Abstract
There is a growing interest for aromatic and biological properties of essential oils as alternatives to synthetic chemicals and drugs. However, essential oils and their components are poorly soluble in aqueous systems and are highly sensitive to degradation and evaporation. These drawbacks can be overcome by encapsulation into cyclodextrins, which are non-toxic cyclic oligosaccharides obtained from the enzymatic degradation of starch. Cyclodextrins inclusion complexes offer solutions to many limitations of the use of essential oils in the food, pharmaceutical and cosmetic industries. This article reviews essential oils encapsulation in cyclodextrins. The strength of binding between cyclodextrins and essential oils components covers a wide range of formation constants with values ranging from 13 to 166,338 M−1. The encapsulation in cyclodextrins can increase the aqueous solubility of essential oils up to 16-fold and reduce their photodegradation rates up to 44-fold, while ensuring a gradual release. We also discuss the effect of encapsulation on biological activities, such as antimicrobial and antioxidant properties of essential oils. Finally, we present novel cyclodextrin-based approaches in the sectors of textiles and nanofibres.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
This article is an abridged version of the chapter published by Kfoury et al. (2018a) in the series Environmental Chemistry for a Sustainable World.
Essential oils are mixtures of volatile organic compounds obtained from aromatic plants (Fig. 1). In addition to their fragrant properties, essential oils possess numerous biological properties, including antimicrobial, anticancer, anti-obesity, anti-inflammatory activities (Costa et al. 2016; da Silveira et al. 2014; Rashed et al. 2017). About 160 essential oils are considered as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA)Footnote 1 (Prakash et al. 2015). Due to their natural origin, essential oils have gained great interest in the food, cosmetic and pharmaceutical industries (Astray et al. 2009).
The global essential oils market size exceeded USD $ 6.0 billion in 2015Footnote 2, and their chemical instability requires additional storage and quality control concern (Turek and Stintzing 2013). Essential oils are also practically insoluble in aqueous systems, highly volatile, and their use is often limited due to flavouring issues (Burt 2004). These drawbacks limit their applications. Encapsulation of essential oils in different systems can solve these problems. Such systems involve emulsions, beads, bioactive films, capsules, liposomes, nanocarriers and inclusion complexes (Crini 2014; Dima and Dima 2015; Pinho et al. 2014; Sherry et al. 2013) (Fig. 2).

(Adapted from Dima and Dima 2015)
Different systems used for the encapsulation of essential oils.
Researchers are conscious of the potential of inclusion encapsulation using cyclodextrins to overcome these limitations in various sectors (Marques 2010; Sherry et al. 2013). Cyclodextrins are cyclic oligosaccharides that do not pose significant safety concern (Brewster and Loftsson 2007; Crini 2014; Gould and Scott 2005). Moreover, they are relatively cheap biodegradable materials, and encapsulation could be performed both in solution and in solid state (Fenyvesi et al. 2005; Loftsson and Duchêne 2007; Szente and Szejtli 2004).
Cyclodextrins are mainly used to increase the solubility of water-insoluble guests (Brewster and Loftsson 2007). The hydrophobic cavity of cyclodextrins also provides a microenvironment that protects the guest from volatilization and against harmful environmental factors (Del Valle 2004; Marques 2010; Szejtli 2004).
Cyclodextrins are inert and do not interfere with the biological properties of guests (Del Valle 2004). Moreover, they provide opportunity for delivering these water-insoluble compounds with reproducible absorption and enhancing bioavailability (Nieddu et al. 2014; Pinho et al. 2014). Several procedures have been developed to prepare inclusion complexes, and various experimental methods have been described to explore and characterize the binding efficiency of cyclodextrins with essential oils (Del Valle 2004; Kfoury et al. 2018b; Mura 2014).
The aim of this review is to summarize the literature data concerning the characterization of cyclodextrin/essential oil inclusion complexes and the main benefits of the encapsulation (solubility, stability and release). It also aims to review the consequences of encapsulation on the biological activities and functionalities of essential oils in order to evaluate the effectiveness of the use of inclusion complexes as bioactive agents. Thus, this paper provides a wealth of information to researchers interested in the application of encapsulated essential oils in food, agriculture, cosmetic and pharmaceutical industries.
Definition and properties of essential oils
Essential oils have been used for thousands of years as incense, perfumes and cosmetics and for their culinary and medical applications (Karapinar and Aktuǧ 1987; Lee et al. 2015). They give spices and herbs their specific scent and flavour and provide flowers and fruits their perfume.
More than 3000 types of essential oils are currently known, of which 300 are of commercial interest. Essential oils contain a mixture of related components; the monoterpenes (C10H16; two isoprene units), sesquiterpenes (C15H24; three isoprene units) and their derivatives being the major constituents. The monoterpene and sesquiterpene derivatives include cyclic and acyclic compounds from different classes, such as alcohols, esters, phenols, ketones, lactones, aldehydes and oxides. Other substantial components of essential oils are phenylpropanoids derived from the carbon skeleton of phenylalanine. Figure 3 reports some examples of the chemical structure of typical essential oil components.
In addition to their aromatic properties, essential oils and their constituents have been shown to possess various biological activities including anti-inflammatory, anticancer, antimicrobial, antidiabetic, anti-ageing and insect repellent (da Silveira et al. 2014; Kfoury et al. 2016a, b; Nazzaro et al. 2013; Raut and Karuppayil 2014). Essential oils have been experiencing a renaissance owing to the progression of alternative medicine practices and to the growing consumer interest in natural products as alternatives for artificial additives and pharmacological drugs. The US Food and Drug Administration (FDA) has considered 160 essential oils as “Generally Recognized As Safe” (GRAS) for the use in food, drugs and cosmetics (Tisserand and Young 2014).
Nonetheless, the major issue is the low aqueous solubility of essential oils and their individual components. The logP (Octanol–water partition coefficients) values for the essential oil components fell generally in the range 1.81–4.48 (Griffin et al. 1999). They are also chemically unstable and susceptible to loss by volatilization, oxidative deterioration when exposed to oxygen, moisture, light, and heat or interaction with other matrix ingredients in food, cosmetic and pharmaceutical formulations (Pavela and Benelli 2016; Turek and Stintzing 2013). These modifications have major drawbacks on the shelf life or the organoleptic and biological properties of the essential oil containing product.
Encapsulation of essential oils in cyclodextrins
Characterization of cyclodextrin/essential oil inclusion complexes in solution
The initial step in the characterization of an inclusion complex is the determination of the stoichiometry and formation constant (Landy et al. 2000). Formation constant is also referred to as binding or association constant; its value indicates the strength of cyclodextrin–guest molecule interactions. The most common stoichiometry of cyclodextrin/guest inclusion complexes is 1:1 but higher stoichiometries can be encountered as well (Landy et al. 2007).
