Abstract
In this study, the potential helper genes were identified through the data analysis of transcriptomic and proteomic profiling in recombinant Pichia pastoris cultured under simulated microgravity (SMG). Co-expressing of four genes PRX1, YAP1, AHA1, and YPT6, involved in the oxidative stress response and protein folding, exhibited promising helper factor effects on the recombinant protein yields in engineered P. pastoris, respectively. When two of the above genes were co-expressed simultaneously, β-glucuronidase (PGUS) specific activity was further increased by 30.3–50.6 % comparing with that of single helper gene, particularly when the oxidative stress response and protein folding genes were both present in the combinations. In addition, co-expressing co-chaperone AHA1 and transcription factor YAP1 not only enhanced PGUS secretion, but also affected its glycosylation. Thus, through deep “omics” analysis of SMG effects, our results provided combined impact of new helper factors to improve the efficacy of recombinant protein secretion and glycosylation in engineered P. pastoris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The methylotrophic yeast Pichia pastoris is the most frequently used yeast system for the production of recombinant protein, due to the capabilities of eukaryotic posttranslational modification, high cell density cultivation, and good secretion capacity with low levels of endogenously secreted proteins [9]. Nevertheless, heterologous protein production with improved yield and quality by P. pastoris due to unsolved bottlenecks involved in the processes of transcription, translation, folding, and secretion, possibly posttranslational modification [1]. As reported, the strong constitutive or inducible promoters, the controlling gene copy numbers, the effective natural or artificial transcription factors, the efficient insertion expression cassettes, the codon-optimized synthetic genes, and the helper genes are all involved in increasing the protein folding or flux and ATP production [8, 9, 19, 20, 22]. They could serve as typical biological components and devices of synthetic biology tools to allow multilevel modifications of host strains with improved yield and quality of recombinant proteins [1]. In recent years, studies based on the N-linked glycosylation modification in P. pastoris have attracted increased attention to meet the need of improving the expression and stability of recombinant protein in biotechnological industry. The approaches of reconstructing N-glycosylation pathways and enhancing the efficiency of preparations by glycan engineering in the P. pastoris play crucial roles in the heterologous protein production [34].
The system biology integrated functional genomics and “omics” analytical platforms have served as valuable tools of finding new targets for yeast engineering strategies [6, 21, 27, 32]. Gasser et al. selected a range of significantly regulated genes based on the transcriptional profile under stress conditions and tested their Saccharomyces cerevisiae homologues for co-expression in a recombinant P. pastoris strain. The helper factors BMH2, BFR2, SSA4, SSE1, CUP5, KIN2, PDI1, ERO1, HAC1, KAR2, and SSO2 were identified to prove their benefits by increasing specific production of an antibody fragment in the P. pastoris [8]. Baumann et al. reported the transcriptome, proteome, and fluoxome of a recombinant P. pastoris strain expressing an Fab antibody fragment under three different conditions of oxygen availability, and the WSC4, which was involved in trafficking through the ER, was identified as a novel potential target gene for strain improvement based on previous transcriptomic analyzing [2]. Prielhofer et al. identified a set of novel regulated promoters of P. pastoris enabling induction without methanol using DNA microarrays analysis [22]. Nocon et al. identified the helper genes which enhanced production of cytosolic human superoxide dismutase by overexpressing or knock outing the targets in pentose phosphate pathway and the TCA cycle in the metabolic model prediction of P. pastoris [21]. The environmental parameters of recombinant protein-producing conditions can affect the host cell physiologies, the volumetric productivities, and the glycosylation of heterologous proteins [15]. The notable cultivation level factors are the temperatures, pH, dissolved oxygen concentration, and composition of the medium [10, 35].
