Abstract
Asteraceae (synonym as Compositae) is one of the largest angiosperm families among flowering plants comprising one-tenth of all agri-horticultural species grown across various habitats except in Antarctica. These are commercially utilized as cut and loose flowers as well as pot and bedding plants in landscape gardens due to their unique floral traits. Consequently, ineffective seed setting and presence of an intraspecific reproductive barrier known as self-incompatibility (SI) severely reduces the effectiveness of hybridization and self-fertilization by traditional crossing. There have been very few detailed studies of pollen-stigma interactions in this family. Moreover, about 63% of Aster species can barely self-fertilize due to self-incompatibility (SI). The chrysanthemum (Chrysanthemum × morifolium) is one of the most economically important ornamental plants in the Asteraceae family which hugely shows incompatibility. Reasons for the low fertility and reproductive capacity of species are still indefinite or not clear. Hence, the temporal pattern of inheritance of self-incompatibility and its effect on reproductive biology needs to be investigated further to improve the breeding efficiency. This review highlights the self-incompatible (SI) system operating in important Astraceous (ornamental) crops which are adversely affected by this mechanism along with different physiological and molecular techniques involved in breaking down self-incompatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have developed a variety of strategies to avoid self-pollination, such as the distinct placement of female and male reproductive parts within the flower or the presence of flowers on various plants to induce unique genetic recombinations (Mraz 2003; Yang et al. 2018b). In most angiosperms, self-incompatibility (SI) is one of the genetic mechanisms that serves as a pre-fertilization barrier where growth of the pollen tube can be inhibited on the stigma or in the style following inbreeding (Ascher 1976; Brennan et al. 2011). Self-incompatibility or self-infertility (SI) is the failure of the pollen grains to germinate on the stigma of the same flower following self-pollination (Brewbaker 1957; Hiscock et al. 2003; Nettancourt 1997; Wang et al. 2018). The recognition and rejection of self-pollen is the rule that prevents inbreeding depression (Vijayakumar et al. 2018) and promotes out-crossing in flowering plants (Hiscock and Kues 1999; Ortiz et al. 2006). Origin of this phenomenon dates back to 90 million years ago and is reported in several eudicot families (Igic et al. 2004). Self-sterility was first reported in Verbascum species. where cross-pollinations were carried out to develop interspecific hybrids (East and Park 1917). Darwin's traditional genetic studies, dating back to the end of the eighteenth century, have documented the presence of these self-inhibitory mechanisms as tactics to increase genetic diversity (Silva and Goring 2001). The majority of self-incompatible systems are regulated by the S-locus with two tightly linked polymorphic genes, one of which regulates the identity of the pollen and the other regulates the identity of the pistil, although the number varies with the crop (Brennan et al. 2011). In pollen, S allele dominance is regulated at the transcription (mRNA) level but in the pistil, it is modulated at the post-transcriptional (protein) level (Hatakeyama et al. 2001; Shiba et al. 2002). Along with that, many other genes linked to the S-locus are also crucial for a fully functioning self-incompatibility trait (Kitashiba and Nasrallah 2014). Genetic studies in diverse crops showed that S genes encode for proteins that necessitates signaling downstream pathways of the S-protein-mediated self-recognition machinery (Jeong et al. 2014). It is noteworthy that many researchers have been able to support this genetic observation over the past two decades through molecular and biochemical studies in several crops that have made a substantial contribution in understanding the intricate web of interactions occurring at the pollen-stigma interface viz.; Crepis foetida and Parthenium argentatum (Gerstel 1950), Cosmos bipinnatus (Crowe 1954), Helianthus annuus (Habura 1957), Chrysanthemum cinerarifolium (Brewer and Parlevliet 1969) and Carthamus flavescens (Imrie and Knowles 1971) among which garden chrysanthemum is a hexaploidy and all other Compositae members with sporophytically determined self-incompatibility are diploids (Drewlow et al. 1973).
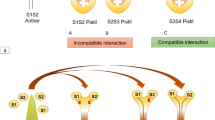
In general, usually for a compatible response, no inhibitory compounds are produced, more so, pollen germination and pollen tube expansion are normal leading to an effective fertilization. However, for self-incompatibility responses, a number of studies have mentioned different systems based on varied interactions. Bateman (1954) classified self-incompatibility into complementary and oppositional systems depending on pollen-pistil interaction following pollination. In a complementary or stimulatory system, when pollen from one self-incompatible group falls on the stigma of another self-incompatible group, specific stimulatory chemicals are produced that promote pollen germination and pollen tube growth which culminates in successful fertilization. On the contrary, in case of an oppositional or inhibitory system, if the stigma and pollen both are members of the same self-incompatible group, no stimulatory chemicals will be produced, preventing pollen germination and further growth of the pollen tube. In the same year, Lewis (1954) classified self-incompatibility into homomorphic and heteromorphic systems based on gene action and floral morphology (Barrett 1992; Nettancourt 1997). The homomorphic self-incompatibility is again sub-classified into gametophytic and sporophytic self-incompatibility (Hiscock and Kues 1999). In a gametophytic system, the haploid pollen genome controls the incompatibility response, whereas, in a sporophytic system, the parental plant's diploid genome controls the incompatibility reaction. Gametophytic self-incompatibility which persists in 17–25 families is the most prevalent of the two types (Charlesworth 1985; Clure 2006; Iwano et al. 2012; Steinbachs and Holsinger 2002; Weller et al. 1995), whereas sporophytic self-incompatibility is less common and is only found in five families viz., Asteraceae (Hiscock 2000; Hughes and Babcock 1950; Wollenweber et al. 2021), Brassicaceae (Bateman 1954), Convolvulaceae (Martin 1968; Mehlenbacher 1997), Betulaceae (Prigoda et al. 2005; Schierup et al. 2006; Thompson 1979) and Caryophyllaceae (Lundqvist 1990). The gametophytic system comprises binucleate pollen characterized by slow growth of pollen tubes in the style (Brewbaker 1957). Trinucleate pollen in the sporophytic system inhibits pollen germination and pollen tube penetration into the stigma. In plants with homomorphic self-incompatibility, specificity is encoded by the S-locus that carries the identity of both the alleles ‘S and s’ from the sporophyte that interact on the stigmatic surface. In heteromorphic self-incompatibility, two types of flowers are present viz., pin and thrum type which differ in their floral morphology (Barrett 1992). Flowers with short stamens and long style are referred as pin type flowers and the opposite condition is termed as thrum type having long stamens and short style being present on different plants. This condition is referred as distyly governed by a single S-locus with two alleles (S and s). The only compatible mating in this case is between pin and thrum flowers. Three varied self-incompatible systems have been observed at molecular levels where sporophytic self-incompatibility (SSI) was elucidated in Brassicaceae and Asteraceae while two divergent types of gametophytic self-incompatibility (GSI) (S-RNase based SI) were studied in Solanaceae and Rosaceae along with programmed cell death (PCD) in the Papaver system (Charlesworth 2010; Sanz et al. 2020).
One-tenth of the angiosperm species belong to the family Asteraceae that owns attractive ornamental plants with unique floral traits for which they are valued at the international market (Nakano 2021). Floral parts of these plants are utilized as cut flowers for making floral arrangements, as loose flowers, ornamental pot plants and also as bedding plants for beautifying the landscape gardens throughout the world (Joshi et al. 2010; Ohmiya 2018). Major flower crops belonging to this family with high ornamental value in both domestic and international markets are chrysanthemum (Chrysanthemum × morifolium Ramat.), gerbera (Gerbera hybrida L.), dahlia (Dahlia × pinnata) and asters (Hao et al. 2011). Among all these, chrysanthemum (Chrysanthemum × morifolium) is a significant floriculture crop with diverse uses (Anderson 2007; Teng et al. 2008) in the floral market. In recent times, a huge increase in demand is evident in the ornamental crops particularly in chrysanthemums (Chrysanthemum × morifolium) because of diverse flower colors, forms and improved vase life. This flower finds its place in making of floral ornaments, garlands, hair decorations and as a bedding plant in landscape gardens (Joshi et al. 2010).