A wide range of chromatographic, spectroscopic and calorimetric methods are used for this purpose (Kfoury et al. 2016b, c). However, the determination of stoichiometry and formation constant values is not a simple approach. In all cases, obtained experimental data should be treated cautiously to ensure final reliable results. It is thoughtful to use nonlinear rather than linear regression analysis. A 1:1 stoichiometry for the inclusion complex is assumed when using linear analysis to determine the formation constant value. However, inclusion complexes could have higher stoichiometry, and at equilibrium, the solution could consist of a mixture of inclusion complexes with different stoichiometries (Landy et al. 2000, 2007). This could be only revealed when nonlinear analysis is applied. Formation constant value is specific for each individual essential oil component and could not be determined for the entire essential oil mixture. Numerous studies evaluated formation constant for cyclodextrin/essential oil component inclusion complexes using various methods. Authors have applied high-performance liquid chromatography (HPLC), static headspace-gas chromatography (SH-GC), UV–Visible spectroscopy, fluorescence spectroscopy, NMR spectroscopy and isothermal titration calorimetry (ITC) (Ciobanu et al. 2012, 2013; Decock et al. 2006; Demian 2000; Kfoury et al. 2016d, e; Liu and Guo 2002; Tanemura et al. 1998). Table 1 summarizes the formation constant values for cyclodextrin/essential oil component inclusion complexes.
Titration experiments, using a constant concentration of essential oil component and increasing amounts of cyclodextrin, are generally employed. Nevertheless, some alternative non-conventional approaches suitable for such low-soluble compounds like essential oil components are now being developed, in particular competitive methods applied to UV–Visible and isothermal titration calorimetry (ITC) (Bertaut and Landy 2014; Landy et al. 2000). The main advantage of the competitive protocols is that they overcome solubility limitations. Moreover, they allow the determination of formation constant values, with a high accuracy, for a) inclusion complexes of essential oil component that lack any chromophore and cannot be studied by direct UV–Visible, and b) for athermic inclusion complexes that cannot be directly detected by isothermal titration calorimetry (ITC) (Bertaut and Landy 2014). Furthermore, a “rapid method” based on static headspace-gas chromatography (SH-GC) technique was developed and successfully validated to study the interactions between cyclodextrin and individual essential oil components present simultaneously in complex mixtures like essential oils (Fourmentin et al. 2013; Kfoury et al. 2015c).
Recently, an NMR method combined with an algorithmic treatment that relies on global analysis was explored to determine formation constants (Kfoury et al. 2016d). This analysis uses simultaneously the chemical shifts (δ) and diffusion coefficients (D) variations for several guest protons to calculate the formation constant value.
The formation constant values for a great number of cyclodextrin/essential oil component inclusion complexes were successfully determined (Table 1). The obtained results cover a wide range of formation constant values from 13 to 166,338 M−1. The binding efficiency could be classified as very weak, weak, moderate, strong and very strong when formation constant values are in the following ranges: 0–500, 500–1000, 1000–5000, 5000–20,000 and greater than 20,000 M−1, respectively (Carrier et al. 2007).
Several studies in the literature showed a high positive correlation between the hydrophobic character of essential oil components (logP) and formation constant values, with the correlation coefficients being 0.986 for β-cyclodextrin (Decock et al. 2008), 0.855 and 0.882, for α-cyclodextrin and β-cyclodextrin, respectively (Astray et al. 2010) and 0.923 for hydroxypropyl-β-cyclodextrin (HP-β-CD) (Kfoury et al. 2014c). However, it is cautious not to establish rules based on a limited number of guests because the correlation becomes weaker when taking into consideration all values from the literature (Table 1) (Kfoury et al. 2016c). This indicates that this correlation could not be universally applicable for estimating or predicting the stability of a cyclodextrin/essential oil component inclusion complex.
Also, other factors, such as the space filling of the cyclodextrin cavity, which is directly related to the structure and geometrical conformation of the guest (Decock et al. 2008; Fourmentin et al. 2013), contribute substantially to the complex stability. Indeed, the essential oil component has to fit entirely or at least partially into the cyclodextrin cavity. The weak residual empty space shows sufficient contact between the cyclodextrin cavity and the essential oil component indicating a tight encapsulation (Eftink et al. 1989).
Preparation and characterization of solid cyclodextrin/essential oil inclusion complexes
The formulation of cyclodextrin/essential oil inclusion complexes can be also achieved in the solid form (Miller et al. 2007). This ensures an easier and safer handling by converting oils into solid dosage forms. The solid cyclodextrin inclusion complexes also help solving the major limitation of the use of essential oils, their chemical instability in the presence of air, light, moisture and heating.
Solid cyclodextrin/essential oil inclusion complexes could be obtained using various techniques such as co-precipitation, freeze-drying, spray-drying, co-grinding, co-evaporation, sealed-heating, complexation by using supercritical carbon dioxide and microwave-assisted encapsulation (Del Valle 2004; Kfoury et al. 2016c). Solid inclusion complexes could be prepared both at the laboratory and industrial scales. The preparation parameters including the temperature, the mixing time and speed, the nature of cyclodextrin, the use of co-solvent or other additives and the drying process should be optimized. These parameters may affect the properties of the obtained complexes particularly their crystallinity and the recovery and encapsulation yields. Thus, the choice of the method depends on encapsulation yield, rapidity, simplicity, cost and the desired characteristics of the final product (Hernández-Sánchez et al. 2017).
The physicochemical properties of an inclusion complex are generally evaluated using thermal analysis, X-ray diffraction, Fourier transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy and transmission electron microscopy (Kfoury et al. 2016c; Mura 2015). Moreover, the quantification of essential oil in solid inclusion complex and the encapsulation yield could be performed. Results are generally expressed as loading capacity and encapsulation efficiency (EE%). The loading capacity is a measure of the encapsulated amount of essential oil per gram of the solid inclusion complex, and the encapsulation efficiency (EE%) is the encapsulated amount of essential oil expressed as a percentage of the quantity initially used to prepare the solid inclusion complex. An appropriate evaluation of these parameters is the most important issue for the application of cyclodextrin inclusion complexes as solid dosage forms. Various techniques could be used for this purpose: UV–Visible spectroscopy, chromatography (HPLC and GC), thermal (TG/DTG) analyses and multiple headspace extraction (MHE) coupled to gas chromatography (Hǎdǎrugǎ et al. 2012, Kfoury et al. 2016f).
Solid inclusion complexes present several advantages: they could improve the handling of oily essential oils and could be used as standardized dosage form. However, the major task is to use inclusion complexes to design bio-based active food and drug packaging systems (Kapetanakou and Skandamis 2016; Ribeiro-Santos et al. 2017). Essential oils could be used in active packaging either as aromatic additives or as bioactive agents. When essential oils are applied as cyclodextrin inclusion complexes, they could benefit from a gradual release into the product (Martina et al. 2013). This release may be influenced by specific factors mainly the temperature and the relative humidity (Ayala-Zavala et al. 2008; Ho et al. 2011; Yamamoto et al. 2012; Yang et al. 2015). Therefore, several studies attempted to evaluate the release performance of essential oils from solid inclusion complexes under specific temperature and humidity conditions. Figure 4 illustrates the release of trans-anethole from β-cyclodextrin inclusion complex as a function of time and temperature in a humid atmosphere (75%).
Both elevated humidity and temperature favour the release of essential oils from inclusion complexes over time. Another crucial factor that influences the release kinetics is the preparation method (Kfoury et al. 2016f).
