Abstract
We examined shear bond strengths (SBSs) of various tooth-coating-materials including the experimental materials to dentin and demineralization resistance of a fractured adhesive surface after the SBS testing. Three resin-type tooth-coating-materials (BC, PRG Barrier Coat; HC, Hybrid Coat II; and SF, Shield force plus) and two glass-ionomer-type tooth-coating-materials (CV, Clinpro XT Varnish; and FJ, Fuji VII) were selected. The experimental PRG Barrier Coat containing 0, 17, and 33 wt% S-PRG filler (BC0, BC17, and BC33, respectively) were developed. Each tooth-coating-material was applied to flattened dentin surfaces of extracted human teeth for SBS testing. After storing in water for 32 days with 4000 thermal cycling, the specimens were subjected to the SBS test. Specimens after SBS testing were subjected to a pH cycling test, and then, demineralization depths were measured using a polarized-light microscope. ANOVA and Tukey’s HSD test were used for statistical analysis. The SBS value of FJ and CV was significantly lower than those of other materials except for BC (p < 0.01). The lesion depth of FJ was significantly shallower than those of other materials (p < 0.01); that of CV was significantly shallower than those of BC, HC, SF, and the control; and those of BC0 and BC17 were significantly shallower than that of the control (p < 0.05). The resin-type tooth-coating-materials demonstrated significantly higher SBS for dentin than the glass-ionomer-type tooth-coating-materials; however, they were inferior to the glass ionomer-type tooth-coating-materials in regards to the acid resistance of the fractured adhesion surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The exposure of the root surface occurs following gingival retraction caused by various factors, including deterioration with age, periodontal treatment, and occlusal trauma [1, 2]. The exposed root surface is covered with cementum, which includes a large quantity of organic substances [3]. Dentin is exposed on the root surface after root scaling and planing and is thereafter susceptible to demineralization, thus resulting in caries or dentin hypersensitivity [4–8]. It is well-known that the critical pH at which demineralization occurs on the root surface is as high as that for deciduous teeth [9, 10]. Recently, elderly population has been increasing, and their natural teeth are retained owing to the development of periodontal treatment and dental homecare. Since root surface caries has also been increasing [11], preventive treatments are equally important for elderly people.
Various resin-type tooth coating materials have been recently used for the prevention and treatment of dentin hypersensitivity, and the protection of exposed dentin surface following gingival retraction or tooth preparation [12–15]. Previous studies reported that a tooth coating material demonstrated a closing effect on open dentinal tubules and an increasing acid resistance effect on the root surface, due to the release of various ions, such as F, Ca, and P ions [14, 16]. A unique resin coating material which includes surface reaction type pre-reacted glass ionomer (S-PRG) fillers has been developed [17, 18]. The S-PRG filler comprises a glass core and glass ionomer phase that is generated by a reaction between a polyacrylic acid and glass powder, including fluoride ions [17, 18]. This filler can release not only fluoride but also various other ions [17–21]. Resistance to acid on the root surface is reinforced by various ions that are gradually released from coating materials. Therefore, to be effective, the coating materials are required to attach to the root surface for a while.
However, both the ion releasing ability and dentin bonding strength of a coating material would be weakened with the passage of time [16, 18–20]. Moreover, thermal and mechanical stresses during eating or tooth brushing may unexpectedly cause an early abrasion and detachment of the tooth coating materials from the root surface [21–23]. Recently, several studies reported that tensile or compressive stress concentrations at the cervical areas of the teeth during mastication may cause a non-caries cervical lesion or abfraction such as a wedge-shaped defect [22–24]. The stress concentrated at the cervical area during mastication may also affect the adhesive interface between the tooth coating material and root surface, and cause an early detachment of the coating material. Nevertheless, no studies reported the bond strength of tooth coating materials to root dentin after long-term storage and the demineralization resistance of the fractured adhesion surface after the bond strength testing.
The purpose of this study was to examine shear bond strengths (SBSs) of two types of tooth coating materials to root dentin after 32 days storage with thermal cycling test, and lesion depths of the fractured surfaces of the dentin site after the SBS testing. Furthermore, the invasion of various ions into the root dentin surface was investigated using an electron probe microanalyzer (EPMA).