It is an attractive field to improve the metabolic conversion efficacy of biopharmaceutical products using yeast as a favorable platform for the production of heterologous recombinant proteins. In recent studies, reduced microgravity condition was reported to have significant effects on the heterologous proteins production [3, 23, 30, 33]. The microgravity effects could promote protein expression, such as the expression of the recombinant β-galactosidase and the glycodelin in human cells, the recombinant β-glucuronidase in Escherichia coli and P. pastoris, and the human monoclonal antibody in Sp2/0 myeloma mouse 200 cell line [13]. These results suggested that microgravity effects could be used for efficient production of recombinant proteins from engineered microorganisms. Simulated microgravity (SMG) is an exceptional environmental condition that can be modeled by special bioreactors in ground-based experiments. One such bioreactor is Rotary Cell Culture System (RCCS, Synthecon Inc., NASA), using high aspect ratio vessel (HARV) to model the environment of microgravity on the ground. The SMG and normal gravity (NG) control environment are created when the HARV is rotated horizontally (with the axis of the vessel perpendicular to the gravitational force) and vertically (with the axis of the vessel parallel to the gravitational force), respectively. Stephen et al. reported that the simulate microgravity (SMG) effects could enhance the production of recombinant proteins of LacZ or glycodelin by the human cell line compared to a stirred bioreactor [30]. Qi et al. reported that the SMG effects had the beneficial impact on the secretion of a recombinant β-glucuronidase in P. pastoris [23]. In our previous study, several helper genes which could be used for strain improvement were identified in the recombinant P. pastoris in response to SMG effects created by RCCS through comparative analysis of the transcriptomic data and the proteomic profiling. Overexpressing the gene encoding detoxifying enzyme of thiol peroxidase was proved to efficiently counteract oxidative stress arising from heterologous protein production and to improve the productivity of recombinant proteins in methylotrophic P. pastors [13]. These studies have provided important platforms to define the molecular mechanisms of response to environmental changes and thus to offer knowledge needed for the improvement of ground-based protein processes and products. Therefore, attempts have been continued to demonstrate the high potential of engineered yeast strains expressing helper genes applied to the normal gravity condition for recombinant proteins production. Additional studies are needed to deeply analyze the “omics” data and further investigate the potential synergetic effects of target helper genes for extended yeast strain engineering strategies [13, 24].
In the present work, we aim to find novel target helper genes related to recombinant proteins secretion and glycosylation for subsequent strain improvement in normal fermentation based on our previous transcriptomic and proteomic data of the recombinant P. pastoris cultured under SMG condition. This study will facilitate engineering the P. pastoris strain by introducing new helper genes and utilize the reduced gravity effects in the fermentation industry.
Materials and methods
Strains and vectors
The P. pastoris GS115 (Invitrogen, Carlsbad, CA) was used in this study. The P. pastoris GS115/pPIC9 K-pgus and the P. pastoris GS115/pPIC9 K-atxyn which produced the extracellular β-glucuronidase (PGUS) and endo-β-1,4-xylanase (AtXYN), respectively, were under control of methanol inducible promoter AOX1. The respective genes were cloned into the pPIC9 K vector (Invitrogen, Carlsbad, CA) and integrated into the P. pastoris GS115 genome at the AOX1 terminator locus. The GenBank accession number of PGUS gene was EU095019, and the GenBank accession number of AtXYN gene was JQ087496.
Prediction of target genes
Identification of overexpressing targets genes for potentially increasing recombinant proteins production was based on the data from the proteomic and the transcriptomic profile of the recombinant P. pastoris GS115/pPIC9 K-pgus in response to SMG effects. Targets genes with differentially expressed changes might have the positive impacts on specific protein synthesis and biomass accumulation [12, 23].
Overexpression of target genes
The target genes were amplified from GS115 genomic DNA and cloned under control of the GAP promoter into the pGAPZB vector (Invitrogen, Carlsbad, CA, USA) containing zeocin (Invitrogen, Carlsbad, CA, USA) resistance cassette. Primers were given in the Supplementary Table S1. The vectors were integrated into the GAP terminator locus of the P. pastoris GS115/pPIC9 K-pgus/atxyn genome, respectively. The co-expressing genes were expressed under control of the constitutive promoter of GAP in vivo. The pGAPZB vector was used for constructing expression cassettes of two target genes. The expression cassettes were introduced into the P. pastoris GS115/pPIC9 K-pgus for PGUS production using the Gibson assembly reaction. See Supplementary Table S2 for details.
Shaker flask cultivation
A single colony of the engineered strain was inoculated in the 50 ml YPD (1 % yeast extract, 2 % peptone, 2 % glucose, and 2 % agar) in a 250 ml shaker flask at 28 °C for 20 h to get the seed liquid cells. The seed yeast cells were first cultured in the BMGY medium (1 % yeast extract, 2 % peptone, and 1 % glycerol) for 12–24 h with the initial optical density (OD600) of 0.5, and grew until OD600 reached 10. The cells were then harvested and suspended in 50 ml BMMY medium [1 % yeast extract, 2 % peptone, 100 mM phosphate buffer saline (pH 6.0), 1.34 % YNB, 1.61 μM biotin, 1 % methanol]. Methanol concentration was maintained around 1 % by feeding methanol at 12 h intervals for producing the reporters at 28 °C, 220 rpm. After 48–96 h of induction, the culture medium was sampled and centrifuged at 5000 rpm for 5 min to determine the specific productivities of the reporters. The engineered strains of harboring the single copy target gene, respectively, were chosen for comparison. Each engineered strain was cultured through triplicate independent experiments.
