Abstract
The solar photodegradation of Sevnol, a commercial pesticide, based on carbaryl as active principle, was studied. Experiments have been carried out at laboratory and at pilot plant scale using titanium dioxide as catalyst. Complete dissappearance of carbaryl was achieved, while total mineralisation required longer irradiation. Active sludge respirometry showed significant detoxification of the solution. Finally, results obtained with commercial Sevnol were consistent with those of pure carbaryl, although the reaction was slower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides constitute an increasing environmental concern, above all in rural areas. Rinse water from fruit, greenhouse plastic and empty pesticide containers, all contain significant concentrations of these compounds. Although the concentration of such pollution in wastewater is not very high, it is very difficult to treat by conventional biological methods, as it is generally very toxic towards microorganisms. Therefore, alternative methods are needed to solve this problem.
Photocatalysis involving titanium dioxide has proved to be a good method for eliminating an important number of pollutants that are recalcitrant to biological treatment. In particular, it seems very effective for detoxification of effluents containing pesticides (Konstantinou and Albanis 2003).
The use of sunlight as the only irradiation source (solar photocatalysis) makes photocatalysis commercially feasible and more environmentally sustainable. Encouraging results have been obtained in this way, not only at laboratory scale, but also using pre-industrial and even industrial plants (Bockelmann et al. 2004).
However, a combination of photocatalysis followed by biological treatment would further decrease operation costs (Esplugas and Ollis 1997). In the first step, photocatalysis destroys toxic pollutants yielding more biodegradable intermediates, which can easily be eliminated in a following classic biological reactor.
The aim of this paper is to confirm the feasibility of this strategy, by using a solar pilot plant to degrade the pollutant measuring the detoxification achieved. Sevnol, a commercial pesticide in which carbaryl (1-naphtyl-N-methylcarbamate) is the active component, was chosen for this purpose, as previous experiments carried out by other authors (Bianco-Prevot et al. 1999; Pramauro et al. 1997) have indicated that carbaryl can be degraded using titanium dioxide as photocatalyst. Those experiments were performed using artificial light as the radiation source. Although decomposition of the substrate could easily be achieved, mineralisation was not complete, and persistent intermediates were detected.
This paper reports on treatment of aqueous solutions of Sevnol with TiO2-mediated solar photocatalysis in a solar wastewater detoxification plant. Active sludge respirometry was employed to measure the toxicity remaining in these solutions throughout the photoreaction (Miranda et al. 2003). Briefly, respirometry measures inhibition of active sludge activity in the presence of the pollutant, by the oxygen uptake rate (OUR) of the microorganisms present in a biological reactor (Checchi and Marsili-Libelli 2005). As the active sludge is taken from a municipal wastewater treatment plant and used without adaptation to the pollutant, a decrease in the OUR is a good parameter for determining the irradiation period required before the effluent can be discharged to the biological reactor.
Experimental
Reagents and reactions
Sevnol and pure carbaryl were obtained from MAFA and employed as received. Sevnol contains 85% (w/w) of the active compound: carbaryl. Experiments were performed in aqueous solutions (Milli-Q grade in experiments up to 4 l total volume, distilled water with conductivity <10 μS/cm for larger-volume experiments). Between 0.2 and 1 g/l of Degussa P-25 titanium dioxide was employed as the photocatalyst. The carbaryl concentration was in the range of 0.05–0.1 g/l.
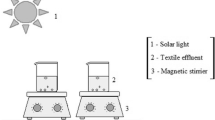
Laboratory-scale experiments under sunlight were carried out in open cylindrical glass vessels (7.5 cm diameter, 8.0 cm high). The reactor was loaded with 250 ml of reaction mixture and magnetic stirring was kept up throughout the reaction. Periodic samples were taken for analysis. The effect of evaporation was corrected by adding the necessary amount of water.
The experiments were scaled up using two different pilot plants, both of them based on compound parabolic concentrators, CPCs (Malato Rodríguez et al. 2004). The small one is able to treat up to 4 l of wastewater and has an irradiated surface of 0.26 m2, corresponding to an irradiated volume of 1.83 l, the UV-A was measured with an Acadus-85 radiometer. The larger plant can be loaded with 40 l, (3.1 m2 irradiated surface, 22 l irradiated volume), and UV radiation was measured by a global UV radiometer (KIPP&ZONEN, model CUV 3). Absorber tube characteristics (internal diameter 29.2 mm; external diameter 32.0 mm.) are the same in both pilot plants.
