Abstract
“Clostridium ragsdalei” is an acetogen that ferments synthesis gas (syngas, predominantly H2:CO2:CO) to ethanol, acetate, and cell mass. Previous research showed that C. ragsdalei could also convert propionic acid to 1-propanol and butyric acid to 1-butanol at conversion efficiencies of 72.3 and 21.0 percent, respectively. Our research showed that C. ragsdalei can also reduce pentanoic and hexanoic acid to the corresponding primary alcohols. This reduction occurred independently of growth in an optimized medium with headspace gas exchange (vented and gassed with CO) every 48 h. Under these conditions, conversion efficiencies increased to 97 and 100 % for propionic and butyric acid, respectively. The conversion efficiencies for pentanoic and hexanoic acid to 1-pentanol and 1-hexanol, respectively, were 82 and 62 %. C. ragsdalei also reduced acetone to 2-propanol at a conversion efficiency of 100 %. Further, we showed that C. ragsdalei uses an aldehyde oxidoreductase-like enzyme to reduce n-fatty acids to the aldehyde intermediates in a reaction that requires ferredoxin and exogenous CO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depletion of petroleum-based energy reserves and effects of petroleum combustion on the global climate have necessitated the development of renewable energy sources to supplement petroleum. Transportation accounts for approximately 74 % of refined crude oil consumption and the demand will only grow with the population at an estimated 0.6 million more barrels of refined crude oil per day from 2010 to 2035 [9]. The chemical products industry also represents a significant fraction of the petroleum demand (16.8 %, [14]) and, like transportation, this demand will also increase with growing populations. Several potential transportation fuel additives and replacements have been investigated, including biologically produced alcohols and biodiesel; however, legislation is beginning to include more defined directives, not just for generating these biologically produced fuels, but also for doing so using sustainable feedstocks and existing infrastructure (Energy Independence and Security Act of 2007). Sustainable feedstocks include perennials grown on agriculturally abandoned land, crop residues, sustainably harvested woody crops, crop rotation/mixed crop systems, and municipal/industrial waste rather than sugar-rich food crops [35]. Synthesis gas (syngas, predominantly H2:CO2:CO), produced via the gasification of organic waste materials, is another sustainable feedstock that can be used, primarily, by mesophilic microorganisms to generate multicarbon compounds [12]. Some acetogenic bacteria (acetogens) produce acetate, ethanol, and cell mass from syngas [8].
While ethanol remains a useful renewable fuel source, there exists a need for products with higher energy yields that are less corrosive than ethanol. The energy density of these products must be comparable with the current transportation fuels and petroleum infrastructures. Fatty acids, such as propionic and butyric acids, are energy-rich, but the high oxygen to carbon ratio makes fatty acids less suitable for fuel applications; converting energy-dense fatty acids to alcohols, however, yields a product more applicable to the fuel industry [7]. Biologically produced C2–C6 alcohols can be blended with gasoline as fuel oxygenates [7]. These solvents are also used in a variety of industrial processes. For example, 1-propanol can be used as a solubility agent in the pharmaceuticals industry [11]. The butanol industry was estimated to be an approximately 7 billion dollar expanding market [16]. Like propanol and other lower molecular weight solvents, butanol is also used in industry as a diluting agent and extraction solvent. According to the PubChem database, pentanol (CID 6276) can be used as a solvent for coatings in the electronics industry. These are just a few examples of the use of alcohols in industrial processes.
Recently, application of the microbial conversion of acetic, propionic, and butyric acids to alcohols as a waste treatment practice was described for mixed bacterial consortia (without the requirement of fermentable sugars or starches) with hydrogen as the electron donor [30]. Hydrogen is produced during anaerobic digestion, which reinforces the waste-based feedstock model of this work; however, the majority of this is consumed by hydrogenotrophic methanogens in the mixed cultures [31]. Alternatively, some solventogenic acetogens can produce alcohols and cell mass from syngas and n-fatty acids. This was demonstrated in the acetogens Clostridium kluyveri [15], Clostridium formicoaceticum [10], Moorella thermoacetica (f. Clostridium thermoaceticum) [28], Clostridium acetobutylicum [13], Clostridium ljungdahlii [22], and Alkalibaculum bacchi strains CP11T, CP13 and CP15 [19].
Both C. ljungdahlii and “Clostridium ragsdalei” (ATCC BAA-622, DSM 15248) were shown to produce 1-propanol and 1-butanol from propionic and butyric acid, respectively, and it was suggested that optimizing fermentation would improve conversion efficiencies for this process [22]. Previously, our lab has shown a four-fold increase in ethanol production by C. ragasdalei in optimized medium [26]. The research presented herein was focused on achieving higher conversion efficiencies of acids to alcohols by C. ragsdalei. This was achieved by developing a better understanding of the enzymes and reductants used by C. ragsdalei to convert acids to aldehyde intermediates and using optimized medium components and incubation conditions for alcohol production described previously [26].
