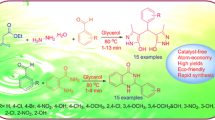
Abstract
1,3-Oxazines have a wide variety of biological activities. Naphthoquinone scaffolds also exhibit several biological responses such as antithrombotic, apoptosis and lipoxygenase inhibitors. There is, therefore, a need to develop efficient green methodologies for hybridizing the two scaffolds in a single entity. Herein, we report a novel protocol for the synthesis of 3-aryl-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones by one-pot three-component condensation of 2-hydroxy-1,4-naphthoquinone, aromatic amines and formaldehyde in glycerol at 50 °C. After separation of products, the glycerol–water layer was extracted using ethyl acetate and the dried glycerol layer was successfully reused several times. The products were obtained in 85–95 % yields in 5–10 min. This environmentally benign protocol holds advantages of high yields, operational simplicity and easy workup over our earlier report.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,3-Oxazine is present in numerous biodynamic heterocycles as the core structure. It provides a focal intermediate for variety of functional group interconversions (Meyers and Smith 1972; Meyers and Malone 1974). 1,3-Oxazine scaffolds exhibit potential biological and pharmacological activities such as anti-tumour (Kuehne and Konopke 1962; Chylinska and Urbanski 1963; Hsu and Lin 1996), antibacterial (Chylinska et al. 1971; Latif et al. 1982), anti-HIV (Pedersen and Pedersen 2000; Cocuzza et al. 2001), analgesic (Kurtz 2005), antihypertensive (Kajino et al. 1991), antithrombotic (Buckman et al. 1998) and antiulcer (Katsura et al. 1991). Moreover, 6-arylbenzoxazines and naphthoxazines possess therapeutic potential for treatment of Parkinson’s disease and as non-steroidal progesterone receptor agonists, respectively (Zhang et al. 2002; Joyce et al. 2003). Some of the biologically active oxazine scaffolds are shown in Fig. 1.
Multicomponent reaction is an efficient economic methodology which allows multiple bond formation between simple starting materials generating highly complex and diverse substrates in a single step. It results in high atom economy and avoids purification processes for the intermediates (Domling and Ugi 2000; Ruijter et al. 2011). Therefore, this approach is advantageous over conventional linear type synthesis.
Glycerol has promising physical and chemical properties, which allows its use in many organic reactions that employ various homogeneous and heterogeneous chemo- and biocatalysts (Wolfson et al. 2006, 2007, 2009). It has high boiling point, negligible vapour pressure, low toxicity, high polarity, biodegradability and also compatibility with most organic and inorganic compounds. Its manufacture from renewable sources makes it a promising solvent. In addition to this, glycerol also allows isolation of products by simple filtration, extraction and distillation processes. Glycerol has emerged as a green solvent for numerous organic reactions including Pd-catalysed Heck and Suzuki cross-couplings, Cu-catalysed cross-coupling of diaryl diselenides with aryl boronic acids, base- and acid-promoted condensations, catalytic hydrogenation, transfer hydrogenation and asymmetrical reduction (Gu and Jerome 2010; Diaz-Alvarez et al. 2011).
Our group has recently reported the synthesis of novel 3-aryl-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones using ionic liquid [Bmim]BF4 as the reaction media (Khanna et al. 2015). However, the use of ionic liquid has several disadvantages such as toxicity because of possible release into the soil or water courses and thus posing a threat to the environment and also high costs make them somewhat impractical for larger industrial applications. Keeping in consideration the need of developing a greener protocol involving environmentally benign, catalyst-free reaction conditions, we investigated the synthesis of 3-aryl-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones from 2-hydroxy-1,4-naphthoquinone (1.0 mmol), aromatic amines (1.0 mmol) and formaldehyde (2.0 mmol) with different hydroxylic solvents.
Experimental
All the chemicals were commercial and purchased from Sigma-Aldrich or Merck and used as received. Thin-layer chromatography (GF254) was used to monitor reaction progress. Melting points were measured on Buchi M-560 melting point apparatus and are uncorrected. IR (KBr) spectra were recorded on a Perkin Elmer FTIR spectrophotometer, and the values are expressed as νmax cm−1. The 1H NMR and 13C NMR spectra were recorded on Jeol JNM ECX-400P at 400 and 100 MHz, respectively, using trimethylsilane as internal standard. The chemical shift values are recorded on δ scale, and the coupling constants (J) are in Hz. Mass spectral data were recorded on Agilent 6520 QT of (ESI-HRMS) mass spectrometer.
