Abstract
Background
Power spectral analysis of heart rate variability is used to assess cardiac autonomic function. The relationship of low frequency (LF) power to cardiac sympathetic tone has been unclear. We reported previously that LF power may reflect baroreflex modulation. In this study we attempted to replicate our findings in additional subject cohorts, taking into account possible influences of respiration and using different methods to measure baroreflex-cardiovagal gain (BCG).
Objective
We assessed relationships of LF power, including respiration-adjusted LF power (LFa), with cardiac sympathetic innervation and baroreflex function in subjects with or without neuroimaging evidence of cardiac sympathetic denervation.
Methods
Values for LF power at baseline supine, seated, and during the Valsalva maneuver were compared between subject groups with low or normal myocardial concentrations of 6-[18F]fluorodopamine-derived radioactivity. BCG was calculated from the slope of cardiac interbeat interval vs. systolic pressure during Phase II of the Valsalva maneuver or after i.v. nitroglycerine injection (the Oxford technique).
Results
LF and LFa were unrelated to myocardial 6-[18F]fluorodopamine-derived radioactivity. During sitting rest and the Valsalva maneuver logs of LF and LFa correlated positively with the log of Phase II BCG (r = 0.61, p = 0.0005; r = 0.47, p = 0.009; r = 0.69, p < 0.0001; r = 0.60, p = 0.0006). Groups with Low BCG (≤3 ms/mmHg) had low LF and LFa regardless of cardiac innervation. The log of LF power during supine rest correlated with the log of Oxford BCG (r = 0.74, p < 0.0001).
Conclusion
LF power, with or without respiratory adjustment, reflects baroreflex modulation and not cardiac sympathetic tone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The autonomic nervous system plays major roles in maintaining cardiovascular homeostasis and in the pathophysiology of a variety of disease states [7]. The system contains parasympathetic cholinergic and sympathetic noradrenergic components. Clinicians and researchers have long sought valid, non-invasive, quantitative means to assess cardiovascular autonomic functions.
One non-invasive mode of autonomic testing is based on power spectral analysis of heart rate variability and quantification of low frequency (LF) and high frequency (HF) power. It is generally accepted that HF power reflects respiratory sinus arrhythmia, which is mediated by the parasympathetic cholinergic system; however, the origin and clinical significance of LF power have aroused persistent controversy.
Both sympathetic and parasympathetic outflows seem to affect LF power [1, 3, 19]. It has been suggested that adjustment for respiratory influences on LF power improves the accuracy of measurement of cardiac sympathetic outflow by power spectral analysis of heart rate variability (2–5). The ANSAR ANX 3.0 system (ANSAR Medical Technologies Inc., Philadelphia, PA, USA) is the only commercially available device that makes this adjustment. The ANX 3.0 uses an algorithm that yields a variable termed LFa. In conjunction with a testing protocol for beat-to-beat heart rate, respiration, and blood pressure during baseline sitting, the Valsalva maneuver, and standing, the ANX 3.0 calculates value for LFa and interprets those values in terms of sympathetic modulation. The ANX 3.0 also generates reports that include diagnostic implications and recommendations about possible therapy associated with autonomic dysfunction.
We and others have reported that LF power of heart rate variability may not provide a measure of cardiac sympathetic tone, because individual values for LF power, with or without adjustment for HF power or total power, are not correlated with the rate of appearance of the sympathetic neurotransmitter, norepinephrine, in coronary sinus plasma [2, 4, 17, 18] and left ventricular myocardial concentrations of the sympathetic imaging agent 6-[18F]fluorodopamine [18]. Moreover, drugs that increase release of norepinephrine from cardiac sympathetic nerves can increase LF power even in patients with neuroimaging evidence of cardiac sympathetic denervation [18]; and patients with heart failure, which is known to be associated with markedly increased cardiac sympathetic outflow [6], have low LF power [5].