Materials and methods
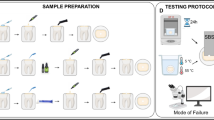
Figure 1 shows the experimental design.
Materials used in the experiment
The materials used in this study are listed in Table 1. Three commercial resin-type tooth coating materials (BC, HC and SF) and two commercial glass ionomer-type tooth coating materials (CV and FJ) were selected. Moreover, the experimental PRG Barrier Coat containing 0, 17, and 33 wt% S-PRG filler (BC0, BC17, and BC33, respectively) were developed and used in the long-term storage experiments.
Preparation of extracted teeth for specimens
A total of 157 extracted human teeth (anterior teeth and premolars) were collected for this study and stored in a solution of 0.1% thymol at 4 °C. The labial and buccal root surfaces close to cement-enamel-junction of anterior teeth and premolars were ground with a 120-grit silicon carbide paper (Bühler Inc., MN, USA) and finished with 600-grit carbide paper (Bühler Inc., MN, USA) using a polishing machine (Refine Tec Ltd, Kanagawa, Japan) under water irrigation to obtain the flat dentin surface. The crowns of 157 anterior teeth and premolars were removed using a #201R diamond point with an air-turbine. Of 157 anterior teeth and premolars, the 144 teeth were divided equally into 8 tooth coating material groups of 18, and the remaining 13 teeth were used as a control.
SBS test
From each tooth coating material group, 16 of 18 sections of anterior teeth and premolars were subjected to the SBS tests (Fig. 1). The tooth section was embedded in the specimen holder ring using a self-curing resin (Province, Shofu Inc., Kyoto, Japan) so that the flat dentin surface was parallel to and projected above the rim of the cylindrical specimen holder ring. A section of a double-coated adhesive tape (0.12 mm thick) with a 3 mm-diameter opening was attached to the flat dentin surface to define the bonding area. After the laminated paper was peeled from the attached adhesive tape, a transparent acrylic tube (3 mm in diameter, 2 mm in height) was placed onto the adhesive tape. Each tooth coating material was applied to the dentin surface according to the instruction of the manufacturer, and then each flowable resin composite of the same manufacturer was placed in the acrylic tube and photopolymerized for 40 s (600 mW/cm2) using a light-curing unit (Candelux, Morita Corp., Tokyo, Japan). Clinically, a thin layer of tooth coating material is applied on the tooth surface. In this study, after each resin-type tooth coating material was applied to the dentin surface, each flowable resin composite of the same manufacturer was placed on the tooth coating material. Fuji VII was placed in the acrylic tube without pre-conditioning the dentin surface.
The specimen holder rings with embedded teeth were stored in distilled water at 37 °C for 32 days, and then the acrylic tubes were removed from the specimens. During the storage for 32 days, the specimens were thermo-cycled using thermo-cycle apparatus (Thermal cycling K178, Tokyo Giken Inc. Tokyo, Japan) between 5 and 55 °C with a dwell time of 30 s. The thermal cycling (500 cycles/day) was conducted every 4 days for 32 days. In total, the specimens were subjected to 4000 thermal cycles (Fig. 1).
The specimen holder rings were placed on a tabletop material tester (EZ Test 500N, Shimadzu Corp, Kyoto, Japan), and the specimens were subjected to SBS testing at a crosshead speed of 1 mm/min using a flat end blade [25].
Failure mode analysis
Fractured surfaces of the specimens were examined using a stereomicroscope (Leica EZ4D, Leica Camera AG, Wetzlar, Germany) at 30× magnification, and the fracture modes were determined according to the evaluation criteria shown in annotation of Table 2.
EPMA analysis
After the 32 days storage, the remaining two specimens in each tooth coating material group were dried well, and then a carbon paste (Colloidal graphite, EM Japan Co, Ltd, Tokyo, Japan) was painted onto the surface of the coating materials (Fig. 1). The specimens were embedded with epoxy resin (Epon812 Resin Embedding Kit, TAAB Laboratories Equipment Ltd, England) using a rubber mold.
