Abstract
Objective
The aim of this study was to report a method that quantifies axon reflex sweating from individual sweat glands with nanoliter precision. Measurement of the axon reflex is generally expressed as a single variable (e.g., the flare area or total sweat volume). High-definition videography enables precise measurement of sweating from single, axon reflex-stimulated sweat glands (SGs).
Methods
The sudomotor axon reflex was activated in healthy subjects and subjects with peripheral neuropathy by iontophoresis of 10% acetylcholine. Sweating was simultaneously imaged for 5 min in a 2.5-cm2 area of iodine-coated skin to one side of the stimulus, using a customized high-resolution camera with starch-coated transparent tape over a rigid viewing screen. A second video then imaged the directly stimulated sweating. The indirect sweat response was quantified in terms of sweat gland number and distance from the stimulation site (radius), sweat rate per gland, and total sweat.
Results
Fifty-two healthy control and twenty subjects with neuropathy underwent testing at the foot, calf, thigh, and hand. Normal ranges were calculated for SG density, mean sweat rate per SG, and total sweat volume. Neuropathy subjects demonstrated reduced sweating, and values differed between body sites.
Interpretation
The described method precisely measures the total and individual sweat output of hundreds of SGs in response to a standard, axon reflex-mediated stimulus, and quantifies alterations in axon reflex sweating seen in peripheral neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
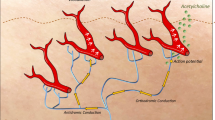
Unmyelinated nerve fibers are selectively affected in some patients with peripheral neuropathy [1,2,3,4,5]. The detection of these unmyelinated nerve fibers is difficult because prevalent clinical tests for peripheral neuropathy, such as electromyography and nerve conduction studies, primarily assess the function of large myelinated nerve fibers. Tests selective for the cutaneous nerve function of small fibers are useful for diagnosis and quantification of peripheral neuropathy. Axon reflex testing, which tests the function of small fiber cutaneous nerves, has a unique clinical utility, and methods include tests of the vasomotor flare [6], pilomotor response [7], and sudomotor axon reflex [8].
The vasomotor flare is the axon reflex-mediated vasodilation response that can be visualized on the skin surface all around a small area following stimulation by iontophoresis of acetylcholine (ACh) or the application of heat. Quantification of the flare is limited to measurement of the area of vasodilation by photography or laser Doppler velocimetry [9,10,11].
Methods quantifying the indirect sweat response to iontophoresis of ACh are commonly encountered in the clinical setting [1, 12,13,14,15,16]. Q-sweat is part of the panel of standardized autonomic tests included in the autonomic reflex screen (ARS) [17, 18]. An evaporimeter is used to measure the total volume of sweat produced by a 1-cm2 area of skin after iontophoresis of ACh into a surrounding ring of skin [16]. The test has been shown to be a sensitive and specific indicator of peripheral neuropathy [12, 13].
We have continued to develop applications for the sensitive sweat test (SST). The SST uses a high-definition video device to analyze nanoliter secretions of sweat produced by all sweat glands, singly and in total, in a 2.5-cm area of skin [19,20,21]. We previously demonstrated the specific capabilities of the test during maximal, direct stimulation by iontophoresis of pilocarpine. The test measures the total volume and rate of sweat, the location and number of excited sweat glands, the chronology of activation, the rate and volume of sweating from individual sweat glands (SGs), and total sweat volume [21]. Pilocarpine acts exclusively on muscarinic cholinergic receptors present on sweat gland tubules [22], but it does not activate synaptic nicotinic cholinergic receptors [23] or nerves involved in activating an axon reflex. Therefore, direct sweat testing with pilocarpine does not assess nerve activity per se.
In order to investigate the use of the SST as an indirect (axon reflex) sweat test, we designed the “sidecar” test, in which SST measures sweating as ACh is iontophoresed into an immediately adjacent area of skin. The camera’s recording of the sidecar area represents one segment of the total axon reflex-activated sweat response, which extends in all directions around the acetylcholine-stimulated area. Herein we describe the test procedures, present normative data, and characterize the differences between normal controls and patients with known neuropathy.