Materials and methods
Cultures, media, and syngas analog
“Clostridium ragsdalei” (ATCC BAA-622, DSM 15248) medium contained (L−1): 20 ml mineral solution [33], modified by the elimination of sodium; 10 ml vitamin solution [33]; 10 ml optimized trace metal solution [26]; 1 g yeast extract (Difco, Becton–Dickinson, Sparks, MD); 10 g 2-(N-morpholino)ethanesulfonic acid (MES), with the pH adjusted to 6.1 using potassium hydroxide; and 3 ml cysteine sulfide solution (0.4 g L−1 stock) as a reducing agent [33]. Clostridium carboxidivorans strain P7T (DSM 15243) medium was prepared in the same manner as C. ragsdalei medium using unmodified trace metal solution [33]. Alkalibaculum bacchi strain CP11T (ATCC BAA-1772) medium was prepared according to Allen et al. [1]. Media were prepared using strict anoxic technique [2]. Incubation vessels contained a headspace of 101.3 kPa N2:CO2 (80:20) and were over-pressurized with carbon monoxide (CO) to a total gas final pressure of 207 kPa. Shaking incubations (60 rpm) were conducted at 37 °C in stoppered and crimp-sealed Balch tubes or bottles depending on the experimental set-up (see below). All experiments were prepared in this manner unless otherwise noted. All chemicals used in this research were obtained from Sigma–Aldrich unless otherwise stated (Sigma–Aldrich Corp.). Additionally, for all studies, the growth phases were detected by measuring the absorbance at 600 nm of cultures used for inoculum. Growth curves for C. ragsdalei in the presence of the different n-fatty acids were determined prior to conducting the experiments described herein (Supplemental Fig. 2).
Nuclear magnetic resonance (NMR) study
C. ragsdalei medium was amended with [2-13C]-acetate or [2-13C]-propionic acid, dispensed as 20 ml aliquots into 160 ml glass bottles (Wheaton), inoculated with mid-log phase C. ragsdalei, and pressurized with CO (207 kPa gauge) in triplicate. Cultures were incubated at 37 °C for 30 days as static incubations (acetate amended) and 9 days at 60 rpm (propionic acid amended), corresponding to consumption of the n-fatty acid. Sterile (autoclaved post-inoculation) and unlabeled controls were implemented. Samples (1.2 ml) were treated with 20 µl washed Chelex 100 Resin to remove paramagnetic ions from the solution that interfere with NMR analysis. Samples were treated with Chelex 100 according to the manufacturer’s instructions (Bio-Rad Laboratories), vortexed for 30 s and centrifuged at 6,000 rpm. The supernatant was stored at −80 °C until analysis. Samples were diluted 1:1 with 2H2O for 13C NMR for a total volume of 1 ml. Chemical shift values were confirmed by adding sodium 3-trimethylsilylpropionate-2,2,3,3,-d 4 (MSD Isotopic Products, Merck Sharp & Dohme of Canada Limited) as an internal standard. Spectra were obtained on a VNMRS 400 MHz spectrometer at a frequency of 100.5577 MHz using an indirect direction probe. Spectra were collected at 25 °C using a single pulse 13C experiment with a 45° pulse width (2.95 µs), a delay time of 2 s (acetate-amended samples) and 1 s (propionate-amended samples), an acquisition time of 1.28 s, and a spectral width of 25,510.2 Hz and 256 acquisitions. Proton decoupling was achieved using a WALTZ-16 pulse sequence. Propionate, propanol, acetate, and ethanol were quantified by GC-FID as described in analytical methods below.
Conversion of propionic acid to 1-propanol by resting cells of C. ragsdalei
In an anaerobic chamber, 50 ml aliquots of medium were dispensed into 500 ml serum bottles that were sealed with butyl-rubber stoppers and aluminum crimp seals, degassed, and then sterilized by autoclaving. Propionic acid was added as a sterile, anoxic stock to a final concentration of 30 mM. A 1 % (v/v) inoculum of log phase C. ragsdalei cells was added to the medium and the bottles were pressurized with CO to a final pressure of 207 kPa. Cells were grown in shaking incubators (60 rpm) at 37 °C and harvested at mid-log phase, corresponding to approximately, 50 % propionic acid consumption. Cells were washed twice in a MES buffered solution (pH 5.3) using strictly anoxic techniques in an anaerobic chamber. A minimal medium (MM), which contained all medium components excluding yeast extract, was amended with 20 mM propionic acid (MMP). Washed C. ragsdalei cells were resuspended in the prepared MMP in the anaerobic chamber, and subsequently added to sterile, anoxic, sealed Balch tubes in 3 ml aliquots. Each tube was pressurized with 207 kPa gauge CO. All resting cell experiments were conducted in triplicate. Along with CO, reduced benzyl viologen (BV), hydrogen, and formate were also tested as potential reductants. N2 headspace controls were also conducted.
