Abstract
The classical use of synthetic dyes is causing issues of environmental pollution and heath risk. As a consequence natural dyes are gaining interest, but the use of natural dyes still includes toxic reagents such as metals as mordants and acids to enhance color and yield. Therefore, we designed a new chitosan-polypropylene imine dendrimer hybrid at 0–2000 mg/L to treat wool before dyeing with cochineal. We compared dye exhaustion, color depth, color characteristics, and color fastness of the new process with dyed pristine and metal mordanted wool. Results show that wool pretreatment improved dye exhaustion from 48 to 88 %, shifted saturation point toward lower dye concentration from 3000 to 1000 mg/L, and improved color depth from 13.68 for pristine wool and 15.17 for metal mordanted wool to 23.89 for the new process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Besides other numerous applications, wool as an important natural fiber is extensively used in textiles, clothing, and hand-knotted carpet and rug production due to various superiority over synthetic fibers such as high elasticity, resilience, stain resistance, flame retardancy, and good dyeability (Gashti et al. 2013). Dyeing is an integral part of wool processing performed to impart color and sophistication and raise product value. Different classes of dyes either synthetic or natural are nowadays being used for wool dyeing. However, synthetic dyes produce hazardous by-products some of which contain carcinogenic intermediates which pose serious environmental hazards and human health risks (Kasiri and Safapour 2013; Shukla and Oturan 2015).
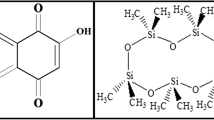
Natural dyes are recently considered as promising alternatives for synthetic dyes (Kasiri and Safapour 2014; Varadarajan and Venkatachalam 2016). Cochineal is an anthraquinone-based natural dye (Bechtold and Mussak 2009). The main colorant of cochineal is carminic acid (Fig. 1a). Rare red–purple color shades used in persian hand-knotted carpets and rugs are obtained from cochineal.
Chemical structures of a carminic acid, the main colorant of cochineal, an anthraquinone-based natural dye. Numerous hydroxyl, carbonyl, and one carboxyl groups in carminic acid are very prone for hydrogen bonding and ionic interactions with protein chains of wool (Bechtold and Mussak 2009) and b chitosan-polypropylene imine dendrimer hybrid used as biomordant for modification of wool. Preparation steps of biomordant along with chemicals used are schematically shown (Sadeghi-Kiakhani et al. 2013a)
Nevertheless, most of natural dyes are nonsubstantive, resulting in low dye exhaustion and poor color fastness. Therefore, salts of transition metals—so-called mordants—are used in natural dyeing. Mordants comprised heavy metal ions such as copper, iron, chromium, cobalt, nickel, or aluminum which their residual in wastewater after dyeing poses serious effluent disposal problem (Kasiri and Safapour 2013). Numerous researchers have examined various technologies and techniques to overcome above drawbacks. However, most of suggested methods bring about serious physical, chemical, or mechanical damage to fiber structure (Gashti et al. 2013).
Chitosan, deacetylated derivative of chitin, possesses numerous useful properties such as biodegradability, nontoxicity, and antimicrobial activity. As a sustainable biopolymer, chitosan is known as excellent candidate for textiles modification. It is extensively used to increase the cationic nature of textiles, thanks to poly-amino groups present in its structure. Chitosan treatment enhances dyeability, antimicrobial activity, and shrink-proof property of textiles (Yang et al. 2010).
Dendrimers, as biologically active macromolecules, possess branched structure, numerous reactive end-groups, and highly ordered and compact shape (Calabrett et al. 2007). Recently, dendrimers and their derivatives have been used in dye removal and modification of dyes and textiles (Sadeghi-Kiakhani et al. 2013a, b; Sadeghi-Kiakhani and Safapour 2015a, b, c; Sadeghi-Kiakhani and Safapour 2016b).