Some applications of these solid inclusion complexes could be found in the literature. For example, the incorporation of β-cyclodextrin/trans-cinnamaldehyde inclusion complex into a chitosan based edible coating improved the shelf life of fresh-cut melon (Moreira et al. 2014) and papaya (Brasil et al. 2012). They can also ensure an optimal flavour and nutritional quality of fruits and vegetables until consumption.
Effect of encapsulation on the physicochemical properties of essential oils
Solubility enhancement
Cyclodextrins are generally preferred to organic solvents to solubilize essential oils, because they are safer. Moreover, unlike cyclodextrins, the solubilizing efficiency of organic solvents is often lost by precipitation when introduced into an aqueous medium (Gould and Scott 2005).
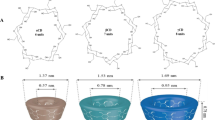
Phase solubility studies are generally carried out to describe and quantify the effect of cyclodextrin concentration on the guest’s solubility (Higuchi and Connors 1965). In general, cyclodextrins solubilize guests as a linear function of their concentration based on the formation of inclusion complexes (Brewster and Loftsson 2007). Phase solubility studies can be performed successfully with a single essential oil component using a variety of techniques mainly the UV–Visible spectroscopy as well as fluorescence spectroscopy and chromatography (HPLC and GC) to determine the amount of solubilized guest (Hill et al. 2013; Kfoury et al. 2014a; Mazzobre et al. 2011; Tao et al. 2014; Waleczek et al. 2003; Zeng et al. 2012). These techniques allow precise measurements leading to an accurate quantification of the essential oil component’s solubility. However, essential oils are mixtures of a great number of components. Therefore, carrying out phase solubility studies with essential oils is a challenge. Nonetheless, phase solubility studies have been commonly carried out to investigate the solubilizing efficiency of cyclodextrins towards different essential oils such as chamomile essential oil (Waleczek et al. 2003), clove essential oil (Hernandez-Sanchez et al. 2012; Hill et al. 2013), cinnamon bark extract (Hill et al. 2013), clary essential oil (Tian 2008) and garlic essential oil (Bai et al. 2010). The authors have used analytical methods based on the assessment of the response of a single essential oil component and concluded on the solubility of the entire essential oil. However, this is not cautious and does not reflect the behaviour of the entire essential oil. A multitude of equilibriums could take place when adding cyclodextrin to essential oil because each essential oil component presents a distinct S0, particular stoichiometry and formation constant value (Kfoury et al. 2015c). Recently, a new total organic carbon (TOC) method was developed to perform phase solubility studies for essential oils (Kfoury et al. 2016g). Total organic carbon provides a precise determination of the solubility of the essential oil because it measures the total organic carbon content leading to the calculation of the massic concentration of encapsulated essential oil. Figure 5 illustrates the phase solubility diagrams for three different essential oils, citronella grass, lemon eucalyptus and winter savory, with hydroxypropyl-β-cyclodextrin (Kfoury et al. 2016g).

Reprinted with permission from Kfoury et al. (2016g)
Phase solubility diagrams of citronella grass, lemon eucalyptus and winter savory essential oils with hydroxypropyl-β-cyclodextrin (HP-β-CD). The profiles show a linear increase in the essential oils solubility as a function of cyclodextrin concentration.
Results from the literature proved that cyclodextrins are efficient to improve the solubility of essential oils. The intrinsic solubility (S0) varied among the essential oils because of their different compositions. These results also lead to the conclusion that the solubilizing potential of cyclodextrins (St/S0) is inversely proportional to the essential oil’s intrinsic solubility (S0) (Kfoury et al. 2014a, 2016g).
Retention
The loss of essential oils and their volatile components by evaporation is still a key drawback in their use in the different formulations (Ciobanu et al. 2012; Marques 2010). Indeed, the chemical composition of an essential oil ensures its specific scent and activity. Thus, the loss of some volatile components may have a strong impact on the flavour and the activity of an essential oil. Hence, essential oils should be retained in formulations. This ensures the stability of the organoleptic and sensorial properties of the product and thus its acceptability by the consumers. Retention of essential oils also provides convenience in maintaining an active dose of essential oils or their components when they are incorporated in active formulations. A recent study showed that, among several additives, cyclodextrins showed the strongest effect on aroma retention by inclusion complexation (Baránková and Dohnal 2016). Several release studies revealed that cyclodextrins reduce the volatility, control and delay the release of essential oils and their components (Ciobanu et al. 2013; Decock et al. 2008; Kfoury et al. 2015c, 2016f). The encapsulation in β-cyclodextrin improved the retention of citral (26-fold enhancement) and menthol (86-fold enhancement) in fruit leathers and hard candies, respectively (Reineccius et al. 2004). Moreover, the retention of essential oils could be beneficial for active essential oils that have strong odour, pungent taste, high flavour impact and low flavour threshold such as clove essential oil (Hernández-Sánchez et al. 2017), garlic essential oil (Wang et al. 2011), oregano and thyme essential oils (Burt 2004).
Stability enhancement
Essential oils are highly volatile compounds susceptible to oxygen, light, heat and humidity (Turek and Stintzing 2013) and prone to hydrolysis, oxidation, heat degradation, evaporation and reaction with the matrix ingredients (Hyldgaard et al. 2012). Degradation of essential oils could lead to the deterioration of their sensory properties as well as their biologic performance. Therefore, developing formulations to preserve essential oils is considered an essential task for their application in the various fields. Despite many years of research, manufactures still struggle with improving the shelf life of essential oil containing formulations.
The protection of essential oils could be achieved by encapsulation. Cyclodextrins offer essential oils a safer and longer life within a protected environment, the apolar cavity while maintaining their properties (Costa et al. 2015; Hill et al. 2013; Pinho et al. 2014). Encapsulation also prevents off note development (off-flavours) and the production of toxic isomers by reducing contact between essential oil components and oxygen, ions or other formulation ingredients and avoiding direct exposure to light (Szejtli and Szente 2005). For example, encapsulation in hydroxypropyl-β-cyclodextrin (HP-β-CD) improved significantly the stability of basil and tarragon essential oils against UV irradiations (Fig. 6) (Kfoury et al. 2015b).

Reprinted with permission from Kfoury et al. 2015b
Photodegradation profiles of pure estragole and estragole present in tarragon and basil essential oils in water and in hydroxypropyl-β-cyclodextrin (HP-β-CD) solution under UVC irradiation. The protective effect of cyclodextrin is clear, and the degradation of estragole was slowed-down by encapsulation. Free estragole was completely disappeared after 40 min of irradiation whereas more than 40% was still remaining in the inclusion complexes solutions after 1 h of irradiation
It has been demonstrated that the addition of β-cyclodextrin preserves the colour intensity and reduces the browning of pear juice without any significant decrease in the aromatic quality (Lopez-Nicolas et al. 2009). This could be explained by the fact that cyclodextrins protect the aromatic compounds from their environment (Lopez-Nicolas et al. 2014). Cyclodextrins prevent the cyclization of citral (Szente and Szejtli 2004) and the isomerization of trans-anethole (Kfoury et al. 2014a), estragole (Kfoury et al. 2015b), eugenol and isoeugenol (Kfoury et al. 2016a) under UV-irradiation.
Thus, encapsulation in cyclodextrins might be also one of the promising tools to protect essential oils against the harmful environment.