Sleight et al. [22] reported evidence that LF power may instead be related to the ability to modulate cardiac autonomic outflows by baroreflexes. They demonstrated that carotid sinus stimulation increases LF power in individuals with normal baroreflex function but not in those with impaired baroreflex sensitivity. In support of their concept, we reported previously that whereas individual values for the log of LF power do not correlate with cardiac sympathetic outflow, they do correlate positively with the log of baroreflex-cardiovagal gain (BCG) [18]. Moreover, patients with baroreflex failure have attenuated responses of LF power to drugs that increase norepinephrine release from sympathetic nerves, independently of cardiac sympathetic innervation.
The previous study by our group did not include LF power adjusted for respiration. Calculations of BCG were based only on the slope of the relationship between cardiac interbeat interval and systolic pressure during the descent of pressure in Phase II of the Valsalva maneuver and not on what has come to be called the “Oxford technique.” In the Oxford technique, BCG is calculated from hemodynamic data after bolus i.v. injection of a vasoconstrictor (e.g., angiotensin or phenylephrine) or a vasodilator (e.g., nitroglycerine or nitroprusside).
Use of the slope of the relationship between cardiac interbeat interval and systolic pressure during Phase II of the Valsalva maneuver to assess baroreflex function was reported by our group more than a quarter century ago [11]. It should be noted that even before then the Oxford group introduced a method for calculating BCG based on the data from the Valsalva maneuver [21].
Baroreceptors are not pressure receptors per se but distortion receptors, and baroreceptor afferent traffic is related not only to mean blood pressure but also to pulse pressure and heart rate. The several clinical approaches to assess baroreflex-cardiovagal function have advantages and disadvantages, and individual values generally agree between techniques but with substantial variability [11]. In the present study, we included data mainly by the Phase II technique but also by the Oxford (nitroglycerine) technique.
One can also quantify BCG from the relationship between interbeat interval and systolic pressure in Phase IV of the Valsalva maneuver [11], when the pressure “overshoots” the baseline value and heart rate falls reflexively. This method is probably invalid in chronic autonomic failure, however, because in this setting Phase IV pressure typically does not exceed the baseline value.
Chronic autonomic failure syndromes differ substantially in the status of cardiac sympathetic innervation. Three well-studied forms are pure autonomic failure, multiple system atrophy, and Parkinson disease with orthostatic hypotension. Pure autonomic failure and Parkinson disease with orthostatic hypotension consistently entail neuroimaging, neurochemical, and postmortem neuropathologic evidence of cardiac sympathetic denervation [10, 12], whereas most patients with multiple system atrophy have intact cardiac sympathetic innervation [20]. All three diseases are associated with baroreflex-cardiovagal failure [13].
In this study we attempted to replicate and extend our findings in additional subject cohorts. Studying groups of chronic autonomic failure patients provided a powerful means to determine if LFa (the respiratory adjusted LF power) is related to cardiac sympathetic innervation, to BCG, or both. We measured LF power at rest and during the Valsalva maneuver with subjects in the seated position to compare with our previous findings in subjects studied during supine rest [18]. We also tested a cohort of subjects who had BCG quantified by both the Phase II and Oxford (nitroglycerine) techniques.
Methods
Subjects
Thirty-three subjects had measurements of power spectral analysis of heart rate variability with and without respiratory adjustment of LF power and also had neuroimaging assessments of cardiac sympathetic innervation. All were evaluated at the NIH Clinical Center after giving informed written consent to participate in clinical research protocols approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke. The population was divided into four groups based on normal or decreased cardiac sympathetic innervation assessed by interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity and normal or decreased baroreflex-cardiovagal gain assessed by the relationship between cardiac interbeat interval and systolic blood pressure during the Valsalva maneuver.
Patient groups included pure autonomic failure (N = 7), multiple system atrophy (N = 10), and Parkinson disease with (N = 3) or without (N = 5) orthostatic hypotension. Controls included referred patients without autonomic failure or evidence of central neurodegeneration (N = 4) and normal volunteers (N = 4).
