Abstract
The quest for quality mineral resources has led to the development of many technologies that can be used to refine minerals. Biohydrometallurgy is becoming an increasingly acceptable technology worldwide because it is cheap and environmentally friendly. This technology has been successfully developed for some sulphidic minerals such as gold and copper. In spite of wide acceptability of this technology, there are limitations to its applications especially in the treatment of non-sulphidic minerals such as iron ore minerals. High levels of elements such as potassium (K) and phosphorus (P) in iron ore minerals are known to reduce the quality and price of these minerals. Hydrometallurgical methods that are non-biological involving the use of chemicals are usually used to deal with this problem. However, recent advances in mining technologies favour green technologies, known as biohydrometallurgy, with minimal impact on the environment. This technology can be divided into two, namely bioleaching and biobeneficiation. This review focuses on Biobeneficiation of iron ore minerals. Biobeneficiation of iron ore is very challenging due to the low price and chemical constitution of the ore. There are substantial interests in the exploration of this technology for improving the quality of iron ore minerals. In this review, current developments in the biobeneficiation of iron ore minerals are considered, and potential solutions to challenges faced in the wider adoption of this technology are proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining, trading, and utilisation of minerals are commercial activities of historic importance and high economic value. Many construction and manufacturing industries have become absolutely dependent on specific minerals. Although talc, asbestos, and elemental sulphur can be used directly after mining without further treatment, most minerals such as iron or gold ores need refinement and reprocessing before they can be utilised industrially [30].
The current pace of global development has led to increases in the demand for minerals and their by-products [16]. This growth has resulted in rapid depletion of quality mineral ores to the extent that most mineral ores are no longer found in economically viable deposits [30]. This has led to investigations on why particular ores from different locations may differ in composition. Long-term natural events such as mineralisation, deposition, solubilisation and weathering were suggested as possible causes of differences in the chemical composition of minerals from different sources [5, 45, 46]. Whilst geologists, microbiologists and biotechnologists may have different ways of defining these processes, the links that exist amongst them cannot be disputed. For example, stages in the weathering process may involve mineralisation and solubilisation. The same weathering can be described as a natural process by which mineral ores could be purified or leached through solubilisation by microbes [6, 45]. Although there are different forms of weathering, biological weathering that usually involves microorganisms such as bacteria and fungi has played a significant role in mineral transformation. Microorganisms play active roles by interacting with the mineral environment. For example, iron ore oxidising bacteria were suggested to have a major role in the formation of the huge Precambrian banded iron formations (BIF) through biologically induced mineralisation in the ancient ocean [32].
Different microbial activities have been exploited to solubilise particular ore constituents commercially. The development of these processes has been achieved on laboratory scale through the application of biohydrometallurgy principles [21, 44, 45]. The term “biohydrometallurgy” has been used widely and interchangeably with “bioleaching” to describe industrial microbial processes such as metal extraction from low grade ores, metal detoxification, the beneficiation of coal and other mineral ores and recovery of metals from waste materials [9, 21, 39].
Biohydrometallurgy as a technology
Biohydrometallurgy is a word that evolved from hydrometallurgy, which means biological hydrometallurgy. In hydrometallurgy, the system relies on dissolution of metallic artefacts by acids or alkalis to produce solubilisation effects that leach metallic ions from minerals into solutions [8, 9]. Meanwhile, leaching conditions are normally adjusted to ensure that the desired part of the mineral remains insoluble. If soluble, additional techniques are introduced to separate the desired portion from other compounds in the solution [8, 9]. Processes involved in biohydrometallurgy are valued for being more environmentally friendly and cheaper compared to most physical and chemical methods of mineral extraction [45, 46]. Generally in biohydrometallurgy, minerals can be categorised into two types, i.e. the sulphidic and non-sulphidic minerals [28]. Chemolithoautotrophic bacteria are in most cases used for bioleaching of sulphidic minerals, whilst heterotrophs, which could be bacteria or fungi, are used for the bioleaching of non-sulphidic minerals [28, 44]. Currently, there is a growing distinction in terms of mechanisms involved in bioleaching and another related process known as biobeneficiation. Biobeneficiation involves the use of microbes to dissolve only unwanted parts of a mineral ore [28, 56]. However, both processes can be referred to as biohydrometallurgy technologies [28, 45]. The focus of this review is Biobeneficiation of iron ore minerals.
Iron ore
Iron is one of the oldest known metals and an essential source of primary iron for the global iron and steel industries. Iron is one of the most common metals on earth that is traded and consumed in different forms in many countries [5]. Iron is mainly desirable because of its hardness, strength, malleability, ductility, durability and the ease with which it alloys with other metals to form different kinds of steel [5, 31].
There are different types of iron-bearing minerals, but the highly exploited ones include: Magnetite—FeO·Fe2O3 (72 % Fe), Haematite—Fe2O3 (70 % Fe), Goethite—α-FeO·OH (61 % Fe), Lepidocrocite Ɣ—FeO·OH (61 % Fe), Siderite—FeO·CO2 (48 % Fe) and Chamosite—3FeO·Al2O3·2SiO2·6H2O (35 % Fe). Iron ore may contain associated gangue minerals such as feldspar, quartz, calcite, dolomite, clays and carbonaceous matter [5]. Iron ore can also contain phosphorus, silica, potassium, zinc, sulphur and sodium [5, 61].