Analysis
The concentration of carbaryl remaining was determined by means of HPLC analysis with a Perkin Elmer XL Autosystem. A LiChrosphere 100 RP-18 column was used with an isocratic mixture of methanol (52%) and a aqueous solution of H2SO4 0.01 M (48%), as the eluent. Absorbance of the eluate was measured at 280 nm by a diode array detector. Cation concentrations were determined with a Dionex DX-120 ion chromatograph equipped with a Dionex Ionpac CS12A 4 mm×250 mm column. Isocratic elution was done with H2SO4 (10 mM) at a flow rate of 1.2 ml/min. Anion concentrations were measured with a Dionex DX-600 ion chromatograph using a Dionex Ionpac AS11-HC 4 mm×250 mm column. The gradient programme was pre-run for 5 min with 20 mM NaOH, followed by an 8 min injection of 20 mM NaOH and 35 mM NaOH for 7 min, the flow rate was 1.5 ml/min. Total organic carbon (TOC) was determined with a Shimadzu model TOC-V CSH apparatus, provided with an autosampler. The sample surface tension was determined by a Krüss K-9 tensiometer.
Respirometric measurements
Inhibition experiments were carried out with a Neurtek BM3-LAB active sludge respirometer provided with a biological reactor and an oxygen sensor (WTW-Cell Ox). The reactor was loaded with the desired amount of active sludge from the biological reactor of Alcoy (Spain) municipal wastewater treatment plant, and continuously saturated with air. In order to make the system as real as possible, sludge was employed without previous adaptation.
The OUR was obtained from the difference in the oxygen concentration in the sludge as it was pumped to the oxygen sensor through two pathways of different length. Sodium acetate (1 g/l) was added to the active sludge to reach the maximum oxygen uptake rate (OURmax). Then, the pesticide was added and the final oxygen consumption (OURf) measured. The inhibition can be calculated by Eq. 1.
As the pollutant was in an aqueous solution, a decrease in OUR could be expected due to sludge dilution (respirometry tests were performed by adding 250 ml of the solution containing the pesticide to 500 ml of active sludge). Around 20% of inhibition can be attributed to this effect, which was determined by a blank experiment (250 ml of distilled water added to the sludge).
Results and discussion
Laboratory-scale experiments: carbaryl
A first series of experiments were carried out to study the possible relationship between the degradation of carbaryl, mineralisation achieved and detoxification of the solution. The concentration of TiO2 was in the range of 0.2–1 g/l.
The first conclusion that can be obtained from Fig. 1 is that results achieved with 0.2, 0.5 and 1 g/l of TiO2 were not significantly different. The existence of an optimum TiO2 concentration and plateau behaviour beyond this point have already been observed and explained elsewhere (Jakob et al. 1993), so it seems that more than 0.2 mg/l of TiO2 does not increase detoxification efficiency.
Solar detoxification of aqueous carbaryl solutions, 0.05 g/l with different amounts of titanium dioxide. Relative concentration of carbaryl remaining, left axis (▴, 0.2 g/l TiO2), (▪, 0.5 g/l TiO2) and (♦, 1 g/l TiO2); Relative TOC decrease, left axis (▵, 0.2 g/l TiO2), (□, 0.5 g/l TiO2) and (⋄, 1 g/l TiO2); Inhibition, right axis, discontinuous lines (▴, 0.2 g/l TiO2), (▪, 0.5 g/l TiO2) and (♦, 1 g/l TiO2)
Degradation of carbaryl (as measured by HPLC) was complete after around 2 h of irradiation, showing that titanium dioxide is an efficient solar photocatalyst. This is in agreement with previous results obtained with simulated sunlight (Xe lamp) that also indicated complete oxidation of this substrate (Pramauro et al. 1997). The disappearance of carbaryl under these conditions fitted a pseudo first-order kinetics, the rate constants obtained were 0.54×10−3, 0.47×10−3 and 0.46×10−3 s−1 for 1, 0.5 and 0.2 g/l of TiO2. Ion chromatography indicates that nitrogen present in the molecule was released as amines and ammonium ions. An unquantified methylamine peak was detected next to the ammonium peak, which could close the incomplete N mass balance observed during the treatment. Only at the end of the process, oxidation of NH3 to give nitrate ion can be observed, although this reaction was very slow.
