Abstract
In aqueous systems, heavy metal ions, when present in excess than permissible limits, are dangerous for human beings and aquatic life. Heavy metals cannot be degraded. Rather, they accumulate in living organisms either directly or through the food chain. Inside the body, metal ions can be converted to more toxic forms or can directly interfere with metabolic processes. As a result of metal toxicity, various disorders and damage due to oxidative stress triggered by metal ions have been witnessed. Toxic effects of metallic pollution coupled with the need of pure water for the survival and sanitation have thus prompted researchers to take every possible step to uphold the quality of water. In this regard, various strategies have been developed for the detection and the removal of metal ions from aqueous systems. Here we review metal-free water and methodologies used for rapid detection at low levels. Also, the application of benign materials and methods for metal removal from aqueous systems is detailed. Electrochemical methods, especially stripping and cyclic voltammetry, are commonly used methods for detection, while adsorption and ion exchange methods are quite effective for removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased anthropogenic activity has led to the release of many hazardous substances into water resources that has put aquatic ecosystem and environment at risk (Nagajyoti et al. 2018). Heavy metal ions are the most serious contributors of water pollution as they are highly toxic, non-degradable and have a tendency to bioaccumulate and biomagnify as a result of food chain (Ayangbenro and Babalola 2017; Ali and Khan 2018; Kahlon et al. 2018). Their presence in aquatic ecosystems is sufficient to affect living systems directly or indirectly. Even in the soil environment, heavy metal ions are quite dangerous to both plants and animals as they are adsorbed by plants and finally reach to animals and humans (Dubey et al. 2018; Mallampati et al. 2013). In the soil environment, sorption of these heavy metals by soil constituents is considered to be a vital process in reducing the mobility of these pollutants to water, crops, vegetables and the threat to human beings and animals (Zhu et al. 2019; Lair et al. 2007). The impacts of this water pollution have forced researchers to develop suitable techniques for the detection and quantification of heavy metal contaminants. Many techniques have been used for the detection of heavy metal ions which can be divided into three main categories; spectroscopic detection techniques, electrochemical detection techniques and optical detection techniques. Spectroscopic methods for heavy metal ion detection include atomic absorption spectroscopy, atomic emission spectroscopy, inductively coupled plasma mass spectrometry (Lebedev et al. 2003), cold vapour atomic fluorescence spectrometry, which are quite sensitive but are expensive and require laborious pre-treatment processes (Harrington et al. 2011). Electrochemical methods on a contrary are more cost-effective, time economic, user-friendly, reliable and suitable for in-field applications and include techniques such as amperometry, voltammetry, potentiometry, impedance measurement and coulometry for heavy metal ion detection (Zhu et al. 2015). These electrochemical techniques allow simple procedures and are also fast in terms of short analytical time as compared to other spectroscopic techniques. Among various electrochemical techniques, anodic stripping voltammetry has been widely used for the analysis of heavy metal ions at trace levels because it possesses good selectivity, portability, low cost, fast analysis speed and excellent sensitivity (Zhu et al. 2012; Zhang et al. 2015; Yao et al. 2014; Liu et al. 2014a, b; Xu et al. 2013). In optical methods of detection, metal ions can be detected by conventional methods of absorption, reflection or luminescence spectrometry. Selective chromogenic reagents and indicator dyes are often used in optical detection methods, where they react with a specific metal ion. In addition, optical fibres, integrated optics, capillary-type devices, etc., are also helpful in the detection of metal ions.
In addition to the detection of heavy metal ion, their removal from aqueous systems is essentially a highly important concern. Water contaminated with highly toxic heavy metal ions such as Hg2+, Pb2+ and Cd2+ is detrimental and hazardous for human health and requires highly efficient and specific methods of removal from aqueous systems. There are many such methods which can be employed for removal process such as precipitation, flocculation, membrane separation, ion exchange, evaporation. Every method has its own advantages and limitations in terms of efficiency, sensitivity, selectivity and specificity (Pujol et al. 2014; Crini and Lichtfouse 2019). Among many of these methods, adsorption is more often a method of choice, because of its low cost, simple design and strong operability, especially its high removal efficiency from dilute solutions (Wang et al. 2013). At several occasions, the surface of materials is modified so as to enhance the removal efficiency towards metal ion uptake (Elfeky et al. 2017; Ojemaye et al. 2017).
Here we present an overview of metal ion pollution scenario in aqueous systems and potential consequences of these increasing levels of pollution on living system and the overall quality of life. Various methodologies that can be used in the detection purpose and the advantages of each method have been highlighted. Finally, some important methods which are quite useful for the removal of heavy metal ions from water have been discussed threadbare in this review paper.
Though metals are important to carry out cellular functions, their concentration window has a great impact on human health. Concentrations below the toxicity range is not going to have many adverse effects, but when it goes beyond the permissible limits, it becomes dangerous and leads to various cytological and physiological effects (Sumner et al. 2005). The effects of heavy metal ions on living beings along with their toxicity range according to World Health Organization (WHO) and Bureau of Indian Standards (BIS) are given in Table 1.
Table 1 lists various sources and effects of some most toxic heavy metal ions like lead (Pb), cadmium (Cd), mercury (Hg), arsenic (As) and chromium (Cr) which are the cause for many of the heavy metal-related diseases (Flora et al. 2008; Flora 2009; Bashir et al. 2016). “Presence” of heavy metal ions is not an issue; rather, it is “excess” that is creating the problem. It has been reported in the literature that in most cases, trace levels of metals are important in the biological functioning of cells such as transportation and cell signalling (Valko et al. 2005). However, when present in excess, these metal ions move out of the main metabolic pathway and can bind with protein sites other than natural binding sites, breaking the cell cascade, thereby, leading to toxicity in living things (Sevcikova et al. 2011; Gemma et al. 2006; Singh et al. 2010).
So considering the toxic effects of heavy metal ions when present in excess than the permissible limits, it is prior requirement to detect, quantify and then remove these heavy metal ions by applying suitable methodological procedures.
Detection
A metal ion detector is a device or instrument designed to detect the presence of metal ions in its surroundings and sometimes may also be useful to quantify these metal ions. As already mentioned the importance of metal ion free water systems, it is very essential to develop a strategy for making water bodies safe to use for living beings. Prior to removal of these toxic metal ions from water samples, we need to have a technique available that could detect their presence and also help in making a quantitative estimate of the level of pollution possible so that a suitable method is chosen for the removal. For this purpose, detection process should be developed which are time- and cost-effective besides being environmentally green. Also a detection technique must be sensitive enough to detect even traces of metal ions with a good accuracy. A number of techniques are available for detection of heavy metal ions, but a particular technique applicable for all the ions is rather missing.
Methods of detection
Heavy metal ion detection techniques can be broadly divided into three main categories:
Spectroscopic detection
Spectroscopic detection of heavy metal ions includes highly sensitive techniques like atomic absorption spectroscopy (Gong et al. 2016; Array and Merkoci 2012), inductively coupled plasma mass spectroscopy (Wang et al. 2015), X-ray fluorescence spectrometry (Sitko et al. 2015), neutron activation analysis and inductively coupled plasma-optical emission spectrometry (Losev et al. 2015; Poikyo and Permki 2003). They are versatile in terms of simultaneous determination of heavy metal ions concentration for a large range of elements with very low detection limits. However, these techniques are quite expensive and require trained personnel to work on the complex equipments. Flame atomic absorption spectroscopic technique has been used to determine Cu, Pb and Cd ions in water where limit of detection has been reported to be 2, 3 and 0.2 μg dm−3 for Cu2+, Pb2+ and Cd2+ ions, respectively (Shirkhanloo et al. 2011). High-resolution surface plasmon resonance spectroscopy combined with anodic stripping voltammetry has been used to detect lead, copper and mercury ions from parts per million down to subparts per billion levels (Wang et al. 2007). Some of the important spectroscopic methods used for the detection of heavy metal ions are explained below.
Atomic absorption spectrometry
In atomic absorption spectroscopy, a specific wavelength is used to excite isolated atoms from ground state to excited state and the amount of energy absorbed during this excitation is measured which is proportional to the concentration of atoms present in the sample. A block diagram of a typical atomic absorption spectrometer is shown in Fig. 1. It consists of a primary light source, atomizer to produce gas-phase atoms or ions for analysis, a monochromator, a detector and an electronic “readout” system.
Atomic absorption spectroscopic determination of elements can only be performed on gaseous medium in which the individual atoms or ions are well separated from each other. Plasmas, flames and electrothermal atomizers are used to generate gas-phase analyte atoms/ions. This process in which sample is volatilized and decomposed in such a way so as to produce gas-phase atoms and ions is called as atomization. A specific light source is used to excite atoms or ions of a particular element and these light sources usually use a hollow cathode lamp or an electrodeless discharge lamp of the same element, which is to be analysed. Many lamps are also available that can be used to determine more than one element without changing the lamp. Solid-state detectors or photomultiplier tubes have been used as detectors in atomic absorption spectroscopy. For the determination of mercury, a modified atomic absorption spectroscopic technique, called as flow injection mercury systems, is used. Spectroscopic detection of some metal ions by the help of atomic absorption spectroscopy is summarized in Table 2.
Graphite furnace atomic absorption spectrometry
Graphitic furnace consists of a cylindrical graphitic tube that is open at both the ends and has a central hole for introduction of samples. The sample is introduced directly into a graphite tube where it is heated to remove the solvent and matrix components, and then atomization of the sample takes place. The atomized sample is then analysed in the same way as reported in flame atomic absorption spectroscopy. In graphitic furnace atomic absorption spectroscopy, atomization occurs in an environment where temperature is not changing rapidly as sample is no longer directly on furnace wall, as a result of which more reproducible signals are obtained. The disadvantages of graphitic furnace atomic absorption spectroscopy as a detection tool for heavy metal ions are in terms of analysis times which are longer than those for flame sampling and the numbers of elements that can be determined by this method are fewer. Graphitic furnace atomic absorption spectroscopy was used for the determination of lead, cadmium, copper, arsenic and mercury with detection limits of 0.28, 0.014, 0.49, 0.19 and 0.061 mg L−1, respectively (Nie et al. 2008). A graphite furnace technique was also used for the analysis of lead in “Yin Qiao Jie Du” tablets where LOD for Pb was found to be of the order of 0.1 ppb (Yuan et al. 2009). Graphitic furnace atomic absorption spectroscopy as an effective method for the detection of some heavy metal ions is reported in Table 2.
Atomic fluorescence spectrometry
Atomic fluorescence spectrometry is different from atomic absorption spectrometry in principle and working. Like atomic absorption spectroscopy, in atomic fluorescence spectrometry a sample solution is first atomized and then the atoms are illuminated with a light source leading to the excitation of atoms. These excited atoms undergo radiative deactivation and emit characteristic radiations that fall on the detection device, and the atomic fluorescence is measured by the detector. For mercury determination, cold vapour atomic fluorescence spectrometry can be used in which carrier gas like argon is used to take free mercury atoms to the cell where these atoms are excited by a collimated ultraviolet light source. The excited atoms re-emit the absorbed energy as fluorescence which is measured by the help of photomultiplier tube detector or a UV photodiode detector. The major advances in the field of metal detection using atomic fluorescence spectrometry are highlighted in Table 3.
X-ray fluorescence spectrometry
When a material is irradiated with X-rays or gamma rays, it leads to the ionization of the material. Such a bombardment of material with high energy radiations can even eject electrons from inner orbitals of K or L shell as shown in Fig. 2. These vacancies are fulfilled by electrons from higher energy shells which is accompanied by the emission of photon or fluorescence called as X-ray fluorescence spectrometry, which is measured.