The need for biobeneficiation of iron ore
Despite its positive potentials, the biobeneficiation of iron ore has not received attention similar to those of other mineral ores due to its low cost [19, 60]. However, fast depletion of sources and difficulties in finding new deposits of high-grade ores have increased the necessity of biobeneficiating iron ore. Problematic elements such as P, K and sodium (Na) can reduce iron ore’s commercial value to a point where the ore becomes valueless [17, 22]. These elements interfere with the processing of iron ore in different ways. Phosphorus is one of the most deleterious elements associated with iron ores. High P levels in iron ore reduce the strength, hardness and ductility of steel and increase its susceptibility to corrosion [36]. High levels of the alkali metals (K and Na) affect the smelting of iron ore in the blast furnace by releasing vapours that react with the refractory lining or burden material to generate unstable compounds. This mainly happens in the cooler regions where oxygen potential is high. The compounds formed progress to the hotter regions where they are reduced and rise again to form a recirculating load of alkalis [7, 29, 61]. Overall, the adverse effects of alkalis on the blast furnace include an increase in coke rate, poor quality of hot metal and mechanical weakening of the furnace lining. They also cause a decrease in production; conditions that are more pronounced when there is low stability of coke and the iron ore [29, 31, 61]. Due to these problems, iron ore with high proportions of P (>0.03 %) and K (>0.24 %) has low value on the international market, hence the need for iron ore industries to find economic and environmentally friendly methods to solve these problems.
Attempts in these regards have relied mainly on the principle of dilution with better quality ores and in-furnace adjustment of temperature, basicity and acidity. For instance, at the Sishen iron ore mine in South Africa, standard (low levels of K and P) and low grade (high levels of K and P) iron ores were mixed to avoid penalty charges and to meet international standards [60], but the method became unsustainable as levels of K and P increased.
Traditionally, chemical- and physical-based processes have been used to get rid of these contaminants with choice of method largely depending on the basic characteristics of the ore and the type or degree of association between the ore and contaminants [19]. For example, Cheng et al. [14] utilised 0.1 M sulphuric acid for the biobeneficiation of Australian iron ore with a P level of 0.126 % resulting in the leaching of >67 % of P within 5 h at 60 °C. Another hydrometallurgical method involving the integrated use of isoamyl alcohol, phosphoric acid and nitric acid to remove P from the iron ore has been suggested by Muhammed and Zhang [38]. Unfortunately, further development of these technologies has been hindered by their high costs and potential negative impacts on the environment [12]. Hence, these technologies are unacceptable to manufacturers. For this reason, there has been a gradual shift in emphasis towards the development of affordable and environmentally friendly iron ore leaching methods. The current focus is more on the use of microorganisms (biobeneficiation) by leaching the unwanted part of iron ore minerals [28].
Iron as metal of interest vs iron as impurity
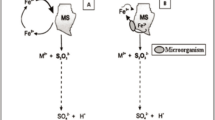
Selection of suitable microorganisms for bioleaching or biobeneficiation of iron-bearing minerals should not only be based on their ability to solubilise the minerals, but on their ability to either release the unwanted part of the ore into solution or removal of iron from the matrix. For this reason, the microbial solubilisation of iron-bearing minerals can be interpreted in two different ways. The two scenarios are quite different, but Fe ions are affected in both processes [3, 4, 19, 20]. Firstly, microbes can be used in situations where the iron is the impurity and the focus is to get rid of the iron. An example of this scenario is the use of microorganisms to remove iron as impurities from kaolin clay and silica sands. These are minerals with vast industrial applications such as paper, ceramics and glass manufacturing. High levels of iron oxide in these minerals affect the quality and value and are hence, unacceptable to manufacturers. Because of technological and environmental disadvantages of physical and chemical methods that have been successfully used in the past to remove iron impurities, microbial leaching is now preferable. Microorganisms such as Shewanella spp. have been successfully used to reduce Fe(III) contained in these minerals [62]. Enzymes are also believed to play a major role in this process. Minerals that are susceptible to oxidation are exposed to direct enzymatic attack by the microorganisms, e.g. Acidithiobacillus ferrooxidans. This can oxidise ferrous ions to ferric ions and involves the transfer of electrons from iron to oxygen [29].
The aim, using enzymatic attack, is to solubilise the Fe ions by getting them into solution, hence it is referred to as bioleaching of iron. This usually happens at low pH as indicated in the Pourbaix diagram in Fig. 1. However, this aspect is not within the scope of the present review.
Eh–pH diagrams for systems: a Fe–H2O and b Fe–H2O–0.21 M H2C2O4 (Sukhotin and Khentov [54])
The main focus of the present discussion is the use of microbes to remove or reduce the unwanted portion of the iron ore such as Na, K and P minerals. Such impurities are detrimental to the quality of the ore, thereby rendering it unusable or non-exportable [1, 18, 20].
In a recent review by Eisele and Gabby [21], the authors described potential biohydrometallurgy solution for the recovery of iron oxides from low grade ores with a high amount of silicate gangue materials. Their suggestion encourages the use of microorganisms to solubilise ore materials, releasing the iron into solution through a reduction process (Fe3+–Fe2+). According to the authors, precipitation as a solid hydroxide or by electrowinning could then be used to recover the dissolved iron. Whilst acknowledging that this method is feasible, it is pertinent to mention that the method may only be applicable when the iron oxide content of the ore is very low. In situations where the iron oxide contents of the ore are high enough, microbial solubilisation of the associated gangue materials could be a better option. Most of the studies on biohydrometallurgy of low grade iron ore minerals have focused on the use of microorganisms to dissolve the unwanted parts of the iron ore minerals.
