Abstract
l-Serine is widely used in pharmaceutical, food and cosmetic industries, and the direct fermentation to produce l-serine from cheap carbon sources such as glycerol is greatly desired. The production of l-serine by engineered Escherichia coli from glycerol has not been achieved so far. In this study, E. coli was engineered to efficiently produce l-serine from glycerol. To this end, three l-serine deaminase genes were deleted in turn, and all of the deletions caused the maximal accumulation of l-serine at 0.06 g/L. Furthermore, removal of feedback inhibition by l-serine resulted in a titer of 1.1 g/L. Additionally, adaptive laboratory evolution was employed to improve glycerol utilization in combination with the overexpression of the cysteine/acetyl serine transporter gene eamA, leading to 2.36 g/L l-serine (23.6% of the theoretical yield). In 5-L bioreactor, l-serine titer could reach up to 7.53 g/L from glycerol, demonstrating the potential of the established strain and bioprocess.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Serine is widely used in the pharmaceutical, food and cosmetic industries. Compared with other amino acids, accumulation of l-serine is more difficult in wild-type strains because it is an intermediate metabolite [2]. Following metabolic engineering of Corynebacterium glutamicum (C. glutamicum) ATCC13032, 36.5 g/L l-serine was produced [15]. In our laboratory, the C. glutamicum SYPS-062-33aΔSSAAI strain was constructed by random mutagenesis and metabolic engineering, which produced 42.62 g/L of l-serine with 21.3% mass yield [20, 26], and this was the highest titer produced by organism.
Compared with C. glutamicum, E. coli has a higher growth rate, and has also been widely used in the synthesis of amino acids [13, 16, 23]. However, l-serine is not accumulated by wild-type E. coli. By deleting the three l-serine deaminase genes, sdaA, sdaB, and tdcG, recombinant E. coli NW-7 could produce 3.8 mg/L l-serine from glucose [6]. Recombinant E. coli DH5α YF-7 was constructed by overexpressing l-serine synthetic genes, blocking the l-serine degradation pathway, and regulating the glyoxylate cycle, and the resulting strain could produce 8.45 g/L l-serine from glucose in fed-batch fermentation [5]. Recently, the recombinant E. coli MG1655 strain was engineered in which l-serine degradation was prevented, and eamA (a cysteine/acetyl serine transporter) were overexpressed, resulting in l-serine titer of 11.7 g/L from glucose with threonine addition in fed-batch fermentation [11]. Further, they obtained an evolved strain using adaptive laboratory evolution, and the strain accumulated 37 g/L l-serine from glucose with a 24% mass yield [12], is the highest l-serine value produced by E. coli. However, the strain was glycine auxotroph, therefore, the addition of glycine was required to maintain the fermentation.
All aforementioned studies were based on the production of l-serine using glucose as a carbon source. The direct fermentative production of l-serine from glycerol is greatly desired (Fig. 1), because glycerol is a renewable resource generated as a by-product from the biodiesel production process [21]. Moreover, glycerol assimilation is initiated by its ATP-dependent phosphorylation via glycerol kinase [4, 14], with no phosphoenolpyruvate (PEP) consumed in this process. It is worthy to note that higher PEP concentration is favourable for l-serine production, since PEP is a precursor of l-serine. In contrast, PEP was consumed when glucose is the carbon source, and glucose is phosphorylated by a glucose-specific phosphotransferase system (PTS) to produce glucose-6-phosphate with PEP as a phosphoryl donor. Using glycerol as a carbon source is a potential strategy for enhancing l-serine production. Inactivation of the PTS to save PEP in C. glutamicum SYPS-062 improved l-serine titer has been done in our laboratory (unpublished data). Glycerol-based production of l-glutamate, l-lysine and succinate has been achieved [7, 14]. Nevertheless, successful production of l-serine by engineered E. coli from glycerol has not been achieved so far.