The existence of the sporophytic self-incompatibility mechanism was first reported in the Asteraceae crop Crepis foetida (Hughes and Babcock 1950) followed by Parthenium argentatum (Gerstel 1950), Cosmos bipinnatus (Crowe 1954), Helianthus annuus (Habura 1957), Chrysanthemum cinerarifolium (Brewer and Parlevliet 1969), Carthamus flavescens (Imrie and Knowles 1971), Cichorium intybus (Eenink 1981; Gonthier et al. 2013) and Senecio squalidus (Allen et al. 2011). Numerous factors are responsible for the failure of self-pollination that include pre-fertilization barriers (lack of pollen germination, presence of trinucleate pollen, unusual pollen tube growth on stigma or style before fertilization, reciprocal crossing differences), and post-fertilization barriers (embryo abortion or hybrid inviability) resulting in low seed setting thereby decreasing breeding efficiency in plants (Deng et al. 2010; Hu 2005; Jing 2000; Sun et al. 2009a, 2011). Garden chrysanthemums are self-incompatible, highly heterozygous aneuploids with complex genetic makeup and unclear trait inheritance patterns. Additionally, the reproductive biology of the chrysanthemum is particular to pollen viability, pistil receptivity, pollen germination on stigma, growth of embryo and seed set percentage, which differs noticeably among various genotypes in relation to their reproductive capacity and affects the shape and size of populations (Rehana and Bala 2021). Nonetheless, most of the chrysanthemum cultivars are unable to get through these pre-fertilization barriers, hence; overcoming self-incompatibility becomes peremptory for successful breeding and crop production (Sun et al. 2011).
In light of the current importance of self-incompatibility acting in the family Asteraceae, an attempt has been made to understand the concept and work done so far to review the recent advances in assessing self-incompatibility. Also, different physiological and molecular techniques involved in breaking down self-incompatibility in important ornamental Asteraceae crops are discussed, thereby giving a holistic approach to the understanding of the trait. Along with that, evolutionary events that led to the transition from self-compatibility (SI) to self-compatibility (SC) are explained as well.
Floral reproductive biology
The anatomy of floral reproductive parts in ornamental crops may participate in bringing complexities like self-incompatibility. The inflorescence of Asteraceae members is a composite head or capitulum consisting of a cluster of small flowers aligned together with both female and perfect flowers aligned in a single flower head. The outer whorls of a flower head comprise of ray florets (pistillate or female) followed by disc florets (perfect or bisexual) located at the center of the inflorescence. Ray florets are attractive and bigger in size with different hues to attract insects for pollination whereas the disc florets are minute and located at the center of the capitulum in gerbera and chrysanthemum respectively (Jaime and Silva 2014; Laitinen et al. 2006). The capitula of Gerbera aurantiaca comprises of 100 central disc (bisexual) florets being surrounded by 100 ray florets (female) and distributed in the outer three whorls (Johnson et al. 2004, 2021). In chrysanthemum, anthesis progress from outer to inner whorls i.e., ray florets open first followed by disc florets that open in the morning hours. Pollen viability is highest from 11:00–14:00 h on the third day after anthesis and the staminate florets start to lose powder eventually from the third day until they gradually wither on the eleventh day (Xu et al. 2012). Stigma receptivity is highest during 01:00–02:00 pm in the afternoon and it is strongest during the fifth to seventh day after flower opening. Four different forms of stigmas are present in which I-shaped stigmas are seen from the third to sixth day after flower opening which gradually turns to Y-shape and eventually develop into horns and wilt type. Among all, Y-type stigmas are highly receptive which could be pollinated between 11:00–14:00 h on sunny days to achieve high pollination efficiency (Yang et al. 2018a). Stigmas located at the center of a capitulum are highly receptive with strong mucus secretions than those situated at the outer edges. Pistil development may get delayed by 1–2 days when emasculation is coupled with wiping off the petals of ray florets. However, emasculating the disc florets during hybridization does not influence the pistil development in ray florets (Zhao et al. 2008). Another Asteraceae flower namely, Senecio squalidus, has an inflorescence that resembles a capitulum, consisting of an outer whorl of carpellate ray florets and interior whorls of bisexual disc florets. The individual disc florets grow in whorls, with the outer whorls of the inflorescence maturing before the inner whorls. The pistil possesses a style, bilobed semi-dry stigma and a single ovary. In immature pistils, two stigmatic lobes are packed tightly to protect the stigmatic papillae. As the pistil matures and develops anthers, sterile pseudo-papillae at the tips of the stigmatic lobes receive pollen from anthers and deliver it to pollinators. When the stigma reaches maturity, two lobes get separate to show the receptive papillae cells (Allen et al. 2011). Figure 1 depicts the floral morphology of general Astereceae species indicating its different flower parts.
Causes of self-incompatibility
Lack of seed set in polyploid crops due to low fertility, inbreeding depression or the existence of self-incompatibility is a major problem faced by breeders (Anderson et al. 1992, 2007; Miler and Wozny 2021). Biparental inbreeding depression after selfing have resulted in F1 seedlings with low survival rate, irregular phenotypic traits and stunted plant growth in Gaillardia pulchella (Heywood 1993). Additionally, this problem has made breeders incorporate other species into the crosses with chrysanthemum (syn. Dendranthema) genome (Smith 1913). Random outcross pollinations between unrelated and non-inbred genotypes have resulted in a low seed set of 36–71% and even lower up to 24.5–38.5% (Ronald 1974) with a maximum not exceeding 50% (Anderson and Ascher 2000). Low seed setting efficiency in Chrysanthemum species may be related to the fact that most of the greenhouse cultivars are developed through induced mutagenesis, which rendered a loss of their natural reproductive abilities (Miler and Kulus 2022). Also, lack of pollen (trinucleate) germination, inhibition of pollen tubes on stigma and reciprocal crossing differences are the main causes of low seed set (Sun et al. 2010). Similarly, both in the case of gerbera and Hymenoxys herbacea, pollen limitation is a major constraint in self-pollination (Campbell and Husband 2007; Johnson et al. 2004). These factors have been linked to the presence of a sporophytic self-incompatible system whose expression is regulated by S loci. The garden chrysanthemum is a hexaploid with 3 loci governing self-incompatibility. If these three loci are active, each clone may contain six S-alleles and the offspring of a compatible cross may include up to 12 distinct S-alleles. When individuals with three separate loci and a compatible cross are produced, only 0.25% of the crosses are predicted to be compatible. In contrast, 2.8% of crosses are compatible when one locus is operational and 25% of compatible crosses with two loci are predicted (Drewlow et al. 1973).