Methods
The study was approved by the University of Minnesota Institutional Review Board. Participants were recruited from the community through flyers and word of mouth. All subjects completed a symptom questionnaire with medical and neurological history and underwent neurologic examination. Inclusion criteria for healthy controls were the absence of risk factors, clinical diagnosis, examination findings, or symptoms of peripheral neuropathy. Neuropathy subjects were included if the medical history and physical examination plus electrodiagnostic testing (nerve conduction studies and electromyography) were supportive of a diagnosis of peripheral neuropathy. Cause and severity of neuropathy was not a factor in inclusion. Subjects who had taken medications with cholinergic or anticholinergic effects during the previous 48 h were excluded. All tests were performed in a dehumidified room with the thermostat set at 21.1 °C.
The SST camera was designed and constructed to have a 29-mm focal length and a 13 × 17.5-mm field of view. The camera is focused on the clear viewing window over the lens that during testing rests firmly against the tested skin. USB-powered LEDs with light diffusers illuminate the viewing screen completely and evenly. Prior to each test, a strip of prepared starch-coated tape is stretched across the viewing window, with the starch side facing out. Collected image data is sent via the USB cable to a laptop computer for analysis with image acquisition software (proprietary NeuroDevices, Minneapolis, MN, USA).
All subjects in our SST undergo testing at four body sites: foot dorsum just distal to the extensor digitorum brevis muscle (Fig. 1); medial–posterior calf, one-third the distance between knee and ankle; distal medial thigh over the motor point of the vastus medialis muscle; and the dorsal hand overlying the first dorsal interosseous muscle. Each body site is cleaned and gently abraded with water-soaked gauze followed by alcohol-soaked gauze. The skin is then dabbed dry, and the test site painted with Povidone-iodine solution, then dabbed dry again. The camera is pressed firmly, face down, on the Povidone-coated site, triggering initiation of the video recording with a pressure-sensitive switch integrated in the camera housing. Only a minimum pressure is applied to trigger the switch—approximately 400 g distributed over the 7-cm area of the camera face, or 0.55 N/cm2. This pressure should not impact the rate of sweating, previously estimated to have a hydrostatic pressure of 7.1 N/cm2 [24,25,26]. Simultaneously, the ACh gel, loaded in the anode iontophoresis capsule, is pressed down immediately adjacent and lateral to the camera. The gel is 2.5 cm in diameter, with a chord cut across one side 0.5 cm from the edge, to form a rounded capital D shape which lies flush with one side of the camera (Fig. 1).
In the SST, iontophoresis of 10% ACh gel is started immediately, at 2 mA for 5 min (10 mA-minute), while the SST records the video of the sweat reaction in the adjacent test site at one frame per second. The video is observed using the image acquisition software on the computer. The first frame of the video shows no sweating. However, soon thereafter sweat exits the sweat duct of each stimulated SG, passing first through the iodine painted on the skin then onto the starch tape, as a small dark spot. The sweat spreads centrifugally by means of a wicking action as an expanding circle within the thin space between the skin and starch tape. Dark areas detected at the beginning of the video which do not increase in size are excluded as noise.
After completion of the video recording, the camera is removed, fresh starch tape is placed across the camera, the iontophoresis site is coated with Povidone and dried, and a second video-recording, 1 min in duration, is acquired.
The area of the dark-blue, almost black, rounded spots on the starch tape accurately corresponds to the volume of sweat in previous calibrations. We then pipetted five sets of drops of known volume (5, 10, 50, 100, and 200 nl) onto an impermeable surface and onto the skin of five control subjects, imaged the results using the SST camera and then measured the imaged areas. The areas closely correlated to known fluid volumes (r2 = 0.9996, p < 0.01) in all measurements, according to the equation: μl = (mm2 × 0.0131) + 0.0068. Therefore, we calculated that 1 μl created a spot with area an 0.48 mm2, which was 1735 pixels using the SST camera [19,20,21].
