Abstract
The aim of this study was to evaluate the biocompatibility of two comparatively new calcium silicate containing sealers (MTA-Fillapex and BioRoot-RCS) with that of two established sealers (AH-Plus, epoxy resin-based; Pulp-Canal-Sealer, zinc oxide eugenol containing). Human periodontal ligament cells (PDL-cells) were brought in contact with eluates from freshly mixed and set sealer. The sealers were mixed strictly according to the manufacturers’ instructions and identically samples were produced. 1:1, 1:2, and 1:10 dilutions of sealers extract were used. Extracts from freshly mixed sealer were added to the PDL-cells on day one to simulate a clinical scenario. Subsequently, at 24 h, 7, 14, and 21 days extracts form set sealers were used for PDL-cell culturing. PDL-cell viability was analyzed by living-cell-count, MTT-assay, and living/dead-staining, cytotoxicity by LDH-assay, and changes by Richardson-staining. All data were statistically evaluated by one way ANOVA and a posthoc analysis with Bonferroni-Holm testing (p < 0.05). In contact with BioRoot-RCS a regeneration of the PDL-cells were observed over time. This sealer showed the lowest toxicity in a freshly mixed and set state (p < 0.05). MTA-Fillapex and Pulp-Canal-Sealer were cytotoxic in a fresh as well as in a set state, whereas AH-Plus was cytotoxic in a freshly mixed state, but not when the sealer was set. BioRoot-RCS is biocompatible and bioactive because it seems to have a positive influence on the PDL-cell metabolism. Pulp Canal Sealer and MTA-Fillapex showed no biocompatibility in contact with PDL-cells at all. Freshly mixed AH Plus is less biocompatible on PDL than in a set state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of a root canal filling is the three dimensional bacteria and fluid-tight seal of the entire root canal system to prevent passage of microorganisms from coronal to apical or vice versa [1, 2]. Hence, root canal filling materials should be more or less insoluble to prevent dissolving by body fluids in the root canal [3]. It is well known, that besides the apical foramen, numerous microscopic and macroscopic communications exist between the root canal system and the periodontal ligament and the surrounding bone, namely dentinal tubules, accessory foramina and lateral canals [4]. Thus, tissue fluid can easily penetrate the root canal system, resulting in degradation of the sealer material and subsequent leaching out various components. Leached substances may then migrate to the periodontal tissues and alveolar bone, generate local periapical inflammatory reactions and adverse effects [5, 6]. If sealer and sealer components come into direct contact with periradicular tissues over extended periods of times, they may cause irritation and may result in delayed wound healing [7]. In addition, overfilled sealer can directly interact with adjacent tissues [5] and extruded into the periradicular tissue sealers can be highly irritating [1].
The contact of the eluents with the periradicular tissue has effects on cell metabolisms and regeneration which are concentration- as well as time-dependent [8]. Hence, since many decades it has been claimed that sealer should be biocompatible and well tolerated by the periradicular tissue [9]. Nevertheless, until today, it is stated in literature that all root canal sealers (regardless of the type) exhibit toxicity in their freshly mixed state, but on setting, their toxicity is greatly reduced and most sealers become relatively inert [1, 2].
To overcome the problem of cytotoxicity, recently tri- or respectively di- and tricalcium silicate based root canal sealers were developed as an offspring of di- and tricalcium silicate cements (e.g., mineral trioxide aggregate; MTA). It is well known, that di- and tricalcium silicate cements are biocompatible and bioactive [10]. However, there are only few data about cytotoxicity or biocompatibility of the new calcium silicate-based sealers. It remains unclear whether these new sealers are really an improvement in terms of biocompatibility compared to conventional sealers. Thus, the aim of the present study was to evaluate the cytotoxicity of four different sealers (two new calcium silicate containing and two conventional) to human periodontal ligament cells (PDL cells) in an unset and set condition. The hypothesis tested was that all sealers perform equally with regard to the effect on human PDL cells.