In silico comparison of aldehyde oxidoreductases (AORs)
Using the Joint Genome Institute’s (JGI) Integrated Genomes and Metagenomes/Expert Review (IMG/ER) comparative analysis software [21], the gene sequence of the proposed AOR in C. ragsdalei was compared to AORs of related organisms that have been shown to reduce carboxylates (n-fatty acids and branched chain fatty acids) and ketones to alcohols. These sequences were translated using the ExPASy Bioinformatics Resource (http://web.expasy.org/translate/) and aligned in ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) to compare the conserved domains as predicted in IMG/ER.
Enzyme activity assays for AOR, carbon monoxide dehydrogenase (CODH), and alcohol dehydrogenase (ADH)
All cell-free extracts were prepared under strictly anaerobic conditions with tightly sealed polypropylene centrifuge bottles in an anaerobic chamber. All plastic materials were kept for at least 48 h in the anaerobic chamber to remove traces of oxygen prior to use. Cells were harvested in the anaerobic chamber when the propionic acid concentration had decreased by approximately, 50 %. Cells were washed with anoxic, sterile 2-[(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]ethanesulfonic acid (TES, pH 7.0) buffered solution. Prior to harvesting, samples were taken to measure acid and alcohol concentrations, growth, and pH as described below. An approximately, 1 g cell pellet (wet weight) was brought to a volume of 3 ml with 100 mM TES (pH 7.0) containing 1 mM dithiothreitol (DTT). The suspension was passed through a French press at 85 MPa under an atmosphere of N2. The crude lysate was transferred to centrifuge vials in the anaerobic chamber, sealed and centrifuged at 15,000×g and 4 °C for 15 min. The supernatant was passed through a desalting column (PD-10, GE Health Care Life Sciences) and used for determining enzyme activities. Enzyme assays were carried out in N2-flushed septum-sealed quartz cuvettes at 37 °C. Reaction mixtures were kept anoxic by flushing cuvettes and syringes with N2 and using strictly anaerobic technique to prepare stock solutions. Substrate-free and boiled cell-free extract controls were implemented and activities presented here were reported relative to these background activities. Linearity of the reactions based on protein concentration was established for all preparations. Enzyme activities represent averages for three replicate cell-extract preparations. Protein concentration was determined by Bradford protein assays using bovine serum albumin (BSA) as the standard [4]. AOR activity was determined by measuring the initial rate of methyl viologen (MV) reduction at 579 nm and production of propionic acid from propionaldehyde as described previously for butyrate production [29]. The assay mixture contained 100 mM TES buffer (pH 7.0), 0.6 mM oxidized MV (MVox), and 10 µM propionaldehyde. The reaction was initiated by the addition of cell-free extract and the activity was determined by measuring changes in the absorbance at 579 nm (A 579) caused by accumulation of reduced MV (MVred).
AOR activity was also determined in the less energetically favorable, aldehyde-forming direction by measuring the initial rate of MVred oxidation at 579 nm as described previously (Simon et al. 1989). The assay mixture contained 100 mM TES buffer (pH 7.0) amended with 0.6 mM MV, reduced drop-wise with 1 mM dithionite, and 10 µM propionic acid. The reaction was initiated by the addition of cell-free extract and the activity was determined by measuring changes in A 579 caused by oxidation of MVred.
ADH activity was determined by measuring the initial rate of NAD+ reduction as a change in A 340. The assay mixture contained 100 mM TES buffer (pH 7.0), 10 µM 1-propanol, and 0.68 mM NAD+. The reaction was initiated by the addition of cell-free extract.
CODH activity was determined as described previously, modified by measuring MV reduction at A 579 rather than A 603 [27].
C. ragsdalei ferredoxin was determined spectrophotometrically as the reduction of metronidazole using the method described previously [6] modified by adding propionaldehyde or CO instead of hydrogen. The assay mixture contained 100 mM TES buffer (pH 7.0), 0.1 mM metronidazole, and 10 mM propionaldehyde or CO. The reaction was initiated by the addition of cell-free extract and the concentration was determined by measuring changes in A 360 caused by reduction of metronidazole.