General procedure for the synthesis of 3-aryl-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones (IVa–n) (using IVa as an example)
A mixture of 2-hydroxy-1,4-naphthoquinone (174.1 mg, 1.0 mmol), 4-fluoroaniline (111.12 mg, 1.0 mmol), formalin (60.06 mg, 37 %, w/v, 2.0 mmol) and glycerol (2 mL) was taken in a 50-mL round-bottomed flask. The contents were stirred magnetically in an oil bath maintained at 50 °C for appropriate time as indicated in Table 2. The progress of the reaction was monitored by TLC using ethyl acetate/petroleum ether (30: 70, v/v) as eluent. After completion of the reaction, the reaction mixture was allowed to cool at room temperature and diluted with water (5 mL). The solid separated was collected by filtration at pump and washed with water followed by 2–3 mL of ethanol. The products were characterized by IR, 1H NMR, 13C NMR and mass spectrometry.
Spectral data
3-(4-Fluorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVa) Khanna et al. (2015)
Yellow solid; m.p. 191–193 °C (Lit. 194–197 °C); IR (νmax cm−1) (KBr): 1676, 1212, 1061; 1H NMR (400 MHz, CDCl3) δ = 8.08–8.02 (m, 2H, ArH), 7.72–7.65 (m, 2H, ArH), 7.09–7.06 (m, 2H, ArH), 6.97–6.92 (m, 2H, ArH), 5.44 (s, 2H, CH2), 4.42 (s, 2H, CH2).
3-(4-Methoxyphenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVb) Khanna et al. (2015)
Yellow solid; m.p. 159–161 °C (Lit. 163–164 °C); IR (νmax cm−1) (KBr): 1681, 1214, 1037; 1H NMR (400 MHz, CDCl3) δ = 8.08–8.01 (m, 2H, ArH), 7.69–7.66 (m, 2H, ArH), 7.07 (d, 2H, ArH, J = 9.2 Hz), 6.80 (d, 2H, ArH, J = 8.4 Hz), 5.44 (s, 2H, CH2), 4.40 (s, 2H, CH2), 3.73 (s, 3H, OCH3).
3-(3,5-Dichlorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVc)
Yellow solid; m.p. 150–152 °C; IR (νmax cm−1) (KBr): 1681, 1214, 1037; 1H NMR (400 MHz, CDCl3) δ = 8.10–8.04 (m, 2H, ArH), 7.74–7.67 (m, 2H, ArH), 6.97–6.95 (m, 3H, ArH), 5.43 (s, 2H, CH2), 4.46 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ = 183.10, 178.67, 155.42, 149.15, 135.82, 134.40, 133.57, 131.43, 130.68, 126.66, 126.56, 126.16, 120.04, 116.63, 79.86, 46.10. HRMS (ESI) m/z calcd. for calcd. for C18H11Cl2NO3: 360.0191, found: 359.0116 [M + H]+.
3-(2-Bromo-4-methylphenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVd)
Yellow solid; m.p. 192–194 °C; IR (νmax cm−1) (KBr): 1682, 1218, 1060; 1H NMR (400 MHz, CDCl3) δ = 8.10–8.02 (m, 2H, ArH), 7.71–7.68 (m, 2H, ArH), 7.39 (s, 1H, ArH), 7.20–7.18 (m, 1H, ArH), 6.99–6.97 (m, 1H, ArH), 5.38 (s, 2H, CH2), 4.35 (s, 2H, CH2), 2.44 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ = 183.20, 179.07, 155.26, 144.08, 136.44, 134.26, 133.33, 131.62, 130.74, 129.01, 126.19, 126.07, 122.44, 119.93, 119.22, 83.11, 46.36, 20.36. HRMS (ESI) m/z calcd. for calcd. for C11H7O3: 187.0395, found: 187.0392.