Self-incompatibility (SI) promoting out crossing in cultivated chrysanthemum (syn. Dendranthema grandiflora) was first reported by Niwa (1931) and it has been documented in the chrysanthemum complex at all ploidy levels, including diploid (Dendranthema boreale), hexaploid (Dendranthema japonense), octaploid (Dendranthema ornatum) and decaploid (Dendranthema shiwogiku). Both garden and greenhouse chrysanthemums are extremely self-sterile (Teynor et al. 1989) and produce very low seed after selfing or outbreeding between genotypes sharing similar S alleles (Anderson and Ascher 2000). Other economically important species viz., octaploid dahlia (Dahlia variabilis) (Lawrence 1931; Broertjes and Ballego 1967), pyrethrum (Chrysanthemum cinerarifolium, syn. Tanacetum cinerariifolium) also possess sporophytic self-incompatibility (Thorpe 1940). Reports on incompatibility are also evident in Cosmos bipinnatus (Little et al. 1940), Parthenium argentatum (Gerstel 1950) and Crepis frtida (Hughes and Babcock 1950) where one incompatibility gene is predicted with multiple alleles which act individually in the style as in nicotiana, but the behaviour of the pollen is determined by the sporophyte and the alleles exhibit dominance, both features being associated with the heteromorphic outbreeding types. Senecio squalidus and Cichorium species are vital model crops for the study of SSI in Asteraceae because their recent population history has been well documented comprising extreme population events that are probable to have an impact on the behaviour of their SSI mating system (Brennan et al. 2011; Palumbo et al. 2023). Self-compatibility and ability for autonomous seed set was studied among twelve invasive Asteraceae species in China by Hao et al. (2011) where in the percentages of self-compatible species (66.7%) and species capable of autonomous seed set (83.3%), including self-fertilizing and apomictic species, were significantly larger than expected from the percentages of such species in the global data sets of Asteraceae (36.8% and 46.0% respectively). These results support the predictions of Baker's Law that self-compatible species, and particularly those capable of autonomous seed production, are more likely to establish and spread in a new range. High polyploidy levels are typically associated with bigger plants since most of the greenhouse chrysanthemums are segmental allohexaploids possessing huge capitula impeding the genetic analysis studies. These constraints have made it impossible to use the cutting-edge breeding techniques utilized for other crops, therefore, the standard breeding method for chrysanthemums still relies on straightforward crossings and clonal multiplication through stem cuttings.
Other causes
There are other morphological factors that bring physiological changes in floral parts following inbreeding that may elucidate the presence of self-incompatibility system in varied species of the Asteraceae family.
Pollen morphology and viability
Seed set is often pollen limited in self-incompatible species because of the inadequate quantity or quality (compatible) of pollen being received by the female (Larson and Barrett 2000). The structural morphology of pollen also affects its germination on stigma as abnormal pollen morphology leads to low pollen germination in chrysanthemum as reported by Wang et al. (2018). The percentage of available viable pollen is also an important factor for a successful fertilization. Figure 2 is showing the visual examination of chrysanthemum pollen as seen under fluorescent microscope.
Stigma morphology
Angiosperm stigmas are classified into three broad categories viz., wet, dry and semi-dry types on the basis of surface extrusions (Harrison 1981; Harrison and Shivanna 1977). In the case of dry type stigma, pollen hydration on the stigma is a regulated mechanism and pollen capture along with its adhesion depends on s gene specificity of species (Dickinson 1995; Zinkl and Preuss 2000; Zinkl et al. 1999). In the case of dry stigmas, presence of a continuous cuticle imposes a major barrier to pollen tube penetration that can be surpassed by pollen secreting hydrolytic enzymes such as cutinase (Allen et al. 2011; Hiscock et al. 2002). On the contrary, in species with wet stigmas, pollen capture is a non-specific mechanism and pollen hydration within the secretion is passive which is not regulated. In this case, epidermal cells of stigmas lack a continuous cuticle that allows for an effortless penetration of the pollen tubes.
As far as Asteraceae species are concerned, stigmas mostly characterized as dry type previously (Allen et al. 2011; Harrison and Shivanna 1977). However, recent studies on pollen-stigma interactions in poppy, cosmos, helianthus and German chamomile have revealed that stigmas of Asteraceae species are not entirely dry as they excrete a small amount of surface secretion (Elleman et al. 1992). These observations were further supported by assessing pollen-stigma interaction in Senecio squalidus, which led to a new classification of the Asteraceae stigma as semi-dry type. This stigma holds a lipid-rich secretion consisting of carbohydrate and protein in the basal regions of stigmatic papillae where the cuticle is absent (Hiscock et al. 2002). Once the pollen comes in association with the stigma, the flow of this secretion gets enhanced irrespective of pollen compatibility (Allen et al. 2011; Hiscock et al. 2002).
Pollen-pistil interactions
Physical separation of female and male gametophytes by the carpel has resulted in the evolution of a number of complex series of cellular and molecular interactions that are together termed as pollen-pistil interactions (Harrison 1975). During this molecular courtship, discrete recognition processes get associated in actively discriminating incompatible pollen at interspecific and intraspecific levels (Hiscock and Allen 2008). On the other hand, in the case of compatible pollinations, ovules synthesis is limited so as to compete with the pollen tubes leading to an additional level of selection on the male gametophyte, a consequence of the carpel which targets the evolutionary success of angiosperms (Hormazo and Herrero 1992).
Incompatible pollen-pistil interactions in Asteraceae species (cosmos, ambrosia and helianthus) marked the development of exine-held pollen coat prior to pollen and stigma association (Dickinson and Lewis 1975; Vithanage and Knox 1977) which eventually led to the inhibition of incompatible pollen at the stigma surface and the deposition of callose in pollen tubes along with its adjacent cells on stigma (Allen et al. 2011). In Ambrosia species (self-compatible) and Cosmos bipinnatus (self-incompatible), incompatible pollinations resulted in the rapid release of pollen wall material through the sexine pores and colpi of pollen grains (containing a diversity of enzymes, carbohydrates and lipids) onto the stigma surface within 10–15 min after pollination (Howlett et al. 1975).
Pollination events in Cosmos bipinnatus and Helianthus annus reported a secretory response of pollen wall material by the stigmas directly after pollination (Elleman et al. 1992). A substantial correlation was also observed between possession of dry stigmas and the presence of complex pollen coatings on pollen grains (Dickinson 1995; Dickinson et al. 2000). These pollen coatings also performed a role similar to that of stigmatic exudates. Currently, new researches are taken upon to identify molecules that could mediate specific events during the pollen-pistil interaction, such as pollen adhesion and hydration, pollen tube growth and navigation through the pistil, particularly self-incompatibility (Hiscock and Allen 2008).
Chemical compounds
When chemical substances secreted from both stigmas and pollen walls do not interact properly, pollen grain germination is usually prevented on stigmas, ultimately hampering pollen tube growth and fertilization (Sun et al. 2010). Callose production was witnessed on stigmatic papillae within 15 min after incompatible mating in Cosmos bipinnatus (Knox 1973). Synthesis of callose plugs were reported by Boyle and Stimart (1986) as a result of inhibition of pollen germination and pollen tube growth on the stigmatic surface suggesting the activity of sporophytic S-gene in reciprocal interpecific crosses between Zinnia angustifolia and Zinnia elegans. Samaha et al. (1989) also tested self-incompatibility in Zinnia angustifolia clones by determining the amount of callose depositions on stigmatic papillae following pollinations. It was inferred that callose fluorescence intensity ranged from 6.4 to 9.9% with high pollen load and little or no callose depositions in stigmatic papillae following compatible crosses. In the case of incompatible crosses, pollen load was low and callose lenticules were deposited on stigmatic papillae with high callose fluorescence intensity that ranged from 47.9 to 62.6%. Before pollen germination and within hours of the pollination, there is an increase in ethylene production in the stigma and style. Although, it is hypothesized that the fundamental pollination signals from plant hormones, auxin, is transmitted directly from pollen to the stigma (Kovaleva and Zakharova 2003). In another investigation on Iberis species, the ß-1, 3-linked glucan, called callose, was shown to accumulate in both stigmatic papillae and pollen tubes within 4–6 h after an incompatible mating.