Reduction of carboxylates to alcohols
Anoxic, sterile stocks of n-fatty acids, lactate, and isobutyrate were individually added to prepared medium to a concentration of 30 mM, a 60 mM addition was used for acetone only, prior to inoculation with C. ragsdalei. C. carboxidivorans and A. bacchi were analyzed for the conversion of propionic acid to 1-propanol only. Solventogenic cells (mid-log phase) were added to media and pressurized with CO to a final pressure of 207 kPa. Samples were collected after inoculation and after 10 d incubation. Experiments were performed in triplicate and repeat experiments were conducted to determine reproducibility. Additionally, carboxylate- and ketone-free and sterile controls were implemented.
Analytical methods
Branched chain fatty acids, n-fatty acids, ketone, n-alcohol, and branched chain alcohol concentrations were quantified by gas chromatography (GC) using the Shimadzu GC-8A (Shimadzu Scientific Instruments, Columbia, MD) equipped with a flame ionization detector (FID). Samples were injected into a glass column (2 m × 5 mm × 2.6 mm) packed with 4 % Carbowax 20 M TPA on Carbopack B 80/120 mesh (Supelco Analytical, Bellefonte, PA). The inlet and detector were both set at 200 °C. Column temperature was held at 155 °C for alcohols containing ≤5 carbons and acids ≤3 carbons in length. Column temperature was held at 185 °C for >C5 alcohols and >C3 acids. Data was analyzed using a C-R8A Chromatopac Integrator (Shimadzu Scientific Instruments, Columbia, MD). Sample pH was determined using the Fisher Accumet Basic pH Meter (Fisher Scientific, Pittsburgh, PA).
Results and discussion
Evidence for the reduction of n-fatty acids to alcohols
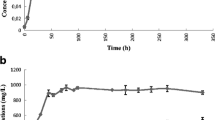
The concentration of acetate and ethanol in “Clostridium ragsdalei” incubations with syngas was monitored over 30 days (Fig. 1). Decreases in the acetate concentration suggested that C. ragsdalei consumed some of the acetate produced earlier in the fermentation. In conjunction with this acetate consumption, was the production of ethanol. Ethanol production did not appear to be growth-dependent and may proceed via the reduction of acetate, though this conclusion cannot be drawn from GC-FID analysis alone given that C. ragsdalei produces both acetate and ethanol from C1 substrates intrinsically. Thus, labeled [2-13C]-acetate was added to fresh medium to evaluate the hypothesis that some of the ethanol produced by C. ragsdalei was derived from the reduction of acetate. The results of this experiment are shown in Fig. 2. The collection of NMR spectra obtained over a 30-day period shows a peak at 23.5 ppm for [2-13C]-acetate and an increasing peak at 16.5 ppm for [2-13C]-ethanol (Fig. 2). The 13C NMR analysis shows that [2-13C]-ethanol is the only labeled end product (Fig. 2). 13CO2, [2-13C]-acetyl-CoA, and [2-13C]-acetyl phosphate were not detected.
The conversion of carboxylates to alcohols catalyzed by resting cells or cell-free extracts of solventogenic Clostridia [10, 28] indicates that these bacteria can convert carboxylates to alcohols independent of growth. A resting cell experiment was conducted with cells of C. ragsdalei harvested at stationary phase of growth. These cells were washed twice and amended with minimal medium containing propionic acid (21.9 ± 0.24 mM). After 5 days, C. ragsdalei consumed 8.71 ± 0.63 mM CO and produced 18.8 ± 3.5 mM 1-propanol (Table 1). This suggested that the oxidation of 1 CO provided 2 of the reducing equivalents required to produce 1-propanol from propionic acid. No change in pH was observed.
The reduction of propionic acid to 1-propanol was further supported by the production of [2-13C]-propanol from [2-13C]-propionic acid (Fig. 3). C. ragsdalei cultures amended with [2-13C]-propionic acid consumed 16 mM of the added propionic acid and produced 15 mM [2-13C]-propanol after 9 d incubation. The only other end products detected by GC-FID were acetate (81.8 ± 17 mM) and ethanol (125 ± 25 mM). In [2-13C]-propionic acid samples, the day 0 spectrum prominently displayed only one peak at 30.5 ppm, corresponding to the 13C-label on the number 2 carbon of propionic acid (Fig. 3). The day 3 spectrum also contained a signal corresponding to the [2-13C]-propionic acid (30.5 ppm); however, this signal had decreased in intensity when compared to the day 0 spectrum. Additionally, a second signal had appeared at 24.6 ppm, which was identified as the 13C-label on the number 2 carbon of 1-propanol. The day 5 spectrum showed a dramatic decrease in [2-13C]-propionic acid (30.5 ppm) and corresponding increase in [2-13C]-propanol (24.6 ppm) signal intensities. The day 9 spectrum was similar to day 5 with a slight increase in the signal intensity at 30.5 ppm, suggesting the oxidation of the [2-13C]-propanol back to [2-13C]-propionic acid. This evidence not only supported the hypothesis that the number 2 carbon of propionic acid is being converted to the number 2 carbon of 1-propanol, but also demonstrated that this reaction is reversible in vivo. Replicate NMR analyses generated similar spectra.