Quantification of recombinant protein concentration
1 ml aliquots of cells were sampled at the time point of cultivation. Cell density was tested periodically by measuring OD600 and the dry cell weight was determined through the standard curve. The reporter protein concentration and correlated total protein content in the supernatant were quantified by SDS–PAGE semi-quantitative determination by Coomassie Protein Assay. Bovine serum albumin was used as an internal standard. All the experiments from the biological samples were carried out in triplicate.
Enzyme assay
The β-glucuronidase activity was determined using para-nitrophenyl β-d-glucuronide (pNPG) (Sigma-Aldrich, St. Louis, USA) as an activated substrate, which converted pNPG to p-nitrophenol and gave a yellow color measured by micro-plate reader at 405 nm. The reaction mixture, consisting of 10 μl of supernatant fermentation broth which contained recombinant PGUS and 40 μl of 1.25 mM pNPG sodium acetate buffer (pH 5.0), was incubated at 40 °C for 5 min. The reaction was halted by adding 200 µl 0.4 M Na2CO3 butter. One unit (U) PGUS activity was defined as 1 nmol p-nitrophenol liberated per minute. The xylanase activity was assayed using birchwood xylan (Sigma-Aldrich, St. Louis, USA) as the substrate [12]. The amount of reducing sugars released was determined by the standard dinitrosalicylic acid method. One unit of AtXYN activity was defined as the amount of enzyme producing 1 μmol of xylose per minute. All the experiments from the biological samples were carried out in triplicate.
Glycosylation analysis by peptide-N-glycosidase F
Peptide-N-glycohydrolase F (NEB, Ipswich, MA, USA) was used for deglycosylating the recombinant PGUS. The PGUS in the supernatant was deposited with the equal volume of acetone, and then was concentrated in phosphate buffer (100 mM, pH 6.0). The reaction mixture of 20 μg PGUS was treated with 100 IU PNGase F and incubated for 24 h at 37 °C according to the manual. The bound fraction and the unbound were denoted as the control and the deglycosylated protein.
RNA extraction and qRT-PCR analysis
Yeast cells were harvested during the exponential growth phase. Approximately 1 × 107 cells were used for the total RNA extraction using the Yeast RNA Kit (OMEGA, Doraville, GA). Genomic DNA contamination was eliminated by DNase I treatment. RNA concentration was quantified by measuring the absorbance at 260 nm using NanoDrop 2000c (Thermo Scientific, Waltham, MA, USA). Five hundred nanogram of RNA from each sample was used as template for the Transcript First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). Quantitative PCR analysis was performed with the LightCycler SYBR Green I Master Kit (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. The reactions were run with the LightCycler 480 real-time System using fast 96 well plates (Roche, Indianapolis, IN, USA). The data were analyzed using the LightCycler Software (v.1.5). The housekeeping gene GADPH was used as the reference gene. The data were normalized using GADPH as the endogenous control. The reaction without reverse transcriptase was used as the negative control. All real-time qPCR reaction assays were performed in biological replicates to allow for statistical confidence in differential gene expression (see Supplementary Tables S3 for details).
Results and discussion
Mining and prediction of potential target helper genes
In the previous studies, we demonstrated that the recombinant PGUS producing strain P. pastoris GS115/pPIC9 K-pgus showed an increased secretion capacity of PGUS under SMG. The transcriptomic and the proteomic profiles of the P. pastoris response changes were compared under the SMG and NG conditions [12, 23, 24]. The predicted target genes were selected for potential effects on improving PGUS expression based on the magnitude of their regulation (relative fold change thresholds) between SMG and NG conditions, also based on their potential relevance for heterogeneous expression. The detailed analysis of data mining could provide a library of potential helper genes for the improvement of P. pastoris. These genes were selected for further analysis of their effects on the recombinant protein production by co-expression methods. Overall, 23 potentially interesting genes that were classified into four functional categories (Table 1): (1) carbon metabolism; (2) oxidative stress response; (3) transcription factors; and (4) protein folding and secretion.
