separating microplastics from foods samples. Outlined below are other examples of studies where membrane technology was used for isolation of microplastics from various food samples (Table 4). The primary drawback of filtration techniques is that if the pore size is too small, the filter can become easily clogged; hence, great care should be taken to use the appropriate equipment to retain all target particles.
Flotation
In order to extract microplastics from materials with nearly full separation, floatation is a widely used, quick, and easy approach (Nguyen et al. 2019). Compared to higher-density plastics like styrene, polyvinyl chloride, plasticizers and additives with a density of 1.40 g/cm3, microplastics like polyethylene and polypropylene have lower densities of 1.10 g/cm3. Although there are various varieties of the floatation technique, each has advantages and disadvantages of its own. To optimize the extraction of plastics, the sample matrix is first pre-digested typically using an H2O2 solution. To create a homogenized solution, the solution is then combined with the floatation medium (Wang et al. 2018). The floating medium is selected based on the plastic's density. For instance, sodium iodide (NaI) (1.6–1.8 g/cm3) can extract lesser-density plastics, while zinc chloride (ZnCl2), which has a greater density of 1.5–1.7 g/cm3, is capable of extracting microplastics (Liebezeit and Dubaish 2012). After a short period of time during which phase separation takes place, the slurry is kept undisturbed, and the supernatant fraction is collected for further investigation (Van Cauwenberghe et al. 2015). For the purpose of understanding floatation experiments in freshwater systems, several studies have been carried out. For example, an average of 66 particles were found per 100 g of the sample after conducting floatation studies in the river Thames, United Kingdom (UK). Plastic pieces made up 91% of these particles (Horton et al. 2017). Similar studies were carried out to determine whether microplastics were present in groundwater samples from 8 water treatment facilities in England, UK. According to the findings, potable water contained 0.00011 microplastic/L, and raw water contained 4.9 microplastic/L (Johnson et al. 2020). Numerous food items have also been the subject of research into the possibility of the flotation technique with diverse media. For instance, in a study conducted in East China, the flotation medium was a solution of potassium hydroxide (20% w/v), sodium iodide (4.4 M), and hydrogen peroxide (30% v/v) for the detection of microplastics in wild fish and crustaceans. According to the findings, 57.5% of the fishes had average microplastic counts of 0.77 to 1.25 in the gills and 0.52 to 0.90 in the digestive system (Zhang et al. 2019a). Similar investigations were made to assess the health of sardines and mussels. With 1.7–2 items per mussel and 1.5–1.9 items for fish using NaCl as floating media, microplastics occurred 47.2% of the time (Dekiff et al. 2014). To comprehend the existence of polyethylene terephthalate (PET) in table sea salts, another study was examined (Zhang et al. 2018). Further evidence of its effectiveness came from floatation investigations conducted after vacuum filtration, which revealed a concentration of 16–63 particles per 50 g of material. The overall process of floatation is often has a high recovery rate (up to 99%), is inexpensive, and is simple to regulate (Tirkey and Upadhyay 2021). The fundamental disadvantage of these materials is that the plastic particles must first be separated from the matrix materials.
Chemical treatment
Chemical treatments have been used to attain the goal of quicker sampling methods for better detection. Physical separation investigations have been beneficial, but they take time and require more accurate detection. Chemical processing is one method for removing microplastics from food systems in an efficient manner. Acid and alkaline treatments can be broadly categorized into two groups when it comes to the chemical digestion of microplastics. Without damaging the plastics, both treatments were able to break down the organic material in foods and tissue samples (Lusher et al. 2020). However, concentration is important for digestion when using alkaline solutions. Other substances have also undergone testing in recent years to determine their benefits and drawbacks. For instance, microscopy was used to investigate the Nile Red dye's potential for quantifying microplastics in bottled water. The results demonstrated Nile Red's ability to adsorb the microplastics from water selectively; propylene was found to be the most common type of polymer (54%) followed by copolymers and lubricants. In addition, 95% of the microplastics ranged in size from 6.5 to 100 µm (Mason et al. 2018). Much research has been done on fish and seafood. A laboratory examination found microplastics in oyster and mussel tissues after 69% HNO3 treatment (mussels: 0.36 ± 0.07 particles/g, oysters: 0.047 ± 0.16 particles/g) (Van Cauwenberghe and Janssen 2014). Similar experiments used 1 M NaOH 35% HNO3 to separate microplastics from mussel soft tissues (Catarino et al. 2017). While the recovery rate was good (93%), extreme responses occurred during HNO3 treatment. Sample pretreatment with H2O2 caused froth and poor tissue digestion. It was found that alkaline digestion (10 M NaOH) resulted in a better efficiency of 91.3 ± 0.4% compared to acidic digestion with HCl (1 M: 82.6 ± 3.7% and 2 M: 72.1 ± 9.2%) (Hidalgo-Ruz et al. 2012). However, excessive amounts may block plant cell walls. To remove plastics from vegetal-rich samples (plant leaves, seagrasses, and seeds), a novel, efficient, and economical digesting process has been suggested six protocols were examined: four acid/alkaline, one with H2O2, and another with ethanol (Herrera et al. 2018). Acid and alkaline treatment with NaOH, KOH, and HCl yielded 9–40% separation efficiency. H2O2 treatment showed an average effectiveness of 64.6 ± 7.1%. The maximum efficiency was 97% with 100 ml of 96% ethanol mixture (Herrera et al. 2018). Alkaline and acid digestion are good for decomposing organic materials and recognizing microplastics, but microplastic treatment needs a systematic approach. High concentrations of chemicals such as concentrated HNO3 (22.5 M) can damage pH-sensitive polystyrene polymers, making microplastic counting and measurement difficult. Moreover, H2O2 treatment causes foaming. However, enzymatic digestion efficiency testing does not damage the food matrix (Primpke et al. 2020a). Conclusively, this technique is suitable for animal tissues and organs; however, it must be tailored to digest all organic debris and could damage or break down plastic particles.
Enzymatic treatment
Enzymatic treatment uses biologically active enzymes to separate better than chemical treatment and is less harmful to the environment. Mild enzymatic digestions take longer and are less effective than chemical digestions. The plastic structure remains after digestion (De Boer et al. 2011). Cellulase, protease, lipase, and chitinase are commonly utilized enzymes (Löder and Gerdts 2015). Because of their selectivity, several enzymes are used to digest a food sample (e.g., proteinase only digests protein). A study recovered PP from marine invertebrates and fish using enzymes and H2O2. With 97% plastic particle recovery, plastic degradation was not observed (Karlsson et al. 2017). As ocean plastic waste increases, plasticizers like bisphenol-A and chemical colors harm aquatic biodiversity. These degraded plastics reach fish guts that humans eat. The growing issue of aquatic life contamination has prompted investigations on sea foods and marine ecosystems. Seawater biota-rich samples were used in early enzyme therapy research (Cole et al. 2014). Proteinase-K was used to separate microplastics in acid, alkaline, and enzymatic methods. Enzymatic digestion achieved > 97% (by weight) digestion efficiency in 2 h at 50 °C for particles over 300 μm. Similar research was done with mussel tissues using papain, collagenase, and trypsin. Trypsin was the most cost-effective and fastest, yielding 88% efficiency in 30 min at 40 °C (Courtene-Jones et al. 2017). An intriguing plant-soil ecosystem study examined how polystyrene microplastics affected broad beans using two enzymes. Using biomass and catalase enzyme activity on plant roots resulted in smaller polystyrene particles (< 5 μm) compared to superoxide dismutase and peroxide enzyme mixture. Microplastics may also clog pores and cell walls during nutrient transport, causing toxicity (Jiang et al. 2019). Conclusively, enzymatic digestion causes less harm to polymers than alkaline or acidic treatments. Although suitable for various biological matrices, it is costly and unpredictable, as enzymatic activity is greatly dependent on the matrix.
Extraction techniques
3.1.1.5.1 Ultrasound extraction Extraction has also been investigated in addition to enzymatic, chemical, and floatation techniques for microplastic separation. For instance, pulse ultrasonic extraction was used in a novel laboratory-scale extraction method to separate microplastics from the intestines of freshwater fish (Wagner et al. 2017). A bath sonicator was used to separate microplastics smaller than 50–100 m containing significant levels of polyethylene, styrene, plasticizers, and fibers from the gut sample for 15 min at 39–41 kHz. As they are less toxic to the sample and more effective, the same novel technique was later suggested for treating microplastics in different marine settings (Wagner et al. 2019). The ultrasound extraction method provides advantages over enzymatic and chemical treatment, such as cost-effectiveness and little sample loss without the need for intricate chemical processes. Improved solvent penetration is another benefit of the ultrasonic technique. In addition, investigations on the processing of vegetables and the treatment of industrial wastewater using ultrasonic extraction techniques, were conducted (Simon et al. 2018; Yu et al. 2021). To comprehend the existence of microplastic pollutants, comparable tests were done with milk, beer, honey, and soft beverages (Diaz-Basantes et al. 2020). The filtered drinks were given a one-hour treatment in an ultrasonic bath. According to the FTIR data, the average microplastic/L for craft honey, industrial honey, milk, industrial beer, and soft drinks, was 67, 54, 40, 47, and 32 microplastic/L, respectively. In summary, it can be observed that these techniques achieved appreciable success for extraction of microplastics based on the previous studies discussed above.
3.1.1.5.2 Solid phase microextraction The Solid-phase microextraction approach has also been investigated for the extraction of microplastics. The methodology has lately received interest for microplastic separation even though it is more frequently used for food product analysis since it combines four phases namely sampling, extraction, concentration, and sample introduction into a single solvent-free step (Kusch and Knupp 2004). In addition to these benefits, the process reduces waste disposal expenses and time. Due to its effectiveness in degrading both polymers and monomers, microextraction in headspace mode has drawn interest as a sample approach. For instance, expanded polystyrene fibers, a polymer typically used for food packaging, were degraded at various temperatures using headspace solid-phase microextraction (Kusch and Knupp 2004). The results showed that when sonication at 60 °C and 15 min of sample collection was coupled, there was strong repeatability with few mistakes (3.2–3.6%). The treatment and identification of polymers and volatile organic compounds from PS contained in soil mixtures comprising of alluvial soil, algae biomass, and organic compost were carried out utilizing HS-SPME in conjunction with gas chromatography-mass spectrometry (GC–MS). The findings demonstrated that the method is particularly effective for identifying both coarser fractions (0.1–1 mm) and larger particles (1–5 mm) (Šunta et al. 2021).
3.1.1.5.3 Magnetic extraction Microplastics in drinking water and environmental samples have been subject to magnetic separation. The straightforward method uses iron nanoparticles, which bind to the plastic in the sample to produce a hydrophobic tail. With recoveries of 92%, the attached iron particles magnetize the polymers, attracting microplastics with particle sizes 10–20 m (Grbic et al. 2019). The isolation of microplastics from food matrices can be accomplished using similar techniques. Although the structural integrity of the sample is not affected by magnetic separation techniques, many gaps are seen when the sample is being separated. Therefore, rather than employing a single separation technique, the bulk of researchers have concentrated on using this method of extraction as a pretreatment step (Zhu and Wang 2020). Furthermore, using extraction techniques like pulse ultrasound or ultrasound only promoted the breakdown of brittle plastic components into smaller microplastic pieces, making it difficult to estimate the total count (Simon et al. 2018). Therefore, additional research is needed to assess microplastic separation.
Microplastics identification techniques
Following the separation using the proper procedure, microplastics in the target component of the food sample are identified and quantified. These identification methods are vital for risk evaluations and sample quality control. The techniques range from low-cost, simple techniques to expensive, complex-equipment procedures that yield reliable outcomes. We present some techniques available in literature in the following subsections.
Optical detection
Due to its simplicity and ease of usage, the naked eye approach has been widely employed to characterize microplastics. According to color, size, and plastic-type, including beads, granules, or fragments, visible-to-the-unaided eye particle sizes (larger than 500 μm) are manually separated before being counted (Renner et al. 2018). Optically observing microplastics has also been done in recent years using microscopy. Although the procedure is cheap and straightforward, it cannot be advised as a routine identification method because microscopic or manual counting may lead to errors and incorrect calculations. Additionally, counting the split microplastics could be difficult because of potential debris in the microplastics.
Scanning electron microscopy
Scanning electron microscopy (SEM) is a visual characterization technique frequently used in the field of study for detecting microplastics. The microplastics' morphology is examined using a scanning electron microscope. An electron beam is focused and utilized to scan the microplastics' surface. These electrons contact the atoms on the microplastics' surface and give the right signals, which, when paired with the beam's position, yield an image showing the sample's size and morphological details (Henini 2000). Several research has been done on the use of SEM for microplastic identification. For instance, the search pattern was divided into five groups in a study to determine the microplastics in freshwater fish, with the primary categories being fragments, micro-pellets, fibers, films, and foams. The SEM results employed an elimination-based methodology and were able to distinguish 23% of the particles from plastic (Anderson et al. 2017).