Effect of encapsulation on the biological properties of essential oils
The exploration and understanding of the benefits of essential oils in nutrition and medicine are still in progress. According to the Biopharmaceutics Classification System, volatile compounds (such as essential oils and essential oil components) belong to class 2. Drugs that belong to this class have low solubility and high permeability; therefore, they mainly undergo passive transport through the biological membranes (Amidon et al. 1995).
Inclusion complexes are essential to design essential oils containing formulations because cyclodextrins do not interfere with their activity (Costa et al. 2015; Lucas-Abellán et al. 2008). Also, cyclodextrins do not permeate hydrophobic membranes via passive diffusion since they are large hydrophilic oligosaccharides with numerous hydrogen bond donors and acceptors (Kurkov and Loftsson 2013; Lipinski 2000, 2004; Loftsson and Brewster 2010). They act as penetration enhancers. They increase the solubility and the concentration of the active agent at the biological membrane. This, consequently, ameliorates its diffusion (Kurkov and Loftsson 2013). One of the primary sites of action of essential oils is the biological membrane (Bakkali et al. 2008; Raut and Karuppayil 2014). Cyclodextrin inclusion complexes enhance the access of active compounds to this region (Kurkov and Loftsson 2013). Accordingly, a lower concentration of active agent could be used to achieve the effective concentration at the target sites. Several studies attempted to evaluate the effect of encapsulation on the antimicrobial and antioxidant properties of essential oils (Arana-Arana-Sánchez et al. 2010; Dima et al. 2014; Hill et al. 2013; Kfoury et al. 2016a; Papajani et al. 2015).
The obtained results can be divided into three categories. Firstly, the encapsulation improved the antimicrobial activity of essential oils by decreasing the active concentration (MIC, MBC, IC50 and LD50) or increasing the inhibition of growth. This improvement in the antimicrobial properties could be due to the increased water solubility of the active compounds upon encapsulation, which in turn enhanced the surface area of contact with the pathogen (Fenyvesi et al. 2014). Essential oils could also bind to the hydrophobic constituents such as lipids and proteins that reduce their activity (Burt et al. 2005; Tao et al. 2014). But, cyclodextrins could reduce these interactions and preserve the inhibitory properties of essential oils (Budryn et al. 2015). Also, the molecular size of the inclusion complex may increase the cellular absorption mechanisms and thus increasing the antimicrobial activity (Rakmai et al. 2017). Secondly, the encapsulation does not affect the antimicrobial activity. Finally, the encapsulation maintained some antimicrobial properties of the essential oils. This could be explained by the fact that cyclodextrins themselves are able to increase the growth of some pathogens since they could be used as a carbohydrate source for the microorganisms (Ayala-Zavala et al. 2008: Kfoury et al. 2016a).
Aiming a potential application of essential oils as antioxidant agents, it is generally intended to reduce their concentration as much as possible while achieving a notable activity, especially when they have a strong pungent taste and smell. The effect of encapsulation on the antioxidant properties of essential oils and their components was evaluated in several studies (Kamimura et al. 2014; Kfoury et al. 2014a, 2015c, 2016d; Miguel et al. 2010).
In general, the encapsulation of essential oils in cyclodextrins either maintained or increased the ability of essential oils to reduce free radicals. However, few studies showed a decrease in the potential of essential oils to scavenge the free radicals. It seems that cyclodextrins could block the functional groups of essential oils, which become less available to reduce the radical species (Kamimura et al. 2014; Rakmai et al. 2017). On the contrary, the preservation and improvement in the antioxidant activity upon encapsulation may be due to the formation of inclusion complexes (Lu et al. 2009; Lucas-Abellán et al. 2008). While inclusion complexes may guarantee excellent protection of essential oils against degradation or evaporation, they do not interfere with the functional groups of the active compounds (Kfoury et al. 2014a; Rakmai et al. 2017). An increase in the antioxidant activity might be observed when intermolecular hydrogen bonds are formed between cyclodextrin and guest resulting in a stable radical formation upon reaction with free radicals (Lucas-Abellán et al. 2008). Also, cyclodextrins could act as secondary antioxidants that enhance the antiradical efficiency of guests (Lopez-Nicolas et al. 2007; Nunez-Delicado et al. 1997).
Overall, these results provide encouraging evidence of the potential effectiveness of cyclodextrin-encapsulated essential oils to be used as stable natural antimicrobial and antioxidant agents. With the increase in consumer concerns regarding limited availability of natural products and environment protection, the use of cyclodextrin/essential oil inclusion complexes will gain further interest in detriment to synthetic materials. Importantly, both cyclodextrins and essential oils are natural and obtained from renewable resources.
Emerging cyclodextrin-based technologies for essential oils encapsulation
Cyclodextrins are also considered as promising carriers in textiles, cosmetotextiles and medical textiles (Radu et al. 2016; Voncina and Vivod 2013). Cyclodextrins, generally grafted to textiles, provide them hosting cavities that can encapsulate a wide variety of functional guest molecules (Szejtli 2003; Voncina and Vivod 2013). The incorporation of slimming, hydrating, or perfuming agents as inclusion complexes allows their controlled release and ensures, consequently, their progressive effect on the skin (Rakshit 2011). Textile material containing β-cyclodextrin/cedar oil, as example, showed a prolonged insect repellent activity compared to textile materials containing free cedar oilFootnote 3. Also the odour release intensity of perfume from PET textile was considerably postponed when the material was treated by cyclodextrins (Voncina and Vivod 2013).
More recently, the electrospinning has attracted considerable attention to produce nanofibres and nanowebs having high surface-to-volume ratio and highly porous structure. The electrospun nanofibres have large applications in food packaging, wound dressing and biomedical use, etc. Cyclodextrins are interesting in this field. The self-assembly and aggregation characteristics of these host molecules in concentrated solutions allow the production of nanofibres without any polymer matrix (Aytac et al. 2016a). Thus, the nanofibres and nanowebs could be effectively functionalized with cyclodextrin/essential oil inclusion complexes.
The electrospinning technology is very simple; a continuous filament is electrospun from concentrated inclusion complex solutions (with or without polymer) under a very high electrical field resulting in nanofibres (Aytac et al. 2016a, b). Electrospun nanofibres were successfully obtained with inclusion complexes of geraniol (Aytac et al. 2016b), vanillin (Celebioglu et al. 2016) and limonene (Aytac et al. 2016a).
These nanofibres have shown fast-dissolving properties, high thermal stability, improved water solubility and enhanced antimicrobial and antioxidant properties. An interesting feature of these electrospun nanofibres is their freestanding nature useful for a simple and longer storage of essential oils. Electrospinning nanofibres from cyclodextrin/essential oil inclusion complexes would be extremely interesting for food application because of the edible nature of native cyclodextrins and for further potential medical, packaging, textile and agriculture use. Electrospun polystyrene textile fibres containing cyclodextrin/menthol inclusion complex retained menthol and enhanced its temperature stability (Uyar et al. 2009).