3-(3-Chlorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVe) Khanna et al. (2015)
Yellow solid; m.p. 173–175 °C (Lit. 173–175 °C); IR (νmax cm−1) (KBr): 1678, 1209, 1052; 1H NMR (400 MHz, CDCl3) δ = 8.12–8.05 (m, 2H, ArH), 7.74–7.68 (m, 2H, ArH), 7.22–7.18 (m, 1H, ArH), 7.12–7.11 (m, 1H, ArH), 7.02–6.95 (m, 2H, ArH), 5.49 (s, 2H, CH2), 4.49 (s, 2H, CH2).
3-(4-Chlorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVf) Khanna et al. (2015)
Yellow solid; m.p. 180–182 °C (Lit. 183–184 °C); IR (νmax cm−1) (KBr): 1676, 1212, 1061; 1H NMR (400 MHz, CDCl3) δ = 8.09–8.03 (m, 2H, ArH), 7.73–7.67 (m, 2H, ArH), 7.25–7.22 (m, 2H, ArH), 7.06–7.04 (m, 2H, ArH), 5.47 (s, 2H, CH2), 4.46 (s, 2H, CH2).
3-(4-Bromophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVg) Khanna et al. (2015)
Yellow solid; m.p. 190–192 °C (Lit. 196–198 °C); IR (νmax cm−1) (Film): 1678, 1213, 1058; 1H NMR (400 MHz, CDCl3) δ = 8.09–8.03 (m, 2H, ArH), 7.73–7.67 (m, 2H, ArH), 7.25–7.22 (m, 2H, ArH), 7.06–7.04 (m, 2H, ArH), 5.47 (s, 2H, CH2), 4.46 (s, 2H, CH2).
3-(3-Acetylphenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVh) Khanna et al. (2015)
Yellow solid; m.p. 179–181 °C (Lit. 183–184 °C); IR (νmax cm−1) (KBr): 1681, 1206, 1061; 1H NMR (400 MHz, CDCl3) δ = 8.07–8.02 (m, 2H, ArH), 7.70–7.67 (m, 3H, ArH), 7.54–7.52 (m, 1H, ArH), 7.38–7.29 (m, 2H, ArH), 5.52 (s, 2H, CH2), 4.52 (s, 2H, CH2), 2.56 (s, 3H, CH3).
3-(4-Iodophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVi)
Yellow solid; m.p. 140–142 °C; IR (νmax cm−1) (KBr): 1682, 1218, 1060; 1H NMR (400 MHz, CDCl3) δ = 8.07–8.02 (m, 2H, ArH), 7.72–7.67 (m, 2H, ArH), 7.54 (d, 2H, ArH, J = 8.4 Hz), 6.88 (d, 1H, ArH, J = 8.4 Hz), 5.45 (s, 2H, CH2), 4.45 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ = 183.20, 178.79, 155.45, 147.15, 138.29, 134.29, 133.43, 131.49, 130.71, 126.57, 126.08, 120.60, 120.08, 85.28, 46.10. HRMS (ESI) m/z calcd. for calcd. for C18H11Cl2NO3: 417.9940, found: 417.9935 [M + H]+.
3-(3-Chloro-4-fluorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVj) Khanna et al. (2015)
Yellow solid; m.p. 162–164 °C (Lit. 162–164 °C); IR (νmax cm−1) (KBr): 1682, 1216, 1062; 1H NMR (400 MHz, CDCl3) δ = 8.09–8.03 (m, 2H, ArH), 7.73–7.66 (m, 2H, ArH), 7.16–7.14 (m, 1H, ArH), 7.05–6.95 (m, 2H, ArH), 5.42 (s, 2H, CH2), 4.42 (s, 2H, CH2).
3-(4-Chloro-3-nitrophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVk) Khanna et al. (2015)
Yellow solid; m.p. 204–206 °C (Lit. 208–211 °C); IR (νmax cm−1) (Film): 1681, 1212, 1058; 1H NMR (400 MHz, CDCl3) δ = 8.11–8.06 (m, 2H, ArH), 7.76–7.69 (m, 2H, ArH), 7.59 (s, 1H, ArH), 7.45–7.42 (m, 1H, ArH), 7.26–7.25 (m, 1H, ArH), 5.50 (s, 2H, CH2), 4.52 (s, 2H, CH2).