Properties of chitosan and dendrimers can be combined via hybridization. Chitosan-polypropylene imine dendrimer hybrid, which is called biomordant hereafter, has been used for dye removal from wastewater (Sadeghi-Kiakhani et al. 2013a), antimicrobial finishing (Sadeghi-Kiakhani et al. 2013b; Sadeghi-Kiakhani and Safapour 2016a), and salt-free reactive dyeing of wool and cotton (Sadeghi-Kiakhani and Safapour 2015b, c).
Thus far, the application of this novel biomordant in modification and dyeing of wool with natural dyes has not been reported. Therefore, in this study, the effect of biomordant treatment on dyeing and fastness properties of wool with cochineal as natural dye was investigated and the feasibility of replacing metal mordant with biomordant as well as acid-free dyeing of wool with cochineal was investigated and deliberated.
Experimental
Materials and methods
Materials and chemicals used were as follows: commercial woolen yarn (200 Tex(gram per 1000 m yarn)/fourfold); chitosan (degree of deacetylation = 85 %, molecular weight = 106 g/mol), Kitotak Co., Iran; polypropylene imine dendrimer (Generation = 2, molecular weight = 770 g/mol), Ciba Ltd; chitosan-polypropylene imine dendrimer hybrid as biomordant was prepared from reaction between chitosan and polypropylene imine dendrimer. The summarized preparation steps of biomordant are schematically shown in Fig. 1b and explained in detail in previous work (Sadeghi-Kiakhani et al. 2013a). Cochineal crude dye (dried insects) was purchased from local dyeing workshop. All other chemicals used were of analytical grade.
Wool dyeing was performed using laboratory high-temperature dyeing machine. Ultraviolet (UV)–Visible spectra of colored solutions were recorded using UV–Visible Spectronic Helios Alpha spectrophotometer. Color characteristics of dyed samples were measured through Color-Eye® handheld portable spectrophotometer, X-Rite Inc., USA (under D65 illumination, 10° standard observer).
Modification of wool with biomordant
Woolen yarns were scoured in solution containing 1000 mg/L nonionic detergent and 250 mg/L sodium carbonate at 50 °C for 30 min. Wool modification was performed using pad-dry-cure method, as follows. Grafting solutions containing biomordant, citric acid as cross-linker, and sodium hypophosphite as catalyst in equal ratios over concentration range of 200–2000 mg/L were prepared. Yarns (2 g each) were immersed in solution with liquor ratio of 30:1, for 6 h at 70 °C. Then, yarns were removed, squeezed, dried for 15 min at 70 °C, cured in oven at 110 °C for 5 min, rinsed with water, squeezed, air-dried, and used for dyeing trials.
Metal mordanting of wool
Before dyeing, wool was mordanted with 2000 mg/L aluminum sulfate as metal mordant, liquor ratio of 50:1, pH 5, 95 °C, 60 min. Then, mordanted wool was removed, rinsed, squeezed, air-dried, and used for dyeing trials.
Extraction of cochineal dye
A weighed finely milled cochineal was extracted with distilled water using liquor ratio of 20:1, 90 °C, 60 min, pH 5. The solution was then filtered and used for dyeing trials.
Dyeing method
All woolen yarns were dyed with cochineal dye over concentration range of 200–10,000 mg/L according to the following procedure. Yarns (1 g each) were wetted for 5 min in dyeing solution at 30 °C and liquor ratio of 50:1 before the addition of dye. As dye added, the temperature was raised to simmering point (~90 °C) with 2 °C/min, kept for 60 min, and then cooled down to 70 °C with the rate of −2 °C/min. Dyed samples were then removed from solution, rinsed with hot and then cold water, air-dried at room temperature, and analyzed.
Optical density of colored solutions was measured at maximum absorption wavelength of dye (492 nm) before and after dyeing. Dye exhaustion percentage (E%) was calculated using Eq. 1
where A 0 and A 1 are the absorbance of dye solutions prior and after dyeing process, respectively.