With this approach, metal solubilisation may occur by an indirect method. This usually involves microbial production of organic acids, amino acids, and other metabolites that can dissolve heavy metals through direct displacement of metal ions from the ore matrix by hydrogen ions or by the formation of soluble metal complexes and chelates [8]. The ultimate aim of this approach is to solubilise the impurities rather than Fe ions, by getting them into solution. This approach is referred to as biobeneficiation of iron ore. The objectives of this review are strongly linked to this approach—biobeneficiation of iron ore.
Although, the two scenarios described above are quite different, Fe ions are affected in both processes.
Kinetics of biobeneficiation of iron ore
Complete kinetics of biobeneficiation of iron ore minerals are not fully understood because of the complex nature of microbial–mineral interactions that involves formation of exopolymeric substances (EPS), such as biofilms and organic acids [19]. An important challenge in this discussion is that biobeneficiation of iron ore minerals is often accompanied by co-dissolution of iron oxide. This is undesirable and may reduce the overall quality of the ore. Iron oxides exhibit different dissolution kinetics with organic acids. The dissolution mechanism consists of a three-stage process that includes adsorption of organic ligands from the solution on the system interface, non-reductive dissolution and reductive dissolution [40].
These steps are indicated in the equations below:
Adsorption of complex to surface
Electron transfer
Desorption
Dissolution
Furthermore, it is also important to understand the fate of impurities (such as phosphates, alkalis etc.) contained in the iron ore after biobeneficiation. In in vitro biobeneficiation experiments of iron ore with high phosphorus content, the extracted phosphate may be incorporated into bacterial cellular material such as phospholipids, cell wall components, DNA as well as co-precipitated with biomineralised secondary Fe oxides [6, 19]. Such a challenge was experienced in the study by Delvasto et al. [19]. In their study, re-precipitation of both phosphates and Fe was evaluated. EPS itself can act as a reservoir of phosphate, as was been demonstrated in the study conducted by Cloete and Oosthuizen [15] in biological waste water treatment processes.
With the abovementioned facts, the unwanted part (phosphates, potassium etc.) of the ore that is usually released into solution during biobeneficiation of iron ore is not equivalent to the amount lost from the mineral surface. Such outcome is possibly not surprising, because a significant proportion of bacteria-solubilised metal is usually incorporated into the biomass during growth [48].
The dynamic processes of re-adsorption of solubilised phosphate onto ore biofilms (i.e. by the EPS and/or by the re-precipitated mineral debris) must be taken into consideration because they may also adversely affect the biobeneficiation process. Solubilised Fe in biobeneficiation experiment could be as low as 10 mg/l, which means that the removal of the impurities is either selective or Fe could be re-precipitated [19].
Knowing the fate of the dissolved impurities during biobeneficiation is as important as the dissolution process itself. For instance, the instability of the Pi reported by Delvasto et al. [19] may pose a significant challenge in the biobeneficiation process. In their study, they demonstrated that soluble-P kinetics in batch cultures of Burkholderia caribensis (strain FeGL3) that was isolated from a Brazilian iron could be linked to the re-precipitation and re-dissolution of an intermediate Ca-phosphate phase. This is an undesirable aspect of biobeneficiation of iron ore minerals. Meanwhile, as suggested by Sashidhar and Podile [51], it is important to consider P incorporation into biomass when estimating solubilisation rates.
Potential factors that can affect biobeneficiation of iron ore minerals and other non-sulphidic minerals
There are various groups of heterotrophs that are involved in the biohydrometallurgy of non-sulphidic minerals [27, 28]. These microbes have varied biohydrometallurgical capabilities in respect of environmental, mineral, and microbial characteristics or factors. It is, however, possible for biobeneficiation of iron ore minerals to proceed either enzymatically or non-enzymatically. Johnson and Mcginness [29] reported the involvement of enzymes in the iron reduction by acidophilic heterotrophic bacteria.
Below are some of the previously investigated factors that can also affect biobeneficiation of iron ore minerals and other non-sulphidic minerals:
Mineralogy
Mineral composition has been found to significantly affect the type of microorganisms that can solubilise mineral constituents, which may interfere with their recovery [52]. In the biobeneficiation of iron ore minerals, the majority of studies carried out were for iron ores that contain phosphates. The phosphatic iron ores give opportunities to utilise phosphate-solubilising microorganisms that are able to dissolve the unwanted part of the mineral such as P and K [1–4, 19, 20].
This process may likely occur through a combination of both enzymatic and non-enzymatic microbial actions. According to Delvasto et al. [20], the most common mechanism in the solubilisation of phosphate minerals may be through the acidification of the medium. This involves the utilisation of a direct oxidase (DO) (Fig. 2) pathway through which glucose is oxidised to gluconic acid and a transmembrane proton motive force is generated that can be used for membrane transport functions. This means the dissociable proton of gluconic acid will be available for phosphate solubilisation [24, 35]. Usually, most phosphate-solubilising bacteria exhibit an additional or second periplasmic oxidation that converts gluconic acid to 2-ketogluconic acid via gluconate dehydrogenase. However, this stage may not be necessary for solubilisation of iron ore minerals [20].
General gluconate pathway (Ramachandran et al. [43])
Nutrient limitation
Either in biohydrometallurgical processes or under normal growth conditions, there is need for microorganisms to survive in order to grow and multiply. Whenever there is a shortage or lack of essential nutrients, competition and adaptation mechanisms set in. Only microbes with special and specific metabolic processes that are relevant to such selective environments are able to make use of the available nutrients [59]. This triggers mechanisms for solubilisation of unavailable nutrients through processes such as increased production of organic acids and scavenging [6, 53].