Another study examined the shape, size distribution, and polymer composition of marine ecosystems using a combination of SEM, infrared spectroscopy, and X-ray tomography. The analysis of the SEM data revealed small fractures 30–40 μm deep, few microplastics (mostly copolymers and polyethylene) of approximately 2 mm, and flat cubical-shaped debris of around 2 mm (Ter Halle et al. 2016). Additionally, 92% of the microplastics were polyethylene, with detritus and polypropylene making up the other 8%. Similar tests were done on commercial Malaysian fish dinners, canned sardines, and sprats (Karbalaei et al. 2020). The research used SEM and energy-dispersive X-ray Analysis (EDX) to combine higher picture resolutions with microplastic elemental compositions. SEM has recently been used to identify microplastics in liquids like milk. To determine the properties of the microplastics present, 23 different milk samples were investigated using SEM (Kutralam-Muniasamy et al. 2020). About 72% of the microplastics (fragments and fibers) were blue in color, and 42% of them were small (< 0.5 mm). Micro-holes and cracks on the surface of microplastics were also noted to have a slight form variation. Similar research was done to comprehend the properties of polystyrene buildup and breakdown in honey (Wang et al. 2021b). However, SEM is always used in accordance with protocols to determine if the separated fragments and granules can be classified as microplastics (Renner et al. 2018). As a result, a more quantitative approach is needed to estimate the concentration of microplastics in food samples.
Fourier-transform infrared spectroscopy
For many years, it has been standard practice to investigate the surface properties of materials using Fourier-transform infrared spectroscopy (FTIR) (Huang et al. 2022). The sample absorbs infrared light at a certain wavelength, which causes a vibration (or disturbances) that can be used to analyze the molecular properties and makeup of the sample. The following stages can be used to describe how FTIR works briefly: The microplastic sample is first placed on a mechanically stable, water-resistant filter substrate. The sample is put in an FTIR setup after drying. The next step in the polymer identification process is to use the system database to quantitatively determine the highest composition of plastics and non-plastics. With spatial resolutions of 5 m, the system can detect samples with a minimum thickness of 150 nm (Nguyen et al. 2019). Transmission, reflection, and attenuated total reflectance (ATR) are the three operational modes of FTIR. ATR-FTIR is typically used to identify larger plastic samples, while micro-FTIR is typically used to identify smaller plastic samples (Li et al. 2018b). A sequential measurement of infrared spectra at various locations on the sample's surface is known as micro-FTIR mapping. To create a chemical image of the sample, thousands of spectra are captured inside the measuring area (Levin and Bhargava 2004). This technique has been used to identify microplastics with success.
Numerous studies have been done recently to assess the microplastics in the food chain. For example, a study was done to assess the microplastics in samples of drinking water (Mintenig et al. 2019). Averaging 7 particles/m3 of water with diameters between 5 and 150 μm, the micro-FTIR analysis with a spectral resolution of 8 cm−1 and polymer determination range between 1430 and 1480 cm−1 came to this conclusion. The capability of FTIR has also been used to identify microplastics in a variety of foods and drinks, including milk, honey, soft drinks, and beer. According to recent study, there were 13.45–6742.48 μm fibers and 2.48–247.54 μm fragments with an overall microplastics concentration of 12% (Diaz-Basantes et al. 2020). The detection of microplastics in bottled drinking water has also been successfully accomplished in an intriguing work that combined FTIR with Nile Red stain (Mason et al. 2018). Nile Red binds to the microplastics' surface, creating a stain that emits fluorescence at a certain wavelength. Therefore, while taking into account things like results precision and time constraints, FTIR is a viable approach. Water in the sample, it should be emphasized, prevents the approach from being used because it can cover up the target spectrum (Primpke et al. 2020a).
Raman spectroscopy
Raman spectroscopy is a particularly popular technique for microplastic identification. The method uses a scattering method to excite the target molecules with a laser of a specific wavelength. The sample's elemental makeup can be determined by measuring the frequency of scattered radiation from the sample. Due to the scattered frequencies produced by the sample, the analytical technique can analyze particles of much smaller sizes with spatial resolutions below 1 μm compared to other techniques like FTIR (Imhof et al. 2016). The possibility of Raman spectroscopy for identifying microplastics in food samples has been examined in a number of studies. For instance, a study was carried out using micro-Raman spectroscopy to comprehend the microplastic concentration in 17 distinct commercial table salts (Karami et al. 2017b). Samples contained 41.6% polymers, 23.6% pigments, and 5.5% amorphous carbon with a laser wavelength of 785 nm and power below 3 mW. Using a combination of microscopic and micro-Raman techniques, similar studies were carried out on Turkish-origin table salts (Gündoğdu 2018). According to the composition tests, polypropylene (19.2%) was followed by polyethylene (22.9%). With particle sizes ranging from 20 to 5 mm (wavelength: 785 nm), the MP concentration in sea, lake, and rock salt was 16–84, 8–102, and 9–16 items/kg, respectively. Micro-Raman spectroscopy was used in another experiment with beach waters (Erni-Cassola et al. 2017). Raman spectroscopy has reportedly been used to analyze microplastics in drinks like wine (Prata et al. 2020). The micro-Raman spectroscopy used to identify polyethylene particles of various sizes (20 m) and colors in white wine had a wavelength of 633 nm and a resolution of 100 cm−1. Crystals of minerals, trash, and fragments of wood were also seen. One of the groundbreaking research was also shown to be true for soft drinks, cold tea, and energy drinks, which after Raman analysis revealed considerable levels of blue pigments and polyamide content (Shruti et al. 2020). Raman spectroscopy was not, however, used as the only approach for identification in the majority of these studies because of the high background fluorescence, sample photodegradation, and weathering-related spectral fluctuation. Many investigations have found success using a Raman and FTIR or Raman and electron microscopy combo. Additionally, only a very tiny number of studies have used Raman spectroscopy to identify microplastics with particle sizes of 1 µm visually. This is such that the scanning filters cannot be handled with tweezers directly (Xu et al. 2019a). Small filter regions are therefore checked, making the entire procedure labor- and time-intensive. The spectral interference that developed during the examination was another roadblock. For instance, it was observed the presence of several additives, colors, and compounds in bottled mineral water that produced higher background signals in opposition to the weak Raman signals (Oßmann et al. 2018). These components included calcium chloride, magnesium fluoride, and silica. Additionally, for encouraging outcomes during MP identification from samples, a more extensive library for polymer identification should be accessible (Xu et al. 2019a).
Thermo-analytical technique
It has been discovered that the thermal degradation of microplastics is advantageous because it can serve the dual purposes of determining the kind of polymer and locating organic substances, gases, and additives in the intricate formulation. To eliminate the problem of background contamination, such approaches can be completed in a single run without the use of solvents like ethanol or water (La Nasa et al. 2020). The method typically makes use of pyrolysis byproducts at a specific temperature and oxygen-free environment. For molecular measurement of polymers, these products are combined with mass spectrometry (MS) and gas chromatography (GC) setups (Fries et al., 2013). GC–MS investigations have proved crucial for examining non-polar components, fatty acids, lipids, and polymers. Due to its dependability, accuracy, and low cost, the technique has proved helpful in the food business in detecting adulteration, pesticides, or other contaminants (Fauzi et al. 2017). The method has been effectively used in a number of food matrices in recent years. As an illustration, a study on fish meal revealed the use of a pyrolysis GC–MS setup with a mass accuracy of 1 ppm and spiked levels of 2.7 g and 2.5 g of polystyrene and polymethylmethacrylate in the sample, respectively (Logemann et al. 2018). The GC–MS equipment also produced a good linear response when recognizing 0.05 g to 50 g of plastic by weight. Another study used pyrolysis GC–MS and transmission electron microscopy (TEM) to show the presence of microplastics in soils grown with wheat (Watteau et al. 2018). The results showed that after pyrolysis treatment at 650 °C with 30 s holding time, microplastics of sizes less than 200 m combined with organic materials were present.
Other than polymers, organo-mineral particles such as titanium, barium, and others were also found. Similar research was done to investigate the possibility of pyrolysis GC–MS and the presence of polystyrene microparticles in mussel tissues at 500 °C (Fabbri et al. 2020). The sensitivity examination revealed bioaccumulation of polystyrene particles in the digestive glands as well as traces of chitin and proteins. A combination of high-performance liquid chromatography (HPLC) and MS has also been used to develop methods for evaluating microplastics in cat and dog food (Zhang et al. 2019b). The study aimed to comprehend the potential hazards of cats and dogs ingesting microplastics. The research found that the range of cat and dog food with the maximum PET detection was 1500 ng/g to 12,000 ng/g after examining 58 pet food samples. According to conclusive correlations, other monomers such as bisphenol-A, polycarbonate, and terephthalic acid were successfully detected using HPLC–MS. The authors of the aforementioned research noted a significant difficulty in quantifying microplastics with regard to particle size, color, and shape. The detection is also in the millimeter range, which results in low efficiencies and resolutions (Primpke et al. 2020b). Although the method is affordable and capable of analyzing complicated matrices, it might be an alternative for quantifying microplastic fragments because it may react with complex sample tissues at a specific temperature, making microplastic identification difficult.
Health impact of microplastic-contaminated foods
The proliferation and adoption of plastics for various uses in daily human endeavors have resulted in a significant increase in microplasticss in the environment (Çıtar Dazıroğlu and Bilici 2023). As already discussed, these microplastics are highly incident and has entered the human food chain through various routes including food packaging, seafood, agricultural produce and food additives (Benson et al. 2022; Alberghini et al. 2022; Çıtar Dazıroğlu and Bilici 2023). The health implication of microplastics pollution may not be limited to food intake, but also from colonizing microorganisms and sorbed contaminants (Udovicki et al. 2022). Although the presence of microplastics in the human food chain has been established, the health implications is understudied, although health risks are enormous (He et al. 2021a, b). Studies show that the ubiquity of microplastics ensure steady inundation of food materials from source to the final consumption (Fig. 3) (He et al. 2021a, b); suggesting a steady increase in the microplastics content in food. This continued contamination of food materials has resulted in variable microplastics content, thus different contributions to the microplastics burden in humans. For example, the microplastics content of salt and the average daily intake suggests a burden range between 0 and 7.3 × 104 MP/person/year (Renzi and Blašković 2018; Wu et al. 2022).
Microplastic ingestion by humans induces severe health implications mainly due to bioavailability and biopersistence. Metabolism is followed by adsorption into the circulatory system and subsequent translocation to various parts. It is the properties of the microplastics that lead to persistence as they are not very degradable, leading to various health implications. Necessary steps need to be taken to remediate this occurrence
Similarly, risk assessment on the microplastics load from seafood shows a global average intake ranging between 11 to 3.5 × 104 MP/year (Hantoro et al. 2019) and about 11,000 microplastics/year in countries of low consumption (Van Cauwenberghe and Janssen 2014). The presence of microplastics in edible parts including muscles at concentrations higher than found in the gut (Karami et al. 2017a; Hantoro et al. 2019) totally cancel this respite. This suggests high bioavailability of microplastics which is an underlying factor to contaminant toxicity (Wu et al. 2022; Okeke et al. 2023b). Further, upon entry into humans, by ingestion, dermal contact or inhalation, these contaminants enter the metabolic pathways, are distributed to other parts and are excreted via urine and stool. Microplastics was detected in the muscles of all sampled fish, with higher concentrations in the benthic compared to pelagic species, posing a serious health risk to consumers (Akhbarizadeh et al. 2018). Similarly, various microplastics have been detected in the gastrointestinal tracts of fish (Cyprinus carpio) with impact on scale morphology and genetics, presenting health implications to the fish and human consumers (Saad et al. 2022). Microplastics have been detected in the liver, blood cells and brain of Oryzias latipes, with the detection in brain proving that microplastics can cross the tough, protective blood–brain barrier; posing significant danger. Overall, seafood including fish and shellfish from oceans has been identified as the most important microplastic source humans (Çıtar Dazıroğlu and Bilici 2023).
There is a lot of premium on increased water intake, drinking up to 2 L of water/day presents many health benefits (Nakamura et al. 2020). But juxtaposed with the health risks due to microplastic contamination, increased water intake may pose a greater health risk. With about 103 microplastics/year available from tap water and more in bottle water, increase water intake appears to present severe health concerns (He et al. 2021b). An average human may drink up to 4.1 × 104 microplastics from drinking water yearly (Wu et al. 2022). Although these risk assessment figures offer an idea of the possible risk faced by humans due to consumption of food contaminated with microplastics, there is need for more reliable and detailed methods of analysis. These methods will go beyond estimation and could capture more microplastics to offer the true information of health impacts of microplastics. Further, information on the availability, metabolism and egestion of microplastics will enable the elucidation of the public health risks.
Obviously, microplastics are incident in food at appreciable levels and present respiratory, reproductive, neurotoxic diseases as well as cancer and disruptions to microbiota (Çıtar Dazıroğlu and Bilici 2023). (Kashiwada 2006). The effects of microplastics on human health remain poorly elucidated, although the presence of this toxicant has been reported in liver, spleen, lungs, heart, kidneys and brain of lower animals with evidence of vector functions from lower animals into humans (Çıtar Dazıroğlu and Bilici 2023; Ziani et al. 2023). The toxic impacts of microplastics in humans have been linked to the translocation and accumulation of microplastics in humans (He et al. 2021b). Ecotoxicity studies on fish has shown that microplastics can enter the blood circulation following adsorption from the gut and can then be translocated to other sites of toxic actions (Guerrera et al. 2021). This translocation made worse by biopersistence of microplastics, cause apoptosis, necrosis, oxidative stress, genotoxicity and inflammation, which could be made worse due to their surface area to volume ratio leading to carcinogenesis (Çıtar Dazıroğlu and Bilici 2023). The contributions of microplastics to immune disruption, neurotoxicity, neoplasm are due to inflammation, particle toxicity and oxidative stress (Prata et al. 2020).