Conclusion
The encapsulation in cyclodextrins is a promising approach for the incorporation of essential oils in food, pharmaceutical, cosmetic, textile and other products. Cyclodextrins are able to overcome the major limitation of essential oils use, the low aqueous solubility, and to increase their chemical stability in the presence of light, oxygen, humidity and heat. Importantly, cyclodextrins can successfully encapsulate essential oils both in solution and in solid state. Moreover, formation of inclusion complexes ensures an easier handling of oily essential oils and solid dosage formulation. Cyclodextrins, also, allow a delayed and controlled release of essential oils under specific conditions of humidity and temperature. In addition, encapsulation in cyclodextrins maintains or even enhances the biological properties and functionalities of essential oils. The use of essential oils coming from natural sources and having relevant biological properties will encourage the transition to a more clean and sustainable environment.
Notes
FDA: Code Fed. Regul. (CFR). Title 21 Food Drugs. Chapter I—Food Drug Adm. Dep. Heal. Hum. Serv. Subchapter B—Food Hum. Consum. (Continued), Part 182–Subst. Gen. Recognized as Safe (GRAS). 2016.
Essential Oil Market Analysis By Product (Orange, Corn Mint, Eucalyptus, Citronella, Peppermint, Lemon, Clove Leaf, Lime, Spearmint), By Application (Medical, Food & Beverage, Spa & Relaxation, Cleaning & Home) And Segment Forecasts To 2024.
See Vraz Kresevic et al. (2008).
References
Amidon GL, Lennernäs H, Shah VP, Crison JR (1995) A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–420. https://doi.org/10.1023/A:1016212804288
Arana-Sánchez A, Estarrón-Espinosa M, Obledo-Vázquez EN, Padilla-Camberos E, Silva-Vázquez R, Lugo-Cervantes E (2010) Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Lett Appl Microbiol 50(6):585–590. https://doi.org/10.1111/j.1472-765X.2010.02837.x
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gándara J (2009) A review on the use of cyclodextrins in foods. Food Hydrocoll 23(7):1631–1640. https://doi.org/10.1016/j.foodhyd.2009.01.001
Astray G, Mejuto JC, Morales J, Rial-Otero R, Simal-Gandara J (2010) Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res Int 43(4):1212–1218. https://doi.org/10.1016/j.foodres.2010.02.017
Ayala-Zavala JF, Soto-Valdez H, Gonzalez-Leon A, Alvarez-Parrilla E, Martin-Belloso O, Gonzalez-Aguilar GA (2008) Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J Incl Phenom Macrocycl Chem 60:359–368. https://doi.org/10.1007/s10847-007-9385-1
Aytac Z, Yildiz ZI, Kayaci-Senirma F, KeskinNO San, Kusku SI, Durgun E, Tekinay T, Uyat T (2016a) Fast-dissolving, prolonged release, and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J Agric Food Chem 64(39):7325–7334. https://doi.org/10.1021/acs.jafc.6b02632
Aytac Z, Yildiz ZI, Kayaci-Senirmak F, San Keskin NO, Tekinayde T, Uyar T (2016b) Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv 6(52):46089–46099. https://doi.org/10.1039/c6ra07088d
Bai Y, Yu B, Xu X, Jin Z, Tian Y, Lu L (2010) Comparison of encapsulation properties of major garlic oil components by hydroxypropyl β-cyclodextrin. Eur Food Res Technol 231(4):519–524. https://doi.org/10.1007/s00217-010-1307-6
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Baránková E, Dohnal V (2016) Effect of additives on volatility of aroma compounds from dilute aqueous solutions. Fluid Phase Equilib 407:217–223. https://doi.org/10.1016/j.fluid.2015.05.038
Bertaut E, Landy D (2014) Improving ITC studies of cyclodextrin inclusion compounds by global analysis of conventional and non-conventional experiments. Beilstein J Org Chem 10:2630–2641. https://doi.org/10.3762/bjoc.10.275
Brasil IM, Gomes C, Puerta-gomez A, Castell-perez ME, Moreira RG (2012) Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. Food Sci Technol 47(1):39–45. https://doi.org/10.1016/j.lwt.2012.01.005
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 59(7):645–666. https://doi.org/10.1016/j.addr.2007.05.012
Budryn G, Zaczyńska D, Rachwał-Rosiak D, Oracz J (2015) Changes in properties of food proteins after interaction with free and β encapsulated hydroxycinnamic acids. Eur Food Res Technol 240:1157–1166. https://doi.org/10.4315/0362-028X-68.5.919
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJA (2005) Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:h7 by addition of food stabilizers. J Food Prot 68(5):919–926. https://doi.org/10.4315/0362-028X-68.5.919
Carrier RL, Miller LA, Ahmed I (2007) The utility of cyclodextrins for enhancing oral bioavailability. J Control Release 123:78–99. https://doi.org/10.1016/j.jconrel.2007.07.018
Celebioglu A, Kayaci-Senirmak F, İpek S, Durgunab E, Uyar T (2016) Polymer-free nanofibers from vanillin/cyclodextrin inclusion complexes: high thermal stability, enhanced solubility and antioxidant property. Food Funct 7:3141–3153. https://doi.org/10.1039/C6FO00569A
Chen H, Ji H, Zhou X, Wang L (2010) Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-β-cyclodextrin. Tetrahedron 66(52):9888–9893. https://doi.org/10.1016/j.tet.2010.10.063
Ciobanu A, Mallard I, Landy D, Brabie G, Nistor D, Fourmentin S (2012) Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr Polym 87(3):1963–1970. https://doi.org/10.1016/j.carbpol.2011.10.005
Ciobanu A, Landy D, Fourmentin S (2013) Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res Int 53(1):110–114. https://doi.org/10.1016/j.foodres.2013.03.048
Costa P, Medronho B, Gonc S, Romano A (2015) Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind Crops Prod 70:341–346. https://doi.org/10.1016/j.indcrop.2015.03.065
Costa G, Gidaro MC, Vulla D, Supuran CT, Alcaro S (2016) Active components of essential Oils as anti-obesity potential drugs investigated by in silico techniques. J Agric Food Chem 64(26):5295–5300. https://doi.org/10.1021/acs.jafc.6b02004
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114(21):10940–10975. https://doi.org/10.1021/cr500081p
da Silveira E, Sá RDC, Andrade LN, De Oliveira RDRB, De Sousa DP (2014) A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 19(2):1459–1480. https://doi.org/10.3390/molecules19021459
Decock G, Fourmentin S, Surpateanu GG, Landy D, Decock P, Surpateanu G (2006) Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins. Supramol Chem 18(6):477–482. https://doi.org/10.1080/10610270600665749
Decock G, Landy D, Surpateanu G, Fourmentin S (2008) Study of the retention of aroma components by cyclodextrins by static headspace gas chromatography. J Incl Phenom Macrocycl 62:297–302
Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochem 39(9):1033–1046. https://doi.org/10.1016/S0032-9592(03)00258-9
Demian BA (2000) Correlation of the solubility of several aromatics and terpenes in aqueous hydroxypropyl-β-cyclodextrin with steric and hydrophobicity parameters. Carbohydr Res 328:635–639. https://doi.org/10.1016/S0008-6215(00)00139-7
Dima C, Dima S (2015) Essential oils in foods: extraction, stabilization, and toxicity. Curr Opin Food 5:29–35. https://doi.org/10.1016/j.cofs.2015.07.003
Dima C, Cotarlet M, Tiberius B, Bahrim G, Alexe P, Dima S (2014) Encapsulation of coriander essential oil in beta-cyclodextrin: antioxidant and antimicrobial properties evaluation. Rom Biotechnol Lett 19(2):9128–9141. https://doi.org/10.1016/j.foodhyd.2016.11.014
Donze C, Coleman W (1993) β-CD inclusion complexes: relative selectivity of terpene and aromatic guest molecules studied by competitive inclusion experiments. J Incl Phenom Macrocycl Chem 62(3–4):297–302. https://doi.org/10.1007/BF00708758
Eftink MR, Andy ML, Bystrom K, Perlmutter HD, Kristol DS (1989) Cyclodextrin inclusion complexes: studies of the variation in the size of alicyclic guests. J Am Chem Soc 111(17):6765–6772. https://doi.org/10.1021/ja00199a041
Fenyvesi E, Gruiz K, Verstichel S, De Wilde B, Leitgib L, Csabai K, Szaniszlo N (2005) Biodegradation of cyclodextrins in soil. Chemosphere 60:1001–1008. https://doi.org/10.1016/j.chemosphere.2005.01.026
Fenyvesi E, Szemán J, Csabai K, Malanga M, Szente L (2014) Methyl-beta-cyclodextrins: the role of number and types of substituents in solubilizing power. J Pharm Sci 103(5):1443–1452. https://doi.org/10.1002/jps.23917
Ferrazza R, Rossi B, Guella G (2014) DOSY-NMR and Raman investigations on the self-aggregation and cyclodextrin complexation of vanillin. J Phys Chem B 118(25):7147–7155
Fourmentin S, Ciobanu A, Landy D, Wenz G (2013) Space filling of β-cyclodextrin and β-cyclodextrin derivatives by volatile hydrophobic guests. Beilstein J Org Chem 9:1185–1191. https://doi.org/10.3762/bjoc.9.133
Gould S, Scott RC (2005) 2-Hydroxypropyl-β-cyclodextrin (HP- β-CD): a toxicology review. Food Chem Toxicol 43(10):1451–1459. https://doi.org/10.1016/j.fct.2005.03.007
Griffin S, Wyllie SG, Markham J (1999) Determination of octanol—water partition coefficient for terpenoids using reversed-phase high-performance liquid chromatography. J Chromatogr A 864:221–228. https://doi.org/10.1016/S0021-9673(99)01009-2
Hǎdǎrugǎ NG, Hǎdǎrugǎ DI, Isengard HD (2012) Water content of natural cyclodextrins and their essential oil complexes : a comparative study between Karl Fischer titration and thermal methods. Food Chem 132:1741–1748. https://doi.org/10.1016/j.foodchem.2011.11.003
Hernandez-Sanchez P, Lopez-Miranda S, Lucas-Abellan C, Nunez-Delicado E (2012) Complexation of eugenol (EG), as main component of clove oil and as pure compound, with β- and HP-β-CDs. Food Nutr Sci 3:716–723. https://doi.org/10.4236/fns.2012.36097
Hernández-Sánchez P, López-Miranda S, Guardiola L, Serrano-Martínez A, Gabaldón JA, Nuñez-Delicado E (2017) Optimization of a method for preparing solid complexes of essential clove oil with β-cyclodextrins. J Sci Food Agric 97(2):420–426. https://doi.org/10.1002/jsfa.7781
Higuchi T, Connors KA (1965) Phase-solubility techniques. Adv Anal Chem Instrum 4:117–212
Hill LE, Gomes C, Taylor TM (2013) Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans -cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. Food Sci Technol 51(1):86–93. https://doi.org/10.1016/j.lwt.2012.11.011
Ho BT, Joyce DC, Bhandari BR (2011) Release kinetics of ethylene gas from ethylene–α-cyclodextrin inclusion complexes. Food Chem 129(2):259–266. https://doi.org/10.1016/j.foodchem.2011.04.035
Hyldgaard M, Mygind T, Meyer RL, Debabov D (2012) Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 3:1–24. https://doi.org/10.3389/fmicb.2012.00012
Jiang S, Li JN, Jiang ZT (2010) Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil. Eur Food Res Technol 230(4):543–550. https://doi.org/10.1007/s00217-009-1192-z
Kamimura JA, Santos EH, Hill LE, Gomes CL (2014) Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. Food Sci Technol 57(2):701–709. https://doi.org/10.1016/j.lwt.2014.02.014
Kapetanakou AE, Skandamis PN (2016) Applications of active packaging for increasing microbial stability in foods: natural volatile antimicrobial compounds. Curr Opin Food Sci 12:1–12. https://doi.org/10.1016/j.cofs.2016.06.001
Karapinar M, Aktuǧ SE (1987) Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int J Food Microbiol 4(2):161–166. https://doi.org/10.1016/0168-1605(87)90023-7
Kfoury M, Landy D, Auezova L, Greige-Gerges H, Fourmentin S (2014a) Effect of cyclodextrin complexation on phenylpropanoids’ solubility and antioxidant activity. Beilstein J Org Chem 10:2322–2331. https://doi.org/10.3762/bjoc.10.241
Kfoury M, Auezova L, Greige-Gerges H, Ruellan S, Fourmentin S (2014b) Cyclodextrin, an efficient tool for trans-anethole encapsulation: chromatographic, spectroscopic, thermal and structural studies. Food Chem 164:454–461. https://doi.org/10.1016/j.foodchem.2014.05.052
Kfoury M, Auezova L, Fourmentin S, Greige-Gerges H (2014c) Investigation of monoterpenes complexation with hydroxypropyl-beta-cyclodextrin. J Incl Phenom Macrocycl Chem 80:51–60. https://doi.org/10.1007/s10847-014-0385-7
Kfoury M, Balan R, Landy D, Nistor D, Fourmentin S (2015a) Investigation of the complexation of essential oil components with cyclodextrins. Supramol Chem 27(9):1–10. https://doi.org/10.1080/10610278.2015.1051977
Kfoury M, Auezova L, Ruellan S, Greige-Gerges H, Fourmentin S (2015b) Complexation of estragole as pure compound and as main component of basil and tarragon essential oils with cyclodextrins. Carbohydr Polym 118:156–164. https://doi.org/10.1016/j.carbpol.2014.10.073
Kfoury M, Auezova L, Greige-Gerges H, Fourmentin S (2015c) Promising applications of cyclodextrins in food: improvement of essential oils retention, controlled release and antiradical activity. Carbohydr Polym 131:264–272. https://doi.org/10.1016/j.carbpol.2015.06.014
Kfoury M, Lounès-Hadj Sahraoui A, Bourdon N, Laruelle F, Fontaine J, Auezova L, Greige-Gerges H, Fourmentin S (2016a) Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem 196:518–525. https://doi.org/10.1016/j.foodchem.2015.09.078
Kfoury M, Borgie M, Verdin A, Ledoux F, Courcot D, Auezova L, Fourmentin S (2016b) Essential oil components decrease pulmonary and hepatic cells inflammation induced by air pollution particulate matter. Environ Chem Lett 14:345–351. https://doi.org/10.1007/s10311-016-0572-4