This microbial adaptation strategy can, therefore, be used to isolate potential bioleaching organisms. For example, K-limited media were used by Hutchens et al. [20] to isolate the Serratia marcescens which can solubilise feldspar by growing the isolate in the same feldspar solubilising medium. Furthermore, Sheng et al. [53] also utilised a K-limited medium to isolate the silicate-solubilising Bacillus globisporus Q12 and observed an increase in the number of cells in the medium and solubilisation rate when using these silicate minerals as the sole source of K. In another study by van Schöll et al. [55], K deficiencies significantly increased the oxalate production by tree seedlings colonised by the fungus Paxillus involutus, whilst magnesium (Mg) deficiencies increased the oxalate production in both mycorrhizal and non-mycorrhizal tree seedlings.
Microbial type
In a mixed population, those organisms that are appropriately endowed genetically will perform bioleaching or biobeneficiation in the presence of an appropriate ore or mineral, provided their nutritional needs for growth and reproduction are met. Those that are not genetically endowed to promote bioleaching or biobeneficiation will or will not grow, depending on whether their growth needs are met. When growing, they may indirectly influence the biohydrometallurgically active population in a positive or negative way. It is difficult to categorise bacteria involved in biobeneficiation of iron ore as Gram negative or positive. In most cases, Gram-negative bacteria are usually involved in biobeneficiation of phosphatic iron ore minerals because they exhibit superior mineral phosphate-solubilising capabilities. For example, Delvasto et al. [19] were able to isolate and utilise B. caribensis FeGL03 for the mobilisation of phosphate contained in the Brazilian high-phosphorus iron ore. On the other hand, Adeleke et al. [4] reported the use of Gram-positive bacteria for a similar purpose. In this situation, Arthrobacter species were utilised in the solubilisation of Sishen iron ore from South Africa.
Microorganisms often exhibit some level of specificity regarding the type and nature of organic acid they produce. For example, a decrease in concentration of organic acids was recorded for Rhodotorula rubra after 15 days of incubation during the dissolution of an aluminosilicate mineral. However, a contrasting result was obtained for Penicillium purpurogenum under the same conditions, where an increase in citric acid production was recorded after 15 days [47]. In addition, there are also recent records of different iron ore-biobeneficiating capabilities of bacteria and fungi. Two different groups, Delvasto et al. [20] and Adeleke et al. [1, 2, 4], have reported that solubilisation of iron ore minerals may differ from one microbial type to the other as described below.
Physicochemical factors
pH (acidification or alkalinisation)
The acidity or alkalinity of the growth medium of bioleaching microbes plays an important role in the bioleaching potential of the microorganisms [13, 28]. Thus, the growth medium is vital and can selectively determine the number of microbes that can grow based on pH. High acidity or alkalinity of the growth medium could easily become an advantage in bioleaching processes as it could allow the leaching experiment to proceed under non-sterile conditions [28, 56]. A study conducted by Vasan et al. [56] revealed that the increase in pH to near-neutral levels of metabolite produced by Paenibacillus polymyxa reduced the calcium (Ca) solubilisation from bauxite. In another example, at pH 3 of the medium, Welch et al. [57] reported increased feldspar dissolution compared to pH 4 whilst Adeleke et al. [4] reported reduction of the K and P content of iron ore minerals by utilising Bacillus sp. in a shake flask experiment.
In biobeneficiation of iron ore minerals, pH plays a significant role in the activities of leaching microorganisms and their metabolites (organic acids). It has been reported that oxalic acid production is improved by a pH close to neutrality, but can only dissolve iron at very acidic pH [11, 12, 33]. For this reason, the conditions for biobeneficiation of iron ore should be carefully optimised to prevent or reduce the co-dissolution of iron oxide when removing the impurities contained in associated gangue materials. A pH close to 7 may be ideal. To ensure appropriate pH is used, there may be a need to use a two-stage biobeneficiation approach as suggested by Camesselle et al. [12] and Adeleke et al. [3] where microbial growth is separated from the leaching process, which involves the use of microbial metabolites (spent medium).
Cultural composition and carbon source
This factor is also related to nutrient limitation and mineralogy in that the chemical constitution of the growth medium determines availability of the substrate for utilisation by microbes for metabolic activities [59]. For example, the source, type or quantity of carbon in the culture may determine the nature and quantity of organic acids to be produced. This invariably affects mineral dissolution because mineral solubilisation is in most cases “organic acid specific”. Various sources of carbon have been used in previous studies to grow the heterotrophs in bioleaching experiments. For instance, Hutchens et al. [20] used 0.2 g of glucose in 100 ml of medium to leach potassium from feldspar whilst Sheng et al. [53] used 1 % sucrose as a carbon source in experimental dissolution of feldspar by Bacillus globisporus Q12. However, developing a good biohydrometallurgical method for non-sulphidic minerals may only be achieved if a cheap carbon source, utilisable by the microbes, is integrated into the process [28].
Temperature
Exposure of minerals to high temperatures before and during bioleaching processes has been found to enhance bioleaching with initial heat treatment of clay minerals prior to the bioleaching process; for example, improving their vulnerability to leaching [32] and reduction in temperature by ~5 °C improving feldspar weathering by a factor of 20 [57].