In addition, 30 other subjects had measurements of power spectral analysis of heart rate variability without respiratory adjustment and measurements of BCG by the Phase II Valsalva and Oxford (nitroglycerine, phenylephrine) techniques. Of the 30 subjects, 14 had head and neck cancer treated with neck irradiation (1 tested both before and after neck irradiation). The remaining subjects had MSA (N = 5), PD + OH (N = 3), or PAF (N = 2) or were control subjects (N = 6).
ANSAR device and testing protocol
Under a clinical trial agreement ANSAR supplied an ANX 3.0 device for clinical evaluation. ANSAR did not participate in any way in the design or conduct of the study or in the interpretation of the results. The testing procedure was conducted according to the company’s instructions and done by or under the supervision of personnel certified by ANSAR to carry out the testing protocol.
Electrocardiographic leads were attached and an automated arm blood pressure cuff applied. The electrocardiogram was used for power spectral analysis of heart rate variability and trans-thoracic electrical impedance for analysis of respiratory rate variability. The ANX 3.0 gives instructions to subjects for the stages of the testing—baseline relaxed breathing for 5 min while sitting, deep breathing for 1 min, return to baseline breathing for 1 min, 5 Valsalva maneuvers, return to baseline for 2 min, and standing for 5 min.
A parameter termed RFa was calculated as the area within a 0.12 Hz bin of the spectrum centered at the fundamental respiratory frequency. The fundamental respiratory frequency, in breaths s−1, is the frequency of the highest peak of the respiratory rate variability spectrum. In a healthy individual this frequency corresponds to the inverse of the respiratory rate during resting breathing. LFa was calculated from the LF component of the heart rate variability after taking into account RFa. This method is described at the web address http://www.ans-hrv.com/ANSparamsDefsWavelet.doc.
Based on the obtained LFa and RFa values, the ANSAR device generates reports that include diagnostic impressions and recommendations about possible treatments. “Sympathetic withdrawal” is reported if the standing:baseline ratio of LFa is less than 0.9. OH is diagnosed if a decrease of more than 20 mmHg in systolic blood pressure or more than 10 mmHg in diastolic pressure is found after 2 min of standing. If there is OH and the heart rate increases during standing, then the OH is reported as non-neurogenic; otherwise the OH is reported as neurogenic. A drop in blood pressure that is insufficient to satisfy criteria for OH is reported as “orthostatic intolerance.” If the blood pressure decreases by less than 5 mmHg or the heart rate increases by less than 30 bpm, the diagnosis is possible “pre-clinical orthostatic intolerance.”
Cardiac sympathetic neuroimaging
Cardiac sympathetic innervation was assessed by positron emission tomographic scanning after i.v. injection of the sympathetic neuroimaging agent 6-[18F]fluorodopamine [9]. The subject was positioned supine feet-first in a General Electric Advance scanner (General Electric, Milwaukee, WI, USA), and approximately 1 mCi of 6-[18F]fluorodopamine was injected at a constant rate over 3 min. Thoracic dynamic scanning data were obtained for 30 min. The image corresponding to the midpoint of the scanning interval at 7.5 min after initiation of the injection was used as described previously [14]. Radioactivity in the interventricular septum that was less than 5,000 nCi-kg/cc-mCi defined cardiac sympathetic denervation.
Baroreflex-cardiovagal gain
For assessment of baroreflex-cardiovagal function each subject was studied while supine with head on pillow. Beat-to-beat blood pressure was measured non-invasively using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) or Nexfin (bmeye, Amsterdam, The Netherlands) device. Hemodynamic data were digitized continuously and recorded using a PowerLab data acquisition system (AD Instruments, Castle Hill, Australia) and stored for later analysis on an Apple PowerBook G4 computer (Apple, Cupertino, CA, USA).