CO as the preferred reductant for the reduction of acids to alcohols
CODH activity was measured in C. ragsdalei cell-free extracts that reduced propionic acid to 1-propanol at the expense of CO. The CODH activity was 1.61 ± 0.8 µmol min−1 mg−1. Even though CODH activity was lower in this study than the activities demonstrated previously [26], it was evident that CO was a preferred reductant based on the activities observed in the presence of alternative reductants (no activity observed with BV and formate). Additionally, C. ragsdalei did not reduce propionic acid to 1-propanol in the presence of hydrogen (data not shown). CO was also the only reductant examined that supported the conversion of propionic acid to 1-propanol by resting cells. The free energy change for the biological conversion of acids to alcohols, though exothermic, is much less than that for the fermentation of glucose to acetate (−225.5 kJ mol−1) [30]. The energetics for acetate reduction to ethanol (Eq. 1), propionic acid to 1-propanol (Eq. 2), and butyric acid to 1-butanol (Eq. 3) with hydrogen as the electron donor are shown below at [34]:
The thermodynamics of acetate reduction (Eq. 4) do not change when CO replaces hydrogen as the reductant. However, the reaction becomes more favorable for the reduction of propionic acid (Eq. 5) and butyric acid (Eq. 6) when CO is used in place of hydrogen [34].
Thermodynamics indicate that the reduction of propionate and butyrate to the corresponding alcohols would be more exothermic with CO as the reductant rather than hydrogen. This analysis was ultimately supported by the use of CO as the preferred electron donor for whole resting cells of C. ragsdalei and measured CODH activity in C. ragsdali cell-free extracts.
Sequence similarity for AOR-mediated n-fatty acid reduction
The reduction of carboxylates to corresponding aldehydes was previously described as being mediated by AOR [37]. The amino acid sequence of AOR in C. ragsdalei DSM 15248 (CragP11_contig00091) was compared to the amino acid sequences of AORs in C. ljungdahlii DSM13528 (NC_014328) [17], C. carboxidivorans strain P7T DSM 15243 (NZ_ACV101000065) [5], and M. thermoacetica DSM 521 [23] using JGI’s IMG comparative analysis software [21] (Fig. 4). All of these acetogens have been shown to reduce n-fatty acids to alcohols at the expense of CO and/or H2. C. ragsdalei AOR exhibited 99 % amino acid sequence identity with C. ljungdahlii AOR. Comparison of the AOR of C. ragsdalei to the AOR of C. carboxidivorans revealed an amino acid sequence similarity of 92 %. By contrast, the amino acid sequence homology of C. ragsdalei AOR to the previously described M. thermoacetica AOR was only 50 % (Fig. 4). Comparatively, M. thermoacetica has a broader substrate range than other acetogens, having reduced acetate, propionic butyric, pentanoic and hexanoic acids, (R)-lactate, (S)-lactate, succinate, glutarate, adipate, suberate, benzoic acid, (2R,S)-2-phenylbutyrate, (E)-2-methyl-2-butenoate, (E)-2-methylcinnamate, vinyl acetate, and sorbate to alcohols [29]. The reduction of acetone, lactate, isobutyrate and propionic, butyric, pentanoic and hexonic acids to alcohols by C. ragsdalei was examined (Table 2). Using CO as a substrate, C. ragsdalei reduced n-fatty acids up to 6 carbons in length with production of the corresponding alcohols. However, when n-fatty acids >6 carbons were initially added to the growth medium, growth was inhibited (Supplemental Fig. 2). C. ragsdalei converted 100 % of the added acetone to 2-propanol with a final isopropanol concentration of 60 mM (Table 2). Acetone conversion was examined due to the application of this ketone as a syngas scrubbing agent prior to fermentation [24]. Although not the first to show the conversion of acetone to 2-propanol by a biocatalyst [24], our research showed complete conversion of twice the amount of acetone to 2-propanol (Table 2). Lactate and isobutyrate did not inhibit growth when added to growth medium, however, 1,2-propanediol and 2-butanol, respectively, were not produced from these substrates (Table 2).