After the epoxy resin was polymerized, the specimens were ground using a 120-grit silicon carbide paper and finished with a 1 µm diamond paste using a polishing machine under water irrigation to obtain the observation surface with the interface between the coating material and dentin. After the observation surface was sputter coated with Au, the concentration of the distribution of the elements (F, Sr, Si, C, Al, and Na) were examined using the EPMA (JXA-8900 WD/ED Combine microanalyzer, JEOL Ltd, Tokyo, Japan).
Demineralization test
Thirteen of 16 specimens which had been used for the SBS tests in each tooth coating material group, and 13 teeth in the control group were subjected to the demineralization test (Fig. 1).
After fracture modes of the specimens were examined, the fractured dentin surfaces in the teeth used for SBS tests and the dentin surface in the control were protected using a masking tape (2 × 2 mm). The entire tooth surface was double coated with Protect Varnish (Kuraray Noritake DENTAL Inc., Tokyo, Japan) and nail varnish. After the varnishes were dried, the masking tape was removed.
The specimens were subjected to pH cycling for 7 days. Thirteen specimens from each group were daily immersed in a demineralizing solution (pH 4.8, containing 0.05 M acetic acid, 2.2 mM calcium, and 2.2 mM phosphate ions) for 18 h, following which they were immersed in a re-mineralizing solution (pH 7.0, containing 0.15 M potassium chloride, 1.5 mM calcium, and 0.9 mM phosphate ions) for 6 h. The solutions were maintained at 37 °C and stirred at 132 rpm. The specimens were irrigated with deionized water for 5 min during transfer between solutions and at the conclusion of the cycling process [26].
Measurement of the lesion depth
Ten of 13 specimens applied with the demineralization tests in each tooth coating material group (Fig. 1) were perpendicularly sectioned to the root surfaces and through the center of each window using a hard-tissue microtome (Isomet, Bühler Inc., MN, USA). Three sections with a thickness of approximately 200 μm were obtained from each window of the specimens. Each section was ground to a thickness of approximately 100 μm using a lapping film sheet.
The sections were examined at 200× magnification using a polarized light microscope (Eclipse LV100POL, Nikon Corp, Tokyo, Japan). Digital photomicrographs were obtained using a CCD camera (DS-L2, Nikon Corp, Tokyo, Japan). The lesion depth in each section was determined by measuring the width between the original dentin surface and the deepest position of the lesion using the camera control software. The mean lesion depth of the three sections was used as the lesion depth for each specimen.
Scanning electron microscopy observation
The remaining 3 specimens without the demineralization tests and 3 specimens with demineralization tests were selected from each tooth coating material group for scanning electron microscopy (SEM) observation (Fig. 1). The fractured surfaces of the selected specimens were sputter coated with palladium and platinum and observed using SEM (S-800; Hitachi Ltd, Tokyo, Japan) at an acceleration voltage of 15 kV.
Statistical analyses
One-way analysis of variance (ANOVA) and Tukey’s HSD post hoc test were used to compare the values between the tooth coating material groups in each storage group at a 5% significant level. All analyses were performed using a statistical analysis in add-in software package for Microsoft Excel (Ekuseru-Toukei 2012, Survey Research Information Co, Ltd, Tokyo, Japan).
This study was conducted after obtaining the approval of the Ethical Review Board of The Nippon Dental University School of Life Dentistry at Niigata, Japan (approval No. ECNG-H-156, 196).
Results
One-way ANOVA indicated that the effect of the tooth coating material on the SBS was significant (p < 0.01). As shown in Fig. 2, Tukey’s HSD post hoc test revealed that the SBS value of FJ was significantly lower than those of other materials except for CV (p < 0.01); and that of CV was significantly lower than those of other materials except for BC and FJ (p < 0.05). There were no significant differences in the SBS values among BC0, BC17, BC33 and BC, which contained S-PRG fillers (p > 0.05). Table 2 shows that the predominant mode of failure is the adhesive failure (71%), followed by the mixed failure (26%).