Videos are saved and named by each subject’s unique de-identified study number, the body site tested and date and time of the test. Videos of the indirect sweating are edited to include one frame for every 5 s, or 12 frames per minute, for 5 min (until iontophoresis of 10 mA-minutes is completed), for a total of 60 frames (Fig. 2). Videos of direct sweating under the iontophoresis electrode are edited to one frame per second for a total of 60 frames.
Software analysis of the videos identifies all expanding sweat spots and their x/y coordinates at the beginning of the video, then the x/y coordinates and the first frame for each stimulated sweat spot as it appears throughout the video. The x coordinate is zero along the edge of the video abutting the ACh gel. The y coordinate is zero along the bottom edge of the video. Based on calibrations using known volumes of water pipetted onto starch tape and imaged by the camera, the blackened area in pixels is converted to volume in nanoliters. The volume (nL) of each individual sweat spot is charted for each frame as they expand, until they merge with a neighboring spot, at which point volume charting for the merged spots ceases.
For each sweat spot, the rate of sweating is calculated as nanoliter per minute, i.e., the area of the spot at the time of merging divided by the time from appearance to merging. The mean sweat rate per spot is calculated among all sweat spots in the video, as well as the median and 5th and 95th‰. Additionally, for indirect videos only, the distance of each sweat spot from the ACh gel is measured using its x-coordinate (the x-value is zero along the stimulating edge of the screen). Total volume of sweat is also charted in each frame, measuring volume as the blackened area of the video at the 15th, 30th, 45th, and 60th frames (15, 30, 45 and 60 s in the direct videos, and 75, 150, 225, and 300 s in the indirect videos).
Distributions are used to calculate the median and 5th and 95th‰ for total sweat, SG density, and rate/SG from each body location and age group of control subjects (Table 1). The degree of distal gradation for all measurements are estimated by a ratio comparing each measurement at a proximal body site to a more distal body site (e.g., hand/foot, thigh/calf, calf/foot, thigh/foot). Ratios are also calculated comparing directly stimulated measures to correlating indirectly stimulated measures.
A total of 52 control and 20 neuropathy subjects were studied. The controls included 21 females and 31 males aged 21–88 years (22 were aged < 45 years, 14 were 45–65 years old, and 16 were > 65 years old). Neuropathy subjects included 13 females and seven males aged 45–82 years (7 were aged 45–65 years and 13 were > 65 years old). Review of the neurologic history of each patient revealed that the cause of neuropathy was diabetes (n = 11), chemotherapy (n = 3), and unknown (n = 6). The onset of neuropathy was at least 5 years prior to testing in all neuropathy subjects. In our comparative analysis, neuropathy subjects were only compared with controls aged ≥ 45 years. In the control subjects, height ranged from 61 to 76 (median 67) in, weight ranged from 100 to 240 (median 168) lb, and the body mass index (BMI) ranged from 15 to 41 (median 26) kg/m2. In the neuropathy subjects, all three parameters were similar, with a median height of 66 (range 61–71) in, median weight of 175 (range 100–248) lb, and median BMI of 27 (range 18–39) kg/m2.
Results
Direct versus indirect stimulated sweating
Results were compared between the indirect and direct stimulated measurements for SG density, Sweat rate/SG and total volume (Table 1). For each sweat measurement, this comparison can be expressed in the form of a quotient: indirect per direct (I/D) sweating. For example, the SG density I/D quotient compares the SG density in the indirectly stimulated skin to that in the directly stimulated skin.
Among the controls, median I/D quotients were < 1.0 (indirect value < direct value) for all three measures at all four body sites, and > 95% of controls had a SG density I/D of < 1.0 at all body sites. Among the controls, sweat rate per SG I/D was < 1.0 at the hand in 100% of subjects, at the thigh in 93% of subjects, at the foot in 74% of subjects, and at the calf in 73% of subjects. Among the controls, total sweat I/D was < 1.0 at the hand in 88% of subjects, at the thigh in 100% of subjects, at the foot in 83% of subjects, and at the calf in 64% of subjects. As a group, neuropathy and control subjects had similar I/D measures for all measures and body sites, with the exception for rate/SG at the foot. Among neuropathy subjects, the indirectly stimulated rate per SG was greater than the directly stimulated rate per SG at the foot (mean I/D = 1.69), whereas in controls, the indirectly stimulated rate/SG was less than that of directly stimulated SGs at the foot (mean I/D = 0.74) (p = 0.0059).