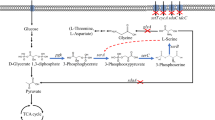
l-serine biosynthesis pathways from glycerol in E. coli. Genes and enzymes: glycerol facilitator (glpF); glycerol kinase (glpK); glycerol 3-phosphate dehydrogenase (glpD); 3-phosphoglycerate dehydrogenase (serA); phosphoserine aminotransferase (serC); phosphoserine phosphatase (serB); cysteine/acetyl serine transporter (eamA); serine hydroxymethyltransferase (glyA); l-serine deaminases (sdaA, sdaB, and tdcG). Intermediates: dihydroxyacetone phosphate (DHAP); phosphoenolpyruvate (PEP); tricarboxylic acid cycle (TCA). Highlighted arrows indicate pathways in which genes were upregulated by overexpression eamA. Red crosses on solid lines (
 ) indicate genes that were deleted. Crosses on dashed lines (
) indicate genes that were deleted. Crosses on dashed lines (
 ) indicate the removal of feedback inhibition (color figure online)
) indicate the removal of feedback inhibition (color figure online)
In this study, E. coli was engineered to efficiently produce l-serine from glycerol. To this end, three l-serine deaminase genes (tdcG, sdaA and sdaB) were deleted in turn. Furthermore, remove of feedback inhibition by l-serine through the mutation (H344A, N346A and N364A) of 3-phosphoglycerate dehydrogenase. Two strategies were attempted to improve glycerol utilization, overexpression of glycerol metabolism pathway genes (glpD, glpK) or adaptive laboratory evolution was employed respectively, to improve l-serine titer further, overexpression of the cysteine/acetyl serine transporter gene eamA was performed. The effects of these modifications on l-serine production and cell growth were investigated and discussed in detail.
Materials and methods
Strains and plasmids
Strains and plasmids used in the work are listed in Table 1. Primers for gene cloning and deleting are listed in Table 2. E. coli JM109 was used for plasmid construction. The vector pDXW-10 [19] was used to provide the tac-M promoter and the corresponding rrnB terminator for eamA expressing, and eamA was amplified from chromosomal DNA of E. coli W3110 using primers pDXW-10-eamA-F and pDXW-10-eamA-R. The eamA PCR product was digested with Not I and BgI II (TaKaRa, Dalian, China) and ligated into pDXW-10 using T4 DNA ligase (TaKaRa, Dalian, China). The resulted pDXW-10-eamA was used as a template for amplifying the tac-M-eamA-rrnB fragment. The compatible pACYCDuet-1 vector was used for overexpression of eamA.
Engineering of strains
All target genes deletion was performed using the λRed system [3]. Plasmid pKD46 was used as the Red recombinase expression vector, pKD3 and pKD4 were used as templates for PCR amplification of disruption cassettes containing the chloramphenicol or kanamycin resistance gene, PCP20 was used to cure the kanamycin or chloramphenicol marker via the flippase mediated-recombination of FRT sequence flaking the antibiotic resistance gene. For sdaA and sdaB deletions, sdaA-F and sdaA-R, sdaB-F and sdaB-R and the pKD4 template were used to obtain linearized PCR products consisting of 50 nt of sequence homologous to the target locus flanked by the Flp recognition target (FRT). These were electroporated into strains containing the pKD46 plasmid. Transformants were selected on kanamycin plates and verified by PCR using the corresponding primers ∆sdaA-F and ∆sdaA-R, or ∆sdaB-F and ∆sdaB-R.
Feedback inhibition of PGDH was prevented by mutating three residues H344, N346 and N364 into alanine [1] using site-directed mutagenesis to generate PGDHdr. The method used for site-specific mutagenesis was the same as described above for genes deletion, but with primers Del-serA-up-P1, Del-serA-up-P2, Del-serA-up-P3, Del-serA-up-P4, serA-FRT-P1, serA-FRT-P2, Del-serA-do-P1, and Del-serA-do-P2.