Shear bond strength. BC PRG Barrier Coat with 50 wt% S-PRG filler, BC0 PRG Barrier Coat with 0 wt% S-PRG filler, BC17 PRG Barrier Coat with 17 wt% S-PRG filler; BC33 PRG Barrier Coat with 33 wt% S-PRG filler; HC Hybrid Coat II, SF Shield foce plus, CV Clinpro XT Varnish, FJ Fuji VII. Means with the same letter are not significantly different each other
One-way ANOVA indicated that the effect of the tooth coating materials on the lesion depth was significant (p < 0.01). As shown in the Fig. 3, Tukey’s HSD post hoc test revealed that the lesion depth of FJ was significantly shallower than those of other materials and the control (p < 0.01); that of CV was significantly shallower than those of BC, HC, SF, and the control (p < 0.05); and those of BC0 and BC17 were significantly shallower than that of the control (p < 0.05). There were no significant differences in the lesion depth among BC0, BC17, BC33 and BC (p > 0.05).
Lesion depth. BC PRG Barrier Coat with 50 wt% S-PRG filler, BC0 PRG Barrier Coat with 0 wt% S-PRG filler, BC17 PRG Barrier Coat with 17 wt% S-PRG filler, BC33 PRG Barrier Coat with 33 wt% S-PRG filler, HC Hybrid Coat II, SF Shield foce plus, CV Clinpro XT Varnish, FJ Fuji VII. Means with the same letter are not significantly different each other
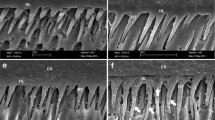
Representative SEM images (1000×) of the specimens after SBS and demineralization testing are shown in Figs. 4 and 5, respectively. As shown in Fig. 4, the positions of dentin tubules were recognized on the SEM images of BC, BC33, and HC, and a slight destruction of the dentin surface was observed on that image of SF. This figure also shows remnants of the coating materials on the SEM images of BC0, BC17, CV, and FJ. Figure 5 shows that the dentin surface with slightly opened dentin tubules was observed on the SEM images of BC17 and CV; some remnants of the coating materials and dentin surface with opened dentin tubules were observed on those of BC, BC33, HC and SF; and only the remnants of the coating materials were observed on those of BC0 and FJ.
Representative SEM images of specimens after SBS tests. BC PRG Barrier Coat with 50 wt% S-PRG filler, BC0 PRG Barrier Coat with 0 wt% S-PRG filler, BC17 PRG Barrier Coat with 17 wt% S-PRG filler, BC33 PRG Barrier Coat with 33 wt% S-PRG filler, HC Hybrid Coat II, SF Shield foce plus, CV Clinpro XT Varnish, FJ Fuji VII. On the SEM images of BC, BC33, and HC, the slightly opened dentin tubules are observed. The SEM image of SF shows a slight destruction of the dentin surface. Some remnants of the coating materials are observed on the SEM images of BC0, BC17, CV, and FJ
Representative SEM images of specimens after pH cycling. BC PRG Barrier Coat with 50 wt% S-PRG filler, BC0 PRG Barrier Coat with 0 wt% S-PRG filler, BC17 PRG Barrier Coat with 17 wt% S-PRG filler, BC33 PRG Barrier Coat with 33 wt% S-PRG filler, HC Hybrid Coat II, SF Shield foce plus, CV Clinpro XT Varnish, FJ Fuji VII. The dentin surface with slightly opened dentin tubules are observed on the SEM images of BC17 and CV. Some remnants of the coating materials and opened dentin tubules are observed on the SEM images of BC, BC33, HC and SF. The SEM images of BC0 and FJ show the only remnants of the coating materials
EPMA analysis revealed that F and Sr ions were predominantly detected in the high level on the dentin surface of the CV and FJ specimens. However, these ions were not clearly detected on the specimens of other groups. Figures 6 and 7 are representative EPMA images of FJ and BC, respectively. The EPMA image of the FJ specimen demonstrates that various ions were detected not only on the dentin surface beneath the coating material but also on the dentin surface around the dentin tubules (Fig. 6). The EPMA image of the BC specimen demonstrates that various ions were rarely detected on the superficial dentin surface beneath the coating material (Fig. 7).