Enzymes
In general, cutinase, esterases and pectinases participate in incompatibility reactions (Christ 1959). Studies on Tropaeolum majus pollen (Takahashi et al. 2010) showed the existence of enzymes belonging to four esterase classes namely, acetylcholine esterase (EC 3.1.1.6), cholinesterase (EC 3.1.1.7), pectin esterase (EC 3.1.1.11) and cutinase (EC 3.1.1.74) where cutinases and pectin esterases were significantly involved in pollen-pistil interaction (Nemaz et al. 2019). Cutinases and serine cutinases were also needed for pollen tube penetration of the wet and dry stigmas after pollen-stigma contact (Hiscock et al. 2002) while pectin esterases were involved in intercellular pollen tube growth in the subsequent phase of pollination.
Proteins
Certain peptides act as pollen signals and identify incompatibility when received by a specific receptor. These include S-locus cysteine rich proteins (SCR) or S-locus protein 11 (SP11). The extremely polymorphic SCR or SP11 is identified by the similar polymorphic S-locus receptor kinase (SRK), which determines the SI specificity (Charlesworth 2010; Ma et al. 2016). The sudden halt of pollen tube elongation is brought by the S-locus specific S-RNase (S-locus ribonuclease), an abundant and extremely polymorphic pistil-specific glycoprotein which is released into the extracellular matrix that lines the course of pollen tube expansion (Sijacic et al. 2004). From previous research, it may be deduced that the S-locus, also known as the S-haplotype that controls self-incompatibility in Asteraceae members sporophytically (Bateman 1955). From research conducted by Takasaki et al. (2000) and Koseva et al. (2017), S-locus glycoprotein (SLG) was considered as a SI-related gene present in stigma which is necessary for full SI response activation in addition to the female S determinant. S-locus receptor kinase (SRK) with bound serine/threonine was observed to be the female S-determinant in the Asteraceae family, which upon interaction with SLG (S-locus glycoprotein) helped to display self-incompatibility. The two essential genes of the SI response were located in the male S-determinant, called as SCR (S-locus cysteine-rich protein)/SP11 (S-locus protein 11) (Charlesworth 2010; Schopfer et al. 1990) a type of pollen coat protein (Koseva et al. 2017; Takasaki et al. 2000; Wang et al. 2018). It is believed that before self-pollen come in contact with the stigma, SRK (S-locus receptor kinase) is inhibited by the thioredoxin H proteins (THL1 and THL2) (Bower 1996; Haffani 2004). Once self-pollen grains occupy space on the stigma, SRK get activated by SCR and is further accompanied by another S-locus cytoplasmic receptor kinase, M-locus protein kinase (MLPK) (Kakita et al. 2007). EXO70A1, a subunit of the exocyst made up of eight subunits, is one of the self-compatibility-related proteins that are phosphorylated by activated CRISPR-Cas9, which causes ubiquitination and proteasomal destruction of the proteins (Samuel et al. 2009; Stone et al. 2003). Safavian et al. (2015) tested the remaining seven subunits, SECRETORY-SEC3, SEC5, SEC6, SEC8, SEC10, SEC15 and exocyst subunit- EXO84, as compatible factors to promote compatible pollen grain acceptance. Many other parallel pathways also work simultaneously other than ARC1 linear ubiquitin–proteasome pathway for SI response (Tantikanjana et al. 2010). Post pollen stigma interaction, hydration helps pollen grains to germinate and pollen tubes to emerge rapidly. To penetrate the stigmatic papilla, the pollen tubes grow through the stigmatic cuticle and then enter the outer layer of the stigmatic cell wall. At this stage, stigmatic cell wall modification is required, which is protein dependent involving EXO70A1, secreted by the stigmatic papilla that is required for the delivery of proteins for pollen tube growth through the stigmatic cuticle. Cell wall abundant ribosome proteins are identified on stigmatic papillae affecting the pollen tube growth and controlling self-incompatibility (Yang et al. 2018b). Considering the case of Cosmos bipinnatus, the rejection or acceptance responses are immediately prevailed within 10 min after pollen arrival on the stigma (Howlett et al. 1973, 1975). The binding of pollen-wall antigens with the stigma surface was augmented by a proteinaceous pellicle formulating a site for pollen or stigma recognition reactions (Mattson et al. 1974).
Gene action
In the Asteraceae (syn. Compositae) family, the reproducing ability of two individuals relies on the alleles they share at a multiallelic S-locus. Stephens et al. (1982) reported that a majority of the sporophytic self-incompatible systems are regulated by a single highly polymorphic S-locus with at least two tightly linked polymorphic genes (S and s) that inherit as a single unit, one out of these genes regulates pollen identity and the other controls pistil identity (Golz et al. 2000; Lai et al. 2002; Lewis 1951). Mating between individuals only occur when both plants share S alleles distinct from their own unless the alleles show dominance in the pistil or pollen (Ferrer and Avila 2007; Nettancourt 1997). The pollen will dehydrate or germinate on the stigmatic surface or the pollen tube may not be able to pass through the stigma if the recipient plant's diploid genotype and the parent plant's genotype that produces the pollen possessing the same S-allele (Brennan et al. 2002; Hiscock and Tabah 2003). Numerous other genes are recognized to exist at the S-locus in addition to these genes which get expressed on the stigmatic surface during anthesis (Jeong et al. 2014; Takayama and Isogai 2005).
Aster furcatus (23 populations) distributed across four geographical regions (Wisconsin, Illinois, Indiana and Missouri) were examined for self-incompatibility by evaluating 22 electrophoretic loci and observed deviation at triose phosphate isomerase (TPI-1) followed by aspartate aminotransferase (AAT-2) and aldolase (ALD-1) (Les et al. 1991). Two rare alleles with variability at S-locus i.e. TPI-1 indicated that maximum genetic variation at the polymorphic locus was due to differentiation among populations. In another species, Taraxacum koksaghyz differentially expressed genes (DEGs) were analysed and three candidate genes (LRX4, TUBB and XTH33) were reported (Wollenweber et al. 2021). Likewise, MDIS1 INTERACTING RECEPTOR LIKE KINASE 2 (MIK 2) was noticed as a crucial candidate gene for SSI in cichorium (Palumbo et al. 2023). After both compatible and incompatible pollinations, there was an expansion in pollen tube growth distinguished by an overall enrichment for biological processes including signaling and different response mechanisms uplifting transcriptional regulation.
External factors
It is generally acknowledged that external influences also have a significant impact on pollen germination as the pH of the stigma varies significantly between genotypes. This genotype-dependent component has been linked to pollen germination response which also affects the fertilization process and eventually the seed set. During pollen tube growth, ionic elements like boron and calcium are engaged in the metabolism and may accelerate or impede the process depending on the concentration (Caser 2017).
Flexibility in self-incompatible reaction
There is an exclusive case among chrysanthemum cultivars which are mostly self-incompatible with a small proportion of self-compatibility. These cultivars project comprehensive separation of self-incompatibility among their progeny and transform to become self-compatible in the succeeding generations. This condition is cultivar dependent and gets affected by environmental conditions (i.e. temperature) during flowering. Chrysanthemum has the capability to showcast this phenomenon via pseudo self-compatibility and end of season pseudo self-compatibility.