Other factors
Other factors include shaking, aeration and time. Most of these remaining factors are interconnected. Franz et al. [23] recorded increased solubilisation of zinc from an industrial filter with increasing shaker speed. The speed of the shaker was related to aeration (oxygen supply) and observed to cause increased dissolution of zinc. Time (period of incubation) is another important factor. Rezza et al. [47] reported a sharp decrease in the concentration of organic acid produced by some heterotrophs after 15 days of incubation. This decrease directly corresponded to a decrease in the dissolution of aluminosilicate by these microbes.
Pulp density
The pulp density of the leaching material is a very important factor as it could disrupt the rate of mineral solubilisation. For instance, Vasan et al. [56] compared the bioleaching rates at two different pulp densities of 5 and 10 % and observed better leaching of Ca from bauxite at densities of 5 %. Pradhan et al. [42] also recorded good bioleaching of copper (Cu) at a low pulp density.
Particle size
Mineral particle size has a great influence on the weathering (solubilisation) rate of minerals [49, 58]. This phenomenon was also reported by Leake et al. [34] who found weathering to be dependent on particle size in Pinus sylvestris colonised by the root microbe Paxillus involutus. In another study by Modak et al. [37], bioleaching of bauxite by Paenibacillus polymyxa was found to be better with finer bauxite particle sizes compared to coarser particle sizes. Similar results were reported by Adeleke et al. [1], who observed more leaching from finer particle sizes of iron ore minerals.
Aeration
Aerated environments have been reported to enhance production of organic acids, thereby promoting the solubilisation of phosphates associated with iron ore minerals. In addition, Delvasto et al. [19] reported low Fe solubilisation in such environments and linked this to the possibility of microbial preference for phosphate content of the ore or the potential re-precipitation of the Fe from solution.
The journey so far: what we know
There are many studies that have been conducted on bioleaching of iron ore minerals. In most cases, fungi and bacteria as well as their metabolites were used to solubilise iron ore minerals. For example, a study conducted by Parks et al. [41] revealed that there was a significant reduction in the phosphorus content of iron ore using metabolites containing itaconic and oxalic acid produced by a Penicillium sp with further reduction of the P content being obtained by addition of a low concentration of hydrochloric acid. In a related research study, the use of ECM fungi for the solubilisation of P from iron ore was reported by Buis [10] in Delvasto et al. [19], where Paxillus involutus, Hebeloma crustuliniforme, Thelephora terrestris and Laccaria bicolor failed to solubilise P from iron ore, despite their ability to solubilise P from hydroxylapatite. However, this is contrary to the result obtained by Adeleke et al. [1] that utilised ECM fungi in an in vitro shake flask experiment for the leaching of P and K from iron ore minerals. The study investigated the potential roles of four different ECM fungi (Pisolithus tinctorius, P. involutus, Phialocephala fortinii, and Suillus tomentosus) in the mobilisation of P and K from Sishen iron ore mineral. Results of the study indicated that these four ECM fungi have the potential to mobilise P and K from the two iron ore types with the mobilisation effect being partially linked to ore type, particle size, organic acid production and attachment of the fungi to the iron ore. In a related study, a pot experiment was conducted by Adeleke et al. [3] to investigate the potential of plants, in association with ECM fungi, to mobilise P and K from iron ore minerals. P. tinctorius, P. involutus, L. bicolor and S. tomentosus were tested in association with Pinus patula. Results indicated that both the ectomycorrhizal and non-ectomycorrhizal plants were able to mobilise P and K from the iron ore.
Delvasto et al. [25] investigated the biobeneficiation of iron ore in greater detail. In this case, after bioactivation of the iron ore samples, one of the phosphate-solubilising fungi isolated from the iron ore (Aspergillus niger) was tested for its ability to solubilise P in the iron ore. Up to 30 % desphosphorisation was attained, signifying a high potential in the use of microorganisms for the biobeneficiation of iron ore. A later study by Delvasto et al. [20] isolated four different phosphate-solubilising bacteria (Leifsonia xyli FeGl 02, Burkholderia cenocepacia FeSu 01, B. caribensis FeGl 03 and Burkholderia ferrariae FeGl 01) from high-phosphorus Brazilian ore using tricalcium phosphate [Ca3(PO4)2] as the insoluble form of P. Further studies were then carried out on B. caribensis FeGl 03 to investigate its potential to dephosphorise iron ore. The results indicated that this bacterium was able to mobilise between 5 and 20 % of the initial iron ore P in 21 days with particle size and the production of organic acid and exopolymeric substances being isolated as possible factors that affected the leaching process [19].
In another study by Adeleke et al. [2], indigenous fungi (one Penicillium, two Alternaria isolates and one Epicoccum isolate) inhabiting the surface of Sishen iron minerals were isolated and used in shake flask experiments. The Penicillium isolate was confirmed as the only phosphate solubiliser and was further used to investigate the iron ore bioleaching potentials of indigenous fungi. In the shake flask experiment, both the fungus and its spent liquid medium were used on the two types of Sishen iron ore materials, namely, conglomerate and shale. Results indicated that the spent liquid medium removed more K than the direct use of the fungus, whilst the latter was more effective in removing P with also the high concentration of gluconic acid being identified as a possible factor that enhanced the mobilisation of P and K.
A similar experiment was conducted by Adeleke et al. [4], but the bioleaching agents were bacteria. In this experiment, twenty-three bacterial strains that belong to Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria were successfully screened on the basis of sequence homology and phylogenetic methods and isolated from the surfaces of Sishen iron ore minerals. Eight isolates were successfully screened on the basis of characteristics such as ability to lower the pH of the growth medium and solubilisation of tricalcium phosphate and utilisation in shake flask experiments in which iron ore minerals were used as sources of K and P. In this experiment, the production of high concentrations of gluconic acid by all isolates confirmed the importance of organic acid in the solubilisation of iron ore minerals.