EPMA images of the representative FJ (Fuji VII) specimen. The EPMA image of the FJ specimen demonstrates that various ions, especially F and Sr ions were detected not only on the dentin surface beneath the coating material but also on the dentin surface around the dentin tubules. Colors of F, Si, Al and Sr level
Discussion
From the results of the SBS test in this study, the resin-type tooth coating materials with flowable resins exhibited higher SBS than the glass ionomer-type tooth coating materials. The adhesive monomer contained in the resin-type coating materials contributed to an increase in the dentin bond strength. The adhesive monomer could penetrate superficial dentin matrix and dentin tubules, and formed hybrid layer and resin tags after photo-polymerization [21]. The formation of resin tags may be useful for increasing the strength of dentin bonds [27]. In contrast, dentin bonding of the glass ionomer-type material is generated by the embedding of polyelectrolyte chains into hydroxyapatite, which is produced by a substitution reaction between the phosphate ion and polyelectrolyte chain [28]. However, the chemical bond strength to dentin with the glass ionomer-type coating materials is low compared with the mechanical bond strength produced by the resin-type coating materials [29]. On the other hand, the previous clinical research reported that resin-modified glass ionomer cement showed higher retention rate than resin composite in class 5 restorations [30]. Thus, the bond strength is not the only factor for retention of restorations, the retention mechanism of the restorative materials must be considered.
Our results showed that the strength of the bonds of all tooth coating materials was relatively high even after 32 days storage with thermal cycling. We also have the data of SBSs of tooth coating materials (BC, HC, SF, CV and FJ) crown dentin after 24 h storage without thermal cycling using extracted human molar. In these data, the SBSs (S.D.) in MPa of BC, HC, SF, CV and FJ were 8.2 (1.7), 10.7 (3.2), 12.1 (3.0), 6.5 (2.7) and 4.3 (1.5), respectively. The data after 32 days storage obtained in the present study cannot significantly compared with the data after 24 h storage, because the kind of tooth and lesion used for adhesion test are different each other. However, the strength of the bonds of the tooth coating materials after 32 days storage with thermal cycling was slightly higher than that after 24 h storage without thermal cycling. These results could corroborate previous studies by Kakuda et al. [31] and Asaka et al. [32]. Kakuda et al. [31] reported that the dentin bond strength of a resin composite with an all-in-one adhesive exhibited no significant decrease after thermal cycling. Asaka et al. [32] showed that the dentin bond strength of resin composite with self-etch adhesive was increased by thermal cycling. Therefore, it is speculated that the resin-type coating materials containing adhesive monomer similar to all-in-one adhesive may demonstrate a slight increase in the strength of the dentin bond after thermal cycling. Hoshika et al. [33] reported that chemical bonding of glass ionomer cement without pre-treatment with a polyalkenoic acid conditioner was increased after 1 month storage in water.
In this study, the coating material with the highest content of S-PRG filler (BC) revealed the lowest dentin bond strength among three experimental and one commercial PRG Barrier Coat with different amounts of this filler. Our results could support the fact that bond strength of resin-type coating materials could depend on the amount of adhesive monomer [17], because the amount of adhesive monomer could be relatively decreased with increasing amount of S-PRG fillers.
Original dentin bond strength of each resin-type tooth coating material used in this study would be slightly different from the data shown in this study, because the surface of the tooth coating material was covered by the flowable resin when preparing specimens for SBS test. However, almost specimens after SBS testing showed adhesive and mixed failure modes and the failure occurred at the interface between the tooth coating material and dentin, and never occurred at the interface between the tooth coating material and the flowable resin. Therefore, the methodology of the SBS test carried out in this study was able to achieve measurement of the bond strength of the resin-type tooth coating materials to dentin. In clinic, we assume that covering the resin-type tooth coating material with thin layer of flowable resin could be effective in staying longer the tooth coating material on the root surface due to increase of wear resistance.
The resin-type coating materials did not demonstrate increasing of the resistance to demineralization for the fractured surface after SBS testing compared with the controls, in this study. Moreover, the glass ionomer-type coating materials exhibited more resistance to demineralization than the resin-type coating materials. This discrepancy of the resistance to demineralization was considered to be due to the differences in the failure modes between the resin-type and glass ionomer-type coating materials. This consideration was based on the results achieved in the present study, in which several specimens in almost all of the resin-type coating materials groups showed mixed failure with dentin chips, although the failure modes of the glass ionomer-type specimens almost exhibited adhesive failure. The exposure of freshly dentin surface occurred by mixed failure might cause to increase the demineralized depth of the specimens in the resin-type coating materials groups. Moreover, the result of EPMA analysis showed that F and Sr ions were not clearly detected on the specimens of the resin-type coating materials groups. This result implies that ability of a resin-type tooth coating material would be low for providing resistance to demineralization on the dentin surface applied the material.