Components of variation
All directly stimulated sweat measures (total sweat, rate/SG, and SG density) decreased significantly with age at all four sites. In contrast, measures of indirectly stimulated sweating did not show a significant association with age at any body site. Among controls, the SG density was greater at all body sites in females and the rate/SG was greater in males. Total sweat did not differ significantly between sexes.
The 95th‰ for radius differed between body sites (analysis of variance, p < 0.0001), suggesting that the axon reflex is most spatially expansive at the calf (mean 15.4 mm) and less so at the thigh (mean 13.1 mm), hand (mean = 12.1 mm), and foot (mean 12.0 mm).
For SG density, rate/SG, and total sweat, indirectly measured sweating was significantly associated with the concordant direct measure for each body site (p < 0.001 for all associations). However, R2 was low for all associations and was > 0.3 for only rate/SG (all body sites) and SG density at the calf.
Neuropathy versus controls
Age, sex, height, weight, and BMI were not significantly different between neuropathy subjects and controls aged ≥ 45 years. All neuropathy subjects had sensory deficits (two-point discrimination, pinprick sensation, and/or vibratory sensation) on neurologic examination, which was limited to the toes in 16 subjects. Seventeen neuropathy subjects described painful paresthesias of the feet. Motor deficits were limited to mild weakness of toe extension in 17 neuropathy subjects, and an additional three reported mild ankle weakness. No neuropathy subject reported proximal muscle weakness or atrophy. Achilles tendon reflexes were reduced in ten neuropathy subjects and absent in ten subjects. Sural sensory nerve action potentials were absent in 15 neuropathy subjects and reduced in five others. Peroneal compound motor action potentials over the extensor digitorum brevis muscle were reduced in 13 neuropathy subjects and absent in three others.
As a group, neuropathy subjects showed a reduction of all sweat measurements at the calf and foot as compared to controls, adjusting for age (p < 0.05) and stimulated both indirectly and directly. Receiver operating characteristic analyses comparing neuropathy subjects and age-adjusted controls showed the best distinction between groups was at the calf and foot, with more overlap between groups at the thigh and hand. The area under the curve (AUC) was 0.9 for the comparison of neuropathy to control subjects for directly and indirectly stimulated rate/SG and total sweat at the calf (Fig. 3). At the foot, the directly stimulated rate/SG and total sweat showed an AUC of 0.80 and 0.84, respectively, while the indirectly stimulated rate/SG showed an AUC of 0.58 and the indirectly stimulated total sweat showed an AUC of 0.74.
Discussion
We previously demonstrated the capabilities of high-definition video sweat testing using direct stimulation of sweating by pilocarpine [21]. In the study reported here, we used ACh to induce indirect stimulation of SGs in neighboring skin via the axon reflex. We also outlined the normal ranges of SG density, rate/SG, and total sweat at four body sites, calculated their association with age and sex, and demonstrated alterations in the results in neuropathy subjects.
The described test using a customized high-resolution video camera with a rigid viewing screen and starch film enabled detailed in situ examination of the sudomotor axon reflex. The test provides multiple quantitative measures for identifying abnormalities of sudomotor function in peripheral neuropathy. The sidecar design was added to our previously reported pilocarpine-stimulated method [21] in order to add the ability to measure ACh-stimulated indirect sweating, while maintaining the ability to measure direct sweating.
A novel feature of our test is the ability to accurately quantify sweat rate from individual SGs in nanoliters per minute. In earlier studies this measurement has been made through the micro-cannulation of dissected sweat glands in vitro [27, 28]. Our results suggest that mean rate/SG is itself a valuable measurement for discerning patients with neuropathy from control subjects. One interesting finding was a relative increase in indirectly stimulated sweat rate/SG compared to directly stimulated sweat rate/SG in neuropathy patients at the foot. This finding might reflect compensatory hyperinnervation of the remaining SGs in skin affected by neuropathy, as we observed in previous histopathologic studies of SGs [29].