In a recent report, microorganisms such as Paenibacillus polymyxa, Bacillus subtilis, Saccharomyces cerevisiae (yeast) and Desulfovibrio desulfuricans (SRB) were reported to be capable of changing the surface chemical behaviour of iron ore minerals such as haematite, corundum, calcite, quartz and apatite. This ultimately depends on mineral attributes such as mineral surface affinities of microbial cells and metabolic products, e.g. proteins and polysaccharides that can be utilised to induce their flotation or flocculation [50].
These are laboratory experiments and their outcomes have not been tested under field conditions.
Way forward
Although greater clarity needs to be obtained on the microbial types that are most suitable for biobeneficiation of iron ore minerals, the examples above strongly suggest that technology in this field is steadily gaining in importance and in acceptance. The most important reason for this level of acceptance is probably because the technology is environmentally friendly. Both fungi and bacteria have been tested in the biobeneficiation process. However, a key challenge that can affect the adoption of this technology is the growth condition of the microorganisms. Heterotrophs are known to grow at average temperatures (that support growth of many microbes) and near-neutral (pH 7, ±2) pH levels that encourage easy contamination of their growth media [28]. Because iron ore is inexpensive, it is not easy to develop biobeneficiation into affordable leaching technologies for broad use. It has, therefore, been suggested that the focus should shift to finding a suitable source of carbon, i.e. food or agricultural and industrial waste. Our laboratory is presently developing a technology that focusses on food and agricultural wastes with high contents of gluconic acid. Information regarding this aspect of biobeneficiation will be made available as soon as the investigation reaches an advanced stage. Another important challenge for full development of biobeneficiation of iron ore minerals is the biofilm production as well as the attachment of fungi to the mineral surface. However, such challenges can be overcome by the use of metabolites produced by the microbes and improved techniques that prevent/reduce fungal attachment to the surface of the mineral as proposed by Adeleke et al. [2].
As discussed above, the dissolution of impurities is often accompanied by the co-dissolution of iron oxides, which is the metal of interest. There is a need to develop a method for the selective dissolution of associated gangue materials by applying mild reducing conditions (ref).
Having discussed potential solutions to the problems associated with biobeneficiation of iron ore, it is time to start thinking about how to take this to the next level—commercial application. Of course, this will not be done on a laboratory scale. Lessons can be learnt from the low-cost dump bioleaching process used in scavenging copper from rocks that could not otherwise be economically processed.
Conclusion
Apart from laboratory scale successes, it has been a difficult challenge to commercialise and fully develop the biohydrometallurgy of iron ore minerals. This can probably be attributed to the non-sulphidic nature of some iron ore minerals [60]. In contrast, there have been numerous investments and studies on sulphidic minerals such as gold and copper, which are notably more economically viable. Hence, there seems to be a strong link between the cost of the technology and its acceptability. It is trusted that the level of acceptability of this technology for sulphidic minerals will increase if the technology is cheap and affordable. Such approach may include the use of waste products as carbon source and carrying out the process under non-sterile conditions. For instance, Hoffmann et al. [26] used domestic wastewater containing Pseudomonas 200 under anaerobic conditions to reduce the ferric iron in iron ore to ferrous iron after which the iron was recovered from the solution through precipitation with the help of a base.
For future development of commercial applications of this technology, there is a need for chemists, microbiologists, chemical engineers, civil engineers and other related disciplines to work together. A better understanding of the mineral surface microbiota will enable us to develop the biological approach of purifying iron ore further.
It is notable that information about gene sequences involved in Mineral Phosphate Solubilisation (MPS) is now available as well as the Direct Oxidation (DO) pathway for glucose metabolism and the involvement of products of direct oxidation in the MPS. With such information, there was the possibility of developing new genetically modified bacterial strains with great biobeneficiation capabilities. As suggested by Sashidhar and Podile [51], biobeneficiation of iron ore minerals can benefit greatly from the membrane-bound glucose dehydrogenase of Gram-negative bacteria being the first enzyme in the DO pathway. It could be worthwhile to explore MPS gene(s) for biobeneficiation application, which is both environmentally friendly and a low-cost process. The major challenge in this area of research lies in not just identifying and isolating the MPS gene(s), but in modifying the gene for superior properties and expression in microbes that thrive under normal environmental conditions with little or no iron oxide dissolution capability.
In conclusion, the following are recommended for consideration in the development of biohydrometallurgical methods for iron ore minerals:
-
1.
Reliable and cheap source(s) of carbon for the biobeneficiating microorganisms (heterotrophs). Such carbon source should be rich in glucose that could be converted into gluconic acid
-
2.
Method development should strive to include microbes that can easily multiply in numbers
-
3.
Method should involve potential re-precipitation of any dissolved Fe oxides
-
4.
It is essential to develop a method that will discourage re-precipitation of impurities. This can probably be controlled with pH and temperature
-
5.
The characteristics of biobeneficiating microbes should include abilities to dissolve phosphates or other undesirable iron ore associated gangue materials and also to participate in the uptake of dissolution products
-
6.
There is a need to develop an integrated technique that will allow easy separation of iron ore minerals, media and the microbes after the leaching process
-
7.
Biobeneficiating technique should be developed to prevent co-dissolution of Fe with the impurities as the concentration of the iron content will define the quality of the ore after biobeneficiation
-
8.