Comparison of the gene sequence for the aldehyde oxidoreductase (AOR) of “Clostridium ragsdalei” DSM 15248(CragP11_contig00091) versus the annotated AORs of Clostridium ljungdahlii DSM 13528 (NC_014328), Clostridium carboxidivorans strain DSM 15243 (NZ_ACV101000065), and Moorella thermoacetica DSM 521. The N-terminal tungsten (W) binding domain was 100 % similar across all species (top to bottom: M. thermoacetica, Mt; C. carboxidivorans, Cc; C. ljungdahlii, Cl; and C. ragsdalei, Cr); whereas the C-terminal substrate binding domain was 71 % similar between C. ragsdalei and M. thermoacetica and 80 % similar among the compared Clostridia
Two conserved domains are described for AOR: an N-terminus described as interacting with the tungsten (W) cofactor and a C-terminus that putatively regulates substrate binding [20]. The N-terminal domain amino acid sequence is 100 % conserved across all of the aligned sequences (Fig. 4). The C-terminal domain, while 80 % similar in the aligned clostridial AOR amino acid sequences, was only 71 % similar to the M. thermoacetica C-terminal amino acid sequence (Fig. 4). This could explain the difference in substrate specificity between C. ragsdalei and M. thermoacetica, since a change in predicted secondary structure of the substrate binding domain is observed in silica due to the amino acid differences. This, however, is speculative and requires further research.
AOR activity was measured in C. ragsdalei cell-free extracts. AOR activity in the propionic acid-reducing direction was 3.65 % of the activity measured in the propionate-producing direction (0.12 ± 0.03 versus 3.28 ± 0.22 µmol min−1 mg−1, respectively) for cell-free extracts of C. ragsdalei (Fig. 5). This is comparable to previous observations in M. thermoacetica cell-free extracts [32], whereby the carboxylic-acid reductase activity of AOR was about 5 % of the aldehyde-dehydrogenase activity. C. ragsdalei produced approximately, 0.31 µM propionic acid when 10 µM propionaldehyde and MVox were amended with cell-free extracts. Unlike acyl kinase, the AOR-like enzyme in C. ragsdalei cell-free extracts appeared to be promiscuous with respect to substrate, as indicated by the reduction of lactate and octanoic acid by cell-free extracts but not whole cells. The absence of kinase activity (Supplemental Table 1) and CoA intermediates (Supplemental Fig. 1) further supports the finding that the primary mechanism of alcohol production from carboxylates occurs via an AOR-like enzyme. In addition to AOR activity, ferredoxin activity was measured as the reduction of metronidazole. The measured ferredoxin activity in C. ragsdalei cell-free extracts using propionaldehyde as the substrate was 0.59 ± 0.13 µmol min−1 mg−1. The proposed mechanism for propionic acid reduction in C. ragsdalei is depicted in Fig. 5.
The proposed mechanism for “Clostridium ragsdalei”-mediated reduction of propionic acid to 1-propanol using aldehyde oxidoreductase (AOR) and alcohol dehydrogenase (ADH). Carbon monoxide dehydrogenase (CODH) activity is also shown. Values represent measured enzyme activity levels in cell-free extracts of C. ragsdalei (µmol min−1 mg−1). The proposed reaction is mediated by ferredoxin (Fd) and nicotinamide adenine dinucleotide (NADH)
Enhanced 1-propanol production from propionic acid
Using C. ragsdalei as a biocatalyst, the conditions for the enhanced conversion of propionic acid to 1-propanol were determined to be fed-batch incubations (CO pressurized to 207 kPa every 48 h) in an optimized medium [26] at an initial pH of 5.3. Under these conditions, C. ragsdalei reduced 97 % of the propionic acid to 1-propanol (Table 3). This represents an improvement in conversion efficiency of 34.7 %. Fed-batch conditions were used to provide a continuous supply of reductant (CO) for the conversion of propionate to 1-propanol. The optimized medium was developed previously to include metals that were required by the metalloenzymes involved in alcohol production [26]. The lower starting pH is reflective of previous work that shows a decrease in initial pH or a lowering of pH during fermentation enhances and can even initiate alcohol production [36]. C. carboxidivorans [18] and A. bacchi [1], two solventogenic syngas-fermenting acetogens, were also assessed for conversion of propionic acid to 1-propanol. C. carboxidivorans and A. bacchi reduced propionic acid to 1-propanol at a conversion efficiency of 100 % (Table 3). While conversion efficiency is used frequently in the literature, this measurement does not account for the amount of 1-propanol produced from the total amount of propionic acid added to the bacterial culture. The percent theoretical maximum, however, is the amount of 1-propanol produced from the total added propionic acid. C. carboxidivorans produced 77 % of the theoretical maximum 1-propanol from the added propionic