Color strength (K/S) was calculated using Kubelka–Munk equation (Eq. 2) (Sadeghi-Kiakhani and Safapour 2016a):
where R is reflectance at maximum absorption wavelength, K and S are absorption and scattering coefficients, respectively. K/S values represent color depth which is directly proportional to the concentration of colorant within substrate. To assure repeatability and accuracy of all experimental data, at least three individual measurements were preformed, averaged, and reported.
Color fastness assay
Wash and light fastnesses were measured according to ISO 105 C06 C2S:1994 (E) and ISO 105 B02:1988 (E) standard procedures, respectively.
Results and discussion
Dyeing characteristics
Effect of biomordant and dye concentration on dye uptake
In Fig. 2a, dye uptake, expressed as percent dye exhaustion, as a function of biomordant concentration used for wool modification is shown. Dye uptake increased by increase in biomordant concentration and reached maximum at around 1400 mg/L of biomordant. This phenomenon could be the direct consequence of increase in the number of free amino groups on wool surface, as dye absorbing sites, and enhanced possibility of ionic (carboxyl groups), hydrogen bonding (carbonyl, carboxyl, and hydroxyl groups), or van der Waals forces and hydrophobic interactions of dye with protein chains of wool giving rise to sequentially improvement in dyeing property (Sadeghi-Kiakhani and Safapour 2015b). The decrease in dye absorption in higher biomordant concentrations may be explained by aggregation of biomordant on wool surface and decrease in specific area for dye adsorption so that some sites become inaccessible to dye molecules (Sadeghi-Kiakhani and Safapour 2016a). Therefore, 1400 mg/L biomordant was selected as optimum concentration for wool modification.
Dye uptake behavior of raw and modified wool as a function of a biomordant concentration used for modification of wool. Remarkable improvement in dye absorbing capacity of treated wool confirms high potential of biomordant for more efficient dyeing process. b Dyeing pH shows the highest uptake for modified wool at pH: 7, suggesting more efficient dyeing in acid-free condition and thus elimination of acid auxiliary material from wool dyeing. Significant improvement in dye uptake of raw wool emphasizes the necessity of the use of an acid in dyeing. Dyeing conditions: pH: 4, dye: 1500 mg/L, time: 60 min, temperature: 95 °C
In another dyeing trial, the impact of dye concentration on dye uptake or dyeability of raw and modified wool was investigated. For both samples, it was found that dye uptake increased with increasing dye concentration until the saturation point was achieved, and afterward no appreciable change was observed. Further, biomordanted wool exhibited higher dye uptake and saturated (reached equilibrium) using 1000 mg/L dye, while untreated wool still required higher amounts of dye (over 3000 mg/L) to be saturated. Such decrease in dyeing saturation concentration is very worthwhile since noticeable dye material and chemicals are saved in this way which is important both in environmental and economical standpoints.
Effect of dyeing pH on dye uptake
The pH is an important factor in wool dyeing that controls the adsorption of dyes (Bechtold and Mussak 2009). Two pH values of 4 and 7, below and above isoelectric point of wool, respectively, were investigated, and dye exhaustion values are shown in Fig. 2b. Data show prominent and completely different role of pH in dye absorption. At pH 7, raw wool showed relatively low dye absorption of 48.62 %, while at pH 4, it increased to 86.4 %. This fact emphasizes the necessity of consumption of an acid auxiliary in dyeing. However, modified wool showed higher dye absorption of 88.2 % at pH 7, more interestingly higher than that of either raw or modified wool dyed at pH 4. This somewhat unusual difference may rely on the different accessibility of sites to dye molecules, interactions, and attachment of dye onto substrate described as follows.