Overexpressing the genes related with the TCA cycle and the pentose phosphate pathway could increase TCA cycle flux to further enhance the recombinant protein production in P. pastoris [32]. The research demonstrated that overexpressing of glucose 6-phosphate dehydrogenase (ZWF1), the initial enzyme of the PPP pathways, and the malate dehydrogenase (MDH1), an important enzyme of TCA cycle, was proved to have the beneficial effect on the hSOD production in a P. pastoris strain [21]. Fructose 1,6-bisphosphate aldolase (FBA), an important enzyme for cofactor regeneration, catalyzed the formation of fructose 1,6-bisphosphate from dihydroxyacetone and glyceradehyde-3-phosphate to replenish the xylulose-5-phosphate in the downstream reactions of the methanol metabolism. The FBA overexpressing strain, leading to a strong impact on PGUS production, was presented in our latest published work [12]. In this study, the genes FBA, GPM, ENO, ICL1, GLO1, and ZWF1 categorized as the carbon metabolism were exploited by data mining.
Protein folding in the ER generally involves a chain of oxidative folding processes [5]. The strategy of balancing cytosolic redox homeostasis was reported to increase the recombinant protein production capacity [5]. In our previous study, we have indicated that co-expressing the gene TPX that encoding the thiol peroxidase might efficiently counteract oxidative stress arising from heterologous protein production [7, 12]. The genes involved in oxidative stress response may be useful to recombinant proteins secretion. In this study, we selected several genes of this category for the first time to evaluate their effects on recombinant protein secretion.
Transcription factors (TFs) as expression helpers could improve the secretion of the recombinant protein when they are overexpressed in engineered strains [32]. One of the transcription factor HAC1, which is involved in the UPR (unfolded protein response), has been demonstrated to overcome expression bottlenecks by Guerfal et al. [11]. In addition, the overexpression of the gene encoding the transcription factor NRG1 is reported to positively influence the secretion of recombinant porcine and human trypsinogen as well as the antibody Fab fragment 2F5 [29]. In addition, AFT1, an activator of ferrous transport, was also proved to be an interesting candidate for improving secretion [4]. Therefore, the biotechnological potential of using transcription factors might improve bioprocesses of heterologous proteins. In this study, we selected the transcription factors PAS_chr4_0169, IRE1, MIG1, YAP1, HSF1, and RPN4 for the first time to test their functions on recombinant protein secretion.
Some of the genes involved in the protein folding and subsequent secretion were identified as novel helper factors in the previous study [8]. In this study, we selected the genes AHA1, SBA1, SIS1, YPT6, and PAS_chr3_0292 to determine general secretion helpers with potential benefits for heterologous proteins.
Comparing the PGUS production in the engineered and the parental strains
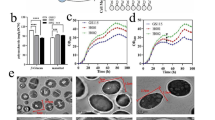
The recombinant protein β-glucuronidase PGUS was used as one of the reporter proteins for screening target helper genes. The PGUS secretion yield was evaluated in five clones per strain that harboring a single copy of co-expressing target gene to preclude the clonal variation. The PGUS specific activities in the supernatant reached a maximum at 48 h of methanol induction, and the PGUS concentration reached a maximum at 96 h of methanol induction (data not shown). The effects of the co-expressing target genes on the PGUS concentration in the supernatant at 96 h of methanol induction were varied (Fig. 1). Among 23 co-expressing target genes, 12 genes displayed significantly positive impacts. These genes were FBA, GPM, TPX, PRX1, RPN4, PAS_chr4_0169, YAP1, AHA1, and YPT6, leading to 20.5–80.4 % increase in the product titers per liter of supernatant, while no significant effects were seen by overexpressing other genes (Table 2). Notably, the genes FBA, TPX, and GPM were proved to have the positive effects on the PGUS secretion in the present study which is in accordance with the previous study [12]. Consequently, the effects on the product titers of co-expressing other helper target genes demonstrated that six of the genes PRX1, YAP1, PAS_chr4_0169, AHA1, YPT6, and RPN4 had the dominant positive impacts (Fig. 2a). The engineered strains overexpressing the above genes significantly increased the PGUS specific activities (Fig. 2b).
PRX1, the gene encoding the mitochondrial peroxiredoxin with the thioredoxin peroxidase activity, is as one of the most important enzymes in the cellular redox homeostasis [28]. YAP1, a transcription factor, plays an important role in response to oxidation and DNA damage. Marizela et al. indicated that overexpressing or engineering of YAP1 could be a promising target to counteract oxidative stress arising from heterologous protein production [16]. In addition, the function of transcription factor PAS_chr4_0169 is controlling expression of many ribosome biogenesis genes. These genes have not been studied in the regulation of protein expression in yeast. The function and interaction of AHA1 of with Hsp90 and its co-chaperones may play roles in modulating RNA splicing and DNA repair to prompt protein correct folding, in addition to other cellular processes [25]. The GTPase YPT6 is involved in the intracellular transport of vesicles and their fusion with the trans-Golgi network and has tolerance under physiological stress conditions [17]. The functions of the transcription factor RPN4 are stimulating the expression of proteasome genes and promoting the regulation of several genes involved in DNA repair, antioxidant response, and glucose metabolism [26]. Notably, it was first reported that these genes except YAP1 could affect recombinant protein secretion in the P. pastoris.