Since each element has a unique set of energy levels, each element produces its own fluorescence spectrum with a unique set of energies associated with multiple peaks of different intensities. Thus, XRF is quite useful technique to determine the elemental composition of a sample quite easily. The block diagram of X-ray fluorescence spectrometer which is composed of an X-ray source, sample chamber, fluorescence detector, data processing and display system is shown in Fig. 3.
XRF technique has been used to screen and identify toxic elements in various FDA-regulated products (Palmer et al. 2009) and many other such sources which are summarized in Table 3.
Electrochemical methods of detection
Electrochemical techniques are economic, user-friendly and reliable and involve simple procedures for monitoring of contaminated samples. The other advantage offered by electrochemical methods is the very short analytical time as compared to other spectroscopic techniques (Pujol et al. 2014). However, these electrochemical techniques have some drawbacks like they possess lower sensitivity and higher limits of detection (LOD) as compared to spectroscopic and optical techniques. Also, these techniques often in certain cases require developments and modifications in the design to improve their performance in detection of heavy metal ions (Bansod et al. 2017). For example, various electrochemical techniques have to be coupled with different biosensing electrodes in order to improve their sensitivity and limits of detection by modifying the electrode material. The presence of heavy metal ion in water usually brings the change in electrical parameters like current, voltage, electrochemical impedance, charge and electroluminescence (Cui et al. 2015) in the electrochemical setup. Based on that electrical signal has been affected by the presence of heavy metal ions, these techniques can be categorized into potentiometric, amperometric, voltammetric, coulometric, impedance measurement and electrochemiluminescent techniques. Some important electrochemical techniques are presented below.
Potentiometry
This technique is based on the measurement of electromotive force (EMF) without applying any electric current for such measurements. Potentiometric techniques are generally used for quantitative analysis of ions in solutions. However, it is also quite effective in the detection of heavy metal ions because of its advantages like low cost, short response time, high selectivity and broad range of response (Array and Merkoci 2012). High detection limits and reduced sensitivity are some drawbacks associated with this technique, and efforts have been carried out in order to overcome such problems. Use of modified electrodes especially with carbon nanotubes and metal nanoparticles has been found promising in this regard (Düzgün et al. 2011; Bakker and Pretsch 2008). Such modified electrodes have been reported with enhanced sensitivity and also lower down the limits of detection for heavy metal ions as shown in Table 4.
Amperometry
Amperometry is a potentiostatic technique in which a fixed potential difference is applied between working and reference electrode in the solution containing electroactive species, and the flow of very small currents due to reduction of these heavy metal ions is measured. The current is recorded as a function of time, and such experiments are called amperometry techniques. By this technique, one particular metal ion is detected among various electroactive species due to fixed potential of the working electrode. The analyte to be detected undergoes a faradic reaction at some desired polarity and magnitude of the potential applied. However, due to less surface area of the working electrode, this faradic reaction is incomplete and only a fraction of analyte reacts. To overcome this problem, electrode surface has been modified by several means. In one such case, a highly sensitive screen-printed electrode modified with a nanostructured carbon black film has been used for the detection of Hg2+ with the detection limit of 5 nM (Arduini et al. 2011). In another case, amperometric biosensors which utilize biologically modified electrodes have also been used in the detection of heavy metal ions (Mohammadi et al. 2005). Electrodes have been modified so as to enhance their sensitivity towards metal ion detection by amperometric technique, as reported in Table 5.
Voltammetry
Among electrochemical techniques, voltammetry is commonly used technique for the detection of heavy metal ions. In voltammetric techniques, current is measured at different applied potentials to obtain a current–voltage curve. Voltammetry is widely used technique for heavy metal ion detection because of its high accuracy, lower limit of detections and high sensitivity. There are various forms of voltammetry, though the basic procedure, i.e. measuring the current by varying the potential, is same for the all. Some of the modes in which voltammetry is conducted for the analysis of heavy metal ions are described below.
Cyclic voltammetry
In cyclic voltammetry, applied potential is swept first in one direction and then in the reverse direction. The current in the forward and reverse scan is recorded and is plotted against applied potential to obtain cyclic voltagram. From this cyclic voltagram, some important parameters like cathodic and anodic peak potentials and corresponding currents are obtained. Gold nanoparticles-thiol functionalized reduced graphene oxide-modified glassy carbon electrode (GCE/rGO-SH/Au nanoparticles) was used for selective detection of Hg2+ by employing cyclic voltammetry (Devi et al. 2018). The sensor showed linear response for Hg2+ detection in concentration range of 1–10 μM in phosphate buffer saline (PBS) solution, and the low detection limit of 0.2 μM was obtained. Cyclic voltammetric response of GCE/rGO-SH/Au nanoparticles was compared to the one with no thiol functionalization GCE/rGO/Au nanoparticles, and a drastic change in the anodic peak potential was obtained as shown in Fig. 4. The higher anodic currents obtained in case of GCE/rGO-SH/Au nanoparticles electrode system may be due to higher loading of Au nanoparticles as the –SH group on the rGO sheets has a strong affinity towards the Au nanoparticles.

Reproduced with permission from Devi et al. (2018). Copyright 2018 Electrochemical Society
Detection of Hg2+ ions by the help of cyclic voltammetry using modified glassy carbon electrode. Cyclic voltagrams of a GCE/rGO/Au nanoparticles and b GCE/rGO/-SH/Au nanoparticles recorded in 0.1 M KCl solution in the potential range from − 0.2 to 0.3 V at a scan rate of 50 mV s−1.
Pulse voltammetry
The use of pulse of voltage signal having different shapes and amplitudes in voltammetric measurement gives rise to another type of voltammetry known as pulse voltammetry. Pulse voltammetry is further subcategorized into normal pulse voltammetry, reverse pulse voltammetry, differential pulse voltammetry, etc. Out of various pulse voltammetric methods, differential pulse is most commonly used due to its high sensitivity towards detection of heavy metal ions. Simultaneous determination of copper, lead and cadmium was carried out at a carbon paste electrode modified with hexagonal mesoporous silica (HMS)-immobilized quercetin (HMS-Qu/CPE) by employing differential pulse voltammetry (Xia et al. 2010). Voltammetric response at querectin-modified carbon paste electrodes (Qu/CPE) and querection/ionic liquid-modified carbon paste electrode (QuIL/CPE) is shown in Fig. 5, and a comparison was drawn between the voltammetric response shown by these modified electrodes towards Cu2+, Pb2+ and Cd2+ ions. The peak currents of copper, lead and Cd at HMS-Qu/CPE showed a good enhancement which apparently may be due to the cooperative effect of HMS and Qu.

Adapted from Xia et al. (2010)
Detection of Cu2+, Pb2+ and Cd2+ ions by differential pulse voltammetric technique. The differential pulse voltammograms of 1.0 μM (each) multicomponent Cu2+/Pb2+/Cd2+ solution at (a) Qu/CPE, (b) QuIL/CPE and (c) HMS-Qu/CPE.
Stripping voltammetry
Stripping voltammetry eventually consists of two main steps: electrodeposition where analyte solution from well-stirred solution is deposited on the electrode and voltammetric step where analyte is stripped off and can be analysed by any of the VM methods. The stripping voltammetry is further characterized into anodic stripping voltammetry (ASV) or cathodic stripping voltammetry (CSV) by applying anodic potential scan or a cathodic potential scan, respectively. In addition to a very low limit of detection, stripping voltammetry requires relatively simple and inexpensive instrumentation, with exceptional suitability for miniaturization (Jothimuthu et al. 2011). Anodic stripping voltammetry for the detection of zinc ions was carried out in acetate buffer by using disposable copper-based electrochemical sensor (Pei et al. 2014). Besides good sensitivity and lower limit of detection, the sensor was able to detect and measure the concentration of Zn2+ in blood serum as well. Anodic stripping voltammograms at various concentrations of Zn2+ ion are shown in Fig. 6.

Reproduced from Pei et al. (2014). Copyright 2014 American Chemical Society
Anodic stripping voltammetric detection of zinc ions by using disposable copper-based electrochemical sensor: a anodic stripping voltagram of Zn2+ samples in a range from 100 nM to 40 μM range, b calibration curves for Zn2+ using peak height and c peak area of stripping voltammograms.
Stripping methods in combination with voltammetric techniques
Stripping methods can be used in combination with various pulse voltammetric techniques that give rise to new detection techniques like linear sweep anodic stripping voltammetry, differential pulse anodic stripping voltammetry, square-wave anodic stripping voltammetry, etc., which are quite effective for trace level detection of heavy metal ions with very low limits of detection. Square-wave anodic stripping voltammetry has been performed for the detection of heavy metal ions such as Zn2+, Cd2+, Pb2+, Cu2+ and Hg2+, on cubic and octahedral Fe3O4 nanocrystal-modified electrodes (Yao et al. 2014). According to Yao et al., in comparison to cubic Fe3O4 nanocrystal-modified electrode, octahedral Fe3O4 nanocrystals show better electrochemical sensing performances towards these investigated heavy metal ions as shown in Fig. 7. Also the electrochemical performance of octahedral Fe3O4 nanocrystals showed dominating performance towards detection of Pb2+ ions with higher sensitivity and lower limit of detection compared to other metal ions.

Adapted with permission from Yao et al. (2014). Copyright 2014 American Chemical Society
Detection of various heavy metal ions by the help of square-wave anodic stripping voltammetry. Square-wave anodic stripping voltammetric response of a octahedral and b cubic Fe3O4 nanocrystals-modified GCE for determination of Pb(II), comparison of c sensitivity and d limit of detection (3σ method) for SWASV detection of Zn2+, Cd2+, Pb2+, Cu2+ and Hg2+ at Fe3O4 cubic and octahedral nanocrystals, respectively (Insets in a and b are the corresponding linear calibration plots of peak current against concentrations).
Other electrochemical methods include linear sweep voltammetry (LSV) which involves conventional electrodes linear sweep of potential (10 mV s−1 to about 1000 mV s−1) to obtain I–V curve and polarography, where one of the electrodes used is polarizable dropping mercury electrode (DME) whose potential changes from its reverse value and the other electrode used is non-polarizable electrode such as calomel electrode. Some of the important electrochemical techniques along with the electrode system used for detection of some toxic heavy metal ions are highlighted in Table 6.
Galvanostatic techniques
In galvanostatic techniques, it is electric potential that is measured while applying electric current. A current source (galvanostat) is used to control the current between the working electrode and counter electrode, and resulting potential is measured across the working and reference electrodes. Galvanostatic technique employees simple instrumentation as compared to the potentiostatic techniques; however, these techniques have the disadvantage of large double-layer charging effects that occur throughout the experiment. Galvanostatic stripping chronopotentiometry (SCP) is one of the most applied galvanostatic techniques for heavy metal ion detection. It has been reported that SCP is less sensitive to the presence of organic matter (Estela et al. 1995), as a result of which SCP has been extensively used for detection of heavy metal ions like cadmium, lead and copper in foods and biological samples (Szlyk and Czerniak-Szydlowska 2004).
Optical methods of detection
The optical effects of the materials may be detected by conventional methods of absorption, reflection or luminescence spectrometry. Optical fibres, integrated optics, capillary-type devices, specific indicator dyes, ionophores, etc., are more often used for optical detection of heavy metal ions. Optical ion sensing, though suitable for detection of some heavy metal ions, has its own limitations (Wolfbeis 2002). There are many non-selective optical indicators that react with more than one metal ion, and also many metal ion indicators combine with hydrogen ions so pH has to be controlled in a proper way so that an appropriate correction factor can be applied. To overcome the poor selectivity of indicator dyes, masking agents may be put into use. For example, fluoride may be co-immobilized in sensor membranes so that undesired interferences by other metal ions are suppressed or to make use of test strips containing reducing agents that convert analyte into a different species or oxidation state for which a selective indicator is available (Oehme and Wolfbeis 1997).