Pseudo self-compatibility (PSC)
Self-incompatibility is widely persistent in the plant kingdom; even then, many flowering plant families have frequently undergone the evolutionary transition from being self-incompatible to self-compatible (Dana and Ascher 1985; Goodwillie 1999; Igic et al. 2004) and sometimes moderately self-incompatible (Stephenson et al. 2000). These species are referred to as pseudo-self-compatible or pseudo-self-fertile population and they are more prevalent in around 10% of the species examined in the family Asteraceae (Cheptou et al. 2002; Stout 1917). The term "partially self-incompatible" refers to species that show quantitative variation in the level of self-incompatibility ranging from being partially self-incompatible to self-compatible (Ferrer and Avila 2007; Levin 1996). The self-incompatible system is temporarily or partially inactive under pseudo-self-compatible (PSC) conditions but resurfaces in succeeding generations or during periods of lower environmental stress. Eschscholtzia californica and Abutilon darwinii, which were self-incompatible in Brazil behaved as pseudo-self-compatible in England and were the first species to record pseudo-self-compatibility (PSC) (Darwin 1876).
The occurrence of self-compatible individuals within self-incompatible populations is relatively frequent and has been observed in Asteraceae species such as Carthamus flavescens (Imrie and Knowles 1971), Stephanomeria exigua sub sp. coronaria (Brauner and Gottlieb 1987), Rutidosis leptorrhynchoides (Young et al. 2000) and Senecio squalidus (Brennan et al. 2002). Although this phenomenon has occurred at a very low frequency in Asteraceae, still the pseudo-self-compatibility (PSC) was also noticed in garden chrysanthemums (Chrysanthemum × morifolium Ramat.) by Ronald and Ascher (1975a). As per recent macro phylogenetic study in the family Asteraceae, pseudo-self-compatibility is not considered a standard condition and it could change back to both self-incompatibility (SI) and self-compatibility (SC) which could transform the garden chrysanthemums to greenhouse types (Ferrer and Avila 2007; Ronald and Ascher 1975c). Keeping this phenomenon in mind, mating of greenhouse (SI) × garden (PSC) cultivars yielded F1 hybrid in 1:1 ratio (PSC: SI) that demonstrated the pseudo-self-compatible occurrence in chrysanthemums producing both SI and PSC progeny (Ronald and Ascher 1975a, b). Transitions from being self-incompatible to partially self-compatible require the breakdown of a genetic self-incompatible (SI) system (Koseva et al. 2017). Pseudo-selfing species generally have flowers that are reduced in size and floral parts similar to their outcrossing relatives, a feature referred as selfing syndrome (Sicard and Lenhard 2011). In addition to a high percentage of self-seed, selfing species have fewer, smaller and less showy flowers, lower pollen: ovule ratios and smaller anther-stigma separation relative to outcrossing relatives. This sort of floral morphology was comparatively reported in Tolpsis coronopifolia (Crawford et al. 2019; Koseva et al. 2017). Ortiz et al. (2006) observed in H. salzmanniana that self-compatible populations had fewer flowers per head, reduced flower head diameter and a shorter period of anthesis than self-incompatible populations. Contrastingly, in a few species of Asteraceae, the self-incompatible plants have bigger heads than the congeneric self-compatible ones. In another relevant study carried out by Gibbs et al. (1975), out of five species of Senecio, three were self-incompatible namely S. joppensis, S. aetnensis and S. squalidus that had a larger head diameter than the self-compatible species (S. viscosus and S. vulgaris). Such a pattern was also reported in Hypochaeris radicata by Parker (1975). Selfing syndrome was also reported in other self-incompatibility systems, as in Eriotheca (Oliveira et al. 1992) with late-acting self-incompatibility and Anagallis (Gibbs and Talavera 2001) possessing gametophytic self-incompatible system etc. These species transition to self-compatibility may be influenced by alterations in the pre-eminence relationships among S-alleles (Brennan et al. 2002; Reinartz and Les 1994), the availability of unlinked modifier loci (Avila et al. 2008), differential rejection of self-pollen in case of interspecific and allozygous populations, and a variety of abiotic factors including temperature, flower age and the occurrence of developing fruits (Lafuma and Maurice 2007; Levin 1996), etc. In Hypochaeris species, self-compatibility has arisen through loss of allelic diversity at the S locus (S alleles with unequal frequencies) due to bottleneck events and genetic drift. In Aster furcatus, self-compatibility has evolved as a result of bottleneck-induced genetical losses of S-alleles. Self-compatibility was correlated with mean number of ovules per inflorescence in Aster furcatus demonstrating that self-compatibility appeared to be under partial environmental influence (Reinartz and Les 1994).
Pseudo-self-compatibility is inherited quantitatively as a continuous distribution in inbred populations. Studies revealed that if inbreeding depression exceeded 0.5, then, there was no mutational enhanced self-compatibility rate (Charlesworth 1980; Lande and Schemske 1985). It was observed that high PSC levels were not highly heritable when realized heritability (RH) ranged from 0.05 to 10.19% in chrysanthemum (Anderson and Ascher 1996). Inbred offspring with greater PSC levels made up 43–50% of the self or crossings between low PSC × low PSC parents. High PSC levels stimulated non-additive gene activity, whereas low to mid PSC selection brought the PSC threshold with additive action of genes (Anderson and Ascher 1996). Continued inbreeding is thought to be the best strategy when homozygosity at S loci is increased, resulting in the segregation of the different incompatibility classes in order to determine the functional relationship of loci to each other (Drewlow et al. 1973).
Factors like the degree of pollen limitation, the strength of the S-linked loci, background genetic load, linkage status of S-locus modifiers and mutations to the functional S-locus could bring changes elsewhere in the genome that can vary quantitatively owing to mutations at multiple, unlinked loci causing a change in the compatibility status of plants (Avila et al. 2008; Ferrer and Avila 2007; Hancock et al. 2003; Levin 1996; Porcher and Lande 2005). Mutations in two candidate genes leading to self-incompatibility breakdown were observed in Tolpsis coronopifolia (Koseva et al. 2017). Each gene had a coding sequence insertion or deletion mutation within the self-compatible species that produced a truncated protein. Homologs of each gene were implicated in pollen development, pollen germination and pollen tube growth in other species. The initial increase in self-compatibility under environmental conditions was strongly influenced by the extent of its heterozygous effect. If plants were heterozygous for an SC mutation, there was an incremental reduction in self-sterility and the plants could generate progeny at a much higher rate by selfing (Koseva et al. 2017). In the family Asteraceae, if the number of S alleles at the S locus becomes low and the mate availability is limited, then self-compatible individuals are selected to make the population strongly self-fertile (Hiscock and Tabah 2003; Imrie and Knowles 1971). Pollen limitation is considered to be another condition favoring the breakdown of self-incompatibility (Charlesworth and Charlesworth 1979). Loss of function due to mutations in genes that are essential for self-incompatibility is the most common genetic mechanism that could collapse self-incompatibility (Nasrallah 2017).
End of season pseudo self-compatibility (ESC)
Sometimes, the self-incompatible reaction weakens towards the completion of their blooming season as seen in nicotiana (East and Park 1917), Beta vulgaris (Owen 1942), trifolium (Townsend 1965) and brassica (Johnson 1971). Additionally, the weaker state of plants and increased temperatures also progress the condition of PSC (Litzow and Ascher 1983). In natural populations, having both SI and PSC serves useful functions by encouraging outcrossing. SI preserves genetic diversity whereas PSC and ESC guards against the extinction of the original parental genotype. This phenomenon of end of season pseudo self-compatibility (ESC) does not persist in chrysanthemum genotypes. According to Anderson and Ascher (1996), exposure to heat can considerably enhance the self-seed set by promoting pseudo self-incompatible condition in chrysanthemum (Ronald and Ascher 1975a).