On the other hand, mineralisation of the substrate requires more illumination time, as only after 5 h of irradiation the TOC measured in the solution is negligible. This can be attributed to the formation of stable organic intermediates during the degradation of carbaryl. Mineralisation is expected to be even more difficult in the case of commercial formulation or real wastewater, as other organic species could be present.
However, as mentioned in “Introduction” section, total mineralisation is not necessary, as once toxic pollutants are eliminated, biological treatment seems more advisable. As a matter of fact, respirometric experiments performed with the treated solutions indicate that there is a considerable decrease in the inhibition of active sludge OUR from an initial value of 50–60% to only around 20% after 3 h of irradiation, clearly indicating that the wastewater could be ready for a biological treatment.
Finally, in blank experiments (irradiation of carbaryl in the absence of TiO2), although there was some degradation of carbaryl after 5 h (around 15%), no decrease in TOC and no change in inhibition of the solution were observed. The disappearance of carbaryl could be due to hydrolysis of the carbamate group, yielding a toxic intermediate, probably a naphthol (Pramauro et al. 1997)
Experiments were repeated with a higher concentration of carbaryl (100 mg/l). The same trends were observed. Total elimination of carbaryl was achieved (although around 4 h of irradiation was needed); decrease in TOC was also very significant, although mineralisation was not complete, and detoxification was even more remarkable in this case, dropping from an initial OUR inhibition of 75% to around 20% after 5 h. This residual inhibition, as indicated in “Experimental” section, could be attributed to either dilution of the sludge or slight remaining inhibition.
Pilot plant experiments: carbaryl
In the previous section solar photocatalysis was shown to be an efficient detoxification method for aqueous solutions of carbaryl, even when complete mineralisation was not achieved. In this section, the reaction is scaled up using a solar pilot plant able to treat larger volumes of effluent.
In the first stage, 50 mg/l solutions of the active component carbaryl were prepared. A total volume of 4 l was used in the smallest pilot plant described in “Experimental” section. Two different amounts of titanium dioxide, 0.2 and 0.5 g/l were tested in parallel experiments, results are shown in Fig. 2.
Solar detoxification of aqueous solutions of Sevnol (0.05 g/l of active compound) in a 35-l solar plant employing 200 mg/l of titanium dioxide. Relative remaining concentration of carbaryl (▪, left axis), relative TOC decrease (▴, left axis) and percentage of inhibition of active sludge (♦, right axis)
First of all, it should be noted that the abscissa of Figs. 2 and 3 are expressed as t 30W. Briefly, this is a convenient method of normalising the irradiation period with respect to the changing irradiation conditions present in solar experiments: first the UV-radiation arriving at the plant is measured with a radiometer, then it is converted into time by considering an average intensity of 30W. This parameter is calculated by the following formula, where t n is the experimental time for each sample, UV the average solar ultraviolet radiation during Δt n , V i the irradiated volume and V t the total volume.
In the pilot plant, 0.2 g/l of titanium dioxide appeared to be also the optimal dosage. These results coincide with laboratory-scale results, very fast degradation of carbaryl (total abatement in around 90 min, while mineralisation requires 300 min of irradiation). Detoxification of the solution could also be achieved, as inhibition of the active sludge dropped to a final value below 30%. Final inhibitions in experiments employing double concentration of carbaryl were also in the range of 20–30%.
Pilot plant experiments: commercial Sevnol
To verify the actual technical convenience of the process, the reaction was scaled up to 35 l. In this case commercial product, Sevnol, was employed to check for possible interference by excipients present in the commercial formulation. Solutions were prepared so that the concentration of active compound was 50 mg/l and that of titanium dioxide was 0.2 g/l. The results achieved can be observed in Fig. 3.