Verification of helper target genes by AtXYN production
A second extracellular model protein, endo-β-1,4-xylanase (AtXYN), was chosen to verify the effect of prediction on protein production to preclude a reporter protein specific effect. The six target helper genes PRX1, YAP1, PAS_chr4_0169, AHA1, YPT6, and RPN4 were thus ligated into the pGAPZB vectors and were transformed into the recombinant AtXYN producing strain P. pastoris GS115/pPIC9 K-atxyn, respectively. Five verified co-expressing clones were randomly chosen and cultured. After 72 h of methanol feeding, the xylanase protein concentration and specific activity were detected. The co-expressing target genes PRX1, YAP1, AHA1, and YPT6 favored the AtXYN concentration and the specific activity in the supernatant, while the other mutants showed a slight increase or negative impacts on AtXYN protein concentration (Fig. 3a). Notably, the genes PRX1, YAP1, AHA1, and YPT6 could promote the AtXYN specific activities prominently (Fig. 3b). Taken together the results of both PGUS and AtXYN, the target helper genes PRX1, YAP1, AHA1, and YPT6, may have universal application for the improving recombinant protein productions and specific activities. These identified helper genes were responsible for regulating oxidative stress response and correct protein folding. Therefore, more target helper genes could be mined out from these two functional categories for recombinant protein production study. The hypothesized cause for the enhancement of recombinant protein expression is the genomic response of yeast cells to the extreme environment of SMG, which may alter the global gene transcription especially those related to oxidative stress response and protein folding.
Gene cassettes designed for the co-expressing two target helper genes
To detect the effects of overexpressing two target genes on the recombinant protein production, several gene cassettes combining two target helper genes were inserted into the pGAPZB vector. The vectors of pGAPZB-AHA1-YAP1, pGAPZB-AHA1-YPT6, pGAPZB-PRX1-YPT6, pGAPZB-PRX1-YAP1, pGAPZB-PRX1-AHA1, and pGAPZB-YPT6-YAP1 were thus constructed and introduced into the recombinant P. pastoris-PGUS strain (Fig. 4a). The strains overexpressed two genes demonstrated promising effects in the PGUS specific activities. The PGUS specific activities were increased by nearly 30.3–50.6 % in the strains of co-expression two genes, comparing with that of single genes (Fig. 4b). The expression levels of recombinant PGUS production were increased particularly when the oxidative stress response genes and protein folding helper genes were present in the combination. PRX1 and YAP1 could provide an antioxidant defense by reducing hydro peroxides, thus higher protein production was achieved. Co-expression of PRX1 and YAP1 might efficiently counteract oxidative stress arising from heterologous protein production. Similar effect can be seen on the co-expression of protein folding and secretion helper genes AHA1 and YPT6. AHA1 (activator of Hsp90 ATPase) acted as an autonomous chaperone and associated with stress-denatured proteins to prevent them from aggregation similar to the chaperonin GroEL [25]. YPT6 may very well function in the yeast Golgi maintenance [17]. Our results indicated that the co-expression of AHA1 and YPT6 might promote disposal of folding defective proteins by the cellular protein quality control. Additional studies should further deeply investigate the potential synergetic actions of the individual helper factors in the future.