Indicator dye-based sensors
Such type of sensor is based on the binding reaction of heavy metal ion with the indicator dye that leads to change in the absorption or fluorescence of such binding reagents. In such type of heavy metal ion sensors, indicator plays the role of a transducer for the heavy metal ion for which direct optical determination is not possible. In another group of indicators, heavy metal ions act as “quenchers” where both static and dynamic quenching of luminescence of indicator dye takes place when the two combine together (Lakowicz 1983). In case of static quenching, a quencher interacts with fluorophore in its ground state, while in dynamic quenching, the interaction between the two occurs in the excited state only. The process of dynamic quenching is reversible in the sense that the dye is not consumed. Fluorescent indicators provide advantage of improved sensitivity and selectivity; however, their disadvantages cannot be ruled out. Many indicators are not selective and can bind with more than one metal ion. Some indicators cannot be used in optical sensing of heavy metal ions because of unfavourable analytical wavelengths, poor stability, the need for additional reagents and their non-availability in pure form required for sensing applications. Many indicators bind irreversibly or only at high or low pH values. The main problem associated with indicator dye-based sensors is that for each particular heavy metal ion, a specific dye, and hence a different analytical wavelength, has to be used every time, which complicates the overall procedure of detection (Oehme and Wolfbeis 1997).
Ionophore-based sensors
Because of many limitations of indicator reagents, ion-complexing organic molecules, ionophores have been employed for heavy metal ion detection by optical means. Ion carrier, complexing properties of these ionophores and their ability to bind with selective ions have made them capable for heavy metal ion specification. There are several sensing schemes where ionophores can be made as effective metal ion sensors like the introduction of chromogenic or fluorogenic moieties into ionophores or combining the ionophores with suitable dyes. Another way which is most suitable for detection of heavy metal ions is the extraction of ions into membranes using ion carriers (Lerchi et al. 1992, 1994: Hisamoto et al. 1995). In such optical sensors, selective binding of heavy metal with ionophores is responsible for recognition of heavy metal ion, while the optical signal is provided by a proton-selective chromo-ionophore. During the binding of heavy metal ions, protons equivalent to the charge of metal ion are released from the chromo-ionophore resulting in a change in the colour or fluorescence of the chromo-ionophore. The heavy metal ions bind to ionophore through coordination bonds with various heteroatoms (nitrogen, oxygen, sulphur) which are present in ionophores.
Review on optical sensors
An optic chemical sensor containing a sensitive layer of 5,10,15,20-tetra(p-sulphonatophenyl) porphyrin covalently immobilized onto a polymeric matrix has been used for the detection of Cd2+, Pb2+ and Hg2+. The complexation of these heavy metal ions with the sensor results in different absorbance spectra and thus changes the reflection behaviour of the sensor (Czolk et al. 1992). A novel Hg2+ ion-selective chemosensor bearing a rhodamine and two tosyl groups was synthesized and was successfully used for the detection of Hg2+ ions (Fig. 8). Interaction of Hg2+ with the sensor induces a visual colour change as well as significant enhancement in fluorescence of the sensor. The selectivity of the sensor lies in its ability of producing no or much smaller spectral changes on interacting with metal ions other than Hg2+ (Lee et al. 2007).

Adapted with permission from Lee et al. (2007). Copyright 2007 American Chemical Society
Detection of Hg2+ ion by the optical method where a “tren-based tripodal chemosensor” depict spectral changes on interaction with Hg2+ ion.
The detection of zinc ions by highly selective optical sensor based on the tris-triazole trimethylamine derivative with three pyrene fluorophores is based on the sharp change in the intensity ratio of the monomer (376 nm) and excimer (465 nm) emissions upon binding of metal cations in acetonitrile (Ingale and Seela 2012). Azobenzene derivative containing two amino groups is able to coordinate to Hg2+ ion. This coordination gives rise to a metal-induced intramolecular charge transfer in which free amino group serves as the electron donor. Coordination of Hg2+ induces a 100 nm bathochromic shift of the absorption maximum and red colouring of the solution. The colour change is specific to Hg2+ ions (Fu et al. 2007). Hemicyanine dye, containing aniline (electron-donating group) and a benzothiazolium (electron-withdrawing group), has been used to perform selective optical detection of Hg2+ in aqueous solutions at neutral pH (Tatay et al. 2006). A naked eye detection of Hg2+ ion was successfully achieved by using a bis(ferrocenyl) azine, as chromogenic molecule, supported on solid cellulose fibre (Fig. 9). The sensor is metal specific and produces a spectacular colour change upon binding with Hg2+ ion. The colour change is also helpful to determine the concentration of the ion either by naked eye or spectroscopically (Díez-Gil et al. 2007).

Reproduced with permission from Díez-Gil et al. (2007)
A highly selective optical detection of Hg2+ ion achieved by using a bis(ferrocenyl) azine, as chromogenic molecule a 1,4-disubstituted azine bearing two ferrocene groups, highly selective and chromogenic mercury sensor, b colour change due to binding of sensor with Hg2+ in comparison with other divalent metal cations.
The addition of Hg2+ to such ligand leads to a hypsochromic shift of the absorption band and also a colour change of solution from pink to green. Dithiacrown ether based on 1, 8-dihydroxyanthraquinone has been used for selective detection of Cd2+ and Hg2+ ions. The detection is based on the increase in the fluorescence intensity (Kadarkaraisamy and Sykes 2007). Crown ether fused to 1, 8-dihydroxyanthraquinone has been used as a fluorescent sensor for Pb2+ ions (Kadarkaraisamy and Sykes 2006). The coordination of Pb2+ to such crown ether changes the energy of excited states and increases the fluorescence intensity by many folds. In addition, a bathochromic shift of the emission band maximum as a result of coordination has also been reported.
Removal of metal ions
Once the presence of a particular metal ion and its quantity in water is tested by any of the above-mentioned detection techniques, it is very essential to remove it so that water can be made safe for use. Removal process is as important as detection because a slight excess of metal ion from its permissible limit can prove dangerous to human health. A proper technique of removal has to be followed for a particular metal ion to ensure its complete removal from the aqueous system; besides, care has to be taken to select the technique which is safe to use, environmental friendly and economical.
Methods of removal
Many methods have been used to remove heavy metal ions from water. Among these, the most remarkable ones are chemical precipitation, adsorption, ion exchange, etc., which are most often used for the removal of metal ions from aqueous systems.
Chemical precipitation
It is one of the effective techniques for removal of heavy metals from wastewater. In this process, chemical reagents are used which react with the heavy metal ions present in wastewaters and form an insoluble precipitates. These precipitates are removed by sedimentation or filtration technique to get toxic metal ion free water. The heavy metal ions may be precipitated either by hydroxide precipitation or sulphide precipitation methods. A variety of hydroxides has been used to precipitate metals from wastewater, based on the low cost and ease of handling. For example, Ca(OH)2 and NaOH were used in removing Cu(II) and Cr(VI) ions from wastewater (Mirbagheri and Hosseini 2005). Sometimes, the addition of coagulants such as alum, iron salts and organic polymers can enhance the removal of heavy metals as hydroxide precipitates from wastewater (Charerntanyarak 1999). Hydroxide precipitation is a pH-dependent process, and for mixed metals, it may create a problem as an ideal pH for one metal may put another metal back into solution. Sulphide precipitation is also an effective process for the treatment of toxic heavy metals ions. The solubility of the metal sulphide precipitate is dramatically lower than hydroxide precipitate, and also by this method metal ion can be removed selectively with faster reaction rate. Sulphide precipitation method has been used to remove Cu2+, Cd2+ and Pb2+ ions using pyrite and synthetic iron sulphide as precipitating agents (Özverdi and Erdem 2006). As an alternative, chelating precipitants like trimercaptotriazine, potassium/sodiumthiocarbonate and sodiumdimethyldithiocarbamate can be used to precipitate heavy metals from aqueous systems (Matlock et al. 2002a). Some new chelating precipitants have also been synthesized as commercial heavy metal precipitants may not possess necessary binding sites. For example, a new thiol-based compound, 1,3-benzenediamidoethanethiol (BDET2) has been synthesized and was used effectively to precipitate mercury ions in wastewater (Matlock et al. 2002b).
Ion exchange method
Ion exchange is another technique of interest for the removal of toxic heavy metal ions from wastewater because of high removal efficiency and fast kinetics of ion exchanger materials (Kang et al. 2004). Ion exchange resins, synthetic or natural, have been used for this purpose; however, synthetic resins are preferred as they are more effective in removing the heavy metals from the solution (Alyüz and Veli 2009). These cation exchangers are either strongly acidic resins with sulphonic acid groups (–SO3H) or weakly acid resins with carboxylic acid groups (–COOH) where H+ ions in the sulphonic group or carboxylic group of the exchanger get exchanged with the heavy metal ions. Factors like pH, temperature, initial metal concentration, contact time and charge of the metal ion have a good role to play in the uptake of heavy metal ions by ion exchange resins (Gode and Pehlivan 2006; Abo-Farha et al. 2009; Pathania et al. 2014). Ion exchange capabilities of thermally stable acrylamide zirconium(IV) sulphosalicylate (AaZrSs) composite material for the removal of various metal ions have been carried out by our research group (Ahad et al. 2016). Out of various metal ions tested, the exchanger was found to have maximum retention potential for Cd2+ ion with the recovery efficiency of around 98%. The effect of doze, contact time, initial metal ion concentration and pH on the adsorption behaviour of the exchanger has also been investigated (Fig. 10).

Adapted with permission from Ahad et al. (2016). Copyright 2016 Royal Society of Chemistry
Removal of various metal ions by ion exchange method while using thermally stable acrylamide zirconium(IV) sulphosalicylate (AaZrSs) as ion exchanger. Effect of a pH, b initial metal ion concentration, c adsorbent dose and d contact time on the percentage removal of Cd2+ by the hybrid material: acrylamide zirconium(IV) sulphosalicylate (AaZrSs).
Ion exchange capabilities of zirconium resorcinol phosphate synthesized by reverse micelle method have been carried out by our research group (Bashir et al. 2016). The nanocomposite was found to be Cd2+ ion selective with high distribution coefficient (Kd) value in aqueous system as well as in other solvents. As a result, the ion exchanger was not only capable of removing Cd2+ ions from wastewater but also can be effectively used to separate binary mixtures like Cd2+/Zn2+ and Cd2+/Ni2+ as shown in Fig. 11.

Adapted with permission from Bashir et al. (2016). Copyright 2016 American Chemical Society
Binary mixture separation capabilities of zirconium resorcinol phosphate nanocomposite. Separation of a Cd2+/Zn2+ ion mixture and b Cd2+/Ni2+ ion mixture.