The test also approximates the functionality of existing tests of sudomotor function, including the measurement of total sweat, as in QSweat [16], and SG density and spatial extent, as in QDIRT [14, 15] and silastic sweat molds [1]. The measurements of SG density, and the difference between sexes and body sites obtained in our test are comparable to those of existing tests. Variations in total sweat between body sites and sexes in our test are concordant with those of existing tests, but the normal ranges are generally less because in our test total sweat values are measured at earlier time points, at frame 45, rather than frame 60. The reason for measuring the sweat values at frame 45 is due to the maximum measurable sweat volume being limited by the area of the viewing screen; thus, an earlier time point is used to avoid a ceiling effect. Earlier time points may also have unique value for discerning neuropathy patients; in our study, better separation between controls and neuropathy patients was obtained from total sweat measurements at frame 15 of the directly stimulated sweat videos. In contrast, indirect sweat values remain very low at frame 15 for indirectly stimulated sweat videos and thus have little value in separating groups; consequently, frame 45 is used for those comparisons. For consistency, frame 45 values are listed for both direct and indirect total sweat normal ranges in Table 1.
Limitations and future work
The test described herein offers the ability to observe axon reflex sweating develop in situ, so that patchy areas of reduced or delayed sweating can be identified. Multifocal nerve fiber loss is a cardinal feature of ischemic and immune nerve damage and has been described in unmyelinated cutaneous nerves [30,31,32]. An analysis of the degree of clustering of reduced, absent, or delayed sweating areas will be a part of future work.
Because the device can measure very small amounts of sweating from individual SGs, it may be possible to better detect minimal end organ neural activity in response to the stimulation of individual c-fibers using microneurography. Identifying the somatotopic extent of individual sudomotor nerves, the “sudomotor unit”, has been previously attempted using open-air starch iodine reaction [33, 34], laser Doppler [35], or closely spaced distal stimulation [36].
It is not known whether the sudomotor axon reflex occurs symmetrically, spreading in all directions equally across the skin. It is possible that reflex sweating spreads more randomly or eccentrically, so that sweat measured only to one side of an ACh-stimulated area might vary from test to test. Future work should attempt to study the full 360° extent of the axon reflex and whether there is variable spatial distribution and geometry with repeat testing.
The SST may be well suited for this, in that it is capable of recognizing very small sweat production from individual SGs over a large, intact area of skin. However, the expansiveness of the sudomotor axon reflex creates challenges in accurate measurement. Distal sites with bony protrusions, such as the hands and feet, do not provide a large, flat, depressible test site for our necessarily rigid, non-distensible viewing screen. This may, in part, explain our test’s superior performance at the calf compared to the foot. The complete, 360° axon reflex area, especially when including all of the slightly stimulated SGs at the axon reflexes margins, can be too large for a flat, rigid screen even at the fleshiest sites. A more compact test apparatus might aid testing on smaller, more distal body sites, such as the foot or toe dorsum. Because rate/SG appears to be at least as robust an indicator of neural function and dysfunction as total sweat and SG density, smaller areas of tested skin might suffice to enable a device with a smaller footprint.
While the test has high fidelity for discerning very small amounts of sweat, one relative weakness is the measurement of larger volumes. The maximum sweat volume recordable is limited by the area of the viewing screen. The volume of sweat produced by an individual SG can only be charted until its sweat spot merges with that of a neighboring SG. The results therefore may be limited by ceiling effects more so than evaporimeter-based tests, in particular the measurement of total sweat volume.
Finally, while we report the ability to measure the density of SGs in the test area, this should not be mistaken for SG nerve fiber density, a histopathologic measurement of sudomotor nerves commonly measured using microscopy of immunostained 3-mm punch biopsy sections. Previous studies have reported a correlation between SG nerve fiber density and physiologic quantitation of thermoregulatory sweating [29] and axon reflex-stimulated sweating [37]. Future work using the SST will aim to correlate its results with pathologic measures of sudomotor nerves in skin biopsies taken from within the test area. SG nerve fiber measurements could potentially be correlated to the sweat rate measured from each corresponding SG.