Previous studies reported that glass ionomer cement releases a large amount of F ions during hardening [34–36]. The EPMA analysis of the dentin surface applied with FJ and CV revealed that F and Sr ions penetrated into the superficial dentin surface in this study. The F and Sr ions released from the glass ionomer-type tooth coating materials might react with hydroxyapatite and fabricate fluoroapatite and strontiumapatite on the dentin surface, thus generating resistance to demineralization. This inference was supported by Thuy et al. [37], who reported that the simultaneous presence of strontium with fluoride at specific concentrations enhances enamel remineralization in vitro. From the results of the failure mode analysis and SEM observation in this study, all specimens of the glass ionomer cement demonstrated adhesive failure and slight remnants of the materials were observed on the failure surface. The remnants may assist in increasing the resistance to the acid of the de-bonded dentin surface. In a clinical situation, it is expected that a glass ionomer cement could provide an acid-resistance layer for the dentin surface after detaching it from the dentin surface.
PRG Barrier Coat contains S-PRG fillers that release various ions, such as F−, SiO3−, and Sr2+ [14]. Several studies have reported that these ions are useful for re-mineralization of the tooth surface [17–21]. It was reported that PRG Barrier Coat released six ions (F−, BO3 3−, Sr2+, Na+, Al3+, and SiO3 2−), and F, Sr, and SiO ions in particular participated in remineralization of the tooth substance [38–42]. In this study, it was hypothesized that application of tooth coating materials that release F, Si and Sr ions would provide resistance to demineralization after detaching from the root surface, and it is was observed. In addition, it was expected that a high amount of S-PRG filler coating material could demonstrate high resistance to pH cycling. However, a remarkable effect of S-PRG filler on the acid resistance of the dentin surface was not recognized in this study. Furthermore, this study revealed an unexpected result in that a higher content of S-PRG filler coating material demonstrated a larger demineralization depth. From our results of EPMA analysis for PRG Barrier Coat, including the experimental ones, the F, Sr, and Si ions were barely detected on the dentin surface, which were applied with PRG Barrier Coat independent of the S-PRG filler content. Therefore, a limitation of this study is that it was assumed that various ions released from S-PRG filler may not be incorporated into the dentin surface. Because our results were contradictory to those of previous studies [13, 43, 44], further study concerning the effects of S-PRG filler on acid resistance of dentin surface would be required.
Conclusion
Within the limitation of this study, the following conclusions were drawn:
-
1.
The SBS values of resin-type coating materials with flowable resin to dentin (approximately 8–12 MPa) were significantly higher than those of glass ionomer-type coating materials (approximately 4–6 MPa) after 32 days storage.
-
2.
The lesion depths of dentin surface detached materials after SBS test in the glass ionomer-type coating material groups (approximately 110–180 μm) were shallower than those in the resin-type coating material groups (approximately 200–260 μm).
-
3.
The amount of S-PRG fillers did not affect the SBS values to dentin and the lesion depths of dentin surface detached materials after SBS test in the experimental tooth coating materials.
References
Fukumoto Y, Horibe M, Inagaki Y, Oishi K, Tamaki N, Ito HO, Nagata T. Association of gingival recession and other factors with the presence of dentin hypersensitivity. Odontology. 2014;102:42–9.
Kassab MM, Cohen RE. The etiology and prevalence of gingival recession. J Am Dent Assoc. 2003;134:220–5.
Nanci A. Periodontium in oral histology: development, structure, and function (Ten Cate, A.T. ed.). 6th ed. St. Louis: The C.V. Mosby Company; 2003. p. 240–3.
Sugihara N, Maki Y, Kurokawa A, Matsukubo T. Cohort study on incidence of coronal and root caries in Japanese adults. Bull Tokyo Dent Coll. 2014;55:125–30.