Target helper genes affect PGUS glycosylation
Recombinant proteins overexpressed in P. pastoris GS115 are often N-linked glycosylated with high mannose structures [34]. In this study, the PGUS gene was cloned from the Penicillium purpurogenum Li-3 and it was determined that PGUS (β-glucuronidase) contains four potential N-glycosylation sites (N28, N251, N383, and N594). The precise molecular masses of glycosylated and completely deglycosylated PGUS enzymes were 78.84 and 67.83 kDa, respectively [36]. The PGUS expressed in the P. pastoris strains of co-overexpressing target helper genes AHA1 and YAP1 was deglycosylated with PNGase-F. The characteristics of deglycosylated PGUS was enlisted in Fig. 5. The PGUS (lane 5 and lane 9) expressed in the P. pastoris strain without any target helper gene was also deglycosylated and was set as one of the control. The PGUS expressed in the E.coli (PGUS-E) (lane 7) and the PGUS with all glycosylation site-directed mutations (PGUS-M, N28Q/N251Q/N383Q/N594Q) (lane 8) were set as another two controls. The PGUS-E and PGUS-M were both non-glycosylated proteins. The bands of PGUS-AHA1 (lane 9) and PGUS-YAP1 (lane 3) were much lower than the band of PGUS before deglycosylation with PNGase-F and were close to the bands of PGUS-E and PGUS-M. The band of PGUS-AHA1 was below the band of PGUS-YAP1. After deglycosylation with PNGase-F, there were obvious migrations of the bands of PGUS (lane 6) and PGUS-YAP1 (lane 3). However, there was no migration of PGUS-AHA1 (lane 2), indicating that no N-linked oligosaccharides of glycosylation occurred when the target helper gene AHA1 is co-overexpressed. In addition, overexpressing the target gene YAP1 made the PGUS glycosylate at a low level. In this study, co-overexpression of AHA1 and YAP1 not only enhanced recombinant protein secretion, but also altered the glycosylation of the recombinant proteins. Other studies reported that the microgravity condition could alter protein post translation, such as phosphorylation and glycosylation [14, 18, 31]. Joshi et al. demonstrated the mechanism of SMG-mediated changes in the eukaryotic N-linked glycosylation pathway of recombinant protein in insect cells. The oligosaccharide structures were similar to those produced in human placental cells. Insect cells cultured in T-flasks only performed incomplete oligosaccharide processing [14]. Notably, this study is known to be first reported that recombinant protein glycosylation could be affected by overexpressing of either target helper genes co-chaperone AHA1 or transcription factor YAP1 in P. pastoris. Our result suggested that genes responding to SMG effects could be applied for recombinant therapeutic glycoproteins production in industrial fermentation.
Transcription level of relative genes
The relative transcription levels of the genes β-glucuronidase (PGUS), alcohol oxidase I (AOX), and protein disulfide isomerase (PDI) were investigated in engineered PGUS expressing P. pastoris strains (shown in Fig. 6). Notably, the mRNA levels of PGUS were down-regulated (ranging from 10.4 to 40.5 %) in the strains co-expressing the helper genes, indicating that the improvement of target protein production was not due to the increasing level of PGUS gene transcription, but solely resulted from the enhancement of protein secretion. Instead, the significant increased transcription levels of AOX across all the engineered helper gene co-expressing strains implied significant physiological changes of methanol consumption in help factor co-expressing strains compared with control. Likewise, the relative transcriptional level of PDI gene encoding the disulfide isomerase, a chaperone that catalyzes the formation of disulfide bonds, was also upregulated in almost all the co-expressing strains. Furthermore, this pattern of upregulation was dominant in the strains co-expressing two genes, especially when oxidative stress response gene PRX1 was combined with either protein folding and secretion genes YPY6 or transcription factor YAP1 (Fig. 6), which is in accordance with the observation of the promising effects of PGUS specific activities when PRX1 gene was present in the combination (Fig. 4b). Together, these results indicate that the strains overexpressing selected target helper genes probably underwent better protein folding and menthol utilization, which facilitated the enhancement of recombinant protein secretion ability in engineered strains.
Conclusions
In this study, we examined the comparative data of transcriptomic and proteomic profiling of recombinant P. pastoris cultured under SMG and NG to identify potential helper genes which could affect both the heterogeneous recombinant protein production and the protein glycosylation in engineered P. pastoris by gene co-expression. These target helper genes are mainly involved in glycolytic pathway, oxidative stress response, transcription factors, and protein folding and secretion. Our results have provided many novel helper factors and the relevance of simulated microgravity on microbial cells as new tools for subsequent strain engineering and microbial fermentation.