Raw wool dye uptake analysis
In the case of raw wool, it seems dye absorption is primarily controlled by ion exchange reactions between carboxyl group of dye and amino groups of wool. Considering isoelectric point of wool (pH 4.2), below this point, wool is positively charged principally, whereas above that point carboxyl groups render a net negative charge on wool. When pH < 6, the amino groups inside wool will be always present in protonated form. At pH > 6, almost all carboxyl groups in wool will be present as carboxylate anions (Jocic et al. 2005). In the case of dye, pKa value for carboxyl group of carminic acid is 2.81 (Rasimas et al. 1996) indicating carminic acid will exist in carboxylate anion form at pH 4. Therefore, weak carboxylate anion of dye replaces that of acid due to its higher affinity (Bechtold and Mussak 2009). The anion of dye has a complex character, and when it is bound on wool, further kinds of interactions happen together with ionic forces which increase the dyeability of wool. Nonetheless, at pH 7, ionic interactions decrease because of loss in number of protonated amino groups and thus dye absorption capacity decreases. In the absence of electrostatic attractions, hydrogen bonding, van der Waals forces, and hydrophobic interaction mostly would provide attraction between dye and wool.
Modified wool dye uptake analysis
As mentioned above, modified wool demonstrated rather unexpected trend in dye absorption. Theoretically, at pH 4, it is expected biomordant to behave as cationic polyelectrolyte due to protonation of the amine groups, but at pH 7 should have very low positive charge. Subsequently, dye absorption capacity of modified wool would increase considerably at pH 4. Nonetheless, not only the fact did not happen, but also dye uptake in acidic pH was comparatively lower than that in neutral condition. This behavior implies the interaction forces between biomordant and cochineal are not essentially electrostatic; meanwhile, other forces must be considered. Carminic acid with numerous hydroxyl and carbonyl groups is very prone for hydrogen bonding. In fact, in acidic medium, due to protonation and loss of pair electrons of amine groups, the possibility of hydrogen bonding formation reduced, and consequently in neutral medium better dye absorption occurred. Therefore, results clearly showed the development of acid-free dyeing through biomordant treatment of wool.
Effect of metal mordant on color depth
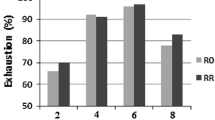
Aluminum salts are extensively used as mordant together with cochineal in wool dyeing. In Fig. 3, color strength (K/S) values representing color depth of three types of dyed wool are shown. It is observed that aluminum mordanting resulted in a small increase in color depth from 13.68 to 15.17, while that of biomordanted wool significantly increased to 23.86 rating. Results show that K/S value of modified wool was 57.48 % higher than that of metal mordanted wool.
Color strength values for dyed raw, metal mordanted, and biomordanted wool. Remarkable enhancement in color depth confirms the effectiveness and superiority of biomordant as promising alternative in place of metal mordant in wool dyeing with cochineal dye. Dyeing conditions: pH: 4, dye: 1500 mg/L, time: 60 min, temperature: 95 °C
This finding approves that the modified wool can be dyed with appreciable higher color depth without incorporation of metal mordant. In other words, using modified wool, not only metal mordant is eliminated, but also lower amount of dye is needed to obtain a given color depth. This finding is worthwhile from ecological standpoint making wool dyeing eco-friendlier than conventional dyeing processes.
Concerning biomordant used in this study, biodegradability of similar hybrid materials is reported in the literature to be dependent mostly on the nature of parent materials. Chitosan biopolymer is biodegradable, but dendrimer cytotoxicity is dependent on the chemistry of the core and mainly influenced by the nature of the dendrimer surface (Duncan and Izzo 2005). As widely documented for other polycations, dendrimers bearing amine (–NH2) termini display concentration and usually generation-dependent cytotoxicity (Duncan and Izzo 2005; Sashiwa et al. 2002). Chitosan-dendrimer hybrids are considered as novel tree-type macromolecules which can provide large variety of materials with diverse functional properties. The biodegradation of some chitosan-dendrimer hybrids has been reported (Sashiwa et al. 2002, 2003). Good biodegradability was observed for these hybrids in which dendrimers decreased somewhat the biodegradability of chitosan. In this study, few amounts of polypropylene imine dendrimer was incorporated, and consequently, it was supposed cytotoxicity of biomordant was in the acceptable range. However, the exact cytotoxicity of chitosan-polypropylene imine dendrimer hybrid needs to be investigated in detail which may be considered in another research work in future.