For calculation of BCG by the Phase II technique, after about a 15 min baseline period each subject performed at least three Valsalva maneuvers. The patient blew into a plastic tube connected to a sphygmomanometer, keeping a pressure of 30 mmHg for 12 S as described previously [14]. The maneuver with the best performance as judged by the delivered pressure was used for analysis. BCG was calculated as the slope of the relationship between cardiac interbeat interval and the beat-to-beat systolic blood pressure (with 1 beat delay) during the descent of pressure in Phase II of the maneuver [11]. A slope of 3 ms/mmHg or less, about one-half normal [8, 18], defined baroreflex-cardiovagal failure.
For calculation of BCG by the Oxford technique, nitroglycerine (2.5 mg dissolved in 50 mL normal saline) was injected as a bolus i.v. (starting dose 50 μg, 1 mL) flushed in with 5 mL normal saline, as published previously [11]. Blood pressure and heart rate were allowed to return to baseline (at least 2 min) between injections. The criterion decrease in systolic pressure after nitroglycerine was 20 mmHg. If the criterion change was not obtained, the dose was increased to 100, 150, and finally 200 μg. BCG was calculated as the slope of the relationship between cardiac interbeat interval and the beat-to-beat systolic blood pressure (with 1 beat delay) during the descent of pressure after nitroglycerine.
Supine LF and HF powers
LF and HF powers (in ms2) during supine testing were calculated using Chart 5.4.2 and the HRV module version 1.03 (PowerLab, AD Instruments, Castle Hill, Australia), as described previously [18]. The LF band was from 0.04 to 0.15 Hz (2.4–9 min−1) and HF band from 0.15 to 0.4 Hz (9–24 min−1).
Data analysis and statistics
Relationships of individual values for the log of LF or log of LFa as the dependent variable and the log of BCG and the septal concentration of 6-[18F]fluorodopamine-derived radioactivity as independent variables were evaluated by linear regression analysis and calculation of Pearson correlation coefficients (Kaleidagraph 4.0.1, Synergy Software, Reading, PA, USA).
In patients with chronic autonomic failure, slopes for the relationship between cardiac interbeat interval and systolic pressure can be near zero, resulting in low correlation coefficients that are not statistically significant. To determine if this phenomenon influenced the obtained results, we analyzed separately data from subjects in whom the correlation coefficient was at least 0.9.
Mean values for HRV parameters across groups with or without cardiac sympathetic denervation (6-[18F]fluorodopamine-derived radioactivity less vs. not less than 5,000 nCi kg/cc-mCi) and with or without baroreflex-cardiovagal failure (BRG less vs. not less than 3.0 ms/mmHg in Phase II of the Valsalva maneuver) were compared using one-way analyses of variance. We used Fisher’s least significant difference method for posthoc comparisons between groups.
Because of skewed distributions, individual data for HRV parameters and BRG were log-transformed for statistical testing. Mean values were expressed ±1 standard error of the mean. A p value of less than 0.05 defined statistical significance.
Results
Individual values for the log of LFa and log of LF power were unrelated to cardiac 6-[18F]-fluorodopamine-derived radioactivity (Fig. 1).
Individual values for a the log of low frequency power without respiration adjustment (log LF) and b the log of respiration-adjusted low frequency power (log LFa) as a function of septal myocardial 6-[18F]fluorodopamine-derived radioactivity. There is no relationship between LF power and cardiac sympathetic innervation, regardless of respiratory adjustment
With subjects seated, the log of LF power unadjusted for respiratory influences was correlated with the log of BCG with subjects supine by the Phase II Valsalva technique (r = 0.61, p = 0.0005; Fig. 2a). Values for log LFa were also positively correlated with the log of BCG (r = 0.47, p = 0.009). The log of LF power at sitting and supine, calculated by ANX 3.0 and Chart respectively, were strongly positively correlated (r = 0.77, p < 0.0001).