Media
Luria–Bertani (LB) medium used for plasmid construction contained (per L) 5.0 g yeast extract, 10.0 g tryptone, and 10.0 g NaCl. When appropriate, chloramphenicol (34 μg/mL) or kanamycin (50 μg/mL) was added. For l-serine fermentation, mineral AM1 medium [9] supplemented with 1 g/L yeast extract and 10 g/L glycerol was used.
Cultivation conditions
E. coli was cultured in 10 mL Luria–Bertani (LB) media with the corresponding antibiotics at 37°C and 220 rpm for 12 h, and 2 mL was inoculated into 50 mL of fermentation medium in 250 mL shake flasks and cultured for 4 h. Expression was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) at the final concentration of 0.5 mM, and culturing continued for a further 30 h at 30°C and 220 rpm. For l-serine production, samples were withdrawn from cultures and used to measure the concentration of amino acids and glycerol, and the biomass.
The batch and fed-batch cultivation were accomplished in a 5-L bioreactor (BIOTECH-50JSA, China) containing 3 L of the fermentation medium with an initial glycerol concentration of 15 g/L at 37 °C. When glycerol was consumed, all the feed was started with feeding medium that contained 300 g/L glycerol. The pH was controlled at 7.0 by automatically feeding concentrated NH3·H2O. Antifoam was added to control foaming.
Adaptive laboratory evolution to improve glycerol utilization
Adaptive laboratory evolution of E. coli strain 4W was performed by culturing cells in LB medium, transferring a 10% (v) inoculum into a fresh AM1 medium containing 10 g/L glycerol, and growing for 10 generations. Then, the cells were inoculated into fresh AM1 medium containing 20 g/L glycerol and grown for genetic screening. Successive rounds of adaptive evolution were carried out with the glycerol concentration increased stepwise (10, 20, 40, 60, and 80 g/L). Among the evolved strains, the most efficient strain was selected for further investigation and named 4WA.
Analytical methods
Cell growth was measured as the optical density at 600 nm (AOE UV-1200S, China) and correlated with the dry cell weight (DCW) using the formula DCW (g/L) = 0.36 × OD600. The concentration of glycerol was determined using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with a Cosmosil Sugar-D column (4.6 × 250 mm) and a refractive index detector (RID). The mobile phase consisted of acetonitrile (80:20, v/v), and the flow rate was adjusted to 1 mL/min. For amino acid analysis, phenylisothiocyanate (PITC) was used as a pre-column derivatization agent. The amount of l-serine and other amino acids was determined by HPLC as previously described [25].
Results
Construction of a l-serine producing stain from glycerol
l-Serine deaminase, encoded by sdaA, sdaB and tdcG in E. coli, converts l-serine into pyruvate, and thus reduces the accumulation of l-serine. In this study, we first knocked out tdcG in E. coli W3110 to generate the G strain, but there was no l-serine detected in the fermentation broth. Deletion of sdaA in the G strain resulted in the double knock out GA strain, but again, l-serine was barely detected in the culture. However, when sdaB was deleted as well, the triple deletion (∆sdaA, ∆sdaB, ∆tdcG) strain GAA accumulated 0.06 g/L l-serine with the biomass (OD600) reached 8.1, and all glycerol was consumed after 18 h (Fig. 2).
Comparison of glycerol consumption, cell growth, and l-serine production of strains W3110, G, GA and GAA. a Profiles of residual glycerol and cell density with strains W3110, G, and GA. Squares represent W3110, circles represent G, triangles represent GA, open symbols represent OD600, closed symbols represent residual glycerol. b Profiles of glycerol consumption, cell growth and l-serine production in GAA. Squares represent residual glycerol, circles represent cell growth, triangles represent l-serine. Values denote the average of three independent experiments, and error bars indicate standard deviation
Furthermore, removal of feedback inhibition by l-serine through mutating three residues (H344A, N346 A and N364A) in strain GAA, resulted in strain 4W. As shown in Fig. 3, strain 4W accumulated 1.1 g/L l-serine at 54 h, which is 18-fold higher than GAA (0.06 g/L l-serine), and the maximal biomass (OD600) reached 4.8. Specific production in the 4W strain was 0.66 g/L/DCW, which is 33-fold higher than the parent strain GAA (0.02 g/L/DCW). However, the rate of glycerol utilization by 4W was decreased significantly, and at 18 h, the glycerol concentration was 4.24 g/L, whereas GAA consumed all glycerol by this time. How to improve glycerol utilization was the key to be solved.