Sugihara N, Maki Y, Okawa Y, Hosaka M, Matsukubo T, Takaesu Y. Factors associated with root surface caries in elderly. Bull Tokyo Dent Coll. 2010;51:23–30.
Imazato S, Ikebe K, Nokubi T, Ebisu S, Walls AWG. Prevalence of root caries in a selected population of older adults in Japan. J Oral Rehabil. 2006;33:137–43.
Yoshiyama M, Masada J, Uchida A, Ishida H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res. 1989;68:1498–502.
Yoshiyama M, Noiri Y, Ozaki K, Uchida A, Ishikawa Y, Ishida H. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J Dent Res. 1990;69:1293–7.
Anderson P, Hector MP, Rampersad MA. Critical pH in resting and stimulated whole saliva in groups of children and adults. Int J Paediatr Dent. 2001;11:266–73.
Hoppenbrouwers PM, Driessens FC, Borggreven JM. The mineral solubility of human tooth roots. Arch Oral Biol. 1987;32:319–22.
Griffin SO, Griffin PM, Swann JL, Zlobin N. Estimating rates of new root caries in older adults. J Dent Res. 2004;83:634–8.
Kaneshiro AV, Imazato S, Ebisu S, Tanaka S, Tanaka Y, Sano H. Effects of a self-etching resin coating system to prevent demineralization of root surfaces. Dent Mater. 2008;24:1420–7.
Tajima K, Nikaido T, Inoue G, Ikeda M, Tagami J. Effects of coating root dentin surfaces with adhesive materials. Dent Mater J. 2009;28:578–86.
Ma S, Imazato S, Chen JH, Mayanagi G, Takahashi N, Ishimoto T, Nakano T. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent Mater J. 2012;31:909–15.
Shiiya T, Tomiyama K, Iizuka J, Hasegawa H, Kuramochi E, Fujino F, Ohashi K, Nihei T, Teranaka T, Mukai Y. Effect of the coating material on root dentin remineralization in vitro. Am J Dent. 2014;27:258–62.
Han L, Cv E, Li M, Niwano K, Ab N, Okamoto A, Honda N, Iwaku M. Effect of fluoride mouth rinse on fluoride releasing and recharging from esthetic dental materials. Dent Mater J. 2002;21:285–95.
Ikemura K, Tay FR, Kouro Y, Endo T, Yoshiyama M, Miyai K, Pashley DH. Optimizing filler content in an adhesive system containing pre-reacted glass-ionomer fillers. Dent Mater. 2003;19:137–46.
Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 2008;27:315–39.
Kamijo K, Mukai Y, Tominaga T, Iwaya I, Fujino F, Hirata Y, Teranaka T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent Mater J. 2009;28:227–33.
Shimazu K, Ogata K, Karibe H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dent Mater J. 2011;30:923–7.
Tay FR, Sano H, Tagami J, Hashimoto M, Moulding KM, Yiu C, Pashley DH. Ultrastructural study of a glass ionomer-based, all-in-one adhesive. J Dent. 2001;29:489–98.
Michael JA, Townsend GC, Greenwood LF, Kaidonis JA. Abfraction: separating fact from fiction. Aust Dent J. 2009;54:2–8.
Sarode GS, Sarode SC. Abfraction: a review. J Oral Maxillofac Pathol. 2013;17:222–7.
Jakupovic S, Cerjakovic E, Topcic A, Ajanovic M, Prcic AK, Vukovic A. Analysis of the abfraction lesions formation mechanism by the finite element method. Acta Inform Med. 2014;22:241–5.
Shimada Y, Sattabanasuk V, Sadr A, Yuan Y, He Z, Tagami J. Shear bond strength of tooth-colored indirect restorations bonded to mid-coronal and cervical dentin. Dent Mater J. 2006;25:7–12.
Gao XL, Pan JS, Hsu CY. Laser-fluoride effect on root demineralization. J Dent Res. 2006;85:919–23.
Gwinnett AJ. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992;5:127–9.
Wilson AD, Prosser HJ, Powis DM. Mechanism of adhesion of polyelectrolyte cements to hydroxyapatite. J Dent Res. 1983;62:590–2.