Various mechanisms are involved in the impacts of microplastics to health. For example, the disruption of immune function and carcinogenicity could involve the upregulation of immunity related genes or reduced expression of immune factors (Çıtar Dazıroğlu and Bilici 2023). Specifically, polyethylene toxicity has been linked to decreased levels of CD4-, T helper- and regulatory T- cells, worsened by intestinal inflammation (Li et al. 2020a). Further, Li and others (Li et al. 2020a) highlight that this PE-induced inflammation led to dysbiosis, with marked increase in abundance of Staphylococcus and decrease in Parabacteroides species plus a corresponding increase in interleukin. Immune cytokines, such as interleukin, was previously reported in response to inflammations even in humans (Nishimoto et al. 2008). In a study on the effect of polystyrene on the epithelial A549 epithelial cells of the human lungs, the easy internalization of microplastics was confirmed to influence apoptosis and cell cycle to eventually disrupt gene transcription and protein expression (Xu et al. 2019b). Further, the consistent exposure to microplastics has been implicated for carcinogenesis due to the increased expression of oncogenes, like asialoglycoprotein receptor 2 (Çıtar Dazıroğlu and Bilici 2023). Neurotoxicity due to exposure to microplastics is the result of their access to the systemic circulation and ability to cross the blood brain barrier to induce oxidative stress (Çıtar Dazıroğlu and Bilici 2023). As the brain is most sensitive to oxidative stress (Dutta and Bandopadhyay 2022), this affects inflammations and consequent diseases. Apart from inflammation due to oxidative stress, microplastics may inhibit acetylcholinesterase expression and change neurotransmitter levels to cause behavioral changes (Prüst et al. 2020). In the long run, the proliferation of plastic materials needs to be downregulated considering the enormous public health consequences. Human exposure to microplastic may cause various diseases including inflammatory, liver, neuro, lung diseases and metabolic imbalance (Prata 2018; Prata et al. 2020; Yan et al. 2022). Further, microplastics release different harmful chemicals such as bisphenol-A, poly aromatic hydrocarbons, chlorinated pesticides, could lead to dysbiosis and other physiological alterations leading to mutation and cancer (De-la-Torre 2020). The need for more studies on these health impacts may need to be more accurate and human-based to provide details on the actual risk.
Impact of microplastic contamination on the global food security
Microplastics particles originating from different sources and having various chemical compositions, shapes and sizes (macro, meso, micro- and nanoparticles) are abundant in the environment (Duis and Coors 2016; Crossman et al. 2020; Wahl et al. 2021; Kuttykattil et al. 2023; Métais et al. 2023; Jiang et al. 2023; Myszka et al. 2023; Chandrakanthan et al. 2023; Rasmussen et al. 2023). In the year 2018, global plastic pollution reached around 380 million tons per year which is predicted to triple by 2050. Mostly these plastic particles originate from various inland sources including domestic and industrial sewage, storm sewers, and runoff from agricultural fields having plastic mulching or treated with sewage sludge and wastewater treatment effluents. The use of polystyrene and polyurethane compost to increase crop productivity and subsequently add to the Microplastics pollution was recently reviewed (Hurley and Nizzetto 2018). This rapidly increasing use of plastic along with paucity proper disposal strategies has resulted in huge waste generation in oceans and land. Microplastic particles are generated by the slow fragmentation of plastic waste as well as from beads and pellets present in personal care products (Wang et al. 2020). These minute particles easily diffuse into different food sources and enter the food chain.
Microplastics are reported to extensively widespread in seafood, fishes such as tuna, sardines, mackerel, haddock, and plaice as well as oysters, mussels, cockles, squids, clams, urchins, cockles, periwinkles, prawns, shrimps and crabs (Markic et al. 2018; Walkinshaw et al. 2020; Ma et al. 2020; Neto et al. 2020; Wootton et al. 2021; Diaz-Basantes et al. 2022; Zitouni et al. 2022; Vitali et al. 2023). These mussels have an ability to thrive under diverse environmental parameters and being filter-feeding organisms, they can concentrate microplastic (Li et al. 2019). Microplastic particles ranging from 200 to 1500 µm were detected from tissue samples of commercially cultivated mussels such as Mytilus galloprovincialis and Mytilus edulis obtained from Belgian supermarkets (De Witte et al. 2014) where the quantity of total microplastics varied from 0.26 to 0.51 fibers/g of soft tissue. The existence of nearly 0.36 and 0.47 microplastic particles/g from Mytilus edulis and Pacific oysters produced in Atlantic Ocean and North Sea was reported (Van Cauwenberghe and Janssen 2014). Similarly, oysters and clams before depuration have also been reported to accumulate a wide amount of microplastics. The presence of microplastic particles in shrimps and crabs was recently demonstrated, these food items which are widely consumed seafood (Daniel et al. 2021). Similar reports of existence of microplastics in the size range of 19.97 to 4976.22 μm from crabs were reported by (Zhang et al. 2021).
The presence of 73 microplastics (48 fibers and 25 fragments) in 26 commercial fish species which consists of particle sizes ranging from 217 to 4810 µm from stomach contents of the commercial fishes available in Portugal (Neves et al. 2015). Another study by Phillips and Bonner (2015) showed the extensive presence of microplastic particles in fish from the Gulf of Mexico. The presence of microplastic particles, mostly polyethylene of size range around < 5 mm in both pelagic fish species such as mackerel, herring and also from demersal fish species such as cod, flounder and dab from Baltic and North Sea was studied (Rummel et al. 2017). In most cases, plastic contamination was more dominant in pelagic species than demersal species. Microplastics were reported in gastrointestinal tracks and dorsal muscles and gills of mackerel and seabass; these microplastics particle sizes ranged from 151 to 5000 μm (Barboza et al. 2020). Several experiments have also been conducted to focus on the transfer of microplastic from one trophic to another trophic level. A study conducted by Farrell and Nelson (2013) highlighted the transfer of microplastic between mussels and crabs. Moreover, fish meals are extensively used as poultry and pig feed which may result in the transfer of microplastic particles in non-marine food sources (Bouwmeester et al. 2015). The presence of microplastics is also reported in poultry products and eggs. In a study made by Huerta Lwanga et al. (2017), it was reported that chicken gizzards, intestinal mucosa and chicken-organs, digestive system showed presence of microplastics. The particle size was reported to vary from 1.5 to 150 μm (EFSA Panel on Contaminants in the Food Chain 2016). Apart from the animal source, microplastic of varying sizes has also been reported in apples, pears, broccoli, lettuce, wax, honey, larvae, corn flour, and carrots (Oliveri Conti et al. 2020; Alma et al. 2023; Shi et al. 2023; Pham et al. 2023). Microplastic contamination in vegetables occurs due to irrigation, plastic items present in covers which are degraded under ultraviolet light, polymers used in the microencapsulation of agrochemicals and the use of sewage as soil fertilizer (Milojevic and Cydzik-Kwiatkowska 2021; Kallenbach et al. 2022; Lwanga et al. 2022). In most cases, the exact mechanism associated with absorption, transportation and accumulation of microplastics from the soil to the aerial part of crop plants is still considered crucial and needs further research.
According to the studies by Li et al. (2021a) and Azeem et al. (2021), it was reported that microplastic of less than 100 nm was able to infiltrate roots and reach stems and leaves of crop plants; on the other hand, the bigger particles fail to penetrate cell wall and remains on the root’s surface. Microplastic of different surface charges and having a diameter of around 100 nm size can accumulate within the crop plants like lettuce and wheat mainly through cracks in the lateral roots (Sun et al. 2020b). In another study by Giorgetti et al. (2020), it was shown that microplastics of size 50 nm were present in the root cells of Allium cepa. A similar accumulation of 100–700 nm polystyrene microplastics was also reported in the cucumber plant by Li et al. (2021c). These accumulations of microplastic and microplastic in plants significantly alter porosity, hydraulic conductivity, bulk density, field capacity, soil water repellency and rhizospheric bacterial communities of crop plants (Qi et al. 2020). The presence of microplastic contamination in nori seaweed widely cultivated in ocean was reported (Li et al. 2020c). A detailed account of microplastic in the food items is enlisted in Table 5.
Remediation of microplastic pollution
The issue of microplastic pollution has caused numerous environmental and human health hazards resulting to global threat to the society. A set of biobased and technologically driven ecofriendly strategies are necessary to address the possible effects of microplastics on global food security.
Microbial enzymes-mediated bioremediation
The circular economy for plastic waste could be achieved via microbial enzyme technology. Because they can produce enzymes for using plastic as a source of energy, making microbes the most ideal for reducing environmental plastic pollution. Till date, potential microbial degraders of plastics have been reported in seven out of the twelve microbial phyla (Gambarini et al. 2022). Further, synthetic plastics can be degraded by microbial enzymes, including lipases, hydrolases, laccases, and peroxidases. Most fungal enzymes present a machinery integrated for depolymerizing and mineralizing plastic (Zhu et al. 2016). The use of microbial enzymes for plastic degradation is preferred for improve stability when compared to their equivalents from animals and plants. For example, Ideonella sakaiensis 201-F6 is a well-known illustration of a bacterial strain that can degrade polyethylene terephthalate (PET). The most widely produced synthetic polymer, PET, is produced annually in excess of 50 million tonnes worldwide (Bornscheuer 2016). Strain 201-F6 produced the serine hydrolases known as IsPETase and IsMHETase, which resemble cutinases (da Costa et al. 2021).
PET breakdown proceeds in two steps; in the first step, an IsPETase causes a nick to form in the PET polymer chain, producing PET chains with hydroxyethyl (HE)- and terephthalic acid (TPA) ends. Later, bis- and mono-(2-hydroxyethyl) terephthalic acid (BHET) and mono-(2-hydroxyethyl) terephthalic acid (MHET) are produced from the two PET chains with those termini (da Costa et al. 2021). Terephthalic acid (TPA) and ethylene glycol (EG) were produced as a result of the subsequent digestion of these compounds (Knott et al. 2020). EG and TPA are converted into water and carbon dioxide through absorption and mineralization, respectively (da Costa et al. 2021). Further, modified/hydrolase fold with a hydrophobic active domain and a lid site was visible in the 3D structure of MGS0156. However, due to its exceptional enzymatic activity against PET, IsPETase has undergone structural changes using a number of biotechnological techniques. It has also been demonstrated that a number of cutinases degrade PET. Cutinases have been discovered in fungus like Fusarium solani pisi and bacteria like Thermobifida fusca (Stavila et al. 2013). Cutinases from both groups belong to the ß-hydrolase superfamily and have comparable spatial configurations. As PET degraders, Thermomonospora and Thermobifida genera have been identified (Ru et al. 2020). PET can be effectively broken down by the catalytic trio of MHETase, tannase, and PET hydrolase (Taniguchi et al. 2019; Bhatt et al. 2021). According to structural studies, these enzymes feature disulfide linkages and/or folds that give them thermal stability. The fungi phyla Humicola and Fusarium have been studied for their cutinases, which degrade PET. The role of PET hydrolases in degradation was investigated in a bioinformatics-based work. The study reported over 800 PET hydrolases from bacteria and archaea from both marine and terrestrial habitats (Danso et al. 2018).
Specific enzymes from different strains have been reported to degrade PETs. For instance, cutinase from Pseudomonas pelagia (De Jesus and Alkendi 2022) and P. pertucinogena (Bollinger et al. 2020) degrades polyester. The genome-based esterases MGS0156 and GEN0105 hydrolyzes polycaprolactone, bis(benzoyloxyethyl)-terephthalate and polylactic acid (Bhatt et al. 2021). Some listed PET-degrading enzymes include TfH from Thermobifida fusca, HiC from Humicola insolens, lsPETase from Ideonell asakaiensis, Tfcut2 from Thermobifida fusca, Cut190 from Saccharomonospora viridis, PsC from Pseudomonas mendocina, PmC, and LC-cutinase from Fusarium solani (Wei et al. 2019). Polyisocyanates and polyols are condensed to produce polyurethane (PU), which is then linked to urethane linkages (Bhatt et al. 2021).
Recent research has shown that biotechnological methods improve the structural stability of enzymes. Protein engineering usually uses a structural-based modeling approach to create enzyme variations with improved enzyme performance and properties that are thermally stable. An IsPETaseS121E/D186H/S242T/N246D strain with improved thermostability and substrate binding efficiency (Son et al. 2020; Meng et al. 2021). When compared to wild-type TfCut2, the mutant T. fusca thermally stable cutinase (TfCut2) had a hydrolyzing action that was 12.7 times better (Furukawa et al. 2019). Therefore, MP bioremediation may be made more effective by the development of a better and more effective microbial strain by site-directed mutagenesis. To create biofuels from microalgae, significant biotechnological research is now being conducted. Additionally, the microalgae's potential for bioremediation has been mentioned in a number of studies. On a diatom and a green alga, numerous functional expression studies have been carried out (Moog et al. 2019; Furukawa et al. 2019; Meng et al. 2021). The ability to clone and express intracellular lipases was discovered by Mohanan et al. in a study (Mohanan et al. 2022). These lipases effectively degraded short- and medium-chain polymers, suggesting a viable bioremediation approach for the biodegradation of microplastics. Nanotechnology and other cutting-edge techniques, such as enzyme immobilization, have begun to spark attention for potential uses to stop microplastics in the near future. The site-directed immobilization method of PETase on magnetic nanoparticles has suggested a potential method for decreasing microplastic (Schwaminger et al. 2021). It is important to pay close attention to how various microbial networks produce enzymes and how microbial associations cooperate with one another. It is expected that further research in this area will quickly reveal useful techniques for biodegradation that can be employed on a bigger scale because microorganisms have almost endless potential and are continually adapting to their surroundings. A detailed account of a few microbes associated with Microplastics particle degradation is enlisted in Table 6. All these bacteria and associated enzymes can be used as a suitable biotechnological approach for the degrade of microplastics particles.