acid, whereas A. bacchi only converted 10 % of the added propionic acid to 1-propanol. C. ragsdalei, on the other hand, converted 86 % of the theoretical maximum propionic acid to 1-propanol. The low theoretical maximum 1-propanol production in A. bacchi could be due to the lack of defined fermentation parameters used in this study, which is an assumption further supported by studies in which defined fermentation parameters were used to successfully increase acid conversion efficiency in A. bacchi strains [19]. In these previous studies, A. bacchi strain CP15 produced approximately, two-fold more 1-propanol than strain CP11T with a conversion efficiency of 37 % (Table 3) [19]. When a mixture of A. bacchi strains and Clostridium propionicum was used the conversion efficiency, again, more than doubled (Table 3) [19]. C. ragsdalei and C. carboxidivorans had similar 1-propanol production rates (3 mM d−1), second only to the well-studied industrial biocatalyst C. acetobutylicum (Table 3) [13]. C. ljungdahlii produced 10.44 ± 1.69 mM 1-propanol from added propionic acid after 400 h incubation at conversion efficiency of 92 % (Table 3) [22]. C. ljungdahlii has also been reported to convert butyric, pentanoic, and hexanoic acids to the corresponding alcohols at conversion efficiencies of 68, 52, and 46 %, respectively [22]. Gas stripping was purportedly responsible for conversion inefficiency, since incubations were conducted under a constant flow of syngas [22]. It remains unclear in the previous studies and this study, why conversion efficiencies decrease as n-fatty acid carbon chain length increases or where the carbons from the consumed n-fatty acids, which are not converted to alcohols, end up. In this research, C. ragsdalei grown on a syngas analog converted propionic, butyric, pentanoic and hexanoic acid, and acetone to the corresponding alcohols at conversion efficiencies of 97, 100, 82, 62 and 100 % (Table 2). This is an improvement in the reduction of n-fatty acids to alcohols by C. ragsdalei from previous reports. The cost of cleaning syngas feedstocks for metal catalysis is typically higher than for biocatalytic processes. Abiotic methods for synthesizing solvents also tend to be performed at much higher temperatures, so energy input requirements are generally larger for these processes versus biological fermentations. However, this higher temperature generally results in faster kinetics [25]. Additionally, recent advances in bioengineering have eliminated some of the pitfalls of using biocatalysts [3]. Developing a better understanding of the biochemical pathways that produce alcohols from n-fatty acids, such as the AOR/ADH pathway presented here, is useful for further advancing bioengineering of sturdy biocatalysts for the production of biofuels.
Conclusions
Whole cells of “Clostridium ragsdalei” reduced acetic, propionic, butyric, pentanoic and hexanoic acid, and acetone to the corresponding alcohols. Acetone and butyric acid were reduced to 2-propanol and 1-butanol, respectively, at a conversion efficiency of 100 %. Resting cells of C. ragsdalei converted propionic acid to 1-propanol with CO as the reductant. An AOR-like enzyme was used by C. ragsdalei to reduce propionic acid to 1-propanol. AOR activity was also measured in C. ragsdalei cell-free extracts for lactic and octanoic acid reduction even though whole cells did not reduce these fatty acids to the corresponding alcohols. Whole cells of C. ragsdalei converted propionic acid to 1-propanol at a conversion efficiency of 97 % with the highest measured final 1-propanol concentration reported in this study and in the literature for acetogenic biocatalysts.
References
Allen TD, Caldwell ME, Lawson PA, Huhnke RL, Tanner RS (2010) Alkalibaculum bacchi gen. nov., sp. nov., a CO-oxidizing, ethanol-producing acetogen isolated from livestock-impacted soil. Int J Syst Evol Microbiol 60:2483–2489
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HSCoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruant G, Lévesque M-J, Peter C, Guiot SR, Masson L (2010) Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7T. PLoS One 5:313033
Chen J-S, Blanchard DK (1979) A simple hydrogenase-linked assay for ferredoxin and flavodoxin. Anal Biochem 93:216–222
Coyle W (2007) The future of biofuels. Economic Res Service/USDA, Washington
Drake HL, Kusel K, Matthies C (2006) Acetogenic prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Shleifer K-H, Stackebrandt E (eds) The Prokaryotes, 3rd edn. Springer, Berlin, pp 354–420
EIA (2012) Annual Energy Outlook 2012. Energy Information Administration. Report No: DOE/EIA-0383(2012)
Fraisse L, Simon H (1988) Observations on the reduction of non-activated carboxylates by Clostridium formicoaceticum with carbon monoxide or formate and the influence of various viologens. Arch Microbiol 150:381–386