Maintaining sterile conditions would be very difficult if not impossible, hence attention should be shifted to the development of a low-cost biobeneficiation process that will function under non-sterile conditions
-
9.
The purity of the final iron ore products of biobeneficiation should reflect what is acceptable internationally and there is need to develop appropriate international standards for different impurities such as the standard for phosphate levels (<0.03 % is acceptable).
Abbreviations
- Ln− :
-
Any organic ligand with oxidation number n, such as oxalate (Ln− = C2O4 2− or HC2O4 −), citrate (Ln−C6H5O7 3− or C6H6O7 2− or C6H7O7 −),
- >:
-
Particle surface
- >Fe′′′:
-
Trivalent lattice iron on the particle surface
- >F′′:
-
Bivalent lattice iron on the particle surface
- [>Fe–L]:
-
Surface complex
- …:
-
Adsorbed species on the particle surface
- II, III:
-
Oxidation number of surface lattice iron
- N+, n − :
-
Valence of aqueous species
References
Adeleke R, Cloete T, Bertrand A, Khasa D (2010) Mobilisation of potassium and phosphorus from iron ore by ectomycorrhizal fungi. W J Microbiol Biotechnol 26:1901–1913
Adeleke R, Cloete T, Bertrand A, Khasa D (2012) Iron ore weathering potentials of ectomycorrhizal plants. Mycorrhiza 1–10
Adeleke R, Cloete T, Khasa D (2010) Isolation and identification of iron ore-solubilising fungus. S Afr J Sci 106:1–6
Adeleke R, Cloete T, Khasa D (2012) Culturable microorganisms associated with Sishen iron ore and their potential roles in biobeneficiation. W J Microbiol Biotechnol 28:1057–1070
Astrup J, Hammerbeck EC (1998) Iron. In: Wilson MG, Anhaeusser CR (eds) The mineral resources of South Africa. Council for Geoscience, South Africa, pp 402–416
Banfield JF, Barker WW, Welch SA, Taunton A (1999) Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci USA 96:3404–3411
Biswas AK (1981) Principles of blast furnace ironmaking. Cootha publishing house, Brisbane
Bodsworth C (1994) The extraction and refining of metals. CRC Press, Florida
Brierley JA, Brierley CL (2001) Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 59:233–239
Buis P (1995). Bioremediation techniques for the removal of phosphorus from iron ore. Ph.D. Dissertation. Mining engineering. Michigan Technological University, USA, p 129
Cameselle C, Bohlmann JT, Nunez MJ, Lema JM (1998) Oxalic acid production by Aspergillus niger, Part I. Influence of sucrose and milk whey as carbon source. Bioprocess Eng 19:247–252
Cameselle C, Ricart MT, Nunez MJ, Lema JM (2003) Iron removal from kaolin. Comparison between ‘‘in situ’’ and ‘‘two stage’’ bioleaching processes. Hydrometallurgy 68:97–105
Cameselle C, Nunez MJ, Lema JM, Pais J (1995) Leaching of iron by a spent fermentation liquor: influence of temperature, pH, agitation and citric acid concentration. J Ind Microbiol 14:288–292
Cheng CY, Misra VN, Clough J, Muni R (1999) Dephosphorisation of western Australian iron ore by hydrometallurgical process. Miners Eng 12:1083–1092
Cloete TE, Oosthuizen DJ (2001) The role of extracellular exopolymers in the removal of phosphorus from activated sludge. Water Res 35:3595–3598
Dale LL (1984) The pace of mineral depletion in the United States. Land Econs 60:255–267
Davies J, Moon JT, Traice FB (1978) Alkalis in the blast furnace. Ironmak Steelmak 5:151–161
Delvasto P, Balleste, A, Muñoz JA, González F, Blázquez ML, García-Balboa C (2005). Exploring the possibilities of biological beneficiation of iron-ores: The phosphorus problem. In: Proceedings of the 15th steelmaking conference, 5th ironmaking conference and 1st environment and recycling symposium IAS (CD-ROM), San Nicolás, Buenos Aires, Argentina, Nov 7–10, 2005. pp 71–82
Delvasto P, Ballester A, Muñoz JA, González F, Blázquez ML, Igual JM, Valverde A, García-Balboa C (2009) Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Miners Eng 22(1):9
Delvasto P, Valverde A, Ballester A, Muñoz JA, González F, Blázquez ML, Igual JM, García-Balboa C (2008) Diversity and activity of phosphate bioleaching bacteria from a high-phosphorus iron ore. Hydrometallurgy 92:124–129
Eisele TC, Gabby KL (2014) Review of reductive leaching of iron by anaerobic bacteria. Min Process Extr Metall Rev Int J 35(2):75–105
Elkasabgy T (1984) Effect of alkalis on reduction behavior of acid iron ore pellets. Trans ISIJ 24:612–621
Franz A, Burgstaller W, Schinner F (1991) Leaching with Penicillium simplicissimum: influence of metals and buffers on proton extrusion and citric acid production. Appl Environ Microbiol 57:769–774
Goldstein AH, Braverman K, Osorio N (1999) Evidence for mutualism between a plant growing in a phosphate-limited desert environment and a mineral phosphate solubilizing (MPS) rhizobacterium. FEMS Microbiol Ecol 30:295–300
Groudev SN, Groudeva VI (1986) Biological leaching of aluminium from clays. Biotechnol Bioeng symp 16:91–99
Hoffmann MR, Arnold RG, Stephanopoulos G (1989) Microbial reduction of iron ore. United States patent 4880740
Hutchens E, Valsami-Jones E, McEldowney S, Gaze W, McLean J (2003) The role of heterotrophic bacteria in feldspar dissolution—an experimental approach. Mineral Mag 67:1157–1170