Scaminaci Russo D, Iuliano V, Franchi L, Ferrari M, Giachetti L. Adhesion to primary dentin: microshear bond strength and scanning electron microscopic observation. Am J Dent. 2013;26:341–6.
Brackett WW, Dib A, Brackett MG, Reyes AA, Estrada BE. Two-year clinical performance of class V resin-modified glass-lonomer and resin composite restorations. Oper Dent. 2003;28:477–81.
Kakuda S, Fu J, Nakaoki Y, Ikeda T, Tanaka T, Sano H. Improved long-term bonding performance of an experimental all-in-one adhesive. Dent Mater J. 2013;32:600–7.
Asaka Y, Yamaguchi K, Inage H, Takamizawa T, Kurokawa H, Rikuta A, Kuroda T, Miyazaki M. Effect of thermal cycling on bond strengths of single-step self-etch adhesives to bovine dentin. J Oral Sci. 2006;48:63–9.
Hoshika S, De Munck J, Sano H, Sidhu SK, Van Meerbeek B. Effect of conditioning and aging on the bond strength and interfacial morphology of glass-ionomer cement bonded to dentin. J Adhes Dent. 2015;17:141–6.
Tay FR, Pashley EL, Huang C, Hashimoto M, Sano H, Smales RJ, Pashley DH. The glass-ionomer phase in resin-based restorative materials. J Dent Res. 2001;80:1808–12.
Creanor SL, Carruthers LM, Saunders WP, Strang R, Foye RH. Fluoride uptake and release characteristics of glass ionomer cements. Caries Res. 1994;28:322–8.
Yip HK, Lam WT, Smales RJ. Fluoride release, weight loss and erosive wear of modern esthetic restoratives. Br Dent J. 1999;187:265–70.
Thuy TT, Nakagaki H, Kato K, Hung PA, Inukai J, Tsuboi S, Nakagaki H, Hirose MN, Igarashi S, Robinson C. Effect of strontium in combination with fluoride on enamel remineralization in vitro. Arch Oral Biol. 2008;53:1017–22.
Featherstone JD, Shields CP, Khademazad B, Oldershaw MD. Acid reactivity of carbonated apatites with strontium and fluoride substitutions. J Dent Res. 1983;62:1049–53.
Chachra D, Vieira AP, Grynpas MD. Fluoride and mineralized tissues. Crit Rev Biomed Eng. 2008;36:183–223.
Tanahashi M, Yao T, Kokubo T, Minoda M, Miyamoto T, Nakamura T, Yamamuro T. Apatite coated on organic polymers by biomimetic process: improvement in its adhesion to substrate by NaOH treatment. J Appl Biomater. 1994;5:339–47.
Forsback AP, Areva S, Salonen JI. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol Scand. 2004;62:14–20.
Saito T, Toyooka H, Ito S, Crenshaw MA. In vitro study of remineralization of dentin: effects of ions on mineral induction by decalcified dentin matrix. Caries Res. 2003;37:445–9.
Shiiya T, Mukai Y, Tomiyama K, Teranaka T. Anti-demineralization effect of a novel fluoride-releasing varnish on dentin. Am J Dent. 2012;25:347–50.
Murayama R, Furuichi T, Yokokawa M, Takahashi F, Kawamoto R, Takamizawa T, Kurokawa H, Miyazaki M. Ultrasonic investigation of the effect of S-PRG filler-containing coating material on bovine tooth demineralization. Dent Mater J. 2012;31:954–9.
Acknowledgements
The authors would like to thank Shofu Inc., Sun Medical Ltd., Tokuyama Dental Corp., 3M Japan Ltd and GC Inc. for the experimental adhesive resins and tooth coating materials they generously provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shofu Inc., Sun Medical Ltd., Tokuyama Dental Corp., 3M Japan Ltd. and GC Inc. provided the materials used in this Study. The authors declare that they have no other conflict of interest.
Rights and permissions
About this article
Cite this article
Arita, S., Suzuki, M., Kazama-Koide, M. et al. Shear bond strengths of tooth coating materials including the experimental materials contained various amounts of multi-ion releasing fillers and their effects for preventing dentin demineralization. Odontology 105, 426–436 (2017). https://doi.org/10.1007/s10266-016-0290-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-016-0290-1