3-(4-Nitrophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVl) Khanna et al. (2015)
Yellow solid; m.p. 216–218 °C (Lit. 216–218 °C); IR (νmax cm−1) (Film): 1679, 1213, 1060; 1H NMR (400 MHz, CDCl3) δ = 8.19–8.16 (m, 2H, ArH), 8.10–8.05 (m, 2H, ArH), 7.75–7.68 (m, 2H, ArH), 7.14–7.12 (m, 2H, ArH), 5.54 (s, 2H, CH2), 4.59 (s, 2H, CH2).
3-(3-Bromophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVm) Khanna et al. (2015)
Yellow solid; m.p. 170–173 °C (Lit. 170–173 °C); IR (νmax cm−1) (Film): 1678, 1212, 1060; 1H NMR (400 MHz, CDCl3) δ = 8.08–8.03 (m, 2H, ArH), 7.72–7.66 (m, 2H, ArH), 7.25 (s, 1H, ArH), 7.14–7.01 (m, 3H, ArH), 5.46 (s, 2H, CH2), 4.47 (s, 2H, CH2).
3-(2-Methylphenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione (IVn) Khanna et al. (2015)
Yellow solid; m.p. 160–162 °C (Lit. 160–162 °C); IR (νmax cm−1) (KBr): 1680, 1222, 1066; 1H NMR (400 MHz, CDCl3) δ = 8.11–8.01 (m, 2H, ArH), 7.71–7.65 (m, 2H, ArH), 7.22–7.17 (m, 2H, ArH), 7.09–7.01 (m, 2H, ArH), 5.37 (s, 2H, CH2), 4.24 (s, 2H, CH2), 2.37 (s, 3H, CH3).
Results and discussion
We report herein a facile and efficient one-pot synthesis of 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione derivatives by one-pot three-component condensation of 2-hydroxy-1,4-naphthoquinone, aromatic amines and formaldehyde in glycerol at 50 °C. The optimum reaction conditions were established using 2-hydroxy-1,4-naphthoquinone (I) (1.0 mmol), 4-fluoroaniline (IIa) (1.0 mmol) and formaldehyde (III) (2.0 mmol), as standard components. The model reactions were performed in various hydroxylic solvents such as MeOH, EtOH, water, ethylene glycol, PEG-400, PEG-600 and glycerol under catalyst-free conditions at varying temperatures. Initially, the reaction was attempted in MeOH under reflux which was complete in 10 min and afforded 52 % of the desired 3-(4-fluorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione as confirmed by spectral analysis (Table 1, entry 1). The reaction in EtOH under reflux was also complete in 10 min and afforded 54 % of the desired product (Table 1, entry 2). The product formation was also observed when the reaction was carried out in EtOH–water and MeOH–water (1:1, v/v) at reflux, though with inferior yields of 40 and 51 %, respectively (Table 1, entries 3–4). Reaction attempted in ethylene glycol at 60 °C gave the desired naphtho[2,3-e][1,3]oxazine-5,10-dione in higher yield (83 %) (Table 1, entry 5). Reaction in ethylene glycol at higher temperatures (80 and 100 °C) did not have much influence on the reaction yield and time (Table 1, entries 6–7). The above reaction when performed in PEG-400 and PEG-600 resulted in a mixture of products even after 8 h as observed by TLC using ethyl acetate/petroleum ether (30: 70, v/v) as eluent (Table 1, entries 8–9). The same reaction was then attempted using glycerol as the solvent which yielded 91 % of the desired 3-(4-fluorophenyl)-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione in just 5 min (Table 1, entry 10).
Therefore, Table 1 clearly shows that the best optimized reaction condition for the one-pot catalyst-free synthesis of naphtho[2,3-e][1,3]oxazine-5,10-diones was using glycerol as the solvent at 50 °C. To realize the generality of this protocol, a series of naphtho[2,3-e][1,3]oxazine-5,10-dione derivatives were synthesized by one-pot condensation of 2-hydroxy-1,4-naphthoquinone, various aromatic amines and formaldehyde in glycerol at 50 °C. Both electron-withdrawing and electron-releasing aromatic amines were employed under the optimized reaction conditions to yield the desired products in good yields (Fig. 2; Table 2).