Late-acting self-incompatibility
A sizeable cohort of Asteraceae species are self-incompatible despite the pollen tube that reaches to ovary and in most cases, penetrates into ovules before they get inhibited prior to fertilization. This phenomenon is called as late-acting self-incompatibility (LSI) (Gibbs 2014) or pistillate sorting (Sage et al. 1994) or ovarian incompatibility. Seavey and Bawa (1986) observed absence of fruit set following selfing despite successful growth of self-pollen tube towards the ovary. This condition operates through two different mechanisms which are discussed as below.
Pre-zygotic self-incompatibility
In this incompatibility system, self-pollen tubes grow into the ovary but cannot penetrate into ovules (Beardsell et al. 1993; Gibbs 2014) although in some cases, if self-pollen tubes penetrate ovules, the pollen tube growth arrests within the micropyle (Gibbs 2014). This causes very few germinated pollen grains on stigmas and abnormal growth of pollen tubes before fertilization.
Post-zygotic self-incompatibility
The failure of syngamy occurs after discharge of male gametes into the embryo sac leading to post-zygotic self-incompatibility causing embryo abortion or hybrid inviability (Cope 1962; Gibbs 2014; Sparrow and Pearson 1948).
Both pre-and post-fertilization barriers are the main factors causing the failure of wide hybridization between Chrysanthemum grandiflorum and Chrysanthemum nankingense (Sun et al. 2009a, b, 2010, 2011). Perhaps, the post-fertilization barriers (embryo abortion) can be partially overcome by means of in vitro ovary and embryo culture in chrysanthemum (Deng et al. 2010; Tang et al. 2009; Sun et al. 2011).
Pre-zygotic or late-acting self-incompatibility has suffered a neglect that is disproportionate to its likely occurrence. It is usually assumed that all plants that fail to set seeds following selfing must be under the possession of self-incompatibility. It is now known that if pistils are rejected after successful penetration into the ovules, then early acting inbreeding depression must be the cause (Dorken and Husband 1999). Early acting inbreeding depression (EID) causes early rejection of self-fertilized ovules due to deleterious recessive alleles in the population as hypothesized by Bittencourt et al. (2003). Contrastingly, another proposal stated no-fusion when both male and female gametes carry the dominant allele (Cope 1962). However, the exact reason for delayed pistil abscission in autogamous pollinations of incompatible species still needs to be investigated (Hao et al. 2012). Hence, further studies are necessary to provide new insights into this enigmatic breeding system.
Table 1 depicts the progressive work carried out by scientists all over the globe in past concerning self- incompatibility and other pre-fertilization barriers in diverse floricultural families that allows us to open new horizons for unveiling the unexplored work needed to carry forward particularly in the family Asteraceae.
Methods to assess self-incompatibility
The majority of cultivars belonging to Asteraceae family are self-incompatible, baring few that set the seed with low frequency when provided with favorable environmental conditions. Therefore, a variety of approaches, including the pollination method, cytological and molecular techniques have been followed to evaluate self-compatibility in flower crops of Asteraceae.
Pollination method
This method involves the bagging of unopened inflorescences with butter paper bags 3 days before anthesis which ensures self-pollination. In some cases, artificial self-pollination is performed with the pollen collected from the same plant (in case of pollen shy cultivars) and the bagged flowers are left for seed formation. Seed set usually occurs 2 months after pollination in chrysanthemum and the bagged inflorescences are generally harvested after 60 days. The amount of self-incompatibility reaction is governed by counting the number of seeds produced per capitulum. When more seeds are produced, it is due to self-compatibility while less seed production is a determinant of self-incompatibility. The major drawback of this method is that it requires long time to evaluate the self-incompatibility reaction (Wang et al. 2014). The number of seeds produced is also influenced by a variety of abiotic and biotic factors (viz., temperature, humidity, the prevalence of pests and diseases and many others). Apart from that, frequency of pollinations also influences seeds in chrysanthemum (Chrysanthemum × morifolium). One report has suggested that repeated pollinations for 1–2 times per week gave high seed set in chrysanthemum which was specific to the structure of the inflorescence (Miler and Kulus 2022). According to Drewlow et al. (1973), inbreeding caused an increase in homozygosity and vigour loss by reducing the amount of heterozygous S loci while sometimes, inbred parents produced more self-seed than outcross seed (Zagorski et al. 1983).
In another research, when autogamy between the self-compatible (SC) species Tolpis coronopifolia and its self-incompatible (SI) relative Tolpis santosii was performed, it was inferred that self-seed set obtained from recombinant plants within two F2 populations gave self-compatibility (Lai et al. 2002). In parallel to this, Wang et al. (2014) observed seed set in 10 out of 24 chrysanthemum genotypes (Chrysanthemum x morifolium) evaluated through selfing where seed set of these genotypes varied between 0.03 and 56.50% and compatible indexes ranged between 0.04 and 87.50 respectively. Highest seed set was noticed in the cultivar “Q10-33–1” (seed set (56.50%) and compatible index 87.50) with ten of its progenies showing a wide range of variation in seed set (0–37.23%) and compatible index (0–68.65) indicating that there was a comprehensive separation of self-incompatibility among progeny from the same self-pollinated self-compatible chrysanthemum cultivar. Self-pollination was observed to be successful in Silphium integrifolium, S. perfoliatum and their interspecific hybrids but the strength of self-incompatibility varied across genotypes and populations (Reinert et al. 2020). Storage conditions can also affect the pollen germination as mentioned by Miler and Wozny (2021) who reported an overall increase in in vitro pollen germinability and seed set efficiency by storing pollen at – 80 ℃ temperature prior to pollination.
Cytological method
This method involves the self-incompatibility reaction being judged by assessing the pollen germination on stigma, pollen tube growth and embryo development after selfing. Pollination is semi-compatible if only one pollen tube penetrates the surface; otherwise, it is compatible if many pollen tubes penetrate the surface and it is incompatible if no pollen tubes penetrate the style (Vijayakumar et al. 2018). In the case of compatible pollination, stigma penetration occurs within 30 min after pollination (Wollenweber et al. 2021). Inhibition of pollen tube growth and improper pistil receptivity are the primary factors preventing chrysanthemums from setting seeds. In the case of compatible pollination, the pollen tube enters the ovary through the style and completes double fertilization to develop an embryo whereas no embryo will be formed in an incompatible case. After self-pollination, the pollen tubes get arrested on the stigma surface and the callose rejection response is detected within 15 min of autogamous pollination (Howlett et al. 1975).
Ovary development is directly related to seed set in chrysanthemum, where ovary abortion has been noticed with the progression of time after self-pollination. A typical compatible ovary produces full ovules that gradually turn dark until the seed forms, whereas incompatible ovaries are smaller and white or lighter in color (Wang et al. 2018). Heart shaped embryos start to develop in the ovaries 12 days after selfing, while torpedo or cotyledon embryos begin to grow in the ovaries 18 days after selfing in compatible pollinations. A nucleus and nucleolus, as well as several common cell organelles such as golgi complex, mitochondria and vacuoles get developed in normal embryonic cells. Tetra nucleate embryo sacs with one egg cell, one central cell and two synergids near to the micropylar end are seen in self-compatible reaction; on the other hand, degraded megaspores are formed in cases of embryo abortion. At the earliest phases of embryo abortion, the cytoplasmic vacuolation, condensed cytoplasm and degenerating cell organelles are seen in the abortive embryonic cells. Incompatible ovaries experience thickening of the integument and degeneration of the embryo sac with the progression of time following self-pollination in Chrysanthemum × morifolium (Wang et al. 2018) and Paeonia ludlowii (Chen et al. 2022). Bivalent formations are highly regular at metaphase-I in the pollen mother cells along with sporadic distribution of univalent and quadrivalents. In subsequent stages of microsporogenesis of self-incompatible cultivars, significant abnormalities, such as lagging chromosomes and chromosome bridges at telophase-I, II and unequal sizes and aberrant number of microspores at tetrad stage (Roxas et al. 1995) are noticed. Wollenweber et al. (2021) conducted self and cross-pollinations between two compatible Russian dandelion (Taracaxum koksaghyz) varieties (TkMS2 and TkMS3) and found pollen swelling at the pollen tube apex in autogamous pollinations which is a classical character governed by sporophytic self-incompatibility. On the contrary, cross-pollinations were characterized by pollen germination and penetration of pollen tubes into stigmas. Figure 3 shows fluorescent microscopic examination of pollen grains on stigma of chrysanthemum, a prominent Asteraceae member.