Al-Othman et al. (2011a, b) have successfully synthesized an organic–inorganic-type composite cation exchanger, poly-o-toluidine Zr(IV) tungstate, and used this hybrid material for the removal of Hg(II) ions. In addition to its high thermal stability and rapid elution of exchangeable H+ ions, the cation exchanger was possessing high selectivity for Hg(II) ion and was quite efficient for metal ion separation in case of binary mixtures. Naushad et al. (2015a, b, c) used Nernst–Planck approximation to study kinetics of Cd2+, Co2+, Cu2+ and Pb2+ ions on the surface of acetonitrile stannic(IV) selenite composite cation exchanger. From these kinetic studies, various physical parameters like fractional attainment of equilibrium, self-diffusion coefficients, energy of activation and entropy of activation were estimated to evaluate the mechanism of ion exchange on the surface of composite ion exchange material. In similar studies, Nernst–Planck equation was applied to study the heavy metal ion exchange kinetics over the surface of nylon 6,6 Zr(IV) phosphate and poly-o-methoxyaniline Zr(IV) molybdate composite cation exchanger (Al-Othman et al. 2011a, b, 2013). Various useful ion exchange kinetic parameters were evaluated in order to validate the practical use of this ion exchanger in the field of wastewater treatment and to predict the ion exchange process occurring on the surface of the cation exchanger. A polymeric–inorganic cation exchanger, acrylonitrile stannic(IV) tungstate with good ion exchange capacity, higher stability, reproducibility and selectivity for heavy metals, is another important ion exchanger that has been used for the removal of metal ions from water (Nabi et al. 2009). Its practical application was demonstrated in the quantitative separation of Fe3+ and Zn2+ contents of a commercially available pharmaceutical, Fefol-Z sample. Zeolites have also been found good cation exchangers for heavy metal ions under different experimental conditions (Motsi et al. 2009). A schematic representation of zeolite involving ion exchange process for the removal of Co(II) ions from wastewaters is shown in Fig. 12.

Reproduced with permission from Sanaeepur et al. (2015). Copyright 2015 Elsevier
Metal ion removal capabilities of zeolites involving ion exchange process. Schematic representation of Co2+ ions distribution on the extra-framework sites in the Co(II)–NaY zeolites.
Clinoptilolite which is a natural zeolite has received extensive attention in this field due to its selectivity towards heavy metal ions. Some other ion exchanger materials used for the removal of heavy metal ion are summarized in Table 7.
Adsorption method
In many cases conventional methods, including chemical precipitation, flocculation, membrane separation, ion exchange, etc., are not desirable because of low capacities and low removal rates for metals other than Hg(II). Adsorption method is considered to be quite attractive in terms of the low cost, simple design and strong operability, especially its high removal efficiency from dilute solutions (Wang et al. 2013). Adsorbents which have been mostly used for heavy metal removal are activated carbon, biomaterials, layered double hydroxide (LDH), carbon nanotubes (CNT)-based materials, etc.
- 1.
Activated carbon.
Activated carbon has proved an efficient adsorbent for the removal of metal contaminants present in the aquatic environment. Because of its high surface areas, it is widely used in the treatment of wastewaters. The effectiveness of activated carbon in cleaning up polluted water is due to its well developed porosity structure as well as the presence of a wide spectrum of surface functional groups. Al-Malack et al. (2017) carried out adsorption studies on activated carbon and obtained adsorption efficiency of 78% and 94% for Cd2+ and Pb2+ ions, respectively. Activated carbon prepared from peanut shell was used to remove Cr(VI) from water where Cr(VI) adsorption was found to be pH dependent (AL-Othman et al. 2012). Effective adsorption of Cr(VI) was found to occur in the pH range of 2–4. Some other examples of activated carbon as adsorbent for metal ions are summarized in Table 8.
Table 8 Removal percentages of different metal ions using activated carbon as adsorbant at neutral pH - 2.
Biosorption
Biosorption of heavy metals from aqueous solutions can be considered as an alternative technology in industrial wastewater treatment; dry biomass is used to extract toxic heavy metals from industrial effluents. In biosorption of heavy metal ions, physisorption and chemical adsorption play an important role in the adsorption mechanisms of metal ions (Sahmoune 2018). The term biosorption is used to describe the passive non-metabolically mediated process of metal binding to living or dead biomass (Rangsayatorn et al. 2002). A variety of low-cost biomass has been developed and commercialized for controlling pollution. Low-cost ash and magnetite-modified ash developed from agricultural products have been used for the removal of Pb2+ ions from water where their highest removal capacities of 25 and 30 mg g−1, respectively, were reported for this toxic metal ion (Ghasemi et al. 2014a, b). Some other important biomaterials used for controlling heavy metal ion pollution include anaerobically digested sludge (Tokcaer and Yetis 2006), fungi (Garcia et al. 2005), algae (Elifantz and Tel-Or 2002), hemp-based biosorbents (Morin-Crini et al. 2019) and bacterial biomass (Oves et al. 2013). In addition, agricultural materials including rice bran, soybean, cottonseed crop milling waste, jute, leaves–derived biosorbents (Anastopoulos et al. 2018), sawdust, spent mushrooms substrates (Kulshreshtha 2018), coconut shell, cellulose-based adsorbents (Varghese et al. 2018), maize cob and groundnut husk (Saeed et al. 2005; Okieimen et al. 1985; Okieimen and Okundaye 1989; Shukla and Pai 2005; Marshall and Johns 1996; Ogunsuyi et al. 2001) have also been found extremely useful in this field. Phytoremediation, a plant-based technology is an efficient, eco-friendly and economic biosorption method to tackle the heavy metal pollution (Muthusaravanan 2018). Biosorption is receiving much more attention in the field of metal ion removal because it offers advantages like cost-effectiveness, selectivity, easy availability, efficient removal of metals even at low concentrations, less production of unwanted secondary sludge, etc. (Bashir et al. 2018; Escudero et al. 2019).
- 3.
Layered double hydroxides
Layered double hydroxides (LDH) are a type of anionic clay mineral with lamellar structures having general formula [M2+1−xM3+x(OH)2]X+ (An–)x/n · mH2O, where M2+ and M3+ represent divalent and trivalent metal cations, respectively, An– represents an interlayer anion, x represents the molar ratio of M3+/(M2+/M3+), and m represents the number of water molecules between the layers (He et al. 2018). Metal cations in the laminates of the LDHs as well as anions in the interlayers can be substituted by other metal cations and anions, respectively (Fig. 12a). As a result, different LDHs with different interlayer spacings can be obtained. The adsorption of heavy metal ion mainly depends on the exchangeability of the interlayer anions of LDHs; besides, type of cations in the laminates, the type of interlayer anions and the nature of surface groups also play an important role in the removal performance of LDHs towards heavy metal ions. Usually small anions, such as ethylenediaminetetraacetate (EDTA), citrate, glutamate, malate, are preferred for interlayer anions as they are effective for the leaching of heavy metals due to the formation of chelate complexes (Mosekiemang and Dikinya 2012). In situ oxidative polymerization was carried out for the synthesis of polyaniline/Mg/Al layered double hydroxide (PANI/LDH) and was used for the efficient removal of Cr(VI) as depicted in Fig. 12b (Zhu et al. 2016). The composite was found to have maximum adsorption capacity of 393.7 mg g−1 for Cr(VI) (Fig. 13).
Fig. 13 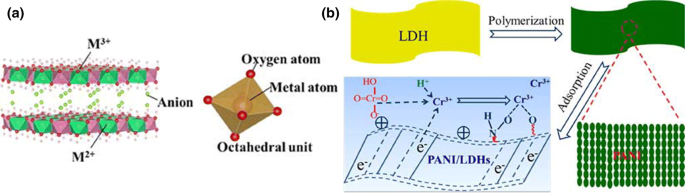
Reproduced with permission from He et al. (2018). Copyright 2018 Taylor & Francis
Use of layered double hydroxides (LDHs) for the removal of heavy metal ions from aqueous systems. a Schematic diagram of LDH, b preparation of PANI/LDHs hybrid material by an in situ oxidative polymerization procedure and its efficient use for Cr(VI).
Table 9 highlights some of the important LDHs having different interlayer anions that have been used to remove various heavy metal ions.
- 4.
Carbon nanotubes (CNTs)
CNTs can be considered as a graphite sheet that has been rolled into a tube. There are two types of CNTs: single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs) (Fig. 14).
Fig. 14 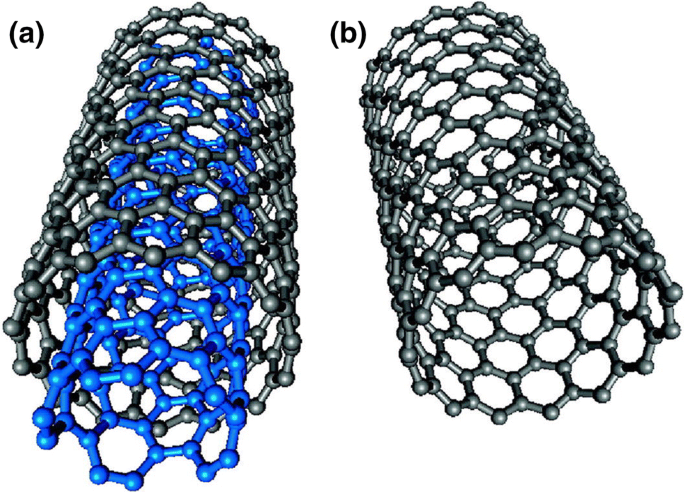
Reproduced with permission from Ihsanullah et al. (2016). Copyright 2015 Elsevier
Highly porous and hollow structure with large specific surface area of carbon nanotubes. Structure representations of a MWCNT and b SWCNT.
CNTs possess highly porous and hollow structure, large specific surface area and light mass density. Also, there are four possible interaction sites present in CNTs: internal sites, interstitial channels, grooves and outside surface (Gadhave and Waghmare 2014). As a result of these additional sites, a strong interaction between CNTs and pollutant molecules can take place very easily (Li et al. 2005). Owing to these properties, CNTs find a role in removal of hazardous pollutants from aqueous systems. Oxygen-containing functionalized carbon nanotubes (F-CNTs) were modified with chitosan so as to obtain chitosan-coated carbon nanotube (CHIT/F-CNTs). The chitosan-modified CNTs were not only found to be a good adsorbant for Cu(II) and Cr(VI) ions but metal-loaded chitosan-coated oxygen-containing functional carbon nanotubes were successfully used to develop a supercapacitor electrodes through a facile heavy metal ion adsorption and carbonization procedure (Hao et al. 2018). The scanning electron micrographs and schematic representation of chitosan-modified CNTs for heavy metal ion adsorption are shown in Fig. 15.
Fig. 15 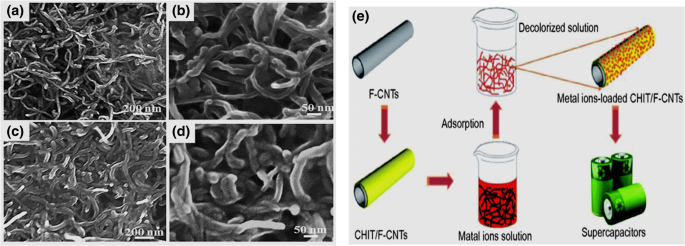
Reproduced with permission from Hao et al. (2018). Copyright 2018 Springer
Use of modified carbon nanotubes for the removal of Cu(II) and Cr(VI) ions. SEM images of a, b oxygen-containing functionalized carbon nanotubes (F-CNTs), c, d chitosan-coated carbon nanotube (CHIT/F-CNTs) and e schematic representation of chitosan-modified CNTs as a heavy metal ion adsorbant.
Some important other CNT-based materials useful for adsorption of heavy metal ions are given in Table 10.
Table 10 Removal of some toxic heavy metal ions by CNT-based materials - 5.