Jain N, Sharma D (2004) Biohydrometallurgy for nonsulfidic minerals—a review. Geomicrobiol J 21:135–144
Johnson DB, McGinness S (1991) Ferric iron reduction by acidophilic heterotrophic bacteria. Appl Environ Microbiol 57:207–211
Klein C, Barbara D (2002) The manual of mineral science. 23rd edn John wiley and sons, inc. Hoboken, New Jersey. USA pp 289–296
Klemic HJ, James HL, Eberlein GD (1973) In: Brobst DA, Pratt WP (eds) Washington. Iron Geol Surv, DC, pp 291–306
Konhauser K, Hamade T, Raiswell R, Morris RC, Ferris FG, Southam G, Canfield DE (2002) Could bacteria have formed the Precambrian banded iron formations? Geology 30:1079–1082
Kubicek CP, Kunar GS, Wohrer W, Rohr M (1988) Evidence for a cytoplasmatic pathway of oxalate biosynthesis in Aspergillus niger. Appl Environ Microbiol 54:633–637
Leake JR, Duran AL, Hardy KE, Johnson I, Beerling DJ, Banwart SA, Smits MM (2008) Biological weathering in soil: the role of symbiotic root-associated fungi biosensing minerals and directing photosynthate-energy into grain-scale mineral weathering. Miner Mag 72:85–89
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-A174 Bioresources Technology 97, pp 957–960
Marshall P (1984) Austenitic stainless steels microstructure and mechanical properties. Elsevier, New York
Modak JM, Vasan SS, Natarajan KA (2001) Calcium removal from bauxite using Paenibacillus polymyxa, In: Kawatra, SK, Natarajan, KA (eds) Minerals and metallurgical processing pp 6–12
Muhammed M, Zhang Y (1989) A hydrometallurgical process for the dephosphorization of iron ore. Hydrometallurgy 21:277–292
Olson GJ, Brierley JA, Brierley CL (2003) Bioleaching review part B. Appl Microbiol Biotechnol 63:249–257
Panias D, Taxiarchou M, Paspaliaris I, Kontopoulos A (1996) Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 42:257–265
Parks EJ, Olson GJ, Brinckman FE, Baldi F (1990) Characterization by high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by a fungus. J Ind Microbiol Biot 5:183–189
Pradhan D, Pal S, Sukla SB, Chaudhury GR, Das T (2008) Bioleaching of low-grade copper ores using indigenous microorganisms. Ind J Chem Technol 15:588–592
Ramachandran S, Fontanille P, Pandey A, Larroche C (2006) Gluconic acid: properties, applications and microbial production. Gluconic acid: a review. Food Technol Biotechnol 44(2):185–195
Rawlings D (2005) Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb Cell Fact 4:1–13
Rawlings DE (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Rawlings DE, Dew D, du Plessis C (2003) Biomineralization of metal-containing ores and concentrates. Trends Biotechnol 21:38–44
Rezza I, Salinas E, Elorza M, Sanz de Tosetti M, Donati E (2001) Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem 36:495–500
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rosling A, Lindahl BD, Finlay RD (2004) Carbon allocation to ectomycorrhizal roots and mycelium colonising different mineral substrates. New Phytol 162:795–802
Sarvamangala H, Natarajan KA, Girisha ST (2012) Biobeneficiation of iron ores. Int J Min Eng Miner Process 1(2):21–30
Sashidhar B, Podile AR (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109:1–12
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Sheng XF, Zhao F, He LY, Qiu G, Chen L (2008) Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol 54(1065):1064–1068
Sukhotin AM, Khentov AI (1980) Anodic behavior of iron oxides and repassivation of iron in acid solutions. Elektrokhimiya 16(7):1037–1041
Van Schöll L, Hoffland E, van Breemen N (2006) Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol 170:153–163
Vasan SS, Modak JM, Natarajan KA (2001) Some recent advances in the bioprocessing of bauxite. Int J Miner Process 62:173–18639
Welch SA, Barker WW, Banfield JF (1999) Microbial extracellular polysaccharides and plagioclase dissolution—effects of pH, CO2, and organic acids. Geochim Cosmochim Ac 63:1415–1419(1415)
White AF, Brantley SL (1995) Chemical weathering rates of silicate minerals: an overview. Rev Miner Geochem 31:1–22
Willey J, Sherwood L, Woolverton C (2007) Prescott’s Microbiology. McGraw-Hill Higher Education, Berkshire SL6 2QL, pp 703–706
Williams PJ (2008) The use of Aspergillus Niger for the removal of potassium and phosphorous from the iron ore of the Sishen iron ore mine, South Africa. University of Pretoria, Pretoria, pp 573–578
Yusfin Y, Chernousov P, Garten V, Karpov Y, Petelin A (1999) The role of alkalis and conserving resources in blast-furnace smelting. Metallurgist 43:54–58
Zegeye A, Yahaya S, Fialips CI, White M, Manning DA, Gray N (2008) Application of bacterial iron reduction for the removal of iron impurities from industrial silica sand and kaolin. American Geophysical Union, Fall Meeting
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adeleke, R.A. Getting rid of the unwanted: highlights of developments and challenges of biobeneficiation of iron ore minerals—a review. J Ind Microbiol Biotechnol 41, 1731–1741 (2014). https://doi.org/10.1007/s10295-014-1514-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1514-4