References
Kennedy WR, Navarro X (1989) Sympathetic sudomotor function in diabetic neuropathy. Arch Neurol 46(11):1182–1186
Hoeldtke RD, Bryner KD, Horvath GG, Phares RW, Broy LF, Hobbs GR (2001) Redistribution of sudomotor responses is an early sign of sympathetic dysfunction in type 1 diabetes. Diabetes 50(2):436–443
Mariani LL, Lozeron P, Theaudin M, Mincheva Z, Signate A, Ducot B, Algalarrondo V, Denier C, Adam C, Nicolas G, Samuel D, Slama MS, Lacroix C, Misrahi M, Adams D, French Familial Amyloid Polyneuropathies Network Study G (2015) Genotype-phenotype correlation and course of transthyretin familial amyloid polyneuropathies in France. Ann Neurol 78(6):901–916. https://doi.org/10.1002/ana.24519
Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, Gordon V, Low PA (2004) Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology 62(4):612–618
Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M (2003) The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 60(1):108–111
Abraham A, Alabdali M, Alsulaiman A, Breiner A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V (2016) Laser doppler flare imaging and quantitative thermal thresholds testing performance in small and mixed fiber neuropathies. PLoS One 11(11):e0165731. https://doi.org/10.1371/journal.pone.0165731
Siepmann T, Gibbons CH, Illigens BM, Lafo JA, Brown CM, Freeman R (2012) Quantitative pilomotor axon reflex test: a novel test of pilomotor function. Arch Neurol 69(11):1488–1492. https://doi.org/10.1001/archneurol.2012.1092
Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN (2013) Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve 48(6):883–888. https://doi.org/10.1002/mus.23891
Sharma S, Venkitaraman R, Vas PR, Rayman G (2015) Assessment of chemotherapy-induced peripheral neuropathy using the LDIFLARE technique: a novel technique to detect neural small fiber dysfunction. Brain Behav 5(7):e00354. https://doi.org/10.1002/brb3.354
Kubasch ML, Kubasch AS, Torres Pacheco J, Buchmann SJ, Illigens BM, Barlinn K, Siepmann T (2017) Laser Doppler assessment of vasomotor axon reflex responsiveness to evaluate neurovascular function. Front Neurol 8:370. https://doi.org/10.3389/fneur.2017.00370
Illigens BM, Siepmann T, Roofeh J, Gibbons CH (2013) Laser doppler imaging in the detection of peripheral neuropathy. Auton Neurosci Basic Clin 177(2):286–290. https://doi.org/10.1016/j.autneu.2013.06.006
Low VA, Sandroni P, Fealey RD, Low PA (2006) Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve 34(1):57–61. https://doi.org/10.1002/mus.20551
Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM (1997) Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 20(12):1561–1568
Gibbons CH, Illigens BM, Centi J, Freeman R (2008) QDIRT: quantitative direct and indirect test of sudomotor function. Neurology 70(24):2299–2304. https://doi.org/10.1212/01.wnl.0000314646.49565.c0
Gibbons CH, Illigens BM, Wang N, Freeman R (2010) Quantification of sudomotor innervation: a comparison of three methods. Muscle Nerve 42(1):112–119. https://doi.org/10.1002/mus.21626
Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ (1983) Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol 14(5):573–580. https://doi.org/10.1002/ana.410140513
Low PA, Vernino S, Suarez G (2003) Autonomic dysfunction in peripheral nerve disease. Muscle Nerve 27(6):646–661. https://doi.org/10.1002/mus.10333
Low PA (1993) Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 68(8):748–752
Kennedy WR, Selim MM, Wendelschaefer-Crabbe G, Blaes AH, Suter F, Nolano M, Provitera V (2013) A device to quantify sweat in single sweat glands to diagnose neuropathy. J Med Devices 7(3):2