Improvement of glycerol utilization
To improve glycerol utilization, two strategies including metabolic engineering and adaptive laboratory evolution were performed separately. Two genes encoding enzymes mediating glycerol dissimilation (glpD and glpK) were overexpressed in strain 4W, resulting in strain 4W-glpD–glpK (Fig. 4a). To our surprise, overexpression of glpD and glpK decreased the cell growth significantly compared with the parental strain 4W, and the maximal biomass (OD600) was only 2.65 at 30 h, while 5.95 g/L glycerol was consumed at 60 h, presumably because the weak cell growth affected glycerol utilization.
Comparison of glycerol consumption, cell growth and l-serine production in strains 4W-glpD–glpK and 4WA. a Profiles of 4W-glpD–glpK. b Profiles 4WA. Squares represent residual glycerol, circles represent cell growth, triangles represent l-serine. Values denote the average of three independent experiments, and error bars indicate standard deviation
Adaptive laboratory evolution was, therefore, performed to improve glycerol utilization, and the overall schematic of the process is shown in Fig. 1. Five populations evolved in this step and the glycerol concentration was increased gradually (10, 20, 40, 60, and 80 g/L) of evolution (Fig. 4b). The cells were continuously transferred into fresh media during the exponential growth phase. At the end of the experiment, the ALE resultant strain, named 4WA exhibited faster growth on glycerol, as shown in Fig. 4b, and reached a maximal biomass (OD600) of 5.2 at 36 h, 1.35 g/L l-serine, with all glycerol consumed at 30 h, while the parental 4W strain consumed 7.76 g/L of glycerol at this time. At the same time, the serine tolerance of those strains were evaluated (Fig. 5).
Comparison of the growth profiles of strains W3110, 4W, 4WR and 4WE in AM1 containing different concentrations of l-serine. a Cell density profile of W3110. b Cell density profile of 4W. c Cell density profile of 4WR. d Cell density profile of 4WE. Values denote the average of three independent experiments, and error bars indicate standard deviation
Overexpression of eamA in 4WA enhances l-serine titer
Overexpression of eamA in the evolved strain 4WA generated strain 4WAE, the cell growth, glycerol consumption and l-serine production in 4WAE were evaluated with glycerol as the sole carbon source (Fig. 6). l-Serine accumulated to 2.36 g/L at 36 h, with a maximal biomass (OD600) of 5.57, a specific production of 1.18 g/L/DCW, and a yield of 23.6%, which was significantly higher than the parent strain 4WA (1.35 g/L l-serine and 0.75 g/L/DCW). Furthermore, all glycerol was consumed at 24 h by 4WAE, and the parent strain finished glycerol at 30 h. This result showed that overexpression of eamA improved l-serine titer.
Production of l-serine by 5-L bioreactor
The fermentation profiles of 4WAE were examined in 5-L bioreactor. As shown in Fig. 7a, the maximal biomass (OD600) of 4WAE reached 9.4 at 24 h, which was 54.1% higher than that obtained under shake-flask conditions. The engineered strain exhibited higher glycerol consumption rate and the highest l-serine titer of 3.23 g/L was achieved. Meanwhile, the l-serine titer was improved by 36.9%, when compared with that obtained under shake-flask conditions.