Materials and methods
Primary human periodontal ligament cells
Human periodontal ligament cells (PDL cells) were harvested from the intact periodontal membrane of two impacted, surgically removed wisdom teeth of the lower jaw. The teeth were removed unseparated and showed no signs of infection or cyst formation. The wisdom teeth were collected anonymously from two patients. Both full-aged participants provided their written informed consent to participate in this study. The Ethical Committee of the Westphalian Wilhelms-University (Münster, Germany) approved the use of human cells (Reg. No. 2010-462-t). The handling of all human samples followed strictly the “Declaration of Helsinki”. The human cells were harvested and cultured according to a standardized protocol. Wisdom teeth were washed three times with Dulbecco’s Phosphate Buffered Saline (DPBS; D8537, Sigma-Aldrich, Munich, Germany) to remove blood and debris. Teeth were placed in a cell culture dish with culturing medium. Dulbelco’s Modified Eagle Medium with high glucose (4.5 g/l) and 4 mM l-glutamine and 1 mM sodium pyruvate (DMEM, 41966-029, gibco by Thermo Fisher Scientific, Darmstadt, Germany), containing 10% fetal bovine serum (FBS) and 1% of penicillin [10.000 U/ml]/streptomycin [10.000 µg/m], and 1% amphotericin b [250 µg/ml] (S0615, A2212, A2612, Biochrom, Berlin, Germany) was used as a culturing medium. Outgrowth of cells was checked for the first time after 7 days to minimize particle floating. After that, outgrowth was controlled daily and teeth were removed after 14 days. Cells were cultivated at 37 °C with 5% CO2, while being fed three times a week and passaged after reaching nearly total confluence. The outgrowing human PDL cells were characterized immunohistochemically by positive expression of collagen I, vimentin, and fibronectin.
The second passage was used for the experiments. PDL cells were seeded with a concentration of 5.300 cells/cm2 in 24-well culturing plates (TPP, Trasadingen, Switzerland) and were allowed to adhere for 24 h. The cells were cultured in their respective cell culture medium DMEM.
Sealers
Following sealers were used in the present study: a zinc oxide eugenol-based (Pulp Canal Sealer; Kerr, Scafati, Italy), an epoxy resin-based (AH Plus; Dentsply DeTrey, Konstanz, Germany), a salicylate-based and MTA containing (MTA Fillapex; Angelus, Londrina, Brazil), and a tricalcium-silicate containing sealer (BioRoot RCS; Septodont, St. Maur-des-Fossés, France). Pulp Canal Sealer and AH Plus are two established sealers, whereas the calcium silicate containing sealers MTA Fillapex and BioRoot RCS are comparatively new.
To produce identical sealer samples, all sealers were mixed according to manufacturers information and applied into silicone molds (diameter 4 mm, height 1.5 mm, volume 18.85 mm3). From all sealers 20 specimens were produced. To ensure complete setting of all sealers, samples were immersed in a physiological solution (Hank’s balanced salt solution) at 37 °C for 48 h [11]. The proper setting was evaluated in a pretest. After setting, the materials were weighed (accuracy ± 0.0001; Sartorius 1801MPS, Göttingen, Germany) three times and the average reading was recorded. The mean weights of test specimens with identical volume were for AH Plus 47.6 mg (± 1.3 mg), MTA-Fillapex 31.6 mg (± 1.3 mg), Pulp Canal Sealer 49.4 mg (± 1.9 mg), and for BioRoot RCS 37.3 mg (± 1.5 mg). The mean weight of one test body for each sealer was defined to be the onefold concentration (single-strength dilution) of the cell culture medium in mL, in which the appropriate sealer was stored.
Determination of cytotoxic sealer concentrations
To evaluate suitable sealer eluate concentration, all sealers were mixed under sterile conditions and added to the medium (DMEM, 41966-029, gibco by Thermo Fisher Scientific, Darmstadt, Germany) without any supplements. To produce sealer eluates, the medium suspension was incubated at 37 °C for 24 h in contact with the sealer samples. After that, the supernatant liquid was filtrated under sterile conditions and stored at minus 20 °C until use. Extracts with fourfold and also single-strength concentration were mixed and diluted to lower concentrations: 4:1, 2:1, 1:1, 1:2, 1:5, 1:10, 1:20, 1:100 dilutions of the cell culture medium were used to determine those concentrations in which the cells will survive. Two kinds of sealer extracts were produced: extracts from freshly mixed or from set sealer. This resulted in [4 different sealers × 8 dilutions × 2 (fresh and set sealer)] 64 cell cultures. These 64 cell cultures were then studied in triplicates (n = 192).