Results indicate that solar photocatalytic treatment of Sevnol can be scaled up to pre-industrial volumes. Again, in this case, complete photo-oxidation of carbaryl is observed, while mineralisation is more difficult to achieve. A clear relationship between the disappearance of the toxic active compound and the decrease in the inhibition of the active sludge by the solutions can be observed, reaching this parameter final values of around 30%. The presence of excipients in commercial Sevnol seems to slow its rate down, as irradiation periods of 500 min are now needed for total abatement of carbaryl, while total mineralisation cannot be achieved even after 700 min.
Surfactants could be present among these excipients. As a matter of fact, surface tension measurement of Sevnol solutions showed values around 58 mN/m (far from that of distilled water 72 mN/m), a strong indication of surfactants presence. This parameter was also monitored during the photocatalytic reaction finding a surface tension of 72 mN/m during the first stages of the process (t 30W=50 min). It may be observed in Fig. 3 that during this first 50 min of treatment, degradation and mineralisation of carbaryl appeared to slow down due to the adsorption of both the catalyst and the compound on the foam.
Conclusion
Solar photocatalysis using titanium dioxide is a very useful method for treating aqueous solutions containing carbaryl, even in commercial formulations. Total elimination of the active component is easily achieved, both at laboratory and pilot plant scale. However, complete mineralisation of the solution requires a longer irradiation time. Respirometric experiments show a relationship between degradation of carbaryl and detoxification of the solution. For this reason, a good strategy may be the use of photocatalysis for detoxification of the solution, followed by a biological treatment to deal with remaining non-toxic organic matter.
References
Bianco-Prevot A, Pramauro E, de la Guardia M (1999) Photocatalytic degradation of carbaryl in aqueous TiO2 suspensions containing surfactants. Chemosphere 39:493–502
Bockelmann D, Dillert R, Dzengel J, Goslich R, Grob E, Higendorff M, Hufschmidt D, Memming R, Sagawe G, Schober M, Schuhmacher H-W, Selzer V, Siemon U, Vollmer S, Theurich J, Bahnemann D (2004). Photocatalysis. Solar Energy 77:445
Checchi N, Marsili-Libelli S (2005) Reliability of parameter estimation in respirometric models. Water Res 39:3686–3696
Esplugas S, Ollis DF (1997) Economic aspects of integrated (chemical + biological) processes for water treatment. J Adv Oxid Technol 2(1):197–202
Hincapié M, Maldonado MI, Oller I, Gernjak W, Sánchez-Pérez JA, Ballesteros MM, Malato S (2005) Solar photocatalytic degradation and detoxification of EU priority substances. Catal Today 101:203–210
Jakob L, Oliveros E, Legrini O, Braun AM (1993) TiO2 photocatalytic treatment of water. Reaction design and optimization experiments. In: Ollis DF, Al-Ekabi H (eds) Photocatalytic purification and treatment of water and air. Elsevier Science Publishers, Amsterdam
Konstantinou IK, Albanis TA (2003). Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl Catal B Environ 42:319–335
Malato Rodríguez S, Blanco Gálvez J, Maldonado Rubio MI, Fernández Ibáñez P, Alarcón Padilla D, Collares Pereira M, Farinha Mendes J, Correia de Oliveira J (2004). Engineering of solar photocatalytic collectors. Solar Energy 77:513–524
Miranda MA, Amat AM, Arques A, Beneyto H, García A, Seguí S (2003) Ozonisation coupled with biological degradation for wastewater treatment: A mechanistically based study. Chemosphere 53:79–86
Pramauro E, Bianco Prevot A, Vincenti M, Brizzolesi G (1997) Photocatalytic degradation of carbaryl in aqueous solutions containing TiO2 suspensions. Environ Sci Technol 31:3126–3131
Acknowledgements
We want to thank Spanish Ministry of Education and Science (project PPQ2003-07596-C03-03 & project PPQ2003-07596-C03-01 “FOTODETOX”) and the European Commission (FEDER projects) for economic support. Ana García and Isabel Oller would like to thank the Ministry of Education and Science for their PhD research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García, A., Amat, A.M., Arques, A. et al. Detoxification of aqueous solutions of the pesticide “Sevnol” by solar photocatalysis. Environ Chem Lett 3, 169–172 (2006). https://doi.org/10.1007/s10311-005-0026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-005-0026-x