Molecular method
In recent times, molecular studies have enabled transformation of self-incompatibility (SI) to self-compatibility (SC) by using marker assisted selection and related techniques established on RNA interference, transcriptome analysis, gene silencing (CRISPR-Cas9) and recombinant technologies related to self-incompatibility factors. To date, coherent findings recognizing the SI linked molecular factors is evident in various families viz., Brassicaceae, Plantaginaceae, Papaveraceae, Rubiaceae, Rosaceae and Solanaceae, yet, a plethora of studies are underway in additional species, particularly in chrysanthemum and many more from the Asteraceae family (Sanz et al. 2020). Even though, in chrysanthemum, some SI-related genes were identified via RNA-sequencing (Wang et al. 2018), still their regulation remain unexplored. Nakano et al. (2019) isolated a natural self-compatible mutant of Chrysanthemum seticuspe named Gojo-0 which was a diploid and self-compatible, pure line developed through repeated selfing and selection. Due to simplicity and homogeneity of its genome, this strain is ideal for front-line breeding including genetic analyses and molecular biological analysis like whole genome sequencing. During the year 2021, Nakano et al. presented a reference genome sequence of 3.05 Gb chromosome that covered 97% of the genome which was amenable for positional cloning. They also reported recent segmental duplication and retrotransposon expansion (SbdRT) accounting 72% in C. seticuspe.
Achieving self-compatibility
There are a few techniques by which sporophytic self-incompatibility can be temporarily overcome such as differential pollination techniques (early bud pollination, mixed or mentor pollination and delayed pollination), high temperature treatment, exposure to ionizing radiations (gamma rays) and chemical sprays (growth hormones, sodium chloridel, amino acids and carbon dioxide) (Kucera et al. 2006). Other mechanical methods include simply removing stigma and washing the surface of stigma with organic solvents (Ockendon 1978) to remove or soften the waxy substances covering the papillae (Roggen and Van 1973). Figure 4 classifies different techniques to break down self-incompatibility in ornamentals of Asteraceae.
Physical treatments
Gamma irradiation
Artificial self-pollination using the pollen, irradiated with gamma rays, can improve the self-compatibility in Asteraceae species. Gamma rays can be widely applied to induce haploidy as such radiations offer uncomplicated application, strong penetration, repeatability, high mutation frequency and minimal disposal issues (Chahal and Gosal 2002; Kundu et al. 2017). Although pollen irradiation is proven to be successful for inducing haploidy, other parameters, including genotype, environmental conditions, irradiation dose, size and shape of the pollen grain and pollen wall thickness are found to have a significant impact on the embryo production (Giles and Prakash 1987). However, so far there is no reporting on usage of gamma irradiation in Asteraceae, but this technique has been frequently used in fruit crops to induce self-compatibility such as the case of x-ray irradiation of pollen employed in a sweet cherry breeding experiment at the John Innes Center in the year 1940. These studies resulted in the creation of numerous self-compatible cultivars with SFB4 allele mutations (Sonneveld et al. 2005; Ushijima et al. 2004). The first diploid pollen part mutations (PPM) were also detected by employing gamma-irradiation to the pollen from the self-incompatible Japanese pear “Kosui,” (Sawamura et al. 2013).
High temperature treatment
At high temperatures, specific temperature sensitive T-genes interact with self-incompatibility expressing S-gene and its alleles to induce high temperature self-compatibility (Townsend 1971). As per the reports from Ronald (1974), high temperature (16 and 21 ℃ in alternating regime) induces partial compatibility in self-incompatible clones of chrysanthemum. Some garden chrysanthemum clones respond to a heat treatment of 35–40 ℃ for 24–48 h by producing selfed seeds as high temperature treatments affect the stylar part of self-incompatibility (Drewlow 1971; Townsend 1966). In addition to it, a stylar protein CLE45 (belonging to the CLE family) could protect pollen tube growth in high-temperature conditions (Endo et al. 2013). Temperature induced self-compatibility is a viable breeding tool for producing compatible clones in inbred progenies but prolonged periods of exposure to high temperatures (21 ℃) may affect both floral and inflorescence development causing compression in the development of floret, thus, lowering the seed yield (Ling et al. 1966). The critical temperatures required to overcome self-incompatibility are genotype-specific and need to be investigated further (Ronald and Ascher 1975a).
Surgical method
In sporophytic systems, the self-incompatibility reaction is expressed at the stigmatic surface in one particular investigation where decapitation or maceration of the stigma removed self-incompatibility (Sears 1937). This technique involved penetration of steel brush on stigmatic surface (Roggen and Van 1973) and pre-pollination electric spark treatments (Roggen and Van 1973) to induce self-compatibility. On the other hand, work carried out by Gerstel and Riner (1950) instigated that stigma amputation had no effect in overcoming self-incompatibility in chrysanthemum as the failures with stigmatic amputation and stylar shortening occurred due to difficulties in lodging pollen directly on the nutritive transmitting tissue of the style (Ascher 1976). Perhaps, treatments to pollen or styles appeared to be unsuccessful in chrysanthemum. The zest of these studies explains the need to bring modifications in these methods so as to generate significant results in hampering self-incompatibility.
Pollination time
Researchers have potentially employed a variety of strategies to overcome the pre-and post-fertilization barriers and boost seed production or breeding efficiency. Among these, unique pollination strategies including early bud pollination, mentor pollination and delayed bud pollinations are extensively practiced.
Early bud pollination
Bud pollination refers to pollination that occurs when the flower is in the bud stage. Generally, it happens in flowers where the stigma and pollen grains develop or mature even before the bud opens. In this case, flower buds are pollinated artificially using fresh pollen at two days prior to anthesis. At this stage, chemicals or factors responsible for incompatibility will not be synthesized on stigmas leading to normal growth of pollen tubes resulting in an effective fertilization (Cabin et al. 1996).
Mentor or mixed pollination
This method involves immersion of fresh pollen from the self-incompatible cultivar in ethanol (70%v/v) for 10 min before being dried in a vacuum evaporator. The fresh pollen of the male parent is mixed with the ethanol treated pollen (also known as mentor pollen) and this pollen mixture is used for artificial pollination (Sun et al. 2011). When a stigma is pollinated with pollen mix, proteins released from the compatible pollen facades the inhibition reaction at the stigmatic surface, thus, making the cross compatible. The mentor pollen provides chemical substances that help incongruent pollen to adhere and germinate (Richards 1997; Sun et al. 2011). The recognition compounds released by pollen grains are what cause the mentor pollen to be stimulating, and the elements contained in the pollen surface's inner layer are connected to pollen germination and pollen tube development on the surface of this stigma (Knox et al. 1972; Pandey 1977). Thus, it may be possible to use mentor pollen to overcome pre-fertilization hurdles for the incompatible cross between the cultivated and the wild species of chrysanthemum. Ronald and Ascher (1975b) noticed an improvement in cross compatibility of chrysanthemum clones by involving self-compatible male parents in the crosses. Self-incompatibility was partially overcome with pollen mixes of compatible and fresh incompatible, self-pollen (Howlett 1975). Chase or cross pollinations were obtained by supplying a mixture of selfed or cross pollen loads on the stigmas with 2–33% viable selfed seeds in Ipomopsis aggregata (Waser and Price 1991). A significant amount of seed yield was achieved in selfed progenies of sunflower by supplying a combination of self and foreign pollen in crosses involving self-incompatible wild species. i.e. Helianthus annuus and Helianthus petiolaris (Desrochers and Rieseberg 1998). Autogamously derived progeny was lower in the genus Pilosella, varying between 6.2% in diploid Pilosella lactucella and 13% in tetraploid Pilosella officinarum (Krahulcova and Krahulec 1999). This technique was successfully adopted in a number of ornamental taxa including populus (Knox et al. 1972), cosmos (Howlett et al. 1975), petunia (Sastri and Shivanna 1976) and Cyrtanthus breviflorus (Vaughton et al. 2010).