In contact to extracts from Pulp Canal Sealer cells survived in a dilution of 1:2 (24.7 mg/ml). No differences between extracts from freshly mixed and set sealer were observed. In contact to extracts from freshly mixed MTA Fillapex cells survived in a dilution of 1:2 (15.8 mg/ml) and onefold concentration from set MTA Fillapex (31.6 mg/ml). Cell survived in contact to extract from freshly mixed BioRoot RCS in a dilution 1:2 (18.5 mg/ml) as well as in a twofold higher concentration from set BioRoot RCS (37.3 mg/ml). Extract from freshly mixed AH Plus was cytotoxic. Cells survived only in the lowest tested dilution of 1:100 (0.48 mg/ml). In contrast, extract from set AH Plus had no cytotoxic effects. Cells survived even in a concentration of 4:1 (190.4 mg/ml).
Alteration of pH induced by added sealer in culturing medium was measured with a pH meter (inoLab pH 7110, WTW, Weilheim, Germany). For all sealer extracts no marked changes of the pH value of the culturing medium were observed, except for BioRoot RCS. Only in the BioRoot RCS samples with a concentration of 4:1 an increase of the pH value to 11 was detected during the first 24 h.
Cell culture studies with sealer extracts
Based on the preliminary study, sealer extracts in a dilution of 1:1, 1:2 and 1:10 from freshly mixed or set sealer were used for the main cell culture test (n = 288). PDL cells were seeded with a concentration of 5.300 cells/cm2 in 24-well culturing plates and were allowed to adhere for 24 h. To simulate a clinical scenario, extracts from freshly mixed sealer were added to the cells on day one. Extracts form set sealers were used for subsequent culturing and renewed every week. The cell culture studies were done in triplicates.
After 24 h, 7, 14, and 21 days cell were evaluated. For this, MTT assay served to demonstrate cell proliferation. To get conclusions about the viability, this test was combined with living cell count and living/dead staining. As evidence of cytotoxicity, the LDH released into the medium was determined by LDH assay. Furthermore, changes in cell morphology were analyzed by Richardson staining.
This resulted in [4 different sealers × 3 dilutions × 2 (fresh and set sealer) × 4 time intervals] 96 experimental groups and 288 cell cultures. Cell growth without sealer extracts in culturing medium [n = 12 cultures (4 time intervals in triplicates)] was used as control.
Cell viability
Living cell count was performed with the CASY1 cell counter (Schärfe System, Reutlingen, Germany). Cell proliferation rates were estimated with a MTT assay. The conversion of the yellow thiazolyl blue tetrazolium bromide (0.5 mg/ml; Sigma-Aldrich) to the purple formazan by the cellular NAD(P) reflux was measured at λ 570 nm. Cytotoxic effects were determined with the Pierce LDH Cytotoxicity Assay (ThermoFisher Scientific, Waltham, MA, USA). All assays were performed according to manufacturers’ protocols and done in triplicates.
The qualitative analysis of cell viability was performed via fluorescein diacetate/propidium iodide (FDA/PI) staining, where FDA (Sigma Aldrich) stains viable cells green, and PI (Fluka, Darmstadt, Germany) stains necrotic and apoptotic cell nuclei red.
Richardson staining
For histological evaluation the cell cultures were fixed in methyl ethanol (Merck, Darmstadt, Germany), air dried, and a Richardson staining was performed. The staining solution I contained 1% methylene blue (Merck) in 1% sodium borate (Merck). The staining solution II contained 1% azure in distilled water. Both solutions were mixed 1:1 before use.
Statistical analysis
Statistical analysis was carried out by one way ANOVA using a modified Levene testing and p < 0.05, and a posthoc analysis with Bonferroni-Holm testing (Daniel’s XL Toolbox version 6.53; http://xltoolbox.sourceforge.net).