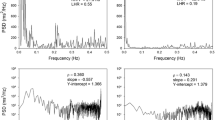
Individual values for the log of low frequency power without respiration adjustment (log LF) as a function of the log of baroreflex-cardiovagal gain (BRG) based on hemodynamic data a in Phase II of the Valsalva maneuver (log BCG Phase II) and b using the Oxford (nitroglycerine) technique (BCG TNG). Dashed lines are the lines of best fit for the linear regression equations. By both the Phase II and Oxford (nitroglycerine) techniques, LF power is related to baroreflex-cardiovagal gain
Individual values for the log of LF power with subjects supine were positively correlated with the log of BCG by the Oxford (nitroglycerine) technique (r = 0.74, p < 0.0001; Fig. 2b). Values for log BCG by the Phase II technique were positively correlated with those for the log of BCG by the Oxford (nitroglycerine) technique (r = 0.85, p < 0.0001).
Figure 3 shows data from a patient with neurogenic orthostatic hypotension who underwent assessment of baroreflex-cardiovagal function by both the Phase II and Oxford (nitroglycerine) techniques. The patient had markedly decreased BCG by both techniques and had extremely low LF power.
Original recordings of heart rate (HR, bpm), blood pressure (BP, mmHg), delivered airway pressure (P, mmHg), and electrocardiogram (ECG) in a patient with neurogenic orthostatic hypotension and very low LF power. a Valsalva maneuver; b nitroglycerine 50 μg injected as a bolus (arrow). By both the Phase II Valsalva and Oxford (nitroglycerine) techniques, the patient has baroreflex-cardiovagal failure, as evidenced by a very low slope for the relationship between cardiac interbeat interval (with one beat delay) and systolic pressure
Across the groups with normal innervation and normal Phase II BCG (Innerv Nl BCG group), normal innervation and Low BCG (Innerv Low BCG group), and cardiac denervation and Low BCG (Denerv Low BCG group), both log LFa and log LF with subjects seated varied highly significantly as a function of patient group (F = 9.6, p = 0.0007; F = 10.9, p = 0.0003). The Denerv Low BCG group had lower mean log LFa than did the Innerv Nl BCG group (p = 0.0002; Fig. 4). Mean LF power was lower in the Denerv Low BCG and Innerv Low BCG groups than in the Innerv Nl BCG group (p = 0.0001, p = 0.005). There were too few patients with cardiac denervation and normal BCG to include in the statistical analyses.
Mean (±SEM) values for a the log of respiration-adjusted low frequency power (log LFa) and b the log of low frequency power without respiration adjustment (log LF) in subject groups with neuroimaging evidence of intact cardiac sympathetic innervation and normal baroreflex slope (Innerv Nl BCG), intact innervation and low baroreflex slope (Innerv low BCG), and cardiac sympathetic denervation and low baroreflex slope (Denerv Low BCG). (*) significant group difference, p < 0.05; (**) p < 0.001 Both LFa and LF are low in groups with baroreflex-cardiovagal failure, regardless of the status of cardiac sympathetic innervation
Values for log LFa during the Valsalva maneuver with subjects seated also varied with subject group (F = 19, p < 0.0001), with mean log LFa in the Innervated-Normal BCG group higher than in the Innervated-Low BCG group (p = 0.0004) and Denervated-Low BCG group (p < 0.0001).
Most subjects had a correlation coefficient of at least 0.9 for the relationship between interbeat interval and systolic pressure in Phase II of the Valsalva maneuver. Among subjects with a correlation coefficient of at least 0.9, the log of LF power supine rest was unrelated to cardiac 6-[18F]fluorodopamine-derived radioactivity (r = −0.08) but was correlated positively with the log of Phase II BCG (r = 0.68, p = 0.007). The log of HF power also correlated positively with the log of BCG (r = 0.64, p = 0.01).
In a minority of patients with chronic autonomic failure, values for the correlation coefficient were less than 0.9 and occasionally near zero. Figure 5 shows data from one such patient. It is evident that in Phase II heart rate failed to change, despite a progressive, large magnitude decline in systolic pressure; that is, the patient had baroreflex-cardiovagal failure. The patient also had extremely low LF power.