All our attempts to prepare bis-derivatives from o- and p-phenylene diamines were unsuccessful.
A study regarding the recovery and reuse of glycerol was also performed. The products were separated by simple filtration from the mixture of glycerol and water. The filtrate so obtained was then extracted with ethyl acetate. Ethyl acetate layer was separated and the solvent was removed. The glycerol–water layer was dried at 90 °C under vacuum and then directly reused for reaction. Marginal loss in the yield (90 and 88 %) of IVa was observed in the second and third cycles. However, the yields decreased gradually in fourth and fifth cycle (85 and 82 %).
A probable mechanism involved in the formation of products is outlined in Fig. 3. Initial condensation of formaldehyde and aromatic amine (ArNH2) gives an imine intermediate ‘A’ which further reacts with 2-hydroxy-1,4-naphthoquinone to form ‘B’. Lastly, condensation of ‘B’ with formaldehyde gives ‘C’ with loss of H2O that undergoes cyclization to give the final product IV.
Conclusion
In conclusion, we have developed an eco-friendly catalyst-free methodology for the synthesis of 3-aryl-3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones from 2-hydroxy-1,4-naphthoquinone, aromatic amines and formaldehyde in glycerol at 50 °C. Inexpensive, environmentally benign reaction media is the advantage of this protocol.
Abbreviations
- CDCl3 :
-
Deuterated chloroform
- 1H-NMR:
-
Proton nuclear magnetic resonance spectroscopy
- 13C-NMR:
-
Carbon nuclear magnetic resonance spectroscopy
- TLC:
-
Thin-layer chromatography
- IR:
-
Infrared spectroscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- ESI-HRMS:
-
Electrospray ionization-high-resolution mass spectrometry
References
Buckman BO, Mohan R, Koovakkat S (1998) Design, synthesis, and biological activity of novel purine and bicyclic pyrimidine factor Xa inhibitors. Bioorg Med Chem Lett 8:2235–2240. doi:10.1016/S0960-894X(98)00386-2
Chylinska JB, Urbanski T (1963) Dihydro-1,3-oxazine derivatives and their antitumor activity. J Med Chem 6:484–487. doi:10.1021/jm00341a004
Chylinska JB, Janowiec M, Urbanski T (1971) Antibacterial activity of dihydro-1,3-oxazine derivatives condensed with aromatic rings in positions 5, 6. J Pharmacol 43:649–657. doi:10.1111/j.1476-5381.1971.tb07194.x
Cocuzza AJ, Chidester DR, Cordova BC, Jeffrey S, Parsons RL, Bacheler LT, Viitanen SE, Trainor GL, Ko SS (2001) Synthesis and evaluation of efavirenz (SustivaTM) analogues as HIV-1 reverse transcriptase inhibitors: replacement of the cyclopropylacetylene side chain. Bioorg Med Chem Lett 11:1177–1179. doi:10.1016/S0960-894X(01)00192-5
Diaz-Alvarez AE, Francos J, Lastra-Barreira B, Crochet P, Cadierno V (2011) Glycerol and derived solvents: new sustainable reaction media for organic synthesis. Chem Commun 47:6208–6227. doi:10.1039/C1CC10620A
Domling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210. doi:10.1002/1521-3773(20000915)
Gu Y, Jerome F (2010) Glycerol as a sustainable solvent for green chemistry. Green Chem 12:1127–1138. doi:10.1039/C001628D
Hsu LY, Lin CH (1996) Synthesis and biological evaluation of 3-hydroxymethylpyrimido[1,6-c][1,3]oxazine derivatives. Heterocycles 43:2687–2699. doi:10.3987/COM-96-7607
Joyce JN, Presgraves S, Renish L (2003) Neuroprotective effects of the novel D3/D2 receptor agonist and antiparkinson agent, S32504, in vitro against 1-methyl-4- phenylpyridinium (MPP) and in vivo against 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP): a comparison to ropinirole. Exp Neurol 184:393–407. doi:10.1016/S0014-4886(03)00353-4