Provitera V, Nolano M, Caporaso G, Stancanelli A, Santoro L, Kennedy WR (2010) Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology 74(1):50–56. https://doi.org/10.1212/WNL.0b013e3181c7da4b
Loavenbruck AJ, Hodges JS, Provitera V, Nolano M, Wendelshafer-Crabb G, Kennedy WR (2017) A device to measure secretion of individual sweat glands for diagnosis of peripheral neuropathy. J Peripher Nerv Syst 22(2):139–148. https://doi.org/10.1111/jns.12212
Lindsay SL, Holmes S, Corbett AD, Harker M, Bovell DL (2008) Innervation and receptor profiles of the human apocrine (epitrichial) sweat gland: routes for intervention in bromhidrosis. Br J Dermatol 159(3):653–660. https://doi.org/10.1111/j.1365-2133.2008.08740.x
Low PA, Opfer-Gehrking TL, Kihara M (1992) In vivo studies on receptor pharmacology of the human eccrine sweat gland. Clin Auton Res 2(1):29–34
Schulz IJ (1969) Micropuncture studies of the sweat formation in cystic fibrosis patients. J Clin Invest 48(8):1470–1477. https://doi.org/10.1172/JCI106113
Sato K, Nishiyama A, Kobayashi M (1979) Mechanical properties and functions of the myoepithelium in the eccrine sweat gland. Am J Physiol 237(3):C177–C184. https://doi.org/10.1152/ajpcell.1979.237.3.C177
Randall WC, Calman C (1954) The surface tension of human sweat; its determination and its significance. J Invest Dermatol 23(2):113–118
Sato K (1980) Pharmacological responsiveness of the myoepithelium of the isolated human axillary apocrine sweat gland. Br J Dermatol 103(3):235–243
Sato K (1973) Sweat induction from an isolated eccrine sweat gland. Am J Physiol 225(5):1147–1152. https://doi.org/10.1152/ajplegacy.1973.225.5.1147
Loavenbruck A, Wendelschaefer-Crabbe G, Sandroni P, Kennedy WR (2014) Quantification of sweat gland volume and innervation in neuropathy: correlation with thermoregulatory sweat testing. Muscle Nerve 50(4):528–534. https://doi.org/10.1002/mus.24185
Waller LA, Sarkka A, Olsbo V, Myllymaki M, Panoutsopoulou IG, Kennedy WR, Wendelschafer-Crabb G (2011) Second-order spatial analysis of epidermal nerve fibers. Stat Med 30(23):2827–2841. https://doi.org/10.1002/sim.4315
Myllymaki M, Panoutsopoulou IG, Sarkka A (2012) Analysis of spatial structure of epidermal nerve entry point patterns based on replicated data. J Microsc 247(3):228–239. https://doi.org/10.1111/j.1365-2818.2012.03636.x
Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E (1999) A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve 22(3):360–371
Schlereth T, Brosda N, Birklein F (2005) Somatotopic arrangement of sudomotor axon reflex sweating in humans. Auton Neurosci Basic Clin 123(1–2):76–81. https://doi.org/10.1016/j.autneu.2005.08.004
Schlereth T, Brosda N, Birklein F (2005) Spreading of sudomotor axon reflexes in human skin. Neurology 64(8):1417–1421. https://doi.org/10.1212/01.WNL.0000158473.60148.FE
Schmidt R, Weidner C, Schmelz M (2011) Time course of acetylcholine-induced activation of sympathetic efferents matches axon reflex sweating in humans. J Peripher Nerv Syst 16(1):30–36. https://doi.org/10.1111/j.1529-8027.2011.00320.x
Schmelz M, Schmidt R, Bickel A, Torebjork HE, Handwerker HO (1998) Innervation territories of single sympathetic C fibers in human skin. J Neurophysiol 79(4):1653–1660
Gibbons CH, Illigens BM, Wang N, Freeman R (2009) Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology 72(17):1479–1486. https://doi.org/10.1212/WNL.0b013e3181a2e8b8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Loavenbruck, A., Sit, N., Provitera, V. et al. High-resolution axon reflex sweat testing for diagnosis of neuropathy. Clin Auton Res 29, 55–62 (2019). https://doi.org/10.1007/s10286-018-0546-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-018-0546-7