References
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98:5301–5317. doi:10.1007/s00253-014-5732-5
Baumann K, Adelantado N, Lang C, Mattanovich D, Ferrer P (2011) Protein trafficking, ergosterol biosynthesis and membrane physics impact recombinant protein secretion in Pichia pastoris. Microb Cell Fact 10:93. doi:10.1186/1475-2859-10-93
Baumstark-Khan C, Hellweg CE, Palm M, Horneck G (2001) Enhanced green fluorescent protein (EGFP) for space radiation research using mammalian cells in the International Space Station. Phys Med 17:210–214
Claudia R, Markus B, Viktorija V, Daniela K, Stefan N, Diethard MH, Brigitte G (2014) Pichia pastoris Aft1-a novel transcription factor, enhancing recombinant protein secretion. Microb Cell Fact 13:120. doi:10.1186/s12934-014-0120-5
Delic M, Rebnegger C, Wanka F, Puxbaum V, Haberhauer-Troyer C, Hann S, Kollensperger G, Mattanovich D, Gasser B (2012) Oxidative protein folding and unfolded protein response elicit differing redox regulation in endoplasmic reticulum and cytosol of yeast. Free Radic Biol Med 52:2000–2012. doi:10.1016/j.freeradbiomed.2012.02.048
Dragosits M, Stadlmann J, Graf A, Gasser B, Maurer M, Sauer M, Kreil D, Altmann F, Mattanovich D (2010) The response to unfolded protein is involved in osmotolerance of Pichia pastoris. BMC Genom 11:207. doi:10.1186/1471-2164-11-207
Fomenko DE, Koc A, Agisheva N, Jacobsen M, Kaya A, Malinouski M, Rutherford JC, Siu KL, Jin DY, Winge DR, Gladyshev VN (2011) Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci USA 108:2729–2734. doi:10.1073/pnas.1010721108
Gasser B, Sauer M, Maurer M, Stadlmayr G, Mattanovich D (2007) Transcriptomics-based identification of novel factors enhancing heterologous protein secretion in yeasts. Appl Environ Microbiol 73:6499–6507
Gasser B, Prielhofer R, Marx H, Maurer M, Nocon J, Steiger M, Puxbaum V, Sauer M, Mattanovich D (2013) Pichia pastoris: protein production host and model organism for biomedical research. Future Microbial 8:191–208. doi:10.2217/fmb.12.133
Gabriel P, Ahmad A, Zhang ZS (2012) Bioprocess engineering aspects of heterologous protein production in Pichia pastoris: a review. Biochem Eng J 64:1591–1605. doi:10.1016/j.biotechadv.2015.05.008
Guerfal M, Ryckaert S, Jacobs PP, Ameloot P, Van CK, Derycke R, Callewaert N (2010) The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb Cell Fact 9:49. doi:10.1186/1475-2859-9-49
Huangfu J, Qi F, Liu H, Zou H, Ahmed MS, Li C (2015) Novel helper factors influencing recombinant protein production in Pichia pastoris based on proteomic analysis under simulated microgravity. Appl Microbiol Biotechnol 99:653–665. doi:10.1007/s00253-014-6175-8
Huangfu J, Zhang G, Li J, Li C (2015) Advances in engineered microorganisms for improving metabolic conversion via microgravity effects. Bioengineered 6(4):251–255. doi:10.1080/21655979.2015.1056942
Joshi L, Shuler ML, Wood HA (2001) Production of a sialylated N-linked glycoprotein in insect cells. Biotechnology Prog 17:822–827
Looser V, Brühlmann B, Bumbak F, Stenger C, Costa M, Camattari A, Fotiadis D, Kovar K (2015) Cultivation strategies to enhance productivity of Pichia pastoris: a review. Biotechnol Adv. doi: 10.1016/j.biotechadv.2015.05.008
Marizela D, Alexandra BG, Gunda K, Christina HT, Stephan H, Diethard M, Brigitte G (2014) Overexpression of the transcription factor Yap1 modifies intracellular redox conditions and enhances recombinant protein secretion. Microbial Cell 1:376–386
Marešová L, Vydarený T, Sychrová H (2012) Comparison of the influence of small GTPases Arl1 and Ypt6 on yeast cells tolerance to various stress factors. FEMS Yeast Res 12:332–340. doi:10.1111/j.1567-1364.2011.00780.x
Muid S, Froemming GR, Ali AM, Nawawi H (2013) Interleukin-6 and intercellular cell adhesion molecule-1 expression remains elevated in revived live endothelial cells following spaceflight. Malays J Pathol 35(2):165–176
Näätsaari L, Mistlberger B, Ruth C, Hajek T, Hartner FS, Glieder A (2012) Deletion of the Pichia pastoris KU70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS One 7:e39720. doi:10.1371/journal.pone.0039720
Norden K, Agemark M, Danielson JÅ, Alexandersson E, Kjellbom P, Johanson U (2011) Increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris. BMC Biotechnol 11:47. doi:10.1186/1472-6750-11-47