Biofilm-mediated remediation
When microorganisms are introduced to an aqueous environment, they quickly colonize the surface to create a permanent biofilm (Okeke et al. 2022c). Certain bacteria in biofilms have the ability to degrade organic pollutants and promote the adhesion of pollutants by microplastics (Rummel et al. 2017). The biological breakdown of the microplastics may be caused by the interaction of microplastics with biofilms, which may alter the chemical and physical properties of the polymer surface. The sorption of heavy metals onto plastic surfaces, the relationship between biofilms and toxins and the impact of biofilm establishment on microplastics were the main topics of early research into microplastics and biofilms (Wu et al. 2021). Research on how biofilms affect the ecologically friendly biodegradation of microplastics has just recently started. On polyethylene (PE) surfaces, Rhodococcus ruber has been demonstrated to colonize and create biofilms (Hadad et al. 2005). The usual molecular mass of the PE samples was reduced to 14% and 21%, respectively (Hadad et al. 2005). Thus, research into whether biofilms' development could alter the microplastics' physicochemical characteristics started (Ganesan et al. 2022) considerable surface degradation of microplastics treated with biofilms has also been observed in an environment with a considerable amount of methane gas; this can support the rise in bacterial aggregation (Faheem et al. 2020). The only byproducts of microplastic degradation that are still present are water and carbon dioxide, neither of which harm the ecosystem (Faheem et al. 2020). In an environment with high levels of methane gas, which may promote the establishment of bacterial aggregation, significant surface degradation of microplastics treated with biofilms has also been observed (Faheem et al. 2020).
Biofilms are abundant and easily accessible in the natural world (Faheem et al. 2020). Scientists have also predicted that biofilms, which act as transporters and may increase the adsorption of microplastics to pollutants in the ecosystem, will amplify the ecological danger posed by microplastics in the environment (Richard et al. 2019; Wang et al. 2021a; Stabnikova et al. 2022). Microplastics are discovered to break down more quickly than natural biofilms when glucose is employed as an external carbon source (Shabbir et al. 2020). While acting as carriers and increasing the ecological risk of microplastics in the ecosystem, other studies have proposed that biofilms can promote the adsorption of microplastics to environmental pollutants (Wang et al. 2021a). In one study, it was discovered that as soon as microplastics are introduced to an aqueous environment, bacteria immediately colonize their surfaces and create biofilms that promote the adsorption of the microplastics to environmental pollutants (Chen et al. 2020). However, the issue can be largely resolved if the biofilm is produced and formed previously under controlled conditions before being treated with microplastics. It is possible to adsorb additional environmental pollutants in addition to breaking down microplastics in the aqueous environment by integrating the technology of biofilm degradation of microplastics into the source treatment of microplastics or applying it to in situ cleanup of microplastics in freshwater sources. Using biofilms to break down microplastics could represent a comparatively environmentally friendly strategy (Faheem et al. 2020).
The two main types of biofilm culture methods used nowadays are laboratory cultivation and in situ cultivation. In situ cultures are frequently used when examining how microplastics behave in the environment after attaching to biofilms. Laboratory cultures have been employed in various research on environmental behavior and for the assessment of wastewater treatment systems for microplastic biofilm breakdown. By removing epiphytes from natural water sources and putting them in a lab, biofilms can be created artificially using these resources. After biofilms or cultures had formed, microplastics were added, and their breakdown was studied (Faheem et al. 2020). The biofilm's flora might not, however, be the same as the in situ culture. In order to control the amount and capacity of biofilm growth to reduce microplastic contamination, a laboratory culture can dramatically shorten the culture period and incorporate external variables. Cultivation in a laboratory is the term used to describe this process. External stimuli can be added to control the rate and precision of biofilm growth in a laboratory setting, but the culture time can also be drastically shortened. The type of plastic polymers and their physicochemical characteristics, such as pH, salinity, temperature, and UV light, are a few of the various factors influencing the growth of biofilm on microplastic surfaces (Faheem et al. 2020). An earlier investigation discovered that a 20% maximum degradation was possible. The fundamental cause of this is that microorganisms require some time to alter the molecular weight, structural stability, surface area, and hydrophobicity of microplastics, all of which are intrinsic features. The biodegradation of microplastics also involves a number of steps, none of which may occur simultaneously. The hydrophobicity and roughness of microplastics' surfaces change as they age. This process initiates the destruction of microplastic biofilms, which is then followed by additional processes. During the following extremely slow biodegradation phase, organisms physically break down plastics by chopping, grinding, or digesting the trash (Faheem et al. 2020). As a result, the entire deterioration process is drawn out. The rate and kind of biofilm growth on the surfaces of microplastics is another factor that, in some ways, also determines how quickly such materials degrade. The level of degradation is still insufficient, despite the possibility of using biofilms to degrade microplastics.
In summary, microplastics contamination has the potential to negatively impact global food security. The accumulation of these particles in the environment can lead to contamination of food items, including crops, seafood, and meat, which can have negative impacts on human health. To address this issue, environmentally friendly and advanced biotechnological approaches and management strategies are needed. By developing innovative solutions for plastic waste management, filtration systems, and biodegradable packaging materials, we can reduce the accumulation of microplastics in the environment and ensure the safety and security of our food supply.
Perspective
Regular release of microplastics particles in the environment is causing huge pollution in water bodies and soil, and there is an urgent requirement for mitigation and management of the plastic waste. Both Physicochemical and microbiological methods can be employed to remove plastic and microplastic debris. However, the problem associated with biological treatment of microplastics particles is it requires specific nutrient source, temperature and pH. Usually combined remediation technologies, circular economy and waste management can be used to reduce Microplastics particles from the environment. Also, there is an urgent need for research on molecular bioremediation techniques which may mitigate microplastics from contaminated environment.
There are different detection, quantification and characterization techniques available which are classified into physical separation, enzymatic decomposition, and chemical both specific and non-specific methods such as chromatography/mass spectrometry, field emission scanning electron microscope, Raman spectrometer, FTIR spectroscopy, X-ray fluorescence, microbalance for Microplastics in the food packaging and stuff.
Researchers used enzymes such as proteinase-K, lipase, cellulase, and chitinase for decomposition. FTIR spectroscopy and Raman spectroscopy are the simplest methods for the characterization of Microplastics in different environmental samples. The SEM hyphenated with Energy-dispersive Atomic X-ray technique gave the elemental composition of polyvinyl chloride particles in fish guts. AFM hyphenated with FTIR good techniques found in the literature for detection of Microplastics in mussel siphons and tap water samples. Micro-FTIR hyphenated with quantum cascade laser for detection of microplastics whose size has < 30 mm.
Based on the literature survey, GC with electron capture detector or GC–MS, X-ray fluorescence or SEM with energy-dispersive X-ray spectroscopy will have the more effective characteristic of microplastics in the food samples. Further, some of the metals accumulated on the microplastics surface in the food samples can be quantified through inductively coupled plasma-MS or atomic absorption spectroscopy.
Conclusion
The enormous increase in plastic production and use has occasion massive anthropogenic plastic pollution in water and soil. This is exacerbated by improper disposal, lack of infrastructure and proper management in most parts of the world. Here, we discuss the potential applications of biotechnological approaches for preventing microplastics contamination of food items, including the use of enzymes and microorganisms to degrade plastic particles. We also highlight the need for increased collaboration and coordination among researchers, policymakers, and industry stakeholders to effectively address the issue of microplastics contamination. In summary, the sources and pathways of microplastics contamination in the food chain are complex and multifaceted, and require further investigation in order to develop effective strategies for mitigating their effects. The subsequent degradation and weathering of larger plastics generate microplastic particles which can enter the food web. Various studies reviewed report the presence of microplastics particles in sea foods like mussels, oyster, fish shrimp, poultry products, vegetables, fruits, honey, salt and water. Overall, these studies implicate seafood and water, especially in plastic bottles for the highest incidence of microplastics in food. Interestingly, food additives such as salt and honey possess an appreciable level of these toxicants. Although the presence in honey needs further elucidation, the high incidence in salt was linked to the original seawater sources of salt, considering the irresponsible disposal of plastic wastes into the sea. Of great concern is the enormous potential health impacts of microplastics on public health, worsened by the near absence of studies to elucidate this aspect. However, ecotoxicity studies have shown that exposure to microplastics could result in many human diseases including reproductive, respiratory and circulatory disorders and even cancers. The presence of precise methods for the quantification of microplastics in food offers some starting points into further studies in this area. Further, ecofriendly methods to reduce the environmental pollution of microplastic are essential in nipping this menace at the bud while further research continues to suggest mitigations including introduction of alternative materials.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Abbasi S, Moore F, Keshavarzi B et al (2020) PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ 744:140984. https://doi.org/10.1016/J.SCITOTENV.2020.140984
Abidli S, Lahbib Y, Trigui El Menif N (2019) Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar Pollut Bull 142:243–252. https://doi.org/10.1016/j.marpolbul.2019.03.048
Adıgüzel AO, Tunçer M (2017) Purification and characterization of cutinase from Bacillus sp. KY0701 isolated from plastic wastes. Prep Biochem Biotechnol 47:925–933. https://doi.org/10.1080/10826068.2017.1365245
Afrin S, Rahman MM, Hossain MN et al (2022) Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci Total Environ 837:155849. https://doi.org/10.1016/J.SCITOTENV.2022.155849
Akan OD, Udofia GE, Okeke ES et al (2021) Plastic waste: Status, degradation and microbial management options for Africa. J Environ Manag 292:112758. https://doi.org/10.1016/j.jenvman.2021.112758
Akhbarizadeh R, Moore F, Keshavarzi B (2018) Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163. https://doi.org/10.1016/j.envpol.2017.09.028
Akhbarizadeh R, Dobaradaran S, Nabipour I et al (2020) Abundance, composition, and potential intake of microplastics in canned fish. Mar Pollut Bull 160:111633. https://doi.org/10.1016/j.marpolbul.2020.111633
Akoueson F, Sheldon LM, Danopoulos E et al (2020) A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ Pollut 263:114452. https://doi.org/10.1016/j.envpol.2020.114452
Al Mamun A, Prasetya TAE, Dewi IR, Ahmad M (2023) Microplastics in human food chains: Food becoming a threat to health safety. Sci Total Environ 858:159834. https://doi.org/10.1016/j.scitotenv.2022.159834
Alberghini L, Truant A, Santonicola S et al (2022) Microplastics in fish and fishery products and risks for human health: a review. Int J Environ Res Public Heal 20:789. https://doi.org/10.3390/IJERPH20010789