Original recording of heart rate (bpm), blood pressure (mmHg), stroke volume (Stroke Vol., cc), forearm circumference (Forearm Circumf., from impedance plethysmography), delivered airway pressure (Val. Pressure, mmHg), laser-Doppler flow in the thumb, and electrocardiogram (ECG) in a patient with neurogenic orthostatic hypotension and very low LF power. The patient has baroreflex-cardiovagal failure, as evidenced by stable heart rate despite a large fall in systolic pressure, and has very low LF power. The correlation coefficient for the slope of the relationship between interbeat interval and systolic pressure is very low
Among 19 patients with neurogenic OH studied using the ANSAR device, all of 13 with neurogenic OH in the setting of Parkinson disease or multiple system atrophy had an increase in heart rate during orthostasis, as did 3 of 6 patients with pure autonomic failure. None of the patients with neurogenic OH was diagnosed correctly in the ANSAR reports. Six of the 19 were reported to have possible non-neurogenic OH, 2 were reported to have orthostatic intolerance, 3 could not tolerate the standing stage of the ANSAR protocol, and 8 were not diagnosed with OH. The ANSAR device also reported that three normal volunteers, one patient with pure autonomic failure, and one patient with Parkinson disease and no OH had possible mild or moderate “autonomic nervous system dysfunction” (e.g., due to low RFa or LFa values at baseline). A possible diagnosis of “chronic autonomic nervous system dysfunction” was given to all ten patients with multiple system atrophy, all three patients with Parkinson disease and OH, one of five patients with Parkinson disease and no OH, five of seven patients with pure autonomic failure, and one control patient. For 29 of the 33 subjects, including 3 of the 4 normal volunteers, alpha lipoic acid was recommended as treatment.
Discussion
The present results relate to the ongoing controversy about the sources and clinical diagnostic meaning of LF power of heart rate variability. In this study LF power, with or without adjustment for influences of parasympathetically-mediated respiratory sinus arrhythmia, was unrelated to cardiac sympathetic innervation as indicated by interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity. These findings replicate and extend on our previously published findings [18] in a different cohort of subjects. Analogously, a recent study found no relationship between heart rate variability assessed in the frequency domain and myocardial uptake of 123I-metaiodobenzylguanidine, another neuroimaging measure of cardiac sympathetic innervation, in patients with Parkinson disease [15]. Other studies have noted no correlation between LF power and the rate of entry of norepinephrine into cardiac venous plasma, a neurochemical index of cardiac sympathetic tone [2, 4, 17, 18].
We tested for the first time whether LF power adjusted for respiratory influences is related to cardiac sympathetic innervation. The ANSAR device applies the concept that LF power is composed of cardiac sympathetic and parasympathetic contributions and that the latter is determined importantly by respiratory sinus arrhythmia. ANSAR reports include LFa as a parameter for respiration-adjusted LF power. Our finding that even LFa was independent of cardiac sympathetic innervation as indicated by myocardial 6-[18F]fluorodopamine-derived radioactivity casts further doubt on the notion that power spectral analysis of heart rate variability provides a measure of cardiac sympathetic tone.
On the other hand, LF power, both during rest sitting and during the Valsalva maneuver while sitting, was related to baroreflex-cardiovagal gain (BCG) calculated from the slope of the linear relationship of cardiac interbeat interval with systolic pressure during Phase II of the Valsalva maneuver during supine rest. The results in subjects tested while sitting replicate and extend on our previous findings from a different cohort of subjects studied during supine rest [18].
In another subject cohort we used the “gold standard” Oxford technique to assess baroreflex-cardiovagal function. LF power was again positively correlated with BCG. Individual values for BCG by the Phase II Valsalva approach were positively correlated with those by the Oxford (nitroglycerine) approach, consistent with both measures estimating cardiovagal responses to baroreceptor unloading.
In patients with congestive heart failure, cardiac sympathetic outflow is known to be markedly increased [6], while BCG is reduced [5]. Such patients have low LF power [5], in agreement with the concept that LF power tracks baroreflex function rather than cardiac sympathetic outflow.