Kfoury M, Hadaruga NG, Hadaruga DI, Fourmentin S (2016c) Cyclodextrins as encapsulation material for flavors and aroma. A volume in nanotechnology in the agri-food industry. ISBN: 978-0-12-804307-3
Kfoury M, Landy D, Ruellan S, Auezova L, Greige-Gerges H, Fourmentin S (2016d) Determination of formation constants and structural characterization of cyclodextrin inclusion complexes with two phenolic isomers: carvacrol and thymol. Beilstein J Org Chem 12(12):29–42. https://doi.org/10.3762/bjoc.12.5
Kfoury M, Landy D, Ruellan S, Auezova L, Greige-gerges H, Fourmentin S (2016e) Nootkatone encapsulation by cyclodextrins : effect on water solubility and photostability. Food Chem 236:41–48. https://doi.org/10.1016/j.foodchem.2016.12.086
Kfoury M, Auezova L, Greige-Gerges H, Larsen KL, Fourmentin S (2016f) Release studies of trans-anethole from β-cyclodextrin solid inclusion complexes by multiple headspace extraction. Carbohydr Polym 151:1245–1250. https://doi.org/10.1016/j.carbpol.2016.06.079
Kfoury M, Auezova L, Greige-Gerges H, Fourmentin S (2016g) Development of a total organic carbon method for the quantitative determination of solubility enhancement by cyclodextrins : application to essential oils. Anal Chim Acta 918:21–25. https://doi.org/10.1016/j.aca.2016.03.013
Kfoury M, Pipkin JD, Antle V, Fourmentin S (2017) Captisol®: an efficient carrier and solubilizing agent for essential oils and their components. Flavour Fragr J 32(5):340–346. https://doi.org/10.1002/ffj.3395
Kfoury M, Auezova L, Greige-Gerges H, Fourmentin S (2018a) Cyclodextrins for essential oils applications. Fundamentals and applications of cyclodextrins. In: Fourmentin S, Crini G, Lichtfouse E (eds) Environmental chemistry for a sustainable world, vol 1. Springer, Berlin, pp 81–123. https://doi.org/10.1007/978-3-319-76159-6_4
Kfoury M, Landy D, Fourmentin S (2018b) Characterization of cyclodextrin/volatile inclusion complexes: a review. Molecules 23:1204–1226. https://doi.org/10.3390/molecules23051204
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453(1):167–180. https://doi.org/10.1016/j.ijpharm.2012.06.055
Landy D, Fourmentin S, Salome M, Surpateanu G (2000) Analytical improvement in measuring formation constants of inclusion complexes between β-cyclodextrin and phenolic compounds. J Incl Phenom 38(1–4):187–198. https://doi.org/10.1023/A:1008156110999
Landy D, Tetart F, Truant E, Blach F, Fourmentin S, Surpateanu G (2007) Development of a competitive continuous variation plot for the determination of inclusion compounds stoichiometry. J Incl Phenom Macrocycl Chem 57:409–413. https://doi.org/10.1007/s10847-006-9226-7
Lee JH, Lee J, Song KB (2015) Development of a chicken feet protein film containing essential oils. Food Hydrocoll 46:208–215. https://doi.org/10.1016/j.foodhyd.2014.12.020
Lipinski CA (2000) Drug-like properties and the cause of poor solubility and poor permeability. J Pharmacol Toxicol Methods 44:235–249. https://doi.org/10.1016/S1056-8719(00)00107-6
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Liu L, Guo QX (2002) The driving forces in the inclusion complexation of cyclodextrins. J Incl Phenom Macrocycl Chem 42(1):1–14. https://doi.org/10.1023/A:1014520830813
Loftsson T, Brewster ME (2010) Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol 62(11):1607–1621. https://doi.org/10.1111/j.2042-7158.2010.01030.x
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications. Int J Pharm 329:1–11. https://doi.org/10.1016/j.ijpharm.2006.10.044
Lopez-Nicolas JM, Perez-Lopez AJ, Carbonell-Barrachina A, Garcia-Carmona F (2007) Use of natural and modified cyclodextrins as inhibiting agents of peach juice enzymatic browning. J Agric Food Chem 55(13):5312–5319. https://doi.org/10.1021/jf070499h
Lopez-Nicolas JM, Andreu-Sevilla AJ, Carbonell-Barrachina ÁA, García-Carmona F (2009) Effects of addition of α-cyclodextrin on the sensory quality, volatile compounds, and color parameters of fresh pear juice. J Agric Food Chem 57(20):9668–9675. https://doi.org/10.1021/jf901919t
Lopez-Nicolas JM, Rodríguez-Bonilla P, García-Carmona F (2014) Cyclodextrins and antioxidants. Crit Rev Food Sci 54(2):251–276. https://doi.org/10.1080/10408398.2011.582544
Lu Z, Cheng B, Hu Y, Zhang Y, Zou G (2009) Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food Chem 113(1):17–20. https://doi.org/10.1016/j.foodchem.2008.04.042
Lucas-Abellán C, Mercader-Ros MT, Zafrilla MP, Fortea MI, Gabaldon JA, Nunez-Delicado E (2008) ORAC-fluorescein assay to determine the oxygen radical absorbance capacity of resveratrol complexed in cyclodextrins. J Agric Food Chem 56:2254–2259. https://doi.org/10.1021/jf0731088
Marques HMC (2010) A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J 25(5):313–326. https://doi.org/10.1002/ffj.2019
Martina K, Binello A, Lawson D, Jicsinszky L, Cravotto G (2013) Recent applications of cyclodextrins as food additives and in food processing. Curr Nutr Food Sci 9(3):167–179. https://doi.org/10.2174/1573401311309030001
Mazzobre MF, dos Santos CI, Buera M (2011) Solubility and stability of β- cyclodextrin-terpineol inclusion complex as affected by water. Food Biophys 6(2):274–280. https://doi.org/10.1007/s11483-011-9208-1
Miguel MG, Dandlen SA, Figueiredo AC, Pedro LG, Barroso JG, Marques MH (2010) Comparative evaluation of the antioxidant activities of thymol and carvacrol and the corresponding β-cyclodextrin complexes. Acta Hort 853:363–368. https://doi.org/10.17660/ActaHortic.2010.853.44
Miller LA, Carrier RL, Ahmed I (2007) Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci 96(7):1691–1707. https://doi.org/10.1002/jps.20831
Moreira SM, de Carvalho W, Alexandrino AC, de Paula HCB, Rodrigues MCP, de Figueiredo RW, Maia GA, de Figueiredo EMAT, Brasil IM (2014) Int J Food Sci Technol 49(10):2192–2203. https://doi.org/10.1111/ijfs.12535
Mura P (2014) Analytical techniques for characterization of cyclodextrin complexes in aqueous solution : a review. J Pharm Biomed Anal 101:238–250. https://doi.org/10.1016/j.jpba.2014.02.022
Mura P (2015) Analytical techniques for characterization of cyclodextrin complexes in the solid state : a review. J Pharm Biomed Anal 113:226–238. https://doi.org/10.1016/j.jpba.2015.01.058
Nazzaro F, Fratianni F, Martino LD (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6(12):1451–1474. https://doi.org/10.3390/ph6121451