Color fastness
Color fastness data against wash and light are presented in Table 1a. Raw and modified wool generally possessed similar color fastness. Small color change was observed at higher biomordant concentration. Light fastness showed very good rating, while wash fastness had good rating with slight improvement in the case of treated samples. Overall, results showed that color fastness was independent from biomordant concentration and wool treatment did not impair color fastness.
Colorimetric properties
More uniform version of Commission Internationale de l’Eclairage (CIE) namely CIEL*a*b* was used to represent color characteristics of dyed samples. L* corresponds to the brightness (100 = white, 0 = black), a* to red–green coordinate (+ = red, − = green), b* to yellow–blue coordinate (+ = yellow, − = blue). C* is color purity or vividness–dullness (100 = vivid, 0 = dull), and h° is hue angle, calculated from the reflectance data (Ford and Roberts 1998; Wyszecki and Stiles 2000). Color values of dyed samples are presented in Table 1b. Dyed samples possessed purple color shade with slight variations. Modified wool showed an increase in yellowness (decrease in blueness) of color shade compared to dyed raw wool. The variations in colorimetric data may be attributed to the presence of more amine groups on the modified wool. Lower L* values denote darker shades which are in agreement with color strength data.
Conclusion
Our results show that chitosan-polypropylene imine dendrimer hybrid can be used as an alternative promising biomordant in place of metal mordant in wool dyeing with cochineal natural dye. Indeed, biomordant treated wool showed better performance in dyeing so that dye exhaustion markedly increased, saturation point of wool shifted to lower dye concentrations, and the amount of dye required to obtain a given color depth decreased. Further, metal mordant and acid auxiliaries were eliminated from cochineal dyeing process and replaced with biomordant. The method suggested in this study makes wool dyeing eco-friendlier over conventional dyeing processes. Therefore, the problems associated with metal mordant and dyeing wastewater hazards in cochineal dyeing of wool are minimized.
References
Bechtold T, Mussak R (2009) Handbook of natural colorants. Wiley. The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom, Online ISBN: 9780470744970. doi: 10.1002/9780470744970
Calabrett MK, Kumar A, McDermott AM, Cai C (2007) Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 8:1807–1811. doi:10.1021/bm0701088
Duncan R, Izzo L (2005) Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev 57:2215–2237. doi:10.1016/j.addr.2005.09.019
Ford A, Roberts A (1998) Colour space conversions. Westminster University, London. http://www.poynton.com/PDFs/coloureq.pdf. Accessed 08 April 2016
Gashti MP, Katozian B, Shaver M, Kiumarsi A (2013) Clay nanoadsorbent as an environmentally friendly substitute for mordants in the natural dyeing of carpet piles. Color Technol 130:54–61. doi:10.1111/cote.12065
Jocic D, Vı´lchez S, Topalovic T, Navarro A, Jovancic P, Julia MR, Erra P (2005) Chitosan/acid dye interactions in wool dyeing system. Carbohydr Polym 60:51–59. doi:10.1016/j.carbpol.2004.11.021
Kasiri MB, Safapour S (2013) Natural dyes and antimicrobials for textiles, in: Lichtfouse E, Schwarzbauer J, Robert D (Eds.) Green materials for energy, products and depollution, 6th Chapter, Springer Science + Business Media, Dordrecht, pp 229–286. doi: 10.1007/978-94-007-6836-9
Kasiri MB, Safapour S (2014) Natural dyes and antimicrobials for green treatment of textiles. Environ Chem Lett 12:1–13. doi:10.1007/s10311-013-0426-2