Ali W, Ali H, Souissi S, Zinck P (2023) Are bioplastics an ecofriendly alternative to fossil fuel plastics? Environ Chem Lett 21:1991–2002. https://doi.org/10.1007/S10311-023-01601-6/FIGURES/3
Alma AM, de Groot GS, Buteler M (2023) Microplastics incorporated by honeybees from food are transferred to honey, wax and larvae. Environ Pollut 320:121078. https://doi.org/10.1016/j.envpol.2023.121078
Anand U, Dey S, Bontempi E et al (2023) (2023) Biotechnological methods to remove microplastics: a review. Environ Chem Lett 213(21):1787–1810. https://doi.org/10.1007/S10311-022-01552-4
Anderson PJ, Warrack S, Langen V et al (2017) Microplastic contamination in Lake Winnipeg, Canada. Environ Pollut 225:223–231. https://doi.org/10.1016/J.ENVPOL.2017.02.072
Asif MB, Hai FI, Singh L et al (2017) Degradation of pharmaceuticals and personal care products by white-rot fungi—a critical review. Curr Pollut Reports 3:88–103. https://doi.org/10.1007/s40726-017-0049-5
Auguet T, Bertran L, Barrientos-Riosalido A et al (2022) Are ingested or inhaled microplastics involved in nonalcoholic fatty liver disease? Int J Environ Res Public Health. https://doi.org/10.3390/ijerph192013495
Auta HS, Emenike CU, Fauziah SH (2017) Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559. https://doi.org/10.1016/j.envpol.2017.09.043
Auta HS, Emenike CU, Jayanthi B, Fauziah SH (2018) Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar Pollut Bull 127:15–21. https://doi.org/10.1016/j.marpolbul.2017.11.036
Avio CG, Pittura L, D’Errico G et al (2020) Distribution and characterization of microplastic particles and textile microfibers in Adriatic food webs: general insights for biomonitoring strategies. Environ Pollut 258:113766. https://doi.org/10.1016/j.envpol.2019.113766
Azeem I, Adeel M, Ahmad MA et al (2021) Uptake and accumulation of nano/microplastics in plants: a critical review. Nanomaterials 11:2935. https://doi.org/10.3390/nano11112935
Barboza LGA, Lopes C, Oliveira P et al (2020) Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ 717:134625. https://doi.org/10.1016/j.scitotenv.2019.134625
Bardají DKR, Furlan JPR, Stehling EG (2019) Isolation of a polyethylene degrading Paenibacillus sp. from a landfill in Brazil. Arch Microbiol 201:699–704. https://doi.org/10.1007/s00203-019-01637-9
Benson NU, Agboola OD, Fred-Ahmadu OH et al (2022) Micro(nano)plastics prevalence, food web interactions, and toxicity assessment in aquatic organisms: a review. Front Mar Sci. https://doi.org/10.3389/fmars.2022.851281
Bhatt P, Pathak VM, Bagheri AR, Bilal M (2021) Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ Res 200:111762. https://doi.org/10.1016/J.ENVRES.2021.111762
Birnstiel S, Soares-Gomes A, da Gama BAP (2019) Depuration reduces microplastic content in wild and farmed mussels. Mar Pollut Bull 140:241–247. https://doi.org/10.1016/j.marpolbul.2019.01.044
Bollinger A, Thies S, Knieps-Grünhagen E et al (2020) A novel polyester hydrolase from the marine bacterium pseudomonas aestusnigri—structural and functional insights. Front Microbiol 11:510772. https://doi.org/10.3389/FMICB.2020.00114/BIBTEX
Bornscheuer UT (2016) Feeding on plastic. Science (80- ) 351:1154–1155. https://doi.org/10.1126/SCIENCE.AAF2853
Bour A, Avio CG, Gorbi S et al (2018) Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ Pollut 243:1217–1225. https://doi.org/10.1016/j.envpol.2018.09.115
Bouwmeester H, Hollman PCH, Peters RJB (2015) Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol 49:8932–8947. https://doi.org/10.1021/acs.est.5b01090
Brandon J, Goldstein M, Ohman MD (2016) Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar Pollut Bull 110:299–308. https://doi.org/10.1016/J.MARPOLBUL.2016.06.048
Bubpachat T, Sombatsompop N, Prapagdee B (2018) Isolation and role of polylactic acid-degrading bacteria on degrading enzymes productions and PLA biodegradability at mesophilic conditions. Polym Degrad Stab 152:75–85. https://doi.org/10.1016/j.polymdegradstab.2018.03.023
Carr Kinnear EJ, Miller KY, Tong AZ (2021) Impacts of brewing time, brewing temperature and brands on the leaching of phthalates and bisphenol A in dry tea. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 38:1755–1766. https://doi.org/10.1080/19440049.2021.1940307
Catarino AI, Thompson R, Sanderson W, Henry TB (2017) Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ Toxicol Chem 36:947–951. https://doi.org/10.1002/ETC.3608
Chandrakanthan K, Fraser MP, Herckes P (2023) Airborne microplastics in a suburban location in the desert southwest: occurrence and identification challenges. Atmos Environ 298:119617. https://doi.org/10.1016/j.atmosenv.2023.119617
Chen X, Chen X, Zhao Y et al (2020) Effects of microplastic biofilms on nutrient cycling in simulated freshwater systems. Sci Total Environ 719:137276. https://doi.org/10.1016/J.SCITOTENV.2020.137276
Chia RW, Lee JY, Kim H (2021) Jang J (2021) Microplastic pollution in soil and groundwater: a review. Environ Chem Lett 196(19):4211–4224. https://doi.org/10.1007/S10311-021-01297-6
Chia RW, Lee JY, Cha J, Rodríguez-Seijo A (2023) Methods of soil sampling for microplastic analysis: a review. Environ Chem Lett 1:1–12. https://doi.org/10.1007/S10311-023-01652-9/TABLES/2
Çıtar Dazıroğlu ME, Bilici S (2023) The hidden threat to food safety and human health: microplastics. Environ Dev Sustain. https://doi.org/10.1007/s10668-023-03565-7
Cole M, Webb H, Lindeque PK et al (2014) (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 41(4):1–8. https://doi.org/10.1038/srep04528
Courtene-Jones W, Quinn B, Murphy F et al (2017) Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal Methods 9:1437–1445. https://doi.org/10.1039/C6AY02343F
Crossman J, Hurley RR, Futter M, Nizzetto L (2020) Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci Total Environ 724:138334. https://doi.org/10.1016/j.scitotenv.2020.138334
Cverenkárová K, Valachovičová M, Mackul’ak T et al (2021) Microplastics in the food chain. Life 11:1349. https://doi.org/10.3390/LIFE11121349
De Boer J, Garrigues P, Gu J-D et al (2011) The handbook of environmental chemistry
da Costa CHS, dos Santos AM, Alves CN et al (2021) Assessment of the PETase conformational changes induced by poly(ethylene terephthalate) binding. Proteins Struct Funct Bioinforma 89:1340–1352. https://doi.org/10.1002/PROT.26155
Dalmau-Soler J, Ballesteros-Cano R, Boleda MR et al (2021) Microplastics from headwaters to tap water: occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain). Environ Sci Pollut Res Int 28:59462–59472. https://doi.org/10.1007/s11356-021-13220-1
Daniel DB, Ashraf PM, Thomas SN (2020) Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala. India Sci Total Environ 737:139839. https://doi.org/10.1016/j.scitotenv.2020.139839
Daniel DB, Ashraf PM, Thomas SN, Thomson KT (2021) Microplastics in the edible tissues of shellfishes sold for human consumption. Chemosphere 264:128554. https://doi.org/10.1016/j.chemosphere.2020.128554
Danso D, Schmeisser C, Chow J et al (2018) New insights into the function and global distribution of polyethylene terephthalate (PET)-degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02773-17/SUPPL_FILE/ZAM008188431S1.PDF
De Jesus R, Alkendi R (2022) A minireview on the bioremediative potential of microbial enzymes as solution to emerging microplastic pollution. Front Microbiol 13:1066133. https://doi.org/10.3389/FMICB.2022.1066133/BIBTEX
De Witte B, Devriese L, Bekaert K et al (2014) Quality assessment of the blue mussel (Mytilus edulis): comparison between commercial and wild types. Mar Pollut Bull 85:146–155. https://doi.org/10.1016/j.marpolbul.2014.06.006
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256. https://doi.org/10.1016/J.ENVPOL.2013.11.019
De-la-Torre GE (2020) Microplastics: an emerging threat to food security and human health. J Food Sci Technol 57:1601–1608. https://doi.org/10.1007/s13197-019-04138-1
De-la-torre GE, De-la-torre GE (2020) Microplastics : an emerging threat to food security and human health. J Food Sci Technol 57:1601–1608. https://doi.org/10.1007/s13197-019-04138-1
Deme GG, Ewusi-Mensah D, Olagbaju OA et al (2022) Macro problems from microplastics: toward a sustainable policy framework for managing microplastic waste in Africa. Sci Total Environ 804:150170. https://doi.org/10.1016/j.scitotenv.2021.150170
Dessì C, Okoffo ED, O’Brien JW et al (2021) Plastics contamination of store-bought rice. J Hazard Mater 416:125778. https://doi.org/10.1016/J.JHAZMAT.2021.125778
Diaz-Basantes MF, Conesa JA, Fullana A (2020) Microplastics in honey, beer, milk and refreshments in ecuador as emerging contaminants. Sustain 12:5514. https://doi.org/10.3390/SU12145514
Diaz-Basantes MF, Nacimba-Aguirre D, Conesa JA, Fullana A (2022) Presence of microplastics in commercial canned tuna. Food Chem 385:132721. https://doi.org/10.1016/j.foodchem.2022.132721
Ding J, Li J, Sun C et al (2020) An examination of the occurrence and potential risks of microplastics across various shellfish. Sci Total Environ 739:139887. https://doi.org/10.1016/j.scitotenv.2020.139887
Du F, Cai H, Zhang Q et al (2020) Microplastics in take-out food containers. J Hazard Mater 399:122969. https://doi.org/10.1016/j.jhazmat.2020.122969
Duis K, Coors A (2016) Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur 28:1–25. https://doi.org/10.1186/S12302-015-0069-Y
Dutta B, Bandopadhyay R (2022) Biotechnological potentials of halophilic microorganisms and their impact on mankind. Beni-Suef Univ J Basic Appl Sci. https://doi.org/10.1186/s43088-022-00252-w
EFSA Panel on Contaminants in the Food Chain (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. https://doi.org/10.2903/j.efsa.2016.4501
Enechi OC, Okeke ES, Awoh OE et al (2021) Inhibition of phospholipase A2, platelet aggregation and egg albumin induced rat paw oedema as anti-inflammatory effect of Peltophorun pterocarpus stem-bark. Clin Phytosci. https://doi.org/10.1186/s40816-021-00310-3
Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA (2017) Lost, but found with nile red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ Sci Technol 51:13641–13648. https://doi.org/10.1021/ACS.EST.7B04512/SUPPL_FILE/ES7B04512_SI_001.PDF
Fabbri D, Rombolà AG, Vassura I et al (2020) Off-line analytical pyrolysis GC–MS to study the accumulation of polystyrene microparticles in exposed mussels. J Anal Appl Pyrolysis 149:104836. https://doi.org/10.1016/J.JAAP.2020.104836
Fadare OO, Okoffo ED, Olasehinde EF (2021) Microparticles and microplastics contamination in African table salts. Mar Pollut Bull 164:112006. https://doi.org/10.1016/j.marpolbul.2021.112006
Faheem M, Shabbir S, Zhao J et al (2020a) Multifunctional periphytic biofilms: polyethylene degradation and Cd2+ and Pb2+ bioremediation under high methane scenario. Int J Mol Sci 21:5331. https://doi.org/10.3390/IJMS21155331
Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis ( L.) to Carcinus maenas ( L.). Environ Pollut 177:1–3. https://doi.org/10.1016/j.envpol.2013.01.046
Fauzi A, Hameed IH, Kadhim MJ (2017) A review: uses of gas chromatography–mass spectrometry (GC–MS) technique for analysis of bioactive natural compounds of some plants forensic nursing view project biochemical analysis view project. Artic Int J Toxicol Pharmacol Res. https://doi.org/10.25258/ijtpr.v9i01.9042
Feng Z, Wang R, Zhang T et al (2020) Microplastics in specific tissues of wild sea urchins along the coastal areas of northern China. Sci Total Environ 728:138660. https://doi.org/10.1016/j.scitotenv.2020.138660
Ferraz M, Bauer AL, Valiati VH, Schulz UH (2020) Microplastic concentrations in raw and drinking water in the sinos river, southern brazil. Water (switzerland) 12:1–10. https://doi.org/10.3390/w12113115
Fries E, Dekiff JH, Willmeyer J et al (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15:1949–1956. https://doi.org/10.1039/C3EM00214D
Furukawa M, Kawakami N, Tomizawa A, Miyamoto K (2019) Efficient degradation of poly(ethylene terephthalate) with thermobifida fusca cutinase exhibiting improved catalytic activity generated using mutagenesis and additive-based approaches. Sci Rep 91(9):1–9. https://doi.org/10.1038/s41598-019-52379-z
Galafassi S, Sighicelli M, Pusceddu A et al (2021) Microplastic pollution in perch (Perca fluviatilis, Linnaeus 1758) from Italian south-alpine lakes. Environ Pollut 288:117782. https://doi.org/10.1016/j.envpol.2021.117782
Gambarini V, Pantos O, Kingsbury JM et al (2022) PlasticDB: a database of microorganisms and proteins linked to plastic biodegradation. Database 2022:1–12. https://doi.org/10.1093/DATABASE/BAAC008
Ganal-Vonarburg SC, Hornef MW, Macpherson AJ (2020) Microbial–host molecular exchange and its functional consequences in early mammalian life. Science (80- ) 368:604–607. https://doi.org/10.1126/SCIENCE.ABA0478/SUPPL_FILE/ABA0478_GANAL-VONARBURG_SM.PDF
Ganesan S, Ruendee T, Kimura SY et al (2022) Effect of biofilm formation on different types of plastic shopping bags: structural and physicochemical properties. Environ Res 206:112542. https://doi.org/10.1016/J.ENVRES.2021.112542
Giorgetti L, Spanò C, Muccifora S et al (2020) Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2020.02.014