Results
Main cell culture tests
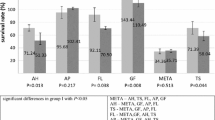
One way ANOVA was performed for each assay and differences were analyzed on a level of significance of p < 0.05, with regard to living cell count (p = 0.0007) proliferation rate (p = 0.0024), and cytotoxicity (p = 0.002). Significant differences were obtained. Statistically significant differences (p < 0.05) within the individual seal groups and in comparison to the controls at the different examination times are indicated in the figures. (Figs. 1, 2, 3, 4). The count of living cells for the dilution 1:2 (n = 96 cell tests) are summarized in Fig. 1 and the results of the MTT assay in Fig. 2. The results of the LDH assay for the 1:2 are summarized in Fig. 3 and for the 1:10 dilution in Fig. 4.
MTT assay of human PDL cells after contact to different endodontic sealers (dilution 1:2) up to 21 days. Asterisk indicates significant differences to the controls; n.s. not significant (p < 0.05). Values marked with the same letters were not statistically different within one sealer group (p > 0.05)
Within the one-fold concentration (n = 96 cell tests), all PDL cells died during the first days irrespective of sealer type. In the MTA Fillapex and the Pulp canal sealer group (n = 24 cell tests per sealer), significantly less PDL cells survived an extract diluted 1:2 compared to the controls (Fig. 1). During the first week in the MTT assay, a conversion was observed which was significantly lower than in the controls (Fig. 2). In a dilution 1:2 a LDH release was detectable until day 7 (Fig. 3) and until day 21 in a dilution 1:10 (Fig. 4) for both sealers.
In the living/dead (Fig. 5) and the Richardson staining (Fig. 6) there were nearly no living PDL cells after 14 days contact to MTA Fillapex and Pulp Canal Sealer in a dilution 1:2. After 21 days in a 1:10 dilution MTA Fillapex and Pulp Canal Sealer showed comparable cell density to the controls in the living/dead staining (Fig. 7). In the Richardson staining these two sealers showed already after 7 days the same PDL cell density compared to the control group (Fig. 8).
At a sealer extract dilution of 1:10 (n = 96 cell tests) all cells in the test groups showed the same survival rate as the cells in the control group, with the exception of AH Plus. In contrast to all other sealers, the PDL cells did not survive the contact to a 1:10 diluted AH Plus extract (n = 24 cell tests) (Figs. 7, 8). The PDL cells died with the lowest added sealer concentration. In contact to AH Plus the morphology of the PDL cells was altered; they become bigger with longer incubation period (Fig. 7, days 14 and 21). There was a high release of LDH with a factor of 4.2 for AH Plus (n = 24 cell tests) during the first day (Fig. 3). Thus, the living/dead and Richardson staining confirmed the results from the living cell count, MTT conversion and LDH release.
In the BioRoot RCS group (n = 24 cell tests), all PDL cells survived the contact with a 1:2 diluted extract and in contrast to all other sealers, a cell proliferation was observed (Fig. 1). The living cell count (Fig. 1) and the MTT assay (Fig. 2) showed comparable results. No cytotoxic effects were observed. A release of LDH was observable until day 21 (Fig. 4). In the 1:2 dilution BioRoot RCS was the only sealer tested where no differences compared to the control group occurred in the living/dead (Fig. 5) and the Richardson staining (Fig. 6).
Discussion
Discussion of methods
Ex vivo cell testing offers some information regarding the biocompatibility of new endodontic sealers in comparison with currently used ones. Hence, in the present study, the reactions of human PDL cells to four different sealers were evaluated in a freshly mixed and in a set state. A critical review of the literature reveals that the present study does not represent a novel approach. However, previous results—although interesting—are incomplete and insufficient to support their conclusions, e.g., all test models working only with set sealer samples have some limitations, because in vivo the surrounding tissue will be exposed to unset material. Furthermore, it was shown that sealers were significantly more toxic immediately after mixing than in set form [12].
Employing human primary cells of a relevant type in studies assessing endodontic materials has been pointed out previously [13] because primary cells derived from human target tissues of endodontic sealers, like PDL cells, are more relevant for biocompatibility studies than other cell lines [5]. For this, human PDL cells have been chosen for cytocompatibility testing here because these cells may get in direct contact with the sealer during root canal obturation. PDL cells are the predominant cell type of the periodontal ligament, and are the most important collagen producers in this tissue. The specific setup for this ex vivo cell test was verified in previous studies [14, 15].