The ANSAR ANX 3.0 generates reports that include suggested diagnoses and treatment options. Although the device correctly identified chronic autonomic nervous system dysfunction in most (but not all) patients with neurogenic OH, it is clear from our results that ANSAR reports often include erroneous diagnoses and consequently inappropriate treatment recommendations. By defining OH in terms of blood pressure changes between the seated position and 2 min upright, the ANSAR device failed to detect OH in patients who had unequivocal OH by generally accepted criteria [16]. The ANSAR device also failed to identify OH as neurogenic in all patients who actually had neurogenic OH, due to the assumption that any increase in heart rate during orthostasis excludes neurogenic OH, whereas virtually all patients with neurogenic OH had at least some orthostatic increase in heart rate. Because of these errors, the ANSAR device inappropriately recommended treatment with alpha lipoic acid for most of the patients in this study who had neurogenic OH.
In conclusion, LF power of heart rate variability does not reflect cardiac sympathetic tone but may reflect the ability to modulate cardiac autonomic outflows via baroreflexes. The relationship between LF power and the baroreflex-cardiovagal gain obtains with or without adjustment of LF power for respiratory influences, whether subjects are evaluated in the supine or sitting position, whether they are evaluated at rest or during the Valsalva maneuver, and whether baroreflex-cardiovagal gain is quantified by the Phase II technique or the Oxford technique.
Abbreviations
- BCG:
-
Baroreflex-cardiovagal gain
- HF:
-
High frequency
- HFa:
-
Adjusted high frequency power
- LF:
-
Low frequency
- LFa:
-
Adjusted low frequency power
References
Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ (1981) Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213:220–222
Alvarenga ME, Richards JC, Lambert G, Esler MD (2006) Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med 68:8–16
Aysin B, Aysin E (2006) Effect of respiration in heart rate variability (HRV) analysis. Conf Proc IEEE Eng Med Biol Soc 1:1776–1779
Baumert M, Lambert GW, Dawood T, Lambert EA, Esler MD, McGrane M, Barton D, Sanders P, Nalivaiko E (2009) Short-term heart rate variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol 297:H674–H679
Creager MA (1992) Baroreceptor reflex function in congestive heart failure. Am J Cardiol 69:10G–15G discussion 15G-16G
Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD (1996) Cardiac sympathetic nerve function in congestive heart failure. Circulation 93:1667–1676
Goldstein DS (2001) The autonomic nervous system in health and disease. Marcel Dekker, New York
Goldstein DS (2003) Dysautonomia in Parkinson’s disease: neurocardiological abnormalities. Lancet Neurol 2:669–676
Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, Holmes C, Kirk KL, Bacharach S, Adams R et al (1993) Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol 22:1961–1971
Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO 3rd (2000) Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med 133:338–347
Goldstein DS, Horwitz D, Keiser HR (1982) Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 66:432–439
Goldstein DS, Orimo S (2009) Cardiac sympathetic neuroimaging: summary of the First International Symposium. Clin Auton Res 19:133–136
Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y (2003) Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42:136–142
Goldstein DS, Tack C (2000) Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res 10:285–291
Haensch CA, Lerch H, Jorg J, Isenmann S (2009) Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson’s disease. Parkinsonism Relat Disord 15:134–137
Kaufmann H (1996) Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 6:125–126
Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD (1994) Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation 90:234–240
Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y (2007) Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 4:1523–1529
Ng J, Sundaram S, Kadish AH, Goldberger JJ (2009) Autonomic effects on the spectral analysis of heart rate variability after exercise. Am J Physiol Heart Circ Physiol 297:H1421–H1428
Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, Nagao T, Yokochi M (2002) Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry 73:776–777
Pickering TG, Sleight P (1969) Quantitative index of baroreflex activity in normal and hypertensive subjects using Valsalva’s manoeuvre. Br Heart J 31:392
Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L (1995) Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 88:103–109
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke. Ms. Tereza Jenkins coordinated patient travel. Division of Intramural Research, NINDS, NIH. The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, F., Pechnik, S., Gross, D. et al. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res 21, 133–141 (2011). https://doi.org/10.1007/s10286-010-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-010-0098-y