Time course of l-serine production in the 4WAE strain during batch and fed-batch fermentation. a Time course of l-serine production in the 4WAE strain during batch fermentation. b Time course of l-serine production in the 4WAE strain during fed-batch fermentation. Squares represent glycerol, circles represent cell growth, triangles represent l-serine. Values denote the average of three independent experiments, and error bars indicate standard deviation
As shown in Fig. 7b, the engineered strain 4WAE exhibited maximal biomass (OD600) of 15.48 at 42 h, which was 90.1% higher than that of batch fermentation, and the highest l-serine titer of 7.53 g/L was reached with a yield of 21.5% at 42 h after overall feeding of 4 times of glycerol, which was 133% higher than that of batch fermentation.
Discussions
Direct fermentative production of l-serine is attracting growing attention, and glycerol is considered as a potentially ideal carbon source to convert to value-added bio-products. In this study, a series of genetic manipulations in E. coli were performed to improve l-serine production from glycerol (Table 3). Overexpression of eamA, deletion of sdaA, sdaB and tdcG, and alleviation of feedback inhibition by serA, combined with adaptive evolution, together enhanced l-serine production to 2.36 g/L, with a yield of 23.6%. The results clearly demonstrate the feasibility of our approach for engineering E. coli for l-serine production from glycerol.
In this study, three l-serine deaminase genes tdcG, sdaA, sdaB was knocked out in turn, we initially knocked out tdcG in E. coli W3110 to generate the G strain, but l-serine accumulation remained undetectable. Deletion of sdaA in the G strain resulted in the double knock out of GA strain, but again, l-serine was not detected (Fig. 2a). Further, when sdaB was also deleted, the triple deletion (∆sdaA, ∆sdaB, ∆tdcG) strain GAA accumulated 0.06 g/L l-serine. Combined with alleviation of the feedback inhibition by serA, resulting in strain 4W, 1.1 g/L l-serine was achieved, However, the glycerol utilization rate in 4W was lower than the parental strain E. coli W3110.
The glpK–glpD pathway mediates glycerol dissimilation under aerobic conditions, overexpressing glpK and glpD can increase glycerol flux in E. coli [10, 18, 22]. In this study, to improve glycerol utilization, glpD and glpK were overexpressed in strain 4W first, resulting in strain 4W-glpD–glpK (Fig. 4a). To our surprise, overexpression of glpD and glpK decreased the cell growth significantly compared with the parental 4W strain, and the maximal biomass (OD600) was only 2.65 at 30 h, while glycerol consumption was 5.95 g/L at 60 h, presumably because the weak cell growth affected glycerol utilization. The negative effect of glpD and glpK overexpression on the cell growth was observed in other study, where E. coli BL21 (DE3) DK DCW achieved a biomass of 4.2 g/L, compared with the parent strain 5.3 g/L [22]. It is possible that the plasmids used for overexpression burdened the host and lowered the growth rate.
Moreover, adaptive laboratory evolution was used to improve glycerol utilization, and the resulting strain 4WA was improved further for l-serine production. To illustrate the mutations causing the increased glycerol utilization, the whole genome of 4WA was sequenced on the Illumina HiSeqX10 platform with over 1.59 million reads. A comparative genomics analysis of 4WA and the parental E. coli W3110 strain was performed. The results revealed single-nucleotide variants, which included one synonymous mutation, nine non-synonymous mutations, and other mutations located in the intergenic region. Further studies will be needed to explore the reason for phenotypic differences.
Compared with sugar, glycerol dissimilation is initiated by its ATP-dependent phosphorylation via glycerol kinase [4, 14], whereas glucose is initially phosphorylated by the glucose-specific PTS to glucose 6-phosphate with phosphoenolpyruvate (PEP) as phosphoryl donor. A high glucose uptake rate promotes a high pyruvate and low PEP concentration in the cell, which is unfavourable for l-serine production in E. coli, because PEP is the substrate for anaplerotic C3 carboxylation via phosphoenolpyruvate carboxylase (PEPCx) or phosphoenolpyruvate carboxykinase (PEPCk) [24]. Conveniently, tremendous growth in the biofuels industry has created a surplus of glycerol, resulting in a dramatic decrease in crude glycerol prices [8]. Thus, using glycerol as a carbon source in the fermentation production of value-added chemicals such as 1,3-propanediol, ethanol, and amino acids is a feasible approach to enhance the economic efficiency of biofuel production processes [17]. Successful production of l-serine by engineered E. coli from glycerol has not been achieved so far.