Other adsorbant materials
In addition to these adsorbant materials for heavy metal ions, there are so many other adsorbants, which have shown excellent performance in the field of wastewater treatment. Some of these adsorbants are not only having high adsorption capacities for the highly toxic metal ions like Hg2+ but can also be regenerated without much loss in their adsorption capacities (Naushad et al. 2016). Since in adsorption process, adsorbents can be recreated by the desorption process because it is reversible technique, adsorption is considered as an environmentally acceptable method for recovery and separation of heavy metal ions (Carolin et al. 2017). Metal ions like Hg2+ possess strong affinity towards O, N and S atoms containing ligands, so the materials coated with polymers or resins can prove effective in increasing their efficiency and selectivity towards various metal ions (Naushad et al. 2015a, b, c). Crystal violet-modified amberlite IR-120 resin have been effectively used for the removal of Co(II) ions from aqueous systems (Naushad et al. 2015a, b, c), while polyaniline Sn(IV) silicate composite has been found very much effective for the removal of Cd(II) ion (Naushad et al. 2013). Some other adsorbants that have been used for the metal ion removal are summarized in Table 11.
Table 11 List of the various adsorbants for heavy metal ion removal
Membrane filtration
Membrane filtration is useful for removal of suspending solids and organic molecules, but its role in metal ion removal can’t be ignored. There are various types of membrane filtration such as ultrafiltration, nanofiltration and reverse osmosis that can be employed for heavy metal removal from wastewater. In ultrafiltration, permeable membrane is used to separate heavy metals or macromolecules on the basis of the pore size and molecular weight (Barakat 2011). Lower driving force and a smaller space requirement due to its high packing density are some of the advantages offered by ultrafiltration. Ultrafiltration can be modified so as to improve its removal efficiency. One such modification involves polymer-supported ultrafiltration where water soluble polymeric ligands bind metal ions and form macromolecular complexes (Rether and Schuster 2003). Many polymeric complexing agents such as pectin, alginate, chitosan, polyacetic acid, polystyrene, polyethyleneimine, polyvinyl alcohol have been found very effective to increase the efficiency of metal removal by ultrafiltration (Garba et al. 2019). This modification provides advantages like low-energy requirements and fast reaction kinetics and also improves selectivity. In reverse osmosis pure solvent is forced through semi-permeable membrane by applying pressure, while solute particles are retained. The membranes used for reverse osmosis have a dense barrier layer in the polymer matrix where most of the separation process takes place. Reverse osmosis has been employed to remove Cu2+ and Cd2+ ions from wastewater where more than 90% removal has been reported (Maximous et al. 2004). In another case ceramic ultrafiltration membrane was used in the recovery of mixtures of Fe2+ and Fe3+, Cu2+ and Cr3+ by using ultrafiltration technique (Crini et al. 2017). Polymer-supported ultrafiltration is a technique of choice where metal ions form stable chelates with enhanced selectivity. The schematic representation of polymer-supported ultrafiltration is shown in Fig. 13a. Resins containing amino and imino groups are well known to form stable chelates with copper, nickel and other transition metal ions. Poly(ethylenimine) (Fig. 13b) is one such example which has been used in connection with polysulphone or polyamide membranes for heavy metal ion removal (Geckeler and Volchek 1996). Molecules of poly(ethylenimine) form chelates with transition metal ions with no interference from the presence of alkali and alkaline earth metals (Fig. 16).

Adapted with permission from Geckeler and Volchek (1996). Copyright 1996 American Chemical Society
Polymer-supported ultrafiltration as a tool for the removal of heavy metal ions from water. a Schematic representation of the polymer-supported ultrafiltration separation technique and b idealized structure of the polymeric complex poly(ethylenimine) with copper (II) ions.
Electrochemical method
Electrolytic recovery is one of the methods used to remove metals from wastewaters. This process uses electricity to pass through an aqueous metal-bearing solution. Electrochemical treatments of wastewater involve electrodeposition, electrocoagulation, electrofloatation and electro-oxidation (Shim et al. 2014). In this process, consumable electrode is used to supply ions into the wastewater, where they neutralize the charges of the particles (Tran et al. 2017). These ions remove the undesirable contaminants by means of chemical reaction, coagulation or precipitation as depicted in Fig. 17. In electroprecipitation method heavy metals present in wastewater are precipitated as hydroxides with the supply of electricity.

Reproduced with permission from Al-Qodah and Al-Shannag (2017). Copyright 2017 Taylor & Francis
Schematic diagram of an electrocoagulation cell showing the main reactions involved in the electrocoagulation process, possessing a carbonaceous material cathode.
Copper, chromium and nickel ions have been removed from wastewater by using electrocoagulation process with 100% removal efficiency in a time span of 20 min (Akbal and Camci 2011). Nearly 100% removal efficiency has also been reported for Cd2+, Cu2+ and Ni2+ ions by electrocoagulation method using batch cylindrical iron reactor (Un and Ocal 2015). The electrochemical method has some advantages over the traditional flotation and coagulation such as better removal rate and larger probability of coagulation as the electricity applied to the system sets the whole process in motion, reduced sludge production, no requirement for chemical use and ease of operation. On the other hand, chemical precipitation requires a large amount of chemicals to reduce metals to an acceptable level for discharge.
Conclusion
Pollution by heavy metal ions is one of the serious environmental problems. Heavy metal ion toxicity has been reported to cause many health issues to living beings which has motivated researchers to develop various strategies for detection and removal of these heavy metal ions from aqueous systems to make water safe for use. Many materials and methods have been adopted earlier for these purposes which have shown promising performance, but are having many drawbacks as well. Such materials and methods have been modified from time to time in order to improve their performance and to overcome the associated limitations. Search for new materials and methods, which are more efficient, environment friendly, easy to operate and cheap, is a continuous ongoing process to lower down the concentrations of these heavy metal ions below the level where they cannot prove dangerous to living beings.
References
Abdulrazak S, Hussaini K, Sani HM (2017) Evaluation of removal efficiency of heavy metals by low-cost activated carbon prepared from African palm fruit. Appl Water Sci 7:3151–3155
Abo-Farha SA, Abdel-Aal AY, Ashourb IA, Garamon SE (2009) Removal of some heavy metal cations by synthetic resin purolite C100. J Hazard Mater 169:190–194
Aglan RF, Saleh HM, Mohamed GG (2018) Potentiometric determination of mercury(II) ion in various real samples using novel modifed screen-printed electrode. Appl Water Sci 8:141–151
Ahad S, Bashir A, Manzoor T, Pandith AH (2016) Exploring the ion exchange and separation capabilities of thermally stable acrylamide zirconium(IV) sulphosalicylate (AaZrSs) composite material. RSC Adv 6:35914–35927
Akbal F, Camci S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalin 269:214–222
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fsh. Risk to human health. Environ Chem Lett 16:903–917
Al-Malack MH, Al-Attas OG, Basaleh AA (2017) Competitive adsorption of Pb2+ and Cd2+ onto activated carbon produced from municipal organic solid waste. Desalin Water Treat 60:310–318
Al-Othman ZA, Inamuddin, Naushad M (2011a) Determination of ion-exchange kinetic parameters for the poly-o-methoxyaniline Zr(IV) molybdate composite cation-exchanger. Chem Eng J 166:639–645
Al-Othman ZA, Naushad M, Inamuddin (2011b) Organic–inorganic type composite cation exchanger poly-o-toluidine Zr(IV) tungstate: preparation, physicochemical characterization and its analytical application in separation of heavy metals. Chem Eng J 172:369–375
AL-Othman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Al-Othman ZA, Alam MM, Naushad M (2013) Heavy toxic metal ion exchange kinetics: validation of ion exchange process on composite cation exchanger nylon 6,6 Zr(IV) phosphate. J Ind Eng Chem 19:956–960
Alqadami AA, Naushad M, Abdalla MA, Ahamad T, AL Othman ZA, Alsehri SM, AA AA (2017a) Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J Clean Prod 156:426–436
Alqadami AA, Naushad M, Alothman ZA, Ghfar AA (2017b) Novel metal − organic framework (MOF) based composite material for the sequestration of U(VI) and Th(IV) metal ions from aqueous environment. ACS Appl Mater Interfaces 9:36026–36037
Al-Qodah Z, Al-Shannag M (2017) Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep Sci Technol 52:2649–2676
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Anastopoulos I, Robalds A, Tran HN, Mitrogiannis D, Giannakoudakis DA, Hosseini-Bandegharaei A, Dotto GL (2018) Removal of heavy metals by leaves-derived biosorbents. Environ Chem Lett 5:4. https://doi.org/10.1007/s10311-018-00829-x
Arduini F, Majorani C, Amine A, Moscone D, Palleschi G (2011) Hg2+ detection by measuring thiol groups with a highly sensitive screen-printed electrode modified with a nanostructured carbon black film. Electrochim Acta 56:4209–4215
Array G, Merkoci A (2012) Nanomaterials application in electrochemical detection of heavy metals. Electrochim Acta 84:49–61
Athanasiadis K, Helmreich B (2005) Influence of chemical conditioning on the ion exchange capacity and on kinetic of zinc uptake by clinoptilolite. Water Res 39:1527–1532
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14:94. https://doi.org/10.3390/ijerph14010094
Badawy NA, El-Bayaa AA, Abdel-Aal AY, Garamon SE (2009) Chromatographic separations and recovery of lead ions from a synthetic binary mixtures of some heavy metal using cation exchange resin. J Hazard Mater 166:1266–1271
Bahr JL, Mickelson ET, Bronikowski MJ, Smalley RE, Tour JM (2001) Dissolution of small diameter single-wall carbon nanotubes in organic solvents. Chem Commun 2:193–194
Bakker E, Pretsch E (2008) Nanoscale potentiometry. Trends Anal Chem 27:612
Bansod BK, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arabian J Chem 4:361–377
Bashir A, Ahad S, Pandith AH (2016) Soft template assisted synthesis of zirconium resorcinol phosphate nanocomposite material for the uptake of heavy-metal ions. Ind Eng Chem Res 55:4820–4829
Bashir A, Malik LA, Ahad S, Manzoor T, Bhat MA, Dar GN, Pandith AH (2018) Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00828-y