Kajino N, Shibout Y, Nishikawa K (1991) Synthesis and biological activities of new 2-substituted 1,4-benzoxazine derivatives. Chem Pharm Bull 39:2896–2905. doi:10.1248/cpb.39.2896
Katsura Y, Nishino S, Takasugi H (1991) Studies on antiulcer drugs. I. Synthesis and antiulcer activities of imidazo[1,2-alpha]pyridinyl-2-oxobenzoxazolidines-3-oxo-2H-1,4-benzoxazines and related compounds. Chem Pharm Bull 39:2937–2943. doi:10.1248/cpb.39.2937
Khanna G, Aggarwal K, Khurana JM (2015) An efficient and confluent approach for the synthesis of novel 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-dione derivation by a three component reaction in ionic liquid. RSC Adv 5:46448–46454. doi:10.1039/C5RA06169E
Kuehne ME, Konopke EA (1962) Dihydro-1, S-oxazines as antitumor agents. J Med Chem 5:257–280. doi:10.1021/jm01237a005
Kurtz T (2005) Synthesis of novel pyrido[2,3-e][1,3]oxazines. Tetrahedron 61:3091–3096. doi:10.1016/j.tet.2005.01.039
Latif N, Mishriky N, Massad F (1982) Carbonyl and thiocarbonyl compounds. XIX. Intramolecular cyclization of (2-nitroetheny1) aryl N-arylcarbamates: synthesis of newer series of 3,4-dihydro-2H-1,3-oxazin-2-ones and their antimicrobial activities. Aust J Chem 35:1037–1043. doi:10.1071/CH9821037
Meyers AI, Malone GR (1974) The chemistry of 2-chloromethyl-5,6-dihydro-1,3-oxazines. Grignard coupling and metalation studies. A synthesis of a-chloro aldehydes and arylacetic acids. J Org Chem 39:618–623. doi:10.1021/jo00919a008
Meyers AI, Smith EM (1972) The synthesis of ketones from dihydro-1,3-oxazines via stepwise alkyl or aryl introduction. J Org Chem 37:4289–4293. doi:10.1021/jo00799a015
Pedersen OS, Pedersen EB (2000) The flourishing syntheses of non- nucleoside reverse transcriptase inhibitors. Synthesis 2000:479–495. doi:10.1055/s-2000-6357
Ruijter E, Scheffelaar R, Orru RVA (2011) Multicomponent reaction design in the quest for molecular complexity and diversity. Angew Chem Int Ed 50:6234–6246. doi:10.1002/anie.201006515
Wolfson A, Dlugy C, Tavor D, Blumenfeld J, Shotland Y (2006) Baker’s yeast catalyzed asymmetric reduction in glycerol. Tetrahedron Asymmetry 17:2043–2045. doi:10.1016/j.tetasy.2006.07.026
Wolfson A, Dlugy C, Shotland Y (2007) Glycerol as a green solvent for high product yields and selectivities. Environ Chem Lett 5:67–71. doi:10.1007/s10311-006-0080-z
Wolfson A, Litvak G, Dlugy C, Shotland Y, Tavor D (2009) Employing crude glycerol from biodiesel production as an alternative green reaction medium. Ind Crops Prod 30:78–81. doi:10.1016/j.indcrop.2009.01.008
Zhang P, Terefenko EA, Fensome A, Zhang Z, Zhu Y, Cohen J, Winneker R, Wrobel J, Yardley J (2002) Potent nonsteroidal progesterone receptor agonists: synthesis and SAR study of 6-aryl benzoxazines. Bioorg Med Chem Lett 12:787–790. doi:10.1016/S0960-894X(02)00025-2
Acknowledgments
SG and GK thank UGC and CSIR, New Delhi, India, for the grant of junior research fellowship and senior research fellowship, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, S., Khanna, G. & Khurana, J.M. A facile eco-friendly approach for the one-pot synthesis of 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones using glycerol as a green media. Environ Chem Lett 14, 559–564 (2016). https://doi.org/10.1007/s10311-016-0570-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-016-0570-6