In the dilution 1:2 a high release of LDH (above 1) indicates that the cells had stress. In contact to AH Plus and MTA Fillapex a high LDH value was only observed after 24 h, thereafter no longer. It can be concluded that these sealers were cytotoxic for the PDL cells and after 7 days all cells were dead and thus LDH was no longer produced. In contrast, in the BioRoot RCS group a LDH release was also visible after 21 days, but not after 1 day.
In the dilution 1:10, LDH was detected in a varying release factors for MTA Fillapex, BioRoot RCS and Pulp Canal Sealer. These sealers still caused stress in the cells but were not lethal. Whereas in the AH Plus group no LDH release could be observed after 7 days even in this lower dilution. It may be assumed that all PDL cells were dead. Nevertheless, the LDH release should only be interpreted in connection with other cell tests like living/dead or Richardson staining.
Pulp Canal Sealer
The cytotoxic and tissue-irritating potencies of zinc oxide-eugenol sealers were confirmed by the results of current publications on human PDL cells. This type of sealer caused significantly decrease of PDL cell proliferation [12, 16,17,18]. A zinc oxide eugenol containing sealer (Tubuli seal) was significantly less toxic in the set form than immediately after mixing [12], which is also in accordance with the present results. The cytotoxicity was associated with necrosis [12]. Zinc oxide eugenol type sealers are irritating mainly because of the eugenol [1]. Eugenol and other ingredients may modulate the immune response and contribute to periapical inflammation and pain [5].
AH Plus
Hitherto, it is known that AH Plus has excellent physical and sealing properties [19,20,21]. On the other hand, AH Plus cause significantly decrease of PDL cell proliferation [16, 22]. However, AH Plus remains popular despite its well-documented mutagenic [23], cytotoxicity and the induction of a severe inflammatory response [24, 25]. The cytotoxicity of AH Plus could be confirmed in the present study. Fresh specimen extracts of AH Plus exerted a marked cytotoxic effect. It was striking that in contact with eluates of AH Plus nearly all PDL cells died. The few surviving PDL cells became bigger (Fig. 7). This can be interpreted as a pathological hydropic cell swelling and is a sign of degeneration [26]. The cytotoxicity of AH Plus was associated with apoptosis by other authors [12].
AH Plus contains epoxy resin, which displays cytotoxic profile, especially at slightly diluted concentrations [27]. The epoxy resin present in AH Plus is mutagen and may cause breaks in the chain of cellular DNA [23]. According to the manufacturer, AH Plus is a formaldehyde-free material. Nevertheless, a minute amount of formaldehyde release (3.9 ppm) was observed in a previous study [28]. This release of formaldehyde in combination with the release of amine and epoxy resin components may explain the cytotoxicity of freshly mixed AH Plus sealer [29].
Here, freshly mixed AH Plus was cytotoxic in a concentration-depending manner. This is in agreement with previous studies that have documented the cytotoxic effect of AH Plus immediately after mixing [12, 29, 30]. Fresh AH Plus was strongly cytotoxic at a high extract concentration (1:2). After setting, AH Plus was no longer cytotoxic [29,30,31]. In contrast, other studies described AH Plus as moderately cytotoxic in fresh conditions, mildly cytotoxic after 1 week, and nontoxic after 2 weeks [32]. Compared to other resin-containing sealers, AH Plus showed the least cytotoxic effects, but lead to a reduction of cell viability of 26% [33]. AH Plus was 10 times more cytotoxic compared to BioRoot RCS [34]. On the other hand in an animal study with an induced apical periodontitis, the periapical tissues adjacent to root canals filled with epoxy-resin showed less inflammation compared to other sealers tested (zinc oxide eugenol and silicone) [35].