Nieddu M, Rassu G, Boatto G, Bosi P, Trevisi P, Giunchedi P, Carta A, Gavini E (2014) Improvement of thymol properties by complexation with cyclodextrins: in vitro and in vivo studies. Carbohydr Polym 102:393–399. https://doi.org/10.1016/j.carbpol.2013.10.084
Numanoglu U, Şen T, Tarimci N, Kartal M, Koo OMY, Önyüksel H (2007) Use of cyclodextrins as a cosmetic delivery system for fragrance materials: linalool and benzyl acetate. AAPS PharmSciTech 8(4):85. https://doi.org/10.1208/pt0804085
Nunez-Delicado E, Sanchez-Ferrer A, Garcia-Carmona F (1997) Cyclodextrins as secondary antioxidants: synergism with ascorbic acid. J Agric Food Chem 45(8):2830–2835. https://doi.org/10.1021/jf9609800
Papajani V, Haloci E, Goci E, Shkreli R, Manfredini S (2015) Evaluation of antifungal activity of Origanum vulgare and Rosmarinus officinalis essential oil before and after inclusion in β-cyclodextrin. Int J Pharm Pharm Sci 7(5):5–8
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides ? Challenges and constraints. Trends Plant Sci 21(12):1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Pinho E, Grootveld M, Soares G, Henriques M (2014) Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr Polym 101:121–135. https://doi.org/10.1016/j.carbpol.2013.08.078
Prakash B, Kedia A, Mishra PK, Dubey NK (2015) Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—potentials and challenges. Food Control 47:381–391. https://doi.org/10.1016/j.foodcont.2014.07.023
Radu CD, Parteni O, Ochiuz L (2016) Applications of cyclodextrins in medical textiles—review. J Control Release 224:146–157. https://doi.org/10.1016/j.jconrel.2015.12.046
Rakmai J, Cheirsilp B, Torrado-agrasar A, Simal Gandara J, Mejuto JC, Torrado-agrasar A (2017) Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: physiochemical characterization and evaluation of bio-efficacies. CyTA Journal of Food. https://doi.org/10.1080/19476337.2017.1286523
Rakshit R (2011) Skin care textiles - A review. Man-Made Textiles in India 39(3):81–85
Rashed AA, Mohd Nawi MN, Sulaiman K (2017) Assessment of essential oil as a potential anti- obesity agent: a narrative review Assessment of essential oil as a potential anti- obesity agent: a narrative review. J Essent Oil Res 29(1):1–10. https://doi.org/10.1080/10412905.2016.1213668
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crops Prod 62:250–264. https://doi.org/10.1016/j.indcrop.2014.05.055
Reineccius TA, Reineccius GA, Peppard TL (2004) Utilization of β-cyclodextrin for improved flavor retention in thermally processed foods. J Food Sci 69(1):58–62. https://doi.org/10.1111/j.1365-2621.2004.tb17856.x
Ribeiro-Santos R, Andrade M, Sanches-Silva A (2017) Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr Opin Food Sci 14:78–84. https://doi.org/10.1016/j.cofs.2017.01.012
Sherry M, Charcosset C, Fessi H, Greige-Gerges H (2013) Essential oils encapsulated in liposomes: a review. J Liposome Res 23(4):268–275. https://doi.org/10.3109/08982104.2013.819888
Szejtli J (2003) Cyclodextrins in the textile industry. Starch 55(5):191–196. https://doi.org/10.1002/star.200390050
Szejtli J (2004) Past, present and future of cyclodextrin research. Pure Appl Chem 76:1825–1844
Szejtli J, Szente L (2005) Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur J Pharm Biopharm 61(3):115–125. https://doi.org/10.1016/j.ejpb.2005.05.006
Szente L, Szejtli J (2004) Cyclodextrins as food ingredients. Trends Food Sci Technol 15(3–4):137–142. https://doi.org/10.1016/j.tifs.2003.09.019
Tanemura I, Saito Y, Ueda H, Sato T (1998) Solubility method using static head-space gas chromatography for determination of the stability constants of fragrance materials with 2- hydroxypropyl-β-cyclodextrin. Chem Pharm Bull 46(3):540–541
Tao F, Hill LE, Peng Y, Gomes CL (2014) Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. Food Sci Technol 59(1):247–255. https://doi.org/10.1016/j.lwt.2014.05.037
Tian XN, Jiang ZT, Li R (2008) Inclusion interactions and molecular microcapsule of Salvia sclarea L. essential oil with β-cyclodextrin derivatives. Eur Food Res Technol 227(4):1001–1007. https://doi.org/10.1007/s00217-007-0813-7
Tisserand R, Young R (2014) Essential oil safety. A guide for health care professionals, 2nd edn. Churchill Livingstone, London, pp 23–38
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci Food Saf 12(1):40–53. https://doi.org/10.1111/1541-4337.12006
Uyar T, Hacaloglu J, Besenbacher F (2009) Reactive & functional polymers electrospun polystyrene fibers containing high temperature stable volatile fragrance/flavor facilitated by cyclodextrin inclusion complexes. React Funct Polym 69(3):145–150. https://doi.org/10.1016/j.reactfunctpolym.2008.12.012
Voncina B, Vivod V (2013) Cyclodextrins in textile finishing. Cyclodextrins in textile finishing. In: Günay M (ed) Textile dyeing. InTech, Tijeka, Chapter 3, p 53 https://doi.org/10.5772/53777
Vraz Kresevic S, Voncina B, Gersak J (2008) Insect resistant and eco-friendly textile materials. In: Dragcevic Zvonko (ed) 4th International textile, clothing & design conference: magic world of textiles: book of proceedings, ITC&DC, 05–08 October, 2008, Faculty of Textile Technology, University of Zagreb, Dubrovnik, Croatia. Zagreb, 2008
Waleczek KJ, Marques HMC, Hempel B, Schmidt PC (2003) Phase solubility studies of pure (–)—α-bisabolol and camomile essential oil with β–cyclodextrin. Eur J Pharm Biopharm 55:247–251. https://doi.org/10.1016/S0939-6411(02)00166-2
Wang J, Cao Y, Sun B, Wang C (2011) Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem 127(4):1680–1685. https://doi.org/10.1016/j.foodchem.2011.02.036
Yamamoto C, Neoh TL, Honbou H, Yoshii H, Furuta T (2012) Kinetic analysis and evaluation of controlled release of D-limonene encapsulated in spray-dried cyclodextrin powder under linearly ramped humidity. Drying Technol 30(11–12):1283–1291. https://doi.org/10.1080/07373937.2012.681089
Yang Z, Xiao Z, Ji H (2015) Solid inclusion complex of terpinen-4-ol/β-cyclodextrin: kinetic release, mechanism and its antibacterial activity. Flavour Frag J 30(2):179–187. https://doi.org/10.1002/ffj.3229
Zeng Z, Fang Y, Jin H (2012) Side chain influencing the interaction between β-cyclodextrin and vanillin. Flavour Fragr J 27:378–385. https://doi.org/10.1002/ffj.3115
Acknowledgements
Authors thank Marc Fourmentin from the University of the Littoral Opal Coast (ULCO), France, for the illustrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kfoury, M., Auezova, L., Greige-Gerges, H. et al. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ Chem Lett 17, 129–143 (2019). https://doi.org/10.1007/s10311-018-0783-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0783-y