Grbic J, Nguyen B, Guo E et al (2019) Magnetic extraction of microplastics from environmental samples. Environ Sci Technol Lett 6:68–72. https://doi.org/10.1021/ACS.ESTLETT.8B00671/SUPPL_FILE/EZ8B00671_SI_001.PDF
Guerrera MC, Aragona M, Porcino C et al (2021) Micro and nano plastics distribution in fish as model organisms: Histopathology, blood response and bioaccumulation in different organs. Appl Sci. https://doi.org/10.3390/app11135768
Guilhermino L, Martins A, Lopes C et al (2021) Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar Pollut Bull 173:113008. https://doi.org/10.1016/J.MARPOLBUL.2021.113008
Gündoğdu S (2018) Contamination of table salts from Turkey with microplastics. Food Addit Contam Part A 35:1006–1014. https://doi.org/10.1080/19440049.2018.1447694
Habib RZ, Al KR, Al SF et al (2022) Microplastic contamination of chicken meat and fish through plastic cutting boards. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph192013442
Hadad D, Geresh S, Sivan A (2005) Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol 98:1093–1100. https://doi.org/10.1111/J.1365-2672.2005.02553.X
Hantoro I, Löhr AJ, Van Belleghem FGAJ et al (2019) Microplastics in coastal areas and seafood: implications for food safety. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36:674–711. https://doi.org/10.1080/19440049.2019.1585581
He D, Zhang Y, Gao W (2021a) ScienceDirect Micro (nano) plastic contaminations from soils to plants: human food risks Curr Opin Food Sci 116–121. https://doi.org/10.1016/.COFS.2021.04.001
He D, Zhang Y, Gao W (2021b) Micro(nano)plastic contaminations from soils to plants: human food risks. Curr Opin Food Sci 41:116–121. https://doi.org/10.1016/j.cofs.2021.04.001
Henini M (2000) Scanning electron microscopy: an introduction. III-Vs Rev 13:40–44. https://doi.org/10.1016/S0961-1290(00)80006-X
Hermabessiere L, Paul-Pont I, Cassone AL et al (2019) Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ Pollut 250:807–819. https://doi.org/10.1016/J.ENVPOL.2019.04.051
Herrera A, Garrido-Amador P, Martínez I et al (2018) Novel methodology to isolate microplastics from vegetal-rich samples. Mar Pollut Bull 129:61–69. https://doi.org/10.1016/J.MARPOLBUL.2018.02.015
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075. https://doi.org/10.1021/ES2031505/ASSET/IMAGES/MEDIUM/ES-2011-031505_0006.GIF
Hidayaturrahman H, Lee TG (2019) A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar Pollut Bull 146:696–702. https://doi.org/10.1016/J.MARPOLBUL.2019.06.071
Horton AA, Svendsen C, Williams RJ et al (2017) Large microplastic particles in sediments of tributaries of the River Thames, UK—abundance, sources and methods for effective quantification. Mar Pollut Bull 114:218–226. https://doi.org/10.1016/J.MARPOLBUL.2016.09.004
Hossain MS, Rahman MS, Uddin MN et al (2020) Microplastic contamination in Penaeid shrimp from the Northern Bay of Bengal. Chemosphere 238:124688. https://doi.org/10.1016/j.chemosphere.2019.124688
Huang Z, Hu B, Wang H (2022) Analytical methods for microplastics in the environment: a review. Environ Chem Lett 211(21):383–401. https://doi.org/10.1007/S10311-022-01525-7
Huerta Lwanga E, Mendoza Vega J, Ku Quej V et al (2017) Field evidence for transfer of plastic debris along a terrestrial food chain. Sci Rep 7:14071. https://doi.org/10.1038/s41598-017-14588-2
Huerta Lwanga E, Thapa B, Yang X et al (2018) Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci Total Environ 624:753–757. https://doi.org/10.1016/j.scitotenv.2017.12.144
Hurley RR, Nizzetto L (2018) Fate and occurrence of micro(nano)plastics in soils: knowledge gaps and possible risks. Curr Opin Environ Sci Heal 1:6–11. https://doi.org/10.1016/j.coesh.2017.10.006
Imhof HK, Laforsch C, Wiesheu AC et al (2016) Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res 98:64–74. https://doi.org/10.1016/J.WATRES.2016.03.015
Jadhav EB, Singh M, Ahmad R, Bhagat DS (2021) Microplastics from food packaging: an overview of human consumption, health threats, and alternative solutions. Environ Nanotechnol Monit Manag 16:100608
Jain K, Bhunia H, Reddy MS (2022) Degradation of polypropylene-poly-l-lactide blends by Bacillus isolates: a microcosm and field evaluation. Bioremediat J 26:64–75. https://doi.org/10.1080/10889868.2021.1886037
Jeon J-M, Park S-J, Choi T-R et al (2021) Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym Degrad Stab 191:109662. https://doi.org/10.1016/j.polymdegradstab.2021.109662
Jiang X, Chen H, Liao Y et al (2019) Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ Pollut 250:831–838. https://doi.org/10.1016/J.ENVPOL.2019.04.055
Jiang J-J, Hanun JN, Chen K-Y et al (2023) Current levels and composition profiles of microplastics in irrigation water. Environ Pollut 318:120858. https://doi.org/10.1016/j.envpol.2022.120858
Johnson AC, Ball H, Cross R et al (2020) Identification and quantification of microplastics in potable water and their sources within water treatment works in England and Wales. Environ Sci Technol 54:12326–12334. https://doi.org/10.1021/ACS.EST.0C03211/ASSET/IMAGES/LARGE/ES0C03211_0005.JPEG
Jung H-W, Yang M-K, Su R-C (2018) Purification, characterization, and gene cloning of an Aspergillus fumigatus polyhydroxybutyrate depolymerase used for degradation of polyhydroxybutyrate, polyethylene succinate, and polybutylene succinate. Polym Degrad Stab 154:186–194. https://doi.org/10.1016/j.polymdegradstab.2018.06.002
Kallenbach EMF, Rødland ES, Buenaventura NT, Hurley R (2022) Microplastics in terrestrial and freshwater environments: Microplastic in the Environment: Pattern and Process 87–130. https://doi.org/10.1007/978-3-030-78627-4_4
Karami A, Golieskardi A, Bin HY et al (2017a) Microplastics in eviscerated flesh and excised organs of dried fish. Sci Rep 7:5473. https://doi.org/10.1038/s41598-017-05828-6
Karami A, Golieskardi A, Keong Choo C et al (2017b) The presence of microplastics in commercial salts from different countries. Sci Rep 71(7):1–11. https://doi.org/10.1038/srep46173
Karbalaei S, Golieskardi A, Watt DU et al (2020) Analysis and inorganic composition of microplastics in commercial Malaysian fish meals. Mar Pollut Bull 150:110687. https://doi.org/10.1016/J.MARPOLBUL.2019.110687
Karlsson TM, Vethaak AD, Almroth BC et al (2017) Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar Pollut Bull 122:403–408. https://doi.org/10.1016/J.MARPOLBUL.2017.06.081
Kashiwada S (2006) Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ Health Perspect 114:1697–1702. https://doi.org/10.1289/ehp.9209
Kedzierski M, Lechat B, Sire O et al (2020) Microplastic contamination of packaged meat: occurrence and associated risks. Food Packag Shelf Life 24:100489. https://doi.org/10.1016/j.fpsl.2020.100489
Keshavarzifard M, Vazirzadeh A, Sharifinia M (2021) Occurrence and characterization of microplastics in white shrimp, Metapenaeus affinis, living in a habitat highly affected by anthropogenic pressures, northwest Persian Gulf. Mar Pollut Bull 169:112581. https://doi.org/10.1016/j.marpolbul.2021.112581
Kim J-S, Lee H-J, Kim S-K, Kim H-J (2018) Global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ Sci Technol 52:12819–12828. https://doi.org/10.1021/acs.est.8b04180
Kirstein IV, Hensel F, Gomiero A et al (2021) Drinking plastics? Quantification and qualification of microplastics in drinking water distribution systems by µFTIR and Py-GCMS. Water Res 188:116519. https://doi.org/10.1016/j.watres.2020.116519
Knott BC, Erickson E, Allen MD et al (2020) Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc Natl Acad Sci USA 117:25476–25485. https://doi.org/10.1073/PNAS.2006753117/SUPPL_FILE/PNAS.2006753117.SM03.MP4
Kosuth M, Mason SA, Wattenberg EV (2018) Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 13:e0194970. https://doi.org/10.1371/JOURNAL.PONE.0194970
Kusch P, Knupp G (2004) Headspace-SPME-GC-MS identification of volatile organic compounds released from expanded polystyrene. J Polym Environ 12:83–87. https://doi.org/10.1023/B:JOOE.0000010053.20382.D7/METRICS
Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC (2020) Branded milks—are they immune from microplastics contamination? Sci Total Environ 714:136823. https://doi.org/10.1016/J.SCITOTENV.2020.136823
Kuttykattil A, Raju S, Vanka KS et al (2023) Consuming microplastics? Investigation of commercial salts as a source of microplastics (MPs) in diet. Environ Sci Pollut Res 30:930–942. https://doi.org/10.1007/s11356-022-22101-0
La Nasa J, Biale G, Fabbri D, Modugno F (2020) A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J Anal Appl Pyrolysis 149:104841. https://doi.org/10.1016/J.JAAP.2020.104841
Lee CW, Hsu LF, Wu IL et al (2022) Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J Hazard Mater 430:128431. https://doi.org/10.1016/J.JHAZMAT.2022.128431
Levin IW, Bhargava R (2004) Fourier transform infrared vibrational spectroscopic imaging: integrating microscopy and molecular recognition*. 56:429–474. https://doi.org/10.1146/ANNUREV.PHYSCHEM.56.092503.141205
Li J, Green C, Reynolds A et al (2018a) Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ Pollut 241:35–44. https://doi.org/10.1016/J.ENVPOL.2018.05.038
Li J, Liu H, Paul Chen J (2018b) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/J.WATRES.2017.12.056
Li J, Lusher AL, Rotchell JM et al (2019) Using mussel as a global bioindicator of coastal microplastic pollution. Environ Pollut 244:522–533. https://doi.org/10.1016/j.envpol.2018.10.032
Li B, Ding Y, Cheng X et al (2020a) Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 244:125492. https://doi.org/10.1016/j.chemosphere.2019.125492
Li D, Shi Y, Yang L et al (2020b) Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat Food 1:746–754. https://doi.org/10.1038/s43016-020-00171-y
Li Q, Feng Z, Zhang T et al (2020c) Microplastics in the commercial seaweed nori. J Hazard Mater 388:122060. https://doi.org/10.1016/j.jhazmat.2020.122060
Li C, Gao Y, He S et al (2021a) Quantification of nanoplastic uptake in cucumber plants by pyrolysis gas chromatography/mass spectrometry. Environ Sci Technol Lett 8:633–638. https://doi.org/10.1021/acs.estlett.1c00369
Li H, Lu X, Wang S et al (2021b) Vertical migration of microplastics along soil profile under different crop root systems. Environ Pollut 278:116833. https://doi.org/10.1016/j.envpol.2021.116833
Li Z, Li Q, Li R et al (2021c) The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ Sci Pollut Res Int 28:16042–16053. https://doi.org/10.1007/s11356-020-11702-2
Li Y, Wang Z, Guan B (2022) Separation and identification of nanoplastics in tap water. Environ Res 204:112134. https://doi.org/10.1016/j.envres.2021.112134
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89:213–217. https://doi.org/10.1007/S00128-012-0642-7/TABLES/1
Liu G, Wang J, Wang M et al (2022a) Disposable plastic materials release microplastics and harmful substances in hot water. Sci Total Environ 818:151685. https://doi.org/10.1016/j.scitotenv.2021.151685
Liu Z, Zhuan Q, Zhang L et al (2022b) Polystyrene microplastics induced female reproductive toxicity in mice. J Hazard Mater 424:127629. https://doi.org/10.1016/J.JHAZMAT.2021.127629
Löder MGJ, Gerdts G (2015) Methodology used for the detection and identification of microplastics—a critical appraisal. Mar Anthropog Litter. https://doi.org/10.1007/978-3-319-16510-3_8/FIGURES/7
Logemann J, Oveland E, Bjorøy Ø et al (2018) Pyrolysis-GC-Orbitrap MS—a powerful analytical tool for identification and quantification of microplastics in a biological matrix
Lopes C, Raimundo J, Caetano M, Garrido S (2020) Microplastic ingestion and diet composition of planktivorous fish. Limnol Oceanogr Lett 5:103–112. https://doi.org/10.1002/lol2.10144
Lu L, Luo T, Zhao Y et al (2019) Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci Total Environ 667:94–100. https://doi.org/10.1016/J.SCITOTENV.2019.02.380
Luissint AC, Parkos CA, Nusrat A (2016) Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151:616–632. https://doi.org/10.1053/J.GASTRO.2016.07.008
Luo Q, Liu ZH, Yin H et al (2018) Migration and potential risk of trace phthalates in bottled water: a global situation. Water Res 147:362–372. https://doi.org/10.1016/J.WATRES.2018.10.002
Lusher AL, Welden NA, Sobral P, Cole M (2020) Sampling, isolating and identifying microplastics ingested by fish and invertebrates*. Anal Nanoplast Microplast Food. https://doi.org/10.1201/9780429469596-8
Lwanga EH, Beriot N, Corradini F et al (2022) Review of microplastic sources, transport pathways and correlations with other soil stressors: a journey from agricultural sites into the environment. Chem Biol Technol Agric 9:20. https://doi.org/10.1186/s40538-021-00278-9
Ma J, Niu X, Zhang D et al (2020) High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou. China Sci Total Environ 744:140679. https://doi.org/10.1016/j.scitotenv.2020.140679
Makhdoumi P, Amin AA, Karimi H et al (2021) Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J Water Process Eng 39:101708. https://doi.org/10.1016/J.JWPE.2020.101708
Markic A, Niemand C, Bridson JH et al (2018) Double trouble in the South Pacific subtropical gyre: Increased plastic ingestion by fish in the oceanic accumulation zone. Mar Pollut Bull 136:547–564. https://doi.org/10.1016/j.marpolbul.2018.09.031