MTA Fillapex
The reactions of PDL cells to MTA Fillapex were comparable to those of the zinc oxide eugenol sealer Pulp Canal Sealer. Like for a zinc oxide eugenol sealer, the cell alterations caused by MTA Fillapex were described mainly as necrotic [12]. In accordance to other studies, MTA Fillapex revealed highly significantly negative impact of PDL cell proliferation and viability [12, 22, 36]. Furthermore, MTA Fillapex exerted adverse effects on the viability of human dental pulp cells [37] as well as on human osteoblasts [38], and exhibited cytotoxic effects on osteogenic and angiogenic cells [8]. Beside its severely cytotoxic effects MTA Fillapex remarkably decreased macrophages viability [6]. The composition of endodontic sealers plays an important role in their biocompatibility. Thus, the severe toxicity of MTA Fillapex may be attributed to the presence of resinous components, mainly salicylate resin, which reduced cell survival rates significantly [6, 30,31,32, 39]. In the present study, the cytotoxicity of MTA Fillapex was related to time and the concentration of the eluates, which is in agreement with other authors [30, 40].
BioRoot RCS
BioRoot RCS was the only sealer tested that showed PDL cell density in the 1:2 dilutions comparable to the controls (Figs. 5, 6). Hence, it may be concluded that BioRoot RCS was not cytotoxic, had a positive influence on the cell metabolism and was bioactive. In agreement with recent studies [17, 18, 34, 41] BioRoot RCS showed good biocompatibility at all extract concentrations as both fresh and set material. In direct contact with cells, BioRoot RCS was not cytotoxic and did not affect cell vitality and morphology. Cell growth was not adversely affected [17, 18, 34, 41]. Concerning biocompatibility, in ex vivo cell tests BioRoot RCS showed better results than other sealers based on epoxy resin or methacrylate [34] or zinc oxide-eugenol based sealers [17, 41] and also better than other sealer based on calcium silicate [18, 34]. BioRoot RCS was the least cytotoxic sealer compared to other sealers with 98.54% cell survival, even when cells were treated with undiluted eluates [34]. In presence of set BioRoot RCS, human PDL cells showed a high degree of proliferation, cell spreading and cell attachment [18].
In contrast to Pulp Canal Sealer, BioRoot RCS did not compromise the osteo-odontogenic differentiation potential of pulpal A4 mouse pulpal stem cells, thus BioRoot RCS did not alter the viability and morphology of these cells. The intrinsic ability of A4 cells to express type 1 collagen, DMP1 or BSP was preserved [41].
In direct contact with human periodontal ligament cells BioRoot RCS showed bioactive effects and induced the secretion of angiogenic and osteogenic growths factors such as VEGF, FGF-2 and BMP-2 from the surrounding tissue, which influence the formation of blood vessels and bone [17].
During the first day in the BioRoot RCS group with a concentration of 4:1 an increase of the pH value to 11 could be observed. This high pH value may influence the material’s cytotoxicity and need to be investigated in another study.
Cytotoxicity
The hypothesis had to be rejected. The results of the current study showed that the different sealers exhibited different levels of cytotoxicity. It must be remembered, however, that molecular leaching, and therefore, cytotoxicity might decrease over time. This depends on the solubility of the sealers. For instance, AH Plus is significantly less soluble than the here tested sealers MTA Fillapex and BioRoot RCS [42]. AH Plus is more or less insoluble after setting [20, 21], which explains that there was a marked difference in cytotoxicity between the freshly mixed and set specimens. Whereas unset samples of AH Plus showed a marked cytotoxicity, cytotoxicity was no longer observed in set AH Plus. MTA Fillapex is more soluble after setting than AH Plus [42], and this may explain the cytotoxicity of set and unset MTA Fillapex samples. Because of the solubility of BioRoot RCS, it may be speculated that BioRoot RCS is not only non-cytotoxic (biocompatibility) but may release some components to the surrounding tissue that might have a beneficial effect on tissue healing (bioactivity). Sealers with good biocompatibility are beneficial to aid or stimulate the repair of injured tissues.
It goes without saying, that the biocompatibility of a root canal sealer is only one of many factors that contribute to success of a root canal treatment. Overall, however, sealers based on calcium silicate can be regarded as an interesting alternative to conventional root canal filling materials.