Granberg RA, Rasmuson ÅC (1999) Solubility of paracetamol in pure solvents. J Chem Eng Data 44:1391–1395
Henstra AM, Sipma J, Rinzema A, Stams AJM (2007) Microbiology of synthesis gas fermentation for biofuel production. Curr Op Biotech 18:200–206
Hüsemann MHW, Papoutsakis ET (1990) Effects of propionate and acetate additions on solvent production in batch cultures of Clostridium acetobutylicum. Appl Environ Microbiol 56:1497–1500
IEA (2009) Key world energy statistics. Internation Energy Agency, Paris
Kenealy WR, Waselefsky DM (1985) Studies on the substrate range of Clostridium kluyveri; the use of propanol and succinate. Arch Microbiol 141:187–194
Kirschner M (2006) n-Butanol. Chem Mark Rep 269:42
Köpke M, Held C, Hujer S, Liesegang H, Wiezer A, Wollherr A, Ehrenreich A, Liebl W, Gottschalk G, Dürre P (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. PNAS 107:13087–13092
Liou JS, Balkwill DL, Drake GR, Tanner RS (2005) Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int J Syst Evol Microbiol 55:2085–2091
Liu K, Atiyeh HK, Stevenson BS, Tanner RS, Wilkins MR, Huhnke RL (2014) Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Biores Tech 152:337–346
Marchler-Bauer A et al (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41:D384
Markowitz VM et al (2014) IMG 4 version of the integrated microbial genomes comparative analysis system. Nucl Acids Res 42:D560–D567. http://img.jgi.doe.gov/er
Perez JM, Richter H, Loftus SE, Angenent LT (2012) Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol Bioeng 110:1066–1077
Pierce E, Xie G, Barabote RD, Saunders E, Han CS, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG, Ragsdale SW (2008) The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Env Microbiol 10:2550–2573
Ramachandriya KD, Wilkins MR, Delorme MJM, Zhu X, Kundiyana DK, Atiyeh HK, Huhnke RL (2011) Reduction of acetone to isopropanol using producer gas fermenting microbes. Biotechnol Bioeng 108:2330–2338
Ridjan I, Mathiesen BV, Connolly D (2014) Synthetic fuel production costs by means of solid oxide electrolysis cells. Energy 76:104–113
Saxena JS, Tanner RS (2010) Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogens, Clostridium ragsdalei. J Ind Microbiol Biotechnol 38:513–521
Sheih J, Whitman WB (1988) Autotrophic acetyl coenzyme A biosynthesis in Methanococcus maripaludis. J Bacteriol 170:3072–3079
Simon H, White H, Lebertz H, Thanos I (1987) Reduction of 2-enoates and alkanoates with carbon monoxide or formate, viologens, and Clostridium thermoaceticum to saturated acids and unsaturated alcohols. Angew Chem Int Ed Engl 26:785–787
Simon H, Lebertz H (1989) Microbial reduction of monocarboxylic and dicarboxylic acids in the presence of carbon monoxide and/or formates plus mediators. United States Patent No. 4, 851, 344
Steinbusch KJJ, Hamelers HVM, Buisman CJN (2008) Alcohol production through volatile fatty acid reduction with hydrogen as electron donor by mixed cultures. Water Res 42:4059–4066
Steinbusch KJJ, Arvaniti E, Hamelers HVM, Buisman CJN (2009) Selective inhibition of methanogenesis to enhance ethanol and n-butyrate production through acetate reduction in mixed culture fermentation. Biores Technol 100:3261–3267
Strobl G, Feicht R, White H, Lottspeich F, Simon H (1992) The tungsten-containing aldehyde oxidreductase from Clostridium thermoaceticum and its complex with a viologen-accepting NADPH oxidoreductase. Biol Chem Hoppe-Seyler 373:123–132
Tanner RS (2007) Cultivation of bacteria and fungi. In: Hurst CJ, Crawford RL, Mills AL, Garland JL, Stetzenbach LD, Lipson DA (eds) Manual of environmental microbiology, 3rd edn. ASM Press, Washington, pp 69–78
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Tilman D, Socolow R, Foley JA, Hill J, Larson E, Lynd L, Pacala S, Reilly J, Searchinger T, Somerville C, Williams C (2009) Beneficial biofuels: the food, energy, and environment trilemma. Science 325:270–271
Wang S, Zhang Y, Dong H, Mao S, Zhu Y, Wang R, Juan G, Li Y (2011) Formic acid triggers the “acid crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Appl Environ Microbiol 77:1674–1680
White H, Huber C, Feicht R, Simon H (1993) On a reversible molybdenum-containing aldehyde oxidoreductase from Clostridium formicoaceticum. Arch Microbiol 159:244–249
Acknowledgments
This research was supported in part by the USDA-CSREES special research grant award. The authors thank Jarrod Warnock and Dr. Ravindranath Garimella for collecting NMR spectra. The authors also thank Dr. Elizabeth Karr for in vitro protein work and Dr. Johannes Kung for collecting acyl-CoA spectra (Supplmental Data).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Isom, C.E., Nanny, M.A. & Tanner, R.S. Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J Ind Microbiol Biotechnol 42, 29–38 (2015). https://doi.org/10.1007/s10295-014-1543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1543-z