Martinelli JC, Phan S, Luscombe CK, Padilla-Gamiño JL (2020a) Low incidence of microplastic contaminants in Pacific oysters (Crassostrea gigas Thunberg) from the Salish Sea, USA. Sci Total Environ 715:136826. https://doi.org/10.1016/J.SCITOTENV.2020.136826
Mason SA, Garneau D, Sutton R et al (2016) Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ Pollut 218:1045–1054. https://doi.org/10.1016/J.ENVPOL.2016.08.056
Mason SA, Welch VG, Neratko J (2018) Synthetic polymer contamination in bottled water. Front Chem 6:389699. https://doi.org/10.3389/FCHEM.2018.00407/BIBTEX
Meng X, Yang L, Liu H et al (2021) Protein engineering of stable IsPETase for PET plastic degradation by Premuse. Int J Biol Macromol 180:667–676. https://doi.org/10.1016/J.IJBIOMAC.2021.03.058
Métais I, Latchere O, Roman C et al (2023) Continuum from microplastics to nanoplastics: effects of size and source on the estuarine bivalve Scrobicularia plana. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-023-25588-3
Miller JD, Young SL (2023) Water security and nutrition. In: Fourth E (ed) Caballero BBT-E of HN. Academic Press, Oxford, pp 706–716
Milojevic N, Cydzik-Kwiatkowska A (2021) Agricultural use of sewage sludge as a threat of microplastic (MP) spread in the environment and the role of governance. Energies 14:6293. https://doi.org/10.3390/en14196293
Mintenig SM, Löder MGJ, Primpke S, Gerdts G (2019) Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ 648:631–635. https://doi.org/10.1016/J.SCITOTENV.2018.08.178
Mohanan N, Wong CH, Budisa N, Levin DB (2022) Characterization of polymer degrading lipases, LIP1 and LIP2 from pseudomonas chlororaphis PA23. Front Bioeng Biotechnol 10:854298. https://doi.org/10.3389/FBIOE.2022.854298/BIBTEX
Moog D, Schmitt J, Senger J et al (2019) Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb Cell Fact 18:1–15. https://doi.org/10.1186/S12934-019-1220-Z/FIGURES/5
Muhib MI, Uddin MK, Rahman MM, Malafaia G (2023) Occurrence of microplastics in tap and bottled water, and food packaging: a narrative review on current knowledge. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.161274
Muroi F, Tachibana Y, Soulenthone P et al (2017) Characterization of a poly(butylene adipate-co -terephthalate) hydrolase from the aerobic mesophilic bacterium Bacillus pumilus. Polym Degrad Stab 137:11–22. https://doi.org/10.1016/j.polymdegradstab.2017.01.006
Myszka R, Enfrin M, Giustozzi F (2023) Microplastics in road dust: a practical guide for identification and characterisation. Chemosphere 315:137757. https://doi.org/10.1016/j.chemosphere.2023.137757
Nakamura Y, Watanabe H, Tanaka A et al (2020) Effect of increased daily water intake and hydration on health in japanese adults. Nutrients. https://doi.org/10.3390/nu12041191
Nalbone L, Cincotta F, Giarratana F et al (2021) Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control 125:108003. https://doi.org/10.1016/j.foodcont.2021.108003
Nematollahi MJ, Keshavarzi B, Moore F et al (2021) Microplastic fibers in the gut of highly consumed fish species from the southern Caspian Sea. Mar Pollut Bull 168:112461. https://doi.org/10.1016/J.MARPOLBUL.2021.112461
Neto JGB, Rodrigues FL, Ortega I et al (2020) Ingestion of plastic debris by commercially important marine fish in southeast-south Brazil. Environ Pollut 267:115508. https://doi.org/10.1016/j.envpol.2020.115508
Neves D, Sobral P, Ferreira JL, Pereira T (2015) Ingestion of microplastics by commercial fish off the Portuguese coast. Mar Pollut Bull 101:119–126. https://doi.org/10.1016/j.marpolbul.2015.11.008
Nguyen B, Claveau-Mallet D, Hernandez LM et al (2019a) Separation and Analysis of microplastics and nanoplastics in complex environmental samples. Acc Chem Res 52:858–866. https://doi.org/10.1021/ACS.ACCOUNTS.8B00602/ASSET/IMAGES/LARGE/AR-2018-00602E_0004.JPEG
Nishimoto N, Terao K, Mima T et al (2008) Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112:3959–3964. https://doi.org/10.1182/blood-2008-05-155846
Nithin A, Sundaramanickam A, Surya P et al (2021) Microplastic contamination in salt pans and commercial salts—a baseline study on the salt pans of Marakkanam and Parangipettai, Tamil Nadu. India Mar Pollut Bull 165:112101. https://doi.org/10.1016/j.marpolbul.2021.112101
Novotna K, Cermakova L, Pivokonska L et al (2019) Microplastics in drinking water treatment—current knowledge and research needs. Sci Total Environ 667:730–740. https://doi.org/10.1016/J.SCITOTENV.2019.02.431
Okeke ES, Atinuke O, Okoye OC et al (2022a) Microplastic burden in Africa: a review of occurrence, impacts, and sustainability potential of bioplastics. Chem Eng J Adv 12:100402. https://doi.org/10.1016/j.ceja.2022.100402
Okeke ES, Okoye CO, Atakpa EO et al (2022b) Microplastics in agroecosystems-impacts on ecosystem functions and food chain. Resour Conserv Recycl 177:105961. https://doi.org/10.1016/j.resconrec.2021.105961
Okeke ES, Prince T, Ezeorba C et al (2022c) Ecotoxicological and health implications of microplastic—associated biofilms: a recent review and prospect for turning the hazards into benefits. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-22612-w
Okeke ES, Ikechukwu K, Izuma C et al (2023a) Micro and nanoplastics ravaging our agroecosystem: a review of occurrence, fate, ecological impacts, detection, remediation, and prospects. Heliyon 9:e13296. https://doi.org/10.1016/j.heliyon.2023.e13296
Okeke ES, Nweze EJ, Ezike TC et al (2023b) Silicon-based nanoparticles for mitigating the effect of potentially toxic elements and plant stress in agroecosystems: a sustainable pathway towards food security. Sci Total Environ 898:165446. https://doi.org/10.1016/j.scitotenv.2023.165446
Okoye CO, Addey CI, Oderinde O et al (2022a) Toxic chemicals and persistent organic pollutants associated with micro-and nanoplastics pollution. Chem Eng J Adv 11:100310. https://doi.org/10.1016/j.ceja.2022.100310
Okoye CO, Okeke ES, Okoye KC et al (2022b) Occurrence and fate of pharmaceuticals, personal care products (PPCPs) and pesticides in African water systems: a need for timely intervention. Heliyon 8:e09143. https://doi.org/10.1016/j.heliyon.2022.e09143
Oliveri Conti G, Ferrante M, Banni M et al (2020) Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ Res 187:109677. https://doi.org/10.1016/j.envres.2020.109677
Osman AI, Hosny M, Eltaweil AS et al (2023) Microplastic sources, formation, toxicity and remediation: a review. Environ Chem Lett 214(21):2129–2169. https://doi.org/10.1007/S10311-023-01593-3
Oßmann BE, Sarau G, Holtmannspötter H et al (2018) Small-sized microplastics and pigmented particles in bottled mineral water. Water Res 141:307–316. https://doi.org/10.1016/J.WATRES.2018.05.027
Othman AR, Hasan HA, Muhamad MH et al (2021) Microbial degradation of microplastics by enzymatic processes: a review. Environ Chem Lett 194(19):3057–3073. https://doi.org/10.1007/S10311-021-01197-9
Padervand M (2017) Reusable porous Na(SiAl)O6·xH2O/NiFe2O4 structure for selective removal of heavy metals from waste waters. U.S. Patent 11,014,082, 25 May 2021
Padervand M, Lichtfouse E, Robert D, Wang C (2020) Removal of microplastics from the environment. A review. Environ Chem Lett 183(18):807–828. https://doi.org/10.1007/S10311-020-00983-1
Panel E, Chain F (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood EFSA Panel on Contaminants in the Food Chain (CONTAM). https://doi.org/10.2903/j.efsa.2016.4501
Park SY, Kim CG (2019) Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 222:527–533. https://doi.org/10.1016/J.CHEMOSPHERE.2019.01.159
Pereira R, Rodrigues SM, Silva D et al (2023) Microplastic contamination in large migratory fishes collected in the open Atlantic Ocean. Mar Pollut Bull 186:114454. https://doi.org/10.1016/J.MARPOLBUL.2022.114454
Periyasamy AP, Tehrani-Bagha A (2022) A review on microplastic emission from textile materials and its reduction techniques. Polym Degrad Stab 199:109901. https://doi.org/10.1016/j.polymdegradstab.2022.109901
Pham DT, Kim J, Lee S-H et al (2023) Analysis of microplastics in various foods and assessment of aggregate human exposure via food consumption in Korea. Environ Pollut 322:121153. https://doi.org/10.1016/j.envpol.2023.121153
Phillips MB, Bonner TH (2015) Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar Pollut Bull 100:264–269. https://doi.org/10.1016/j.marpolbul.2015.08.041
Phuong NN, Zalouk-Vergnoux A, Kamari A et al (2018) Quantification and characterization of microplastics in blue mussels (Mytilus edulis): protocol setup and preliminary data on the contamination of the French Atlantic coast. Environ Sci Pollut Res Int 25:6135–6144. https://doi.org/10.1007/s11356-017-8862-3
Pironti C, Ricciardi M, Motta O et al (2021) Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics. https://doi.org/10.3390/toxics9090224
Pittura L, Avio CG, Giuliani ME et al (2018) Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the mediterranean mussels. Mytilus Galloprovincialis Front Mar Sci 5:351215. https://doi.org/10.3389/FMARS.2018.00103/BIBTEX
Piyawardhana N, Weerathunga V, Sen CH et al (2022) Occurrence of microplastics in commercial marine dried fish in Asian countries. J Hazard Mater 423:127093. https://doi.org/10.1016/J.JHAZMAT.2021.127093
Prata JC (2018) Airborne microplastics: consequences to human health? Environ Pollut 234:115–126. https://doi.org/10.1016/J.ENVPOL.2017.11.043
Prata JC, da Costa JP, Lopes I et al (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/J.SCITOTENV.2019.134455
Praveena SM, Shamsul Ariffin NI, Nafisyah AL (2022) Microplastics in Malaysian bottled water brands: Occurrence and potential human exposure. Environ Pollut 315:120494. https://doi.org/10.1016/j.envpol.2022.120494
Primpke S, Christiansen SH, Christiansen SH et al (2020a) Critical assessment of analytical methods for the harmonized and cost-efficient analysis of microplastics. Appl Spectrosc 74:1012–1047
Primpke S, Godejohann M, Gerdts G (2020b) Rapid Identification and quantification of microplastics in the environment by quantum cascade laser-based hyperspectral infrared chemical imaging. Environ Sci Technol 54:15893–15903. https://doi.org/10.1021/acs.est.0c05722
Prüst M, Meijer J, Westerink RHS (2020) The plastic brain: neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol 17:1–16. https://doi.org/10.1186/s12989-020-00358-y
Qi Y, Beriot N, Gort G et al (2020) Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ Pollut 266:115097. https://doi.org/10.1016/j.envpol.2020.115097
Rasmussen LA, Lykkemark J, Andersen TR, Vollertsen J (2023) Permeable pavements: a possible sink for tyre wear particles and other microplastics? Sci Total Environ 869:161770. https://doi.org/10.1016/j.scitotenv.2023.161770
Reinold S, Herrera A, Saliu F et al (2021) Evidence of microplastic ingestion by cultured European sea bass (Dicentrarchus labrax). Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2021.112450
Renner G, Schmidt TC, Schram J (2018) Analytical methodologies for monitoring micro(nano)plastics: which are fit for purpose? Curr Opin Environ Sci Heal 1:55–61. https://doi.org/10.1016/J.COESH.2017.11.001
Renzi M, Blašković A (2018) Litter & microplastics features in table salts from marine origin: Italian versus Croatian brands. Mar Pollut Bull 135:62–68. https://doi.org/10.1016/j.marpolbul.2018.06.065
Ribeiro F, Okoffo ED, O’Brien JW et al (2021) Out of sight but not out of mind: size fractionation of plastics bioaccumulated by field deployed oysters. J Hazard Mater Lett 2:100021. https://doi.org/10.1016/j.hazl.2021.100021
Richard H, Carpenter EJ, Komada T et al (2019) Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci Total Environ 683:600–608. https://doi.org/10.1016/J.SCITOTENV.2019.04.331
Ru J, Huo Y, Yang Y (2020) Microbial degradation and valorization of plastic wastes. Front Microbiol 11:507487. https://doi.org/10.3389/FMICB.2020.00442/BIBTEX
Rummel CD, Jahnke A, Gorokhova E et al (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267. https://doi.org/10.1021/ACS.ESTLETT.7B00164
Saad D, Chauke P, Cukrowska E et al (2022) First biomonitoring of microplastic pollution in the Vaal river using Carp fish (Cyprinus carpio) “as a bio-indicator.” Sci Total Environ 836:155623. https://doi.org/10.1016/j.scitotenv.2022.155623
Samandra S, Mescall OJ, Plaisted K et al (2022) Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci Total Environ 837:155329. https://doi.org/10.1016/j.scitotenv.2022.155329
Sarmah P, Rout J (2018) Efficient biodegradation of low-density polyethylene by cyanobacteria isolated from submerged polyethylene surface in domestic sewage water. Environ Sci Pollut Res 25:33508–33520. https://doi.org/10.1007/s11356-018-3079-7
Schwaminger SP, Fehn S, Steegmüller T et al (2021) Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv 3:4395–4399. https://doi.org/10.1039/D1NA00243K
Schymanski D, Goldbeck C, Humpf HU, Fürst P (2018) Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res 129:154–162. https://doi.org/10.1016/J.WATRES.2017.11.011
Sewwandi M, Wijesekara H, Upamali A (2023) Microplastics and plastics-associated contaminants in food and beverages: global trends, concentrations, and human exposure. Environ Pollut 317:120747. https://doi.org/10.1016/j.envpol.2022.120747