Conclusions
Within the limitations of this ex vivo study, it can be concluded that contact of freshly mixed AH Plus and Pulp Canal Sealer or MTA Fillapex, respectively, in freshly mixed and set state lead to cytotoxic effects on PDL cells. In contrast, BioRoot RCS had a positive influence on the PDL cell metabolism and is biocompatible. Thus, besides biocompatibility, BioRoot RCS seems to be bioactive and may be recommended for root canal obturation. Further investigations, e.g., in an animal model are necessary to prove the result of the present study.
References
Suresh Chandra B, Gopikrishna V. Obturation of the radicular space. In: Suresh Chandra B, Gopikrishna V, editors. Grossman’s Endodntic Practice. 13th ed. New Dehli: Wolters Kluwer Health; 2014. pp. 343–73.
Johnson W, Kulild JC, Tay F. Obturation of the cleaned and shaped root canal system. In: Hargreaves KH, Berman LH, editors. Cohen’s Pathway of the Pulp. 11th ed. St. Louis: Elsevier; 2016. pp. 280–323.
Granchi D, Stea S, Ciapetti G, Cavedagna D, Stea S, Pizzoferrato A. Endodontic cements induce alterations in the cell cycle of in vitro cultured osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:359–66.
Dammaschke T, Witt M, Ott K, Schäfer E. Scanning electron microscopic investigation of incidence, location, and size of accessory foramina in primary and permanent molars. Quintessence Int. 2004;35:699–705.
Geurtsen W. Biocompatibility of root canal filling materials. Aust Endod J. 2001;27:12–21.
Braga JM, Oliveira RR, de Castro Mantins R, Vieira LQ, Sobrinho AP. Assessment of the cytotoxicity of a mineral trioxide aggregate-based sealer with respect to macrophage activity. Dent Traumatol. 2015;31:390–5.
Tepel J, Darwisch el Sawaf M, Hoppe W. Reaction of inflamed periapical tissue to intracanal medicaments and root canal sealers. Endod Dent Traumatol. 1994;10:233–8.
Costa F, Sousa Gomes P, Fernandes MH. Osteogenic and angiogenic response to calcium silicate-based endodontic sealers. J Endod. 2016;42:113–9.
Spångberg L. Biological effects of root canal filling materials. 7. Reaction of bony tissue to implanted root canal filling material in guinea pigs. Odontol Tidskr. 1969;77:133–59.
Parirokh M, Torabinejad M. Calcium silicate-based cements. In: Torabinejad M, editor. Mineral Trioxide Aggregate. Properties and clinical applications. Ames: Wiley Blackwell; 2014. pp. 281–332.
Xuereb M, Vella P, Damidot D, Sammut CV, Camilleri J. In situ assessment of the setting of tricalcium silicate-based sealers using a dentin pressure model. J Endod. 2015;41:111–24.
Szczurko G, Pawińska M, Łuczaj-Cepowicz KA, Marczuk-Kolada G, Hołownia A. Effect of root canal sealers on human periodontal ligament fibroblast viability: ex vivo study. Odontology. 2018;106:245–56.
Huang FM, Chang YC. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:361–5.
Jung S, Mielert J, Kleinheinz J, Dammaschke T. Human oral cells’ response to different endodontic restorative materials: an in vitro study. Head Face Med. 2014;10:55. https://doi.org/10.1186/s13005-014-0055-4.
Jung S, Sielker S, Hanisch MR, Libricht V, Schäfer E, Dammaschke T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS One. 2018;13:e0194467. https://doi.org/10.1371/journal.pone.0194467.
Willershausen I, Callaway A, Briseño B, Willershausen B. In vitro analysis of the cytotoxicity and the antimicrobial effect of four endodontic sealers. Head Face Med. 2011;7:15. https://doi.org/10.1186/1746-160X-7-15.
Camps J, Jeanneau C, El Ayachi I, Laurent P, About I. Bioactivity of a calcium silicate-based endodontic cement (BioRoot RCS): interaction with human periodontal ligament cells in vitro. J Endod. 2015;41:1469–73.
Collado-González M, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodríguez-Lozano FJ. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J. 2017;50:875–84.
Schäfer E, Olthoff G. Effect of three different sealers on the sealing ability of both Thermafil obturators and cold laterally compacted gutta-percha. J Endod. 2002;28:638–42.