Beltran B, Leal LO, Ferrer L, Cerd V (2015) Determination of lead by atomic fluorescence spectrometry using an automated extraction/preconcentration flow system. J Anal At Spectrom 30:1072–1079
Bernard E, Jimoh A, Odigure JO (2013) Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res J Chem Sci 3:3–9
Cabrera-Vique C, Teissedre P, Cabanis M, Cabanis J (1997) Determination and levels of chromium in french wine and grapes by graphite furnace atomic absorption spectrometry. J Agric Food Chem 45:1808–1811
Carolin CF, Kumar PS, Saravanan A, Joshib GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799
Celebi MS, Ozyörük H, Yildiz A, Abaci S (2009) Determination of Hg2+ on poly(vinylferrocenium) (PVF+)-modified platinum electrode. Talanta 78:405–409
Charerntanyarak L (1999) Heavy metals removal by chemical coagulation and precipitation. Wat Sci Technol 39:135–138
Chen J, Xiao S, Wu X, Fang K, Liu W (2005) Determination of lead in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction. Talanta 67:992–996
Chen H, Qian GR, Ruan XX, Frost RL (2016) Removal process of nickel(II) by using dodecyl sulfate intercalated calcium aluminum layered double hydroxide. Appl Clay Sci 132:419–424
Chiarle S, Ratto M, Rovatti M (2000) Mercury removal from water by ion exchange resins adsorption. Water Res 34:2971–2978
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155
Crini G, Morin-Crini N, Fatin-Rouge Deon S, Fievet P (2017) Metal removal from aqueous media by polymer-assisted ultrafiltration with chitosan. Arabian J Chem 10:3826–3839
Cui L, Wu J, Ju H (2015) Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron 63:276–286
Cvetkovi J, Arpadjan S, Karadjova I, Stafilov T (2006) Determination of cadmium in wine by electrothermal atomic absorption spectrometry. Acta Pharm 56:69–77
Czolk R, Reichert J, Ache HJ (1992) An optical sensor for the detection of heavy metal ions. Sens Actuators B 7:540–543
de Greogi I, Quiroz W, Pinochet H, Pannier F, Potin-Gautier M (2007) Speciation analysis of antimony in marine biota by HPLC-(UV)-HG-AFS: extraction procedures and stability of antimony species. Talanta 73:458–465
Devi NR, Sasidharan M, Sundramoorthy AK (2018) Gold nanoparticles-thiol-functionalized reduced graphene oxide coated electrochemical sensor system for selective detection of mercury ion. J Electrochem Soc 165:3046–3053
Díez-Gil C, Caballero A, Ratera I, Tárraga A, Molina P, Veciana J (2007) Naked-eye and selective detection of mercury (II) ions in mixed aqueous media using a cellulose-based support. Sensors 7:3481–3488
Dong SF, Zhu ZG (2002) Determination of the contents of Ca, Mg, Fe, Cu and Zn in suxiao jiuxin pill and the analysis of Ca/Mg and Cu/Zn values. Guang Pu Xue Yu Guang Pu Fen Xi 22:478–479
Dong SF, Zhu ZG (2003) Determination of the content of inorganic elements in taponin tablet recipe. Guang Pu Xue Yu Guang Pu Fen Xi 23:201–202
Dubey S, Shri M, Gupta A, Rani V, Chakrabarty D (2018) Toxicity and detoxifcation of heavy metals during plant growth and metabolism. Environ Chem Lett 16:1169–1192
Durmuşkahya C, Alp H, Hortooglu ZS, Toktas U, Kayalar H (2016) X-ray fluorescence spectroscopic determination of heavy metals and trace elements in aerial parts of Origanum sipyleum L from Turkey. Trop J Pharm Res 15:1013–1015
Düzgün A, Zelada-Guillén GA, Crespo GA, Macho S, Riu J, Rius FX (2011) Nanostructured materials in potentiometry. Anal Bioanal Chem 399:171–181
El-Bahi SM, Sroor AT, Arhoma NF, Darwish SM (2013) XRF analysis of heavy metals for surface soil of Qarun Lake and Wadi El Rayan in Faiyum, Egypt. Open J Metal 3:21–25
Elfeky SA, Mahmoud SE, Youssef AF (2017) Applications of CTAB modified magnetic nanoparticles for removal of chromium(VI) from contaminated water. J Adv Res 8:435–443
Elifantz H, Tel-Or E (2002) Heavy metal biosorption by plant biomass of the macrophyte Ludwigia stolonifera. Water Air Soil Pollut 141:207–218
Ene A, Bosneaga A, Georgescu L (2010) Determination of heavy metals in soils using XRF technique. Romanian J Phys 55:815–820
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427
Eshaq G, Rabie AM, Bakr AA, Mady AH, ElMetwally AE (2016) Cr(VI) adsorption from aqueous solutions onto Mg–Zn–Al LDH and its corresponding oxide. Desalin Water Treat 57:20377–20387
Estela JM, Tomas C, Cladera A, Cerda V (1995) Potentiometric stripping analysis: a review. Crit Rev Anal Chem 25:91
Flora SJS (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2:191–206
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its possible reversal by chelationtherapy. Indian J Med Res 128:501–523
Fu Y, Li H, Hu W (2007) Small molecular chromogenic sensors for Hg2+: a strong, “push–pull” system exists after binding. Eur J Org Chem 2007:2459–2463
Gadhave A, Waghmare J (2014) Removal of heavy metal ions from wastewater by carbon nanotubes (CNTs). Int J Chem Sci Appl 5:56–67
Garba MD, Usman M, Mazumder MAJ, Al-Ahmed A, Inamuddin (2019) Complexing agents for metal removal using ultrafltration membranes: a review. Environ Chem Lett 5:4. https://doi.org/10.1007/s10311-019-00861-5
Garcia MA, Alonso J, Melgar MJ (2005) Agaricus macrospores as a potential bioremediation agent for substrates contaminated with heavy metals. J Chem Technol Biotechnol 80:325–330
Geckeler KE, Volchek K (1996) Removal of hazardous substances from water using ultrafiltration in conjunction with soluble polymers. Environ Sci Technol 30:725–734
Gemma F, Juan ML, Ana B, Jose DL (2006) Daily intake of arsenic, cadmium, mercury, and lead by consumption of edible marine species. J Agric Food Chem 54:6106–6112
Ghasemi M, Naushad M, Ghasemi N, Khosravi-fard Y (2014a) A novel agricultural waste based adsorbent for the removal of Pb(II) from aqueous solution: kinetics, equilibrium and thermodynamic studies. J Ind Eng Chem 20:454–461
Ghasemi M, Naushad M, Ghasemi N, Khosravi-fard Y (2014b) Adsorption of Pb(II) from aqueous solution using new adsorbents prepared from agricultural waste: adsorption isotherm and kinetic studies. J Ind Eng Chem 20:2193–2199
Gode F, Pehlivan E (2006) Removal of chromium (III) from aqueous solutions using Lewatit S 100: the effect of pH, time, metal concentration and temperature. J Hazard Mater 136:330–337
Gong T, Liu J, Liu X, Liu J, Xiang J, Wu Y (2016) A sensitive and selective platform based on CdTe QDs in the presence of l-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem 213:306–312
Gumpu MB, Sethuramanb S, Krishnanb UM, Rayappana JBB (2015) A review on detection of heavy metal ions in water—an electrochemical approach. Sens Actuators B 213:515–533
Gumpu MB, Krishnan UM, Rayappan JBB (2017) Design and development of amperometric biosensor for the detection of lead and mercury ions in water matrix-a permeability approach. Anal Bioanal Chem 409:4257–4266
Hafuka A, Takitani A, Suzuki H, Iwabuchi T, Takahashi M, Okabe S, Satoh H (2017) Determination of cadmium in brown rice samples by fluorescence spectroscopy using a fluoroionophore after purification of cadmium by anion exchange resin. Sensors 17:2291–2300
Hao P, Ma X, Xie J, Lei F, Li L, Zhu W, Cheng X, Cui G, Tang B (2018) Removal of toxic metal ions using chitosan coated carbon nanotube composites for supercapacitors. Sci China Chem 61:797–805
Harrington CF, Clough R, Drennan-Harris LR, Hill SJ, Tyson JF (2011) Atomic spectrometry update. Elemental speciation. J Anal At Spectrom 26:1561–1595
He X, Qiu X, Hu C, Liu Y (2018) Treatment of heavy metal ions in wastewater using layered double hydroxides: a review. J Dispers Sci Technol 39:792–801
Hisamoto H, Nakagawa E, Nagatsuka K, Abe Y, Sato S, Siswanta D, Suzuki K (1995) Silver ion selective optodes based on novel thia ether compounds. Anal Chem 67:1315–1321
Hutton LA, ONeil GD, Read TL, Ayres Z, Newton ME, Macpherson JV (2014) Electrochemical X-ray fluorescence spectroscopy for trace heavy metal analysis: enhancing X-ray fluorescence detection capabilities by four orders of magnitude. Anal Chem 86:4566–4572
Ihsanullah AA, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161
Inamuddin, Naushad M, Rangreeza TA, AL-Othman ZA (2015) Ion-selective potentiometric determination of Pb(II) ions using PVC-based carboxymethyl cellulose Sn(IV) phosphate composite membrane electrode. Desalin Water Treat 56:806–813
Ingale SA, Seela F (2012) A ratiometric fluorescent on–off Zn2+ chemosensor based on a tripropargylamine pyrene azide click adduct. J Org Chem 77:9352–9356
Inglezakis VJ, Stylianou MA, Gkantzou D, Loizidou MD (2007) Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210:248–256
Innuphata C, Chootoa P (2017) Determination of trace levels of Cd(II) in tap water samples by anodic stripping voltammetry with an electrografted boron-doped diamond electrode. ScienceAsia 43:33–41
Ismaiel AA, Aroua MK, Yusoff R (2012) Potentiometric determination of trace amounts of mercury(II) in water sample using a new modified palm shell activated carbon paste electrode based on kryptofix®5. Am J Anal Chem 3:859–865
Javanbakht M, Divsar F, Badiei A, Ganjali MR, Norouzi P, Mohammadi ZG, Chaloosi M, Abdi JA (2009) Potentiometric detection of mercury(II) ions using a carbon paste electrode modified with substituted thiourea-functionalized highly ordered nanoporous silica. Anal Sci 25:789–794
Jian-Qua L, Xi-We H, Xian-Shun Z, Hai-Li Z, Zheng-Zh Z (2003) Anodic stripping voltammetric determination of lead (II) using glassy carbon electrode modified with novel Calix[4] arene. Chin J Chem 21:687–692
Jothimuthu P, Wilson RA, Herren J, Haynes EN, Heineman WR, Papautsky I (2011) Lab-on-a-chip sensor for detection of highly electronegative heavy metals by anodic stripping voltammetry. Biomed Micro 13:695–703
Kadarkaraisamy M, Sykes AG (2006) Luminescence detection of transition and heavy metals by inversion of excited states: synthesis, spectroscopy, and X-ray crystallography of Ca, Mn, Pb, and Zn complexes of 1,8-anthraquinone-18-crown-5. Inorg Chem 45:779–786
Kadarkaraisamy M, Sykes AG (2007) Selective luminescence detection of cadmium(II) and mercury(II) utilizing sulfur-containing anthraquinone macrocycles (part 2) and formation of an unusual Hg2 2+-crown ether dimer via reduction of Hg(II) by DMF. Polyhedron 26:1323–1330
Kahlon SK, Sharma G, Julka JM, Kumar A, Sharma S, Stadler FJ (2018) Impact of heavy metals and nanoparticles on aquatic biota. Environ Chem Lett 16:919–946
Kanchana P, Sudhan N, Anandhakumar S, Mathiyarasu J, Manisankar P, Sekar C (2015) Electrochemical detection of mercury using biosynthesized hydroxyapatite nanoparticles modified glassy carbon electrodes without preconcentration. RSC Adv 5:68587–68594
Kang SY, Lee JU, Moon SH, Kim KW (2004) Competitive adsorption characteristics of Co2+, Ni2+, and Cr2+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 56:141–147
Kapolna E, Gerely V, Dernovics M, Illes A, Fodor P (2007) Fate of selenium species in sesame seeds during simulated bakery process. J Food Eng 79:494–501
Karimi M, Aboufazeli F, Zhad HRLZ, Sadeghi O, Najafi E (2012) Determination of cadmium(II) ions in environmental samples: a potentiometric sensor. Curr World Environ 7:201–206
Karnib M, Kabbani A, Holail H, Olama Z (2014) Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 50:113–120