Recently, Mundhada [12] obtained a strain accumulated 37 g/L l-serine from glucose with 24% mass yield, which was the highest l-serine production by E. coli so far. Although the l-serine titer achieved by 4WAE in the present study was not significantly higher than the previous one, the resulting yield (23.6%, mass yield) was similar to highest yield (24%) reported by the previous study, in which glucose was used as a substrate.
The present study demonstrates the feasibility of engineering the l-serine biosynthetic pathway to make use of the abundant, renewable and affordable glycerol substrate. At present, microbial fermentative production of glycerol remains more expensive than chemical production due to a lower product yield, but if the cost of raw materials drops even further, the fermentation directly using glycerol as a carbon source could become competitive [8]. Moreover, the crude glycerol is much cheaper than glucose right now, therefore, it is a prime candidate substrate for future fermentation production processes.
Change history
26 April 2019
Unfortunately, the order of the figures 1-4 has been positioned wrongly in the print published article
References
Al-Rabiee R, Lee EJ, Grant GA (1996) The mechanism of velocity modulated allosteric regulation in D-3-phosphoglycerate dehydrogenase cross-linking adjacent regulatory domains with engineered disulfides mimics effector binding. J Biol Chem 271:13013–13017. https://doi.org/10.1074/jbc.271.22.13013
Becker J, Wittmann C (2012) Systems and synthetic metabolic engineering for amino acid production–the heartbeat of industrial strain development. Curr Opin Biotechnol 23:718–726. https://doi.org/10.1016/j.copbio.2011.12.025
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645. https://doi.org/10.1073/pnas.120163297
Durnin G, Clomburg J, Yeates Z, Alvarez PJ, Zygourakis K, Campbell P, Gonzalez R (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103:148–161. https://doi.org/10.1002/bit.22246
Gu P, Yang F, Su T, Li F, Li Y, Qi Q (2014) Construction of an l-serine producing Escherichia coli via metabolic engineering. J Ind Microbiol Biotechnol 41:1443–1450. https://doi.org/10.1007/s10295-014-1476-6
Li Y, Chen G-K, Tong X-W, Zhang H-T, Liu X-G, Liu Y-H, Lu F-P (2012) Construction of Escherichia coli strains producing l-serine from glucose. Biotechnol Lett 34:1525–1530. https://doi.org/10.1007/s10529-012-0937-0
Litsanov B, Brocker M, Bott M (2013) Glycerol as a substrate for aerobic succinate production in minimal medium with Corynebacterium glutamicum. Microb Biotechnol 6:189–195. https://doi.org/10.1111/j.1751-7915.2012.00347.x
Luo X, Ge X, Cui S, Li Y (2016) Value-added processing of crude glycerol into chemicals and polymers. Bioresour Technol 215:144–154. https://doi.org/10.1016/j.biortech.2016.03.042
Martinez A, Grabar T, Shanmugam K, Yomano L, York S, Ingram L (2007) Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol Lett 29:397–404. https://doi.org/10.1007/s10529-006-9252-y
Mazumdar S, Blankschien MD, Clomburg JM, Gonzalez R (2013) Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb Cell Fact 12:7. https://doi.org/10.1186/1475-2859-12-7
Mundhada H, Schneider K, Christensen HB, Nielsen AT (2016) Engineering of high yield production of l-serine in Escherichia coli. Biotechnol Bioeng 113:807–816. https://doi.org/10.1002/bit.25844
Mundhada H, Seoane JM, Schneider K, Koza A, Christensen HB, Klein T, Phaneuf PV, Herrgard M, Feist AM, Nielsen AT (2017) Increased production of l-serine in Escherichia coli through adaptive laboratory evolution. Metab Eng 39:141–150. https://doi.org/10.1016/j.ymben.2016.11.008