Schäfer E, Zandbiglari T. Solubility of root canal sealers in water and artificial saliva. Int Endod J. 2003;36:660–9.
Schäfer E, Bering N, Bürklein S. Selected physicochemical properties of AH-Plus, EndoREZ and RealSeal SE root canal sealers. Odontology. 2015;103:61–5.
Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano J. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow 2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod. 2017;43:816–22.
Schweikl H, Schmalz G, Federlin M. Mutagenicity of the root canal sealer AH Plus in the Ames test. Clin Oral Investig. 1998;2:125–9.
Azar NG, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod. 2000;26:462–6.
Sousa CJA, Montes CRM, Pasco EA, Loyola AM, Versiami MA. Comparison of the intraosseous biocompatibility of AH Plus, Endo REZ, and Epiphany root canal sealer. J Endod. 2006;32:656–62.
Zatloukal K, Roth J, Denk H. Zell- und Gewebereaktionen. In: Böcker W, Denk H, Heitz PU, editors. Pathologie. 3rd ed. München: Urban & Fischer; 2004. pp. 39–76.
Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–20.
Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. Formaldehyde from endodontic materials. Oral Health. 1998;88:37–9.
Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329–37.
Zhou H-M, Du T-F, Shen Y, Wang Z-J, Zheng Y-F, Haapasalo M. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod. 2015;41:56–61.
Silva EJ, Accorsi-Mendonça T, Pedrosa AC, Granjeiro JM, Zaia AA. Long-term cytotoxicity, pH and dissolution rate of AH Plus and MTA Fillapex. Braz Dent J. 2016;27:419–23.
Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013;39:274–7.
Al-Hiyasat AS, Tayyar M, Darmani H. Cytotoxicity evaluation of various resin based root canal sealers. Int Endod J. 2010;43:148–53.
Eldeniz AU, Shehata M, Högg C, Reichl FX. DNA double-strand breaks caused by new and contemporary endodontic sealers. Int Endod J. 2016;49:1141–51.
Dammaschke T, Schneider U, Stratmann U, Yoo J-M, Schäfer E. Reaction of inflamed periapical tissue to three different root canal sealers. Dtsch Zahnärztl Z. 2006;61:15–26.
Rodríguez-Lozano FJ, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Forner L, Moraleda JM. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int Endod J. 2017;50:67–76.
Mestieri LB, Gomes-Cornélio AL, Rodrigues EM, Salles LP, Bosso-Martelo R, Guerreiro-Tanomaru JM, Tanomaru-Filho M. Biocompatibility and bioactivity of calcium silicate-based endodontic sealers in human dental pulp cells. J Appl Oral Sci. 2015;23:467–71.
Scelza MZ, Linhares AB, da Silva LE, Granjeiro JM, Alves GG. A multiparametric assay to compare the cytotoxicity of endodontic sealers with primary human osteoblasts. Int Endod J. 2012;45:12–8.
Assmann E, Böttcher DE, Hoppe CB, Grecca FS, Kopper PM. Evaluation of bone tissue response to a sealer containing mineral trioxide aggregate. J Endod. 2015;41:62–6.
Yoshino P, Nishiyama CK, da Silva Modena KC, Santos CF, Sipert CR. In vitro cytotoxicity of white MTA, MTA Fillapex® and Portland cement on human periodontal ligament fibroblasts. Braz Dent J. 2013;24:111–6.
Dimitrova-Nakov S, Uzunoglu E, Ardila-Osorio H, Baudry A, Richard G, Kellermann O, Goldberg M. In vitro bioactivity of BioRoot™ RCS, via A4 mouse pulp stem cells. Dent Mater. 2015;31:1290–7.
Prüllage R-K, Urban K, Schäfer E, Dammaschke T. Material Properties of a tricalcium silicate-containing, a mineral trioxide aggregate-containing, and an epoxy resin-based root canal sealer. J Endod. 2016;42:1784–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jung, S., Libricht, V., Sielker, S. et al. Evaluation of the biocompatibility of root canal sealers on human periodontal ligament cells ex vivo. Odontology 107, 54–63 (2019). https://doi.org/10.1007/s10266-018-0380-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-018-0380-3