Shabbir S, Faheem M, Ali N et al (2020) Periphytic biofilm: an innovative approach for biodegradation of microplastics. Sci Total Environ 717:137064. https://doi.org/10.1016/J.SCITOTENV.2020.137064
Sharma VK, Ma X, Lichtfouse E, Robert D (2023) Nanoplastics are potentially more dangerous than microplastics. Environ Chem Lett 21:1933–1936. https://doi.org/10.1007/S10311-022-01539-1/TABLES/1
Sheik S, Chandrashekar KR, Swaroop K, Somashekarappa HM (2015) Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegrad 105:21–29. https://doi.org/10.1016/j.ibiod.2015.08.006
Shi Y, Yi L, Du G et al (2023) Visual characterization of microplastics in corn flour by near field molecular spectral imaging and data mining. Sci Total Environ 862:160714. https://doi.org/10.1016/j.scitotenv.2022.160714
Shruti VC, Pérez-Guevara F, Elizalde-Martínez I, Kutralam-Muniasamy G (2020) First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—future research and environmental considerations. Sci Total Environ 726:138580. https://doi.org/10.1016/J.SCITOTENV.2020.138580
Simon M, van Alst N, Vollertsen J (2018) Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res 142:1–9. https://doi.org/10.1016/J.WATRES.2018.05.019
Skariyachan S, Taskeen N, Kishore AP et al (2021) Novel consortia of enterobacter and pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J Environ Manag 284:112030. https://doi.org/10.1016/j.jenvman.2021.112030
Sobhani Z, Lei Y, Tang Y et al (2020) Microplastics generated when opening plastic packaging. Sci Rep 10:1–7. https://doi.org/10.1038/s41598-020-61146-4
Son HF, Joo S, Seo H et al (2020) Structural bioinformatics-based protein engineering of thermo-stable PETase from Ideonella sakaiensis. Enzyme Microb Technol 141:109656. https://doi.org/10.1016/J.ENZMICTEC.2020.109656
Sowmya HV, Ramalingappa KM, Thippeswamy B (2015) Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ Dev Sustain 17:731–745. https://doi.org/10.1007/s10668-014-9571-4
Sridhar A, Ponnuchamy M, Kumar PS et al (2021) (2021) Techniques and modeling of polyphenol extraction from food: a review. Environ Chem Lett 194(19):3409–3443. https://doi.org/10.1007/S10311-021-01217-8
Sridhar A, Kannan D, Kapoor A, Prabhakar S (2022) Extraction and detection methods of microplastics in food and marine systems: a critical review. Chemosphere 286:131653
Stabnikova O, Stabnikov V, Marinin A et al (2022) The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J Microbiol Biotechnol 387(38):1–16. https://doi.org/10.1007/S11274-022-03293-6
Stavila E, Arsyi RZ, Petrovic DM, Loos K (2013) Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur Polym J 49:834–842. https://doi.org/10.1016/J.EURPOLYMJ.2012.12.010
Sun Q, Ren SY, Ni HG (2020a) Incidence of microplastics in personal care products: an appreciable part of plastic pollution. Sci Total Environ 742:140218. https://doi.org/10.1016/j.scitotenv.2020.140218
Sun X, Yuan X, Jia Y et al (2020b) Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat Nanotechnol 15:755–760. https://doi.org/10.1038/s41565-020-0707-4
Šunta U, Trebše P, Kralj MB (2021) Simply applicable method for microplastics determination in environmental samples. Molecules 26:1840. https://doi.org/10.3390/MOLECULES26071840
Taniguchi I, Yoshida S, Hiraga K et al (2019) Biodegradation of PET: current status and application aspects. ACS Catal 9:4089–4105. https://doi.org/10.1021/ACSCATAL.8B05171/ASSET/IMAGES/MEDIUM/CS-2018-05171U_0012.GIF
Teng J, Wang Q, Ran W et al (2019) Microplastic in cultured oysters from different coastal areas of China. Sci Total Environ 653:1282–1292. https://doi.org/10.1016/j.scitotenv.2018.11.057
Ter Halle A, Ladirat L, Gendre X et al (2016) Understanding the fragmentation pattern of marine plastic debris. Environ Sci Technol 50:5668–5675. https://doi.org/10.1021/ACS.EST.6B00594/SUPPL_FILE/ES6B00594_SI_001.PDF
Tirkey A, Upadhyay LSB (2021) Microplastics: An overview on separation, identification and characterization of microplastics. Mar Pollut Bull 170:112604. https://doi.org/10.1016/J.MARPOLBUL.2021.112604
Tong H, Jiang Q, Hu X, Zhong X (2020) Occurrence and identification of microplastics in tap water from China. Chemosphere 252:126493. https://doi.org/10.1016/j.chemosphere.2020.126493
Udovicki B, Andjelkovic M, Cirkovic-Velickovic T, Rajkovic A (2022) Microplastics in food: scoping review on health effects, occurrence, and human exposure. Int J Food Contam 9:1–16. https://doi.org/10.1186/s40550-022-00093-6
Uurasjärvi E, Sainio E, Setälä O et al (2021) Validation of an imaging FTIR spectroscopic method for analyzing microplastics ingestion by Finnish lake fish (Perca fluviatilis and Coregonus albula). Environ Pollut 288:117780. https://doi.org/10.1016/J.ENVPOL.2021.117780
Van Cauwenberghe L, Janssen CR (2014) Microplastics in bivalves cultured for human consumption. Environ Pollut 193:65–70. https://doi.org/10.1016/J.ENVPOL.2014.06.010
Van Cauwenberghe L, Devriese L, Galgani F et al (2015) Microplastics in sediments: a review of techniques, occurrence and effects. Mar Environ Res 111:5–17. https://doi.org/10.1016/J.MARENVRES.2015.06.007
Vickers NJ (2017) Animal communication: when I’m calling you, will you answer too? Curr Biol 27:R713–R715. https://doi.org/10.1016/J.CUB.2017.05.064
Vitali C, Peters RJB, Janssen H, Nielen MWF (2023) Trends in analytical chemistry microplastics and nanoplastics in food, water, and beverages; part I. Occurrence. Trends Anal Chem 159:116670. https://doi.org/10.1016/j.trac.2022.116670
Waddell EN, Lascelles N, Conkle JL (2020) Microplastic contamination in Corpus Christi Bay blue crabs, Callinectes sapidus. Limnol Oceanogr Lett 5:92–102. https://doi.org/10.1002/LOL2.10142
Wagner J, Wang ZM, Ghosal S et al (2017) Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal Methods 9:1479–1490. https://doi.org/10.1039/C6AY02396G
Wagner J, Wang ZM, Ghosal S et al (2019) Nonestructive extraction an ientification of microplastics from freshwater sport fish stomachs. Environ Sci Technol 53:14496–14506. https://doi.org/10.1021/ACS.EST.9B05072/SUPPL_FILE/ES9B05072_SI_001.PDF
Wahl A, Le Juge C, Davranche M et al (2021) Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere 262:127784. https://doi.org/10.1016/j.chemosphere.2020.127784
Walkinshaw C, Lindeque PK, Thompson R et al (2020) Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol Environ Saf 190:110066. https://doi.org/10.1016/J.ECOENV.2019.110066
Wang Z, Taylor SE, Sharma P, Flury M (2018) Poor extraction efficiencies of polystyrene nano- and microplastics from biosolids and soil. PLoS ONE 13:e0208009. https://doi.org/10.1371/JOURNAL.PONE.0208009
Wang F, Wang B, Duan L et al (2020) Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: a case study in Changzhou, China. Water Res 182:115956. https://doi.org/10.1016/j.watres.2020.115956
Wang J, Guo X, Xue J (2021a) Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ Sci Technol 55:12780–12790. https://doi.org/10.1021/ACS.EST.1C04466/SUPPL_FILE/ES1C04466_SI_001.PDF
Wang K, Li J, Zhao L et al (2021b) Gut microbiota protects honey bees (Apis mellifera L.) against polystyrene microplastics exposure risks. J Hazard Mater 402:123828. https://doi.org/10.1016/J.JHAZMAT.2020.123828
Watteau F, Dignac MF, Bouchard A et al (2018) Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Front Sustain Food Syst 2:407866. https://doi.org/10.3389/FSUFS.2018.00081/BIBTEX
Wei R, Breite D, Song C et al (2019) Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv Sci 6:1900491. https://doi.org/10.1002/ADVS.201900491
Wen S, Zhao Y, Wang M et al (2022) Micro (nano) plastics in food system: potential health impacts on human intestinal system. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2022.2116559
Winkler A, Santo N, Ortenzi MA et al (2019) Does mechanical stress cause microplastic release from plastic water bottles? Water Res 166:115082. https://doi.org/10.1016/j.watres.2019.115082
Wootton N, Reis-Santos P, Dowsett N et al (2021) Low abundance of microplastics in commercially caught fish across southern Australia. Environ Pollut 290:118030. https://doi.org/10.1016/j.envpol.2021.118030
Wu M, Liu W, Liang Y (2019) Probing size characteristics of disinfection by-products precursors during the bioavailability study of soluble microbial products using ultrafiltration fractionation. Ecotoxicol Environ Saf 175:1–7. https://doi.org/10.1016/J.ECOENV.2019.02.077
Wu RT, Cai YF, Chen YX et al (2021) Occurrence of microplastic in livestock and poultry manure in South China. Environ Pollut 277:116790. https://doi.org/10.1016/J.ENVPOL.2021.116790
Wu P, Lin S, Cao G et al (2022) Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J Hazard Mater 437:129361. https://doi.org/10.1016/j.jhazmat.2022.129361
Xu JL, Thomas KV, Luo Z, Gowen AA (2019a) FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal Chem 119:115629. https://doi.org/10.1016/J.TRAC.2019.115629
Xu M, Halimu G, Zhang Q et al (2019b) Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ 694:133794. https://doi.org/10.1016/J.SCITOTENV.2019.133794
Xu X, Wong CY, Tam NFY et al (2020) Microplastics in invertebrates on soft shores in Hong Kong: Influence of habitat, taxa and feeding mode. Sci Total Environ 715:136999. https://doi.org/10.1016/J.SCITOTENV.2020.136999
Yan M, Li W, Chen X et al (2021) A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J Hazard Mater 408:124882. https://doi.org/10.1016/j.jhazmat.2020.124882
Yan Z, Liu Y, Zhang T et al (2022) Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol 56:414–421. https://doi.org/10.1021/acs.est.1c03924
Yu L, Di ZJ, Liu Y et al (2021) Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci Total Environ 756:143860. https://doi.org/10.1016/J.SCITOTENV.2020.143860
Zabel F, Delzeit R, Schneider JM et al (2019) Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nat Commun 10:2844. https://doi.org/10.1038/s41467-019-10775-z
Zhang J, Tian K, Lei C, Min S (2018) Identification and quantification of microplastics in table sea salts using micro-NIR imaging methods. Anal Methods 10:2881–2887. https://doi.org/10.1039/C8AY00125A
Zhang F, Wang X, Xu J et al (2019a) Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar Pollut Bull 146:173–182. https://doi.org/10.1016/J.MARPOLBUL.2019.05.061
Zhang J, Wang L, Kannan K (2019b) Polyethylene terephthalate and polycarbonate microplastics in pet food and feces from the United States. Environ Sci Technol 53:12035–12042. https://doi.org/10.1021/ACS.EST.9B03912/SUPPL_FILE/ES9B03912_SI_001.PDF
Zhang T, Sun Y, Song K et al (2021) Microplastics in different tissues of wild crabs at three important fishing grounds in China. Chemosphere 271:129479. https://doi.org/10.1016/j.chemosphere.2020.129479
Zhang Q, Du F, Liang W et al (2022) Microfiber fallout during dining and potential human intake. J Hazard Mater 430:128477. https://doi.org/10.1016/J.JHAZMAT.2022.128477
Zhao K, Wei Y, Dong J et al (2022) Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ Pollut 297:118773. https://doi.org/10.1016/J.ENVPOL.2021.118773
Zhu J, Wang C (2020) Recent advances in the analysis methodologies for microplastics in aquatic organisms: current knowledge and research challenges. Anal Methods 12:2944–2957. https://doi.org/10.1039/D0AY00143K
Zhu N, Liu J, Yang J et al (2016) Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol Biofuels 9:1–22. https://doi.org/10.1186/S13068-016-0461-X/TABLES/8
Ziani K, Ioniță-Mîndrican CB, Mititelu M et al (2023) Microplastics: a real global threat for environment and food safety: a state of the art review. Nutrients. https://doi.org/10.3390/nu15030617
Zitouni N, Cappello T, Missawi O et al (2022) Metabolomic disorders unveil hepatotoxicity of environmental microplastics in wild fish Serranus scriba (Linnaeus 1758). Sci Total Environ 838:155872. https://doi.org/10.1016/j.scitotenv.2022.155872
Acknowledgements
The authors are thankful to their respective departments/institutes/universities for providing space and other necessary facilities, which helped draft this manuscript.
Funding
This article did not receive any form of funding.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed to the literature survey, manuscript writing, writing—review and editing. Emmanuel Sunday Okeke conceptualized the study, and Chidiebele Emmanuel Nwankwo significantly contributed to the study idea and review structure. Chukwuebuka Gabriel Eze, Chidiebele Emmanuel Nwankwo, Satarupa Dey, Suresh Sundaramurthy, and Emmanuel Sunday Okeke were contributed to investigation, methodology, project administration, resources, and software. The revision and modification of the article were done by Emmanuel Sunday Okeke and Chidiebele Emmanuel Nwankwo. All authors contributed substantially to the discussion of the content, figures, and tables. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eze, C.G., Nwankwo, C.E., Dey, S. et al. Food chain microplastics contamination and impact on human health: a review. Environ Chem Lett 22, 1889–1927 (2024). https://doi.org/10.1007/s10311-024-01734-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-024-01734-2