Nocon J, Steiger MG, Pfeffer M, Sohn SB, Kim TY, Maurer M, Rußmayer H, Pflügl S, Ask M, Haberhauer-Troyer C, Ortmayr K, Hann S, Koellensperger G, Gasser B, Lee SY, Mattanovich D (2014) Model based engineering of Pichia pastoris central metabolism enhances recombinant protein production. Metab Eng 24:129–138. doi:10.1016/j.ymben.2014.05.011
Prielhofer R, Maurer M, Klein J, Wenger J, Kiziak C, Gasser B, Mattanovich D (2013) Induction without methanol: novel regulated promoters enable high-level expression in Pichia pastoris. Microb Cell Factories 12:5. doi:10.1186/1475-2859-12-5
Qi F, Kaleem I, Lv B, Guo XX, Li C (2011) Enhancement of recombinant β-glucuronidase production under low-shear modeled microgravity in Pichia pastoris. J Chem Technol Biotechnol 86:505–511
Qi F, Wang C, Liu Y, Kaleem I, Li Q, Li C (2011) Transcriptional profiling of protein expression related genes of Pichia pastoris. PLoS One 6:e26613. doi:10.1371/journal.pone.0026613
Sun L, Prince T, Manjarrez JR, Scroggins BT, Matts RL (2012) Characterization of the interaction of Aha1 with components of the Hsp90 chaperone machine and client proteins. Biochim Biophys Acta 1823:1092–1101. doi:10.1016/j.bbamcr.2012.03.014
Shirozu R, Yashiroda H, Murata S (2015) Identification of minimum Rpn4-responsive elements in genes related to proteasome functions. FEBS Lett 589:933–940. doi:10.1016/j.febslet.2015.02.025
Sohn SB, Graf AB, Kim TY, Gasser B, Maurer M, Ferrer P, Mattanovich D, Lee SY (2010) Genome-scale metabolic model of methylotrophic yeast Pichia pastoris and its use for in silico analysis of heterologous protein production. Biotechnol J 5:705–715. doi:10.1186/1475-2859-9-50
Srinivasa K, Kim NR, Kim J, Kim M, Bae JY, Jeong W, Kim W, Choi W (2012) Characterization of a putative thioredoxin peroxidase prx1 of Candida albicans. Mol Cells 33:301–307. doi:10.1007/s10059-012-2260-y
Stadlmayr G, Benakovitsch K, Gasser B, Mattanovich D, Sauer M (2010) Genome-scale analysis of library sorting (GALibSo): isolation of secretion enhancing factors for recombinant protein production in Pichia pastoris. Biotechnol Bioeng 105:543–555. doi:10.1002/bit.22573
Stephen N (2007) Rotary bioreactor for recombinant protein production. Cell Technol Cell Prod 3:567–569
Tash JS, Bracho GE (1999) Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J 13:S43–S54
Vogl T, Glieder A (2013) Regulation of Pichia pastoris promoters and its consequences for protein production. N Biotechnol 30:385–404. doi:10.1016/j.nbt.2012.11.010
Wang J, Liu C, Liu J, Fang X, Xu C, Guo Y, Chang D, Su L (2014) Space mutagenesis of genetically engineered bacteria expressing recombinant human interferon α1b and screening of higher yielding strains. World J Microbiol Biotechnol 30:943–949. doi:10.1007/s11274-013-1512-0
Yang M, Yu XW, Zheng H, Sha C, Zhao C, Qian M, Xu Y (2015) Role of N-linked glycosylation in the secretion and enzymatic properties of Rhizopus chinensis lipase expressed in Pichia pastoris. Microb Cell Fact 14:40. doi:10.1186/s12934-015-0225-5
Zhong Y, Yang L, Guo Y, Fang F, Wang D, Li R, Jiang M, Kang W, Ma J, Sun J, Xiao W (2014) High-temperature cultivation of recombinant Pichia pastoris increases endoplasmic reticulum stress and decreases production of human interleukin-10. Microb Cell Fact 13:163. doi:10.1186/s12934-014-0163-7
Zou S, Huang S, Kaleem I, Li C (2013) N-Glycosylation enhances functional and structural stability of recombinant β-glucuronidase expressed in Pichia pastoris. J Biotechnol 164:75–81. doi:10.1016/j.jbiotec.2012.12.015
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21425624, 21376028, and 21566032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing financial interests exist.
Additional information
J. Huangfu and Y. Xu contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huangfu, J., Xu, Y., Li, C. et al. Overexpressing target helper genes enhances secretion and glycosylation of recombinant proteins in Pichia pastoris under simulated microgravity. J Ind Microbiol Biotechnol 43, 1429–1439 (2016). https://doi.org/10.1007/s10295-016-1817-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1817-8