Kempegowda RG, Malingappa P (2012) A binderless, covalently bulk modified electrochemical sensor: application to simultaneous determination of lead and cadmium at trace level. Anal Chim Acta 728:9–17
Kojuncu Y, Bundalevska JM, Ay U, Cundeva K, Stafilov T, Akcin G (2004) Atomic absorption spectrometry determination of Cd, Cu, Fe, Ni, Pb, Zn, and Tl traces in seawater following flotation separation. Sep Sci Technol 39:2751–2765
Kulshreshtha S (2018) Removal of pollutants using spent mushrooms substrates. Environ Chem Lett 5:5. https://doi.org/10.1007/s10311-018-00840-2
Lair GJ, Gerzabek MH, Haberhauer G (2007) Sorption of heavy metals on organic and inorganic soil constituents. Environ Chem Lett 5:23–27
Lakowicz JR (1983) Principles of fluorescence spectroscopy. Plenum, New York
Lebedev A, Sinikova N, Nikolaeva S, Poliakova O, Khrushcheva M, Pozdnyakov S (2003) Metals and organic pollutants in snow surrounding an iron factory. Environ Chem Lett 1:107–112
Lee MH, Wu J, Lee JW, Jung JH, Kim JS (2007) Highly sensitive and selective chemosensor for Hg2+ based on the rhodamine fluorophore. Org Lett 9:2501–2504
Lee HJ, Lagger G, Pereirac CM, Silvac AF, Girault HH (2009) Amperometric tape ion sensors for cadmium(II) ion analysis. Talanta 78:66–70
Lerchi M, Bakker E, Rusterholz B, Simon W (1992) Lead-selective bulk optodes based on neutral ionophores with subnanomolar detection limits. Anal Chem 64:1534–1540
Lerchi M, Reitter E, Simon W, Pretsch E, Chowdhury DA, Kamata S (1994) Bulk optodes based on neutral dithiocarbamate ionophores with high selectivity and sensitivity for silver and mercury cations. Anal Chem 66:1713–1717
Li YH, Di Z, Ding J, Wu D, Luan Z, Zhu Y (2005) Adsorption thermodynamic kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res 39:605–609
Li X, Zhou D, Xu J, Chen H (2007) In-channel indirect amperometric detection of heavy metal ions for electrophoresis on a poly(dimethylsiloxane) microchip. Talanta 71:1130–1135
Li Y, Gao B, Wu T, Sun D, Li X, Wang B, Lu F (2009) Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res 43:3067–3075
Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Smalley RE (1998) Fullerene pipes. Science 280:1253–1256
Liu ZG, Chen X, Jia Y, Liu JH, Huang XJ (2014a) Role of Fe(III) in preventing humic interference during As(III) detection on gold electrode: spectroscopic and voltammetric evidence. J Hazard Mater 267:153–160
Liu ZG, Chen X, Liu JH, Huang XJ (2014b) Robust electrochemical analysis of As(III) integrating with interference tests: a case study in groundwater. J Hazard Mater 278:66–74
Logar M, Horvat M, Akagi H, Pihlar B (2002) Simultaneous determination of inorganic mercury and methylmercury compounds in natural waters. Anal Bioanal Chem 374:1015–1021
Losev VN, Buyko OV, Trofimchuk AK, Zuy ON (2015) Silica sequentially modified with polyhexamethylene guanidine and arsenazo I for preconcentration and ICPOES determination of metals in natural waters. Micro Chem J 123:84–89
Luo L, Wang X, Ding Y, Li Q, Jia J, Deng D (2010) Voltammetric determination of Pb2+ and Cd2+ with montmorillonite-bismuth-carbon electrodes. Appl Clay Sci 50:154–157
Lutfullah Rashid M, Rahman N (2012) Potentiometric sensor for the determination of lead(II) ion based on zirconium(IV) iodosulphosalicylate. Sci Adv Mater 4:1–6
Ma L, Wang Q, Islam SM, Liu Y, Ma S, Kanatzidis MG (2016) Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS4 2− ion. J Am Chem Soc 138:2858–2866
Ma L, IslamSM Liu H, Zhao J, Sun G, Li H, Ma S, Kanatzidis MG (2017) Selective and efficient removal of toxic oxoanions of As(III), As(V), and Cr(VI) by layered double hydroxide intercalated with MoS4 2−. Chem Mater 29:3274–3284
Majid S, El-Rhazi M, Amine A, Brett CMA (2002) An amperometric method for the determination of trace mercury(II) by formation of complexes with l-tyrosine. Anal Chim Acta 464:123–133
Mallampati SR, Mitoma Y, Okuda T, Sakita S, Kakeda M (2013) Total immobilization of soil heavy metals with nano-Fe/Ca/CaO dispersion mixtures. Environ Chem Lett 11:119–125
Marguí E, Kregsamer P, Hidalgoc M, Tapias J, Queralt I, Streli C (2010) Analytical approaches for Hg determination in wastewater samples by means of total reflection X-ray fluorescence spectrometry. Talanta 82:821–827
Marshall WE, Johns MM (1996) Agricultural by-products as metal adsorbents: sorption properties and resistance to mechanical abrasion. J Chem Technol Biotechnol 66:192–198
Matlock MM, Henke KR, Atwood DA (2002a) Effectiveness of commercial reagents for heavy metal removal from water with new insights for future chelate designs. J Hazard Mater 92:129–142
Matlock MM, Howerton BS, Aelstyn MAV, Nordstrom FL, Atwood DA (2002b) Advanced mercury removal from gold leachate solutions prior to gold and silver extraction: a field study from an active gold mine in Peru. Environ Sci Technol 36:1636–1639
Maximous NN, Nakhla GF, Wan WK (2004) Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination 164:105–110
Mazloum-ardakani M, Amini MK, Dehghan M, Kordi E, Sheikh-mohsen MA (2012) Nanomolar determination of Pb(II) ions using a selective templated electrode. J Serb Chem Soc 77:899–910
Minami T, Atsumi K, Ueda J (2003) Determination of cobalt and nickel by graphite-furnace atomic absorption spectrometry after coprecipitation with scandium hydroxide. Anal Sci 19:313–315
Mirbagheri SA, Hosseini SN (2005) Pilot plant investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Desalination 171:85–93
Mittal A, Naushad M, Sharma G, ALothman ZA, Wabaidur SM, Alam M (2016) Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin Water Treat 57(46):21863–21869
Mohammadi H, Amine A, Cosnier S, Mousty C (2005) Mercury–enzyme inhibition assays with an amperometric sucrose biosensor based on atrienzymatic-clay matrix. Anal Chim Acta 543:143–149
Moraes PM, Santos FA, Cavecci B, Padilha CC, Vieira JC, Roldan PS, Padilha PM (2013) GFAAS determination of mercury in muscle samples of fish from Amazon, Brazil. Food Chem 141:2614–2617
Morin-Crini N, Loiacono S, Placet V, Torri G, Bradu C, Kostić M, Cosentino C, Chanet G, Martel B, Lichtfouse E, Crini G (2019) Hemp-based adsorbents for sequestration of metals: a review. Environ Chem Lett 17:393–408
Mosekiemang T, Dikinya O (2012) Efficiency of chelating agents in retaining sludge-borne heavy metals in intensively applied agricultural soils. Int J Environ Sci Technol 9:129–134
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92:42–48
Mugheri AQ, Tahira A, Sirajuddin Sherazi STH, Abro MI, Willander M, Ibupoto ZH (2016) An amperometric indirect determination of heavy metal ions through inhibition of glucose oxidase immobilized on cobalt oxide nanostructures. Sens Lett 14:1–9
Muniz-Naveiro O, Dominguez-Gonzalez R, Bermejo-Barrera A, Bermejo-Barrera P, Cocho JA, Fraga JM (2007) Selenium speciation in cow milk obtained after supplementation with different selenium forms to the cow feed using liquid chromatography coupled with hydride generation-atomic fluorescence spectrometry. Talanta 71:1587–1593
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359
Nabi SA, Naushad M, Bushra R (2009) Synthesis and characterization of a new organic–inorganic Pb2+ selective composite cation exchanger acrylonitrile stannic(IV) tungstate and its analytical applications. Chem Eng J 152:80–87
Nagajyoti PC, Lee KD, Sreekanth TVM (2018) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Naushad M (2014) Surfactant assisted nano-composite cation exchanger: development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem Eng J 235:100–108
Naushad M, AL-Othman ZA, Islam M (2013) Adsorption of cadmium ion using a new composite cationexchanger polyaniline Sn(IV) silicate: kinetics, thermodynamic and isotherm studies. Int J Environ Sci Technol 10:567–578
Naushad M, Rangreez TA, Inamuddin (2014) Potentiometric determination of Cd(II) ions using PVC based polyaniline Sn(IV) silicate composite cationexchanger ion-selective membrane electrode. Desalin Water Treat 55:1–8
Naushad M, AL-Othman ZA, Sharma G, Inamuddin (2015a) Kinetics, isotherm and thermodynamic investigations for the adsorption of Co(II) ion onto crystal violet modified amberlite IR-120 resin. Ionics 21:1453–1459
Naushad M, Vasudevan S, Sharma G, Kumar A, AL-Othman ZA (2015b) Adsorption kinetics, isotherms, and thermodynamic studies for Hg2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalin Water Treat 57:21863–21869
Naushad M, Mittal A, Rathore M, Gupta V (2015c) Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin Water Treat 54:2883–2890
Naushad M, Ahamad T, Sharma G, Al-Muhtaseb AH, Albadarin AB, Alam MM, AL-Othman ZA, Alshehri SM, Ghfar AA (2016) Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem Eng J 300:306–316
Naushad M, Ahamad T, Basheer Al-Maswari M, Alqadami AA, Alshehri SM (2017) Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem Eng J 330:1351–1360
Nie LX, Jin HY, Wang GL, Tian JG, Lin RC (2008) Study of determination method for heavy metals and harmful elements residues in four traditional Chinese medicine injections. Zhongguo Zhong Yao Za Zhi 33:2764–2767
Odonchimeg S, Oyun J, Javkhlantugs N (2016) Determination of plantinum in rocks by graphite furnace atomic absorption spectrometry after separation on sorbent. Int Res J Eng Technol 03:753–757
Oehme I, Wolfbeis OS (1997) Optical sensors for determination of heavy metal ions. Mikrochim Acta 126:177–192
Ogunsuyi HO, Ipinmoroti KO, Amoo IA, Ajayi OO (2001) Adsorption of Cu(II) ions from aqueous solution on thiolated and activated cellulose adsorbents developed from agricultural wastes. J Technosci 5:75–83
Ojemaye MO, Okoh OO, Okoh AI (2017) Surface modified magnetic nanoparticles as efficient adsorbents for heavy metal removal from wastewater: progress and prospects. Mater Express 7:439–456
Okieimen FE, Okundaye JN (1989) Removal of cadmium and copper ions from aqueous solutions with thiolated maize (Zea mays) cob meal. Biol Wastes 30:225–230
Okieimen FE, Ogbeifun DE, Nwala GN, Kumsah CA (1985) Binding of cadmium, copper and lead ions by modified cellulosic materials. Bull Environ Contam Toxicol 34:866–870
Oliveira V, Sarmiento AM, Gomez-Ariza JK, Nieto JM, Sanchez-Rodas D (2006) New preservation method for inorganic arsenic speciation in acid mine drainage samples. Talanta 69:1182–1189
Othman AM (2006) Potentiometric determination of mercury(II) using a tribromomercurate–rhodamine B PVC membrane sensor. Int J Environ Anal Chem 86:367–379
Oves M, Khan MS, Zaidi A (2013) Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci 20:121–129
Özverdi A, Erdem M (2006) Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J Hazard Mater 137:626–632
Palmer PT, Jacobs R, Baker PE, Ferguson K, Webber S (2009) Use of fieldportable XRF analyzers for rapid screening of toxic elements in FDA regulated products. J Agric Food Chem 57:2605–2613
Pathania D, Sharma G, Naushad M, Kumar A (2014) Synthesis and characterization of a new nanocomposite cation exchanger polyacrylamide Ce(IV) silicophosphate: photocatalytic and antimicrobial applications. J Ind Eng Chem 20:3596–3603