Rasimas JP, Berglund KA, Blanchard GJ (1996) A molecular lock-and-key approach to detecting solution phase self-assembly. A fluorescence and absorption study of carminic acid in aqueous glucose solutions. J Phys Chem 100(17):7220–7229. doi:10.1021/jp953743t
Sadeghi-Kiakhani M, Safapour S (2015a) Improvement of the dyeing and fastness properties of a naphthalimide fluorescent dye using poly(amidoamine) dendrimer. Color Technol 131:142–148. doi:10.1111/cote.12132
Sadeghi-Kiakhani M, Safapour S (2015b) Eco-friendly dyeing of treated wool fabrics with reactive dyes using chitosanpoly(propylene imine) dendrimer hybrid. Clean Tech Environ Policy 17:1019–1027. doi:10.1007/s10098-014-0855-z
Sadeghi-Kiakhani M, Safapour S (2015c) Salt-free reactive dyeing of the cotton fabric modified with chitosan-poly(propylene imine) dendrimer hybrid. Fibers Polym 16(5):1078–1081. doi:10.1007/s12221-015-1075-9
Sadeghi-Kiakhani M, Safapour S (2016a) Improvement of dyeing and antimicrobial properties of nylon fabrics modified using chitosan-poly(propylene imine) dendreimer hybrid. J Ind Eng Chem 33:170–177. doi:10.1016/j.jiec.2015.09.034
Sadeghi-Kiakhani M, Safapour S (2016b) Functionalization of polyamidoamine dendrimers-based nano-architectures using a naphthalimide derivative and their fluorescent, dyeing and antimicrobial properties on wool fibers. Luminescence. doi:10.1002/bio.3065
Sadeghi-Kiakhani M, Arami M, Gharanjig K (2013a) Dye removal from colored textile wastewater using chitosan-PPI dendrimer composite as a biopolymer: optimization, kinetic and isotherm studies. J Appl Polym Sci 127:2019–2607. doi:10.1002/app.37615
Sadeghi-Kiakhani M, Arami M, Gharanjig K (2013b) Application of a biopolymer chitosan-poly(propylene) imines dendrimer hybrid as an antimicrobial agent on the wool. Iran Polym J 22:931–940. doi:10.1007/s13726-013-0193-8
Sashiwa H, Kawasaki N, Nakayama A, Muraki E, Yamamoto N, Zhu H, Nagano H, Omura Y, Saimoto H, Shigemasa Y, Aiba S (2002) Chemical modification of chitosan. 13.1 Synthesis of organosoluble, palladium adsorbable, and biodegradable chitosan derivatives toward the chemical plating on plastics. Biomacromolecules 3:1120–1125. doi:10.1021/bm0200478
Sashiwa H, Yajima H, Aiba S (2003) Synthesis of a chitosan-dendrimer hybrid and its biodegradation. Biomacromolecules 4:1244–1249. doi:10.1021/bm030021w
Shukla S, Oturan MA (2015) Dye removal using electrochemistry and semiconductor oxide nanotubes. Environ Chem Lett 13(2):157–172. doi:10.1007/s10311-015-0501-y
Varadarajan G, Venkatachalam P (2016) Sustainable textile dyeing processes. Environ Chem Lett 14(1):113–122. doi:10.1007/s10311-015-0533-3
Wyszecki G, Stiles WS (2000) Color science concepts and methods, quantitative data and formulae, 2nd edn. Wiley, New York. ISBN: 978-0-471-39918-6
Yang HC, Wang WH, Huang KS, Hon MH (2010) Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr Polym 79:176–179. doi:10.1016/j.carbpol.2009.07.045
Acknowledgments
This paper has been extracted from thesis submitted for master degree in “Carpet materials and dyeing” in Faculty of Carpet of Tabriz Islamic Art University. Hereby, the authors would like to express their gratitude for “Tabriz Islamic Art University” for all the supports. Also, cordial thanks go to “Institute for Color Science and Technology” for sincere collaboration throughout this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehrparvar, L., Safapour, S., Sadeghi-Kiakhani, M. et al. Chitosan-polypropylene imine dendrimer hybrid: a new ecological biomordant for cochineal dyeing of wool. Environ Chem Lett 14, 533–539 (2016). https://doi.org/10.1007/s10311-016-0559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-016-0559-1