Park JH, Kim TY, Lee KH, Lee SY (2011) Fed-batch culture of Escherichia coli for l-valine production based on in silico flux response analysis. Biotechnol Bioeng 108:934–946. https://doi.org/10.1002/bit.22995
Rittmann D, Lindner SN, Wendisch VF (2008) Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum. Appl Environ Microbiol 74:6216–6222. https://doi.org/10.1128/AEM.00963-08
Stolz M, Peters-Wendisch P, Etterich H, Gerharz T, Faurie R, Sahm H, Fersterra H, Eggeling L (2007) Reduced folate supply as a key to enhanced l-serine production by Corynebacterium glutamicum. Appl Environ Microbiol 73:750–755. https://doi.org/10.1128/AEM.02208-06
Wang Y, Li Q, Zheng P, Guo Y, Wang L, Zhang T, Sun J, Ma Y (2016) Evolving the l-lysine high-producing strain of Escherichia coli using a newly developed high-throughput screening method. J Ind Microbiol Biotechnol 43:1227–1235. https://doi.org/10.1007/s10295-016-1803-1
Wendisch VF, Lindner SN, Meiswinkel TM (2011) Use of glycerol in biotechnological applications. In: Montero G (ed) Biodiesel-quality, emissions and by-products. InTech. https://doi.org/10.5772/25338
Wong MS, Li M, Black RW, Le TQ, Puthli S, Campbell P, Monticello DJ (2014) Microaerobic conversion of glycerol to ethanol in Escherichia coli. Appl Environ Microbiol 80:3276–3282. https://doi.org/10.1128/AEM.03863-13
Xu D, Tan Y, Shi F, Wang X (2010) An improved shuttle vector constructed for metabolic engineering research in Corynebacterium glutamicum. Plasmid 64:85–91. https://doi.org/10.1016/j.plasmid.2010.05.004
Xu G, Zhu Q, Luo Y, Zhang X, Guo W, Dou W, Li H, Xu H, Zhang X, Xu Z (2015) Enhanced production of l-serine by deleting sdaA combined with modifying and overexpressing serA in a mutant of Corynebacterium glutamicum SYPS-062 from sucrose. Biochem Eng J 103:60–67. https://doi.org/10.1016/j.bej.2015.06.009
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol Biofuels 5:13. https://doi.org/10.1186/1754-6834-5-13
Yang Y, Yuan C, Dou J, Han X, Wang H, Fang H, Zhou C (2014) Recombinant expression of glpK and glpD genes improves the accumulation of shikimic acid in E. coli grown on glycerol. World J Microbiol Biotechnol 30:3263–3272. https://doi.org/10.1007/s11274-014-1753-6
Zhang X, Jantama K, Moore JC, Shanmugam KT, Ingram LO (2007) Production of l-alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77:355–366. https://doi.org/10.1007/s00253-007-1170-y
Zhang X, Jantama K, Shanmugam K, Ingram L (2009) Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol 75:7807–7813. https://doi.org/10.1128/AEM.01758-09
Zhang X, Xu G, Li H, Dou W, Xu Z (2014) Effect of cofactor folate on the growth of Corynebacterium glutamicum SYPS-062 and l-serine accumulation. Appl Biochem Biotechnol 173:1607–1617. https://doi.org/10.1007/s12010-014-0945-8
Zhu Q, Zhang X, Luo Y, Guo W, Xu G, Shi J, Xu Z (2015) l-Serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:1665–1673. https://doi.org/10.1007/s00253-014-6243-0
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 31300027, 31600044), the Natural Science Foundation of Jiangsu Province (No. BK20150151).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, D., Zhu, J. et al. High-yield production of l-serine from glycerol by engineered Escherichia coli. J Ind Microbiol Biotechnol 46, 221–230 (2019). https://doi.org/10.1007/s10295-018-2113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2113-6