Pei X, Kang W, Yue W, Bange A, Heineman WR, Papautsky I (2014) Disposable copper-based electrochemical sensor for anodic stripping voltammetry. Anal Chem 86:4893–4900
Poikyo R, Permki P (2003) Acid dissolution methods for heavy metals determination in pine needles. Environ Chem Lett 1:191–195
Pujol L, Evrard D, Serrano KG, Freyssinier M, Cizsak AR, Gros P (2014) Electrochemical sensors and devices for electrochemical assay in water: the French groups contribution. Front Chem Anal Chem 19:1–24
Radu T, Diamond D (2009) Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J Hazard Mater 171:1168–1171
Radulescu C, Dulama ID, Stihi C, Ionita I, Chilian A, Necula C, Chelarescu ED (2014) Determination of heavy metal levels in water and therapeutic mud by atomic absorption spectrometry. Romanian J Phys 59:1057–1066
Rahman MM, Adil M, Yusof AM, Kamaruzzaman YB, Ansary RH (2014) Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells materials 7:3634–3650
Rahmanian O, Amini S, Dinari M (2018) Preparation of zinc/iron layered double hydroxide intercalated by citrate anion for capturing lead(II) from aqueous solution. J Mol Liq 256:9–15
Rangsayatorn N, Upatham ES, Kruatrachue M, Pokethitiyook P, Lanza GR (2002) Phytoremediation potential of Spirulina (Arthrospira) platensis: biosorption and toxicity studies of cadmium. Environ Pollut 119:45–53
Ratner N, Mandler D (2015) Electrochemical detection of low concentrations of mercury in water using gold nanoparticles. Anal Chem 87:5148–5155
Rether A, Schuster M (2003) Selective separation and recovery of heavy metal ions using water-soluble N-benzoylthiourea modified PAMAM polymers. React Funct Polym 57:13–21
Ruiz-Chancho MJ, Sabe R, Lopez-Sanchez JF, Rubio R, Thomas P (2005) New approaches to the extraction of arsenic species from soils. Microchim Acta 151:241–248
Saeed A, Iqbal M, Akhtar MW (2005) Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (Black gram husk). J Haz Mater 117:65–73
Sahmoune MN (2018) Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00819-z
Sanaeepur H, Kargari A, Nasernejad B, Amooghin AE, Omidkhah M (2015) A novel Co2+ exchanged zeolite Y/cellulose acetate mixed matrix membrane for CO2/N2 separation. J Taiwan Inst Chem Eng 60:403–413
Sanchez-Rodas D, GomezAriza JL, Oliveira V (2006) Development of a rapid extraction procedure for speciation of arsenic in chicken meat. Anal Bioanal Chem 385:1172–1177
Sankararamakrishnan N, Jaiswal M, Verma N (2014) Composite nanofloral clusters of carbon nanotubes and activated alumina: an efficient sorbent for heavy metal removal. Chem Eng J 235:1–9
Schaeffer R, Soeroes C, Ipolyi I, Fodor P, Thomaidis NS (2005) Determination of arsenic species in seafood samples from the Aegean Sea by liquid chromatography–(photo-oxidation)–hydride generation–atomic fluorescence spectrometry. Anal Chim Acta 547:109–118
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med 56:537–546
Shahat A, Awual MR, Khaleque MA, Alam MZ, Naushad M, Chowdhury AMS (2015) Large-pore diameter nano-adsorbent and its application for rapid lead(II) detection and removal from aqueous media. Chem Eng J 273:286–295
Shaidan NH, Eldemerdash U, Awad S (2012) Removal of Ni(II) ions from aqueous solutions using fixed-bed ion exchange column technique. J Taiwan Inst Chem Eng 43:40–45
Shim HY, Lee KS, Lee DS, Jeon DS, Park MS, Shin JS, Lee YK, Goo JW, Kim SB, Chung DY (2014) Application of electrocoagulation and electrolysis on the precipitation of heavy metals and particulate solids in washwater from the soil washing. J Agric Chem Environ 3:130–138
Shirkhanloo H, Mousavi HZ, Rouhollahi A (2011) Preconcentration and determination of heavy metals in water, sediment and biological samples. J Serb Chem Soc 76:1583–1595
Shukla SR, Pai RS (2005) Removal of Pb(II) from solution using cellulose containing materials. J Chem Technol Biotechnol 80:176–183
Silva DH, Costa DA, Takeuchi RM, Santos AL (2011) Fast and simultaneous determination of Pb2+ and Cu2+ in water samples using a solid paraffin-based carbon paste electrode chemically modified with 2-aminothiazole-silica-gel. J Braz Chem Soc 22:1727–1735
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of food stuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48:611–619
Sitko R, Janik P, Zawisza B, Talik E, Margui E, Queralt I (2015) Green approach for ultra trace determination of divalent metal ions and arsenic species using totalreflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal Chem 87:3535–3542
Sumner ER, Shanmuganathan A, Sideri TC, Willetts SA, Houghton JE, Avery SV (2005) Oxidative protein damage causes chromium toxicity in yeast. Microbiology 151:1939–1948
Szłyk E, Czerniak-Szydłowska A (2004) Determination of cadmium, lead, and copper in margarines and butters by galvanostatic stripping chronopotentiometry. J Agric Food Chem 52:4064–4071
Taha K (2017) Heavy elements analyses in the soil using X-ray fluorescence and inductively coupled plasma-atomic emission spectroscopy. Int J Adv Sci Eng Technol 5:118–120
Talat M, Tripathi P, Srivastava ON (2018) Highly sensitive electrochemical detection of mercury present in the beauty creams using graphene modified glassy carbon electrode. Innov Corros Mater Sci 8:24–31
Tareen AK, Sultan IN, Parakulsuksatid P, Shafi M, Khan A, Khan MW, Hussain S (2014) Detection of heavy metals (Pb, Sb, Al, As) through atomic absorption spectroscopy from drinking water of District Pishin, Balochistan, Pakistan. Int J Curr Microbiol App Sci 3:299–308
Tatay S, GavinÄa P, Coronado E, Palomares E (2006) Optical mercury sensing using a benzothiazolium hemicyanine dye. Org Lett 8:3857–3860
Tautkus S, Steponeniene L, Kazlauskas R (2004) Determination of iron in natural and mineral waters by flame atomic absorption spectrometry. J Serb Chem Soc 69:393–402
Tokcaer and Yetis (2006) Pb(II) biosorption using anaerobically digested sludge. J Hazard Mater 137:1674–1680
Tran T, Leu H, Chiub K, Lin C (2017) Electrochemical treatment for wastewater contained heavy metal the removing of the COD and heavy metal ions. J Chin Chem Soc 64:493–502
Tsade HK (2016) Atomic absorption spectroscopic determination of heavy metal concentrations in Kulufo River, Arbaminch, Gamo Gofa, Ethiopia. J Environ Anal Chem 3:2380–2391
Un UT, Ocal SE (2015) Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. Int J Environ Sci Dev 6:425. https://doi.org/10.7763/ijesd.2015.v6.630
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Varghese AG, Paul SA, Latha MS (2018) Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00843-z
Vukovic GD, Marinkovic AD, Skapin SD, Ristic MD, Aleksic R, Peric-Grujic AA, Uskokovic PS (2011) Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem Eng J 173:855–865
Wang S, Forzani ES, Tao N (2007) Detection of heavy metal ions in water by high-resolution surface plasmon resonance spectroscopy combined with anodic stripping voltammetry. Anal Chem 79:4427–4432
Wang XH, Deng WY, Xie YY, Wang CY (2013) Selective removal of mercury ions using a chitosan-poly(vinyl alcohol) hydrogel adsorbent with three-dimensional network structure. Chem Eng J 228:232–242
Wang H, Wu ZK, Chen BB, He M, Hu B (2015) Chip-based array magnetic solid phase micro extraction on-line coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in cells. Analyst 140:5619–5626
Wierzbicki T, Pyrzynska K (2002) Determination of vanadium content in wine by GF AAS. Chem Anal 47:449–455
Wolfbeis OS (2002) Fiber optic chemical sensors and biosensors. Anal Chem 74:2663–2678
Xia F, Zhang X, Zhou C, Sun D, Dong Y, Liu Z (2010) Simultaneous determination of copper, lead, and cadmium at hexagonal mesoporous silica immobilized quercetin modified carbon paste electrode. J Autom Methods Manag Chem. https://doi.org/10.1155/2010/824197
Xu RX, Yu XY, Gao C, Liu JH, Compton RG, Huang XJ (2013) Enhancing selectivity in stripping voltammetry by different adsorption behaviors: the use of nanostructured Mg-Al-layered double hydroxides to detect Cd(II). Analyst 138:1812–1818
Yang S, Li J, Shao D, Hu J, Wang X (2009) Adsorption of Ni(II) on oxidized multiwalled carbon nanotubes: effect of contact time, pH, foreign ions and PAA. J Hazard Mater 166:109–116
Yanming S, Dongbin L, Shifeng L, Lihui F, Shuai C, Haque MA (2017) Removal of lead from aqueous solution on glutamate intercalated layered double hydroxide. Arabian J Chem 10:2295–2301
Yao X, Guo Z, Yuan Q, Liu Z, Liu J, Huang X (2014) Exploiting differential electrochemical stripping behaviors of Fe3O4 nanocrystals toward heavy metal ions by crystal cutting. ACS Appl Mater Interfaces 6:12203–12213
Yuan S, Chen W, Hu S (2004) Simultaneous determination of cadmium (II) and lead (II) with clay nanoparticles and anthraquinone complexly modified glassy carbon electrode. Talanta 64:922–928
Yuan X, Koh HL, Chui WK (2009) The analysis of heavy metals in Chinese herbal medicine by flow injection–mercury hydride system and graphite furnace atomic absorption spectrometry. Phytochem Anal 20:293–297
Yuan-Zhen P, Yong-Ming H, Dong-Xing Y, Yan L, Zhen-Bin G (2012) Rapid analysis of heavy metals in coastal seawater using preconcentration with precipitation/co-precipitation on membrane and detection with X-ray fluorescence. Chin J Anal Chem 40:877–882
Zarazúa G, Girón-Romero K, Tejeda S, León CC, Avila-Pérez P (2014) Total reflection X-ray fluorescence analysis of toxic metals in fish tissues. Am J Anal Chem 5:805–811
Zewail TM, Yousef NS (2015) Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex Eng J 54:83–90
Zhang QX, Wen H, Peng D, Fu Q, Huang XJ (2015) Interesting interference evidences of electrochemical detection of Zn(II), Cd(II) and Pb(II) on three different morphologies of MnO2 nanocrystals. J Electroanal Chem 739:89–96
Zhao G, Wang H, Liu G (2017) Direct quantification of Cd2+ in the presence of Cu2+ by a combination of anodic stripping voltammetry using a bi-film-modified glassy carbon electrode and an artificial neural network. Sensors 17:1558–1572
Zhong W, Ren T, Zhao L (2016) Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal 24:46–55
Zhu YH, Hu J, Wang JL (2012) Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J Hazard Mater 221:155–161
Zhu C, Yang G, Li H, Du D, Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:230–249
Zhu K, Gao Y, Tan X, Chen C (2016) Polyaniline-modified Mg/Al Layered Double Hydroxide composites and their application in efficient removal of Cr(VI). ACS Sustain Chem Eng 4:4361–4369
Zhu J, Fu Q, Qiu G, Liu Y, Hu H, Huang Q, Violante A (2019) Influence of low molecular weight anionic ligands on the sorption of heavy metals by soil constituents: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-019-00881-1
Acknowledgements
We are thankful to the Head of the department for providing timely encouragement and other valuable suggestions. Also LAM and AB would like to thank CSIR, New Delhi, for their financial help in the form of Senior Research Fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malik, L.A., Bashir, A., Qureashi, A. et al. Detection and removal of heavy metal ions: a review. Environ Chem Lett 17, 1495–1521 (2019). https://doi.org/10.1007/s10311-019-00891-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-019-00891-z