Delayed pollination
This method allows for artificial pollination on the eighth day after emasculation using fresh pollen from the male parent. Withholding the pollination for few days following anthesis degrades the activity of pollen-inhibiting chemicals secreted by stigmas resulting in an effective pollination (Jing 2000). As the selectivity of pollen recognizing and inhibiting chemicals on the stigmatic surface is partially or completely degraded in delayed pollination, this method significantly overcomes the reproductive barriers and increases the pollen grain germination and seed set (Sun et al. 2011).
Chemical treatments
Coupling with different chemical reagents and growth hormones allow for the possibility to break self-incompatibility in a variety of members of the Asteraceae family. The role of enlisted chemicals is defined in this section.
NaCl treatment
This treatment is easy to operate as well as cost effective and helps to develop self-compatible lines for hybrid breeding (Yang et al. 2018b). In Senecio squalidus, a member of Asteraceae forced inbreeding using 5% salt treatment helped in overcoming self-incompatibility by producing pseudo-self-compatible individuals with reduced self-incompatibility along with weakened stigmatic S-specific discrimination (Hiscock 2000).
Gibberellic acid (GA3) treatment
GA3 treatment prior to pollination improves the physiological environment on stigma for pollen germination and pollen tube growth on the stigmatic surface leading to high seed set (Chen and Zhang 2004; Hu 2005). It has been observed that treating emasculated flower stigmas with gibberellic acid @ 50 mg L−1 at anthesis would induce self-fertility in chrysanthemum. Post 1 h, artificial pollination is carried out using fresh pollen from the male parent which will uplift physiological conditions for better pollen germination and pollen tube growth on stigmas (Sun et al. 2011).
Amino acid sprays
The fact that proteins perform a crucial role in self-incompatibility has led to the use of agents that can disturb protein synthesis, structure, stability and function to promote self-compatibility. Researchers have observed an increase in amino acids i.e., r-amino butyric acid, alanine and a decrease in glutamic acid following compatible pollination in chrysanthemum. A similar tendency of elevated levels of valine, leucine and asparagine (Nasrallah and Wallace 1967) was observed following compatible reactions by Anderson and Ascher (1996) (where S-allele specific proteins might differ by amino acid substitution. The presence or synthesis of serine or related compounds in pistils increases the activities of pollen tube growth and its penetration through the style by inhibiting the mechanism of self-incompatibility. A higher seed set was reported by spraying with amino acids viz., serine, arginine, lysine in chrysanthemum but the effect was not higher than cross combination (Sachiko 1985).
Polyploidy (colchicine doubling)
In some cases, seeds are difficult to obtain due to a mismatch in the ploidy levels of parents. This type of ploidy barrier usually occurs due to abnormal endosperm formation and hampered development of seeds. This sort of pre-fertilization barrier can be overcome by manipulating the ploidy levels of one or both parents to match before the hybridization (Bharadwaj 2015). Colchicine doubling may increase self-compatibility in hexaploid chrysanthemum species, a member of Asteraceae family, involving crosses with single allele differences. It causes production of homozygous S alleles as a result of the centromere and S-locus crossing over during quadrivalent association and double reduction. Dowrick (1953) reported that quadrivalent associations exist in chrysanthemum species. In garden chrysanthemums, double reduction might reduce the diversity of S alleles and increase its compatibility with more of its siblings (Sibs). In order to achieve the success in cross between Dendranthema indicum var. Aromaticum (diploid, 2n = 2x = 18) and Dendranthema × grandiflora (polyploid, 2n = 6x = 54), chromosome doubling has been employed through colchicine in D. indicum var. Aromaticum, to maximize the chances of success in the cross between both the parents (He et al. 2016).
Use of polyamines
The most abundantly found polyamines in plants are putrescine (Put), spermine (Spm) and spermidine (Spd). They play an important role in fertilization by acting either as signaling molecules or by performing structural roles after cross-linking to proteins and cell wall components with the help of the transglutaminase (TGase) enzyme (Aloisi et al. 2016). An increase in polyamine content appears to be crucial during emergence and elongation of pollen tubes (Antognoni and Bagni 2008). Polyamines act as a substrate for polyamine oxidases and regulate cell wall deposition and wall stiffening during fertilization (Aloisi et al. 2015). When stigmas are sprayed with naturally occurring polyamines like spermine, pollen growth and extension of the pollen tubes is promoted in many floriculture crops (Caser 2017).
Use of phosphatidic acid (PA)
Stigmatic lipids are vital for pollen development and pollen tube growth on the stigma. After entering inside the pistil, other molecules such as receptor kinases and their ligands, lipid-transfer proteins (LTPs) and arabinogalactan glycoproteins significantly influences the growth of the pollen tube (Mayfield et al. 2001). Phosphatidic acid is a minor phospholipid constituting 1% of total glycerophospholipids whose level changes in response to stimuli (Vu et al. 2012). During SI response, Phospholipase Da1 (PLD a1) may be targeted for down regulation (Scandola and Samuel 2019). Hence, exogenous supplementation of phosphatidic acid (PA) helps to uplift PLDa1 levels to rescue pollination defects and halt the SI response, thereby, favoring pollen adhesion and subsequent pollen tube growth.
In vitro techniques
Tissue culture can be successfully used to overcome pre and post fertilization barriers by carrying out in vitro pollination (Kanta 1962). Placental pollination by culturing the ovules in optimum nutrient medium helps to promote both pollen development as well as the fertilization of ovules. This procedure yields better results as the ovules are cultured intact in placental tissue. Germination media containing agar, boric acid (H3BO3), putrescine and sucrose at pH 5.0 is favorable to use for in vitro pollen germination of rose. This method involves direct loading of the media in petri dishes and sprinkling the fresh pollen straight onto sterile media. Later, optimal conditions for in vitro pollen germination and pollen tube elongation i.e., temperature @ 23–30 ℃ and relative humidity @ 60–65% are maintained. The average percentage of pollen germination in hybrid tea roses is positively associated with the average proportion of normal pollen (Caser 2017). In vitro experiments have shown that SRNase governing self-incompatibility is inhibited by supplementing with mixtures of copper and divalent zinc ions in nutrient media (Kim et al. 2001).
Molecular methods
Self-compatibility has been introduced in ornamental crops by utilizing both traditional breeding and molecular techniques that directly influencing S-gene expression. Different molecular techniques are used to engineer breakdown of self-incompatibility that include CRISPR-Cas9 technology, gene silencing, mutations in non-S-locus factors, pollen part mutation etc. CRISPR-Cas9 technology can be efficiently utilized to generate self-compatible lines by knocking out SRNase genes. Using self-compatible lines could help in avoiding the linkage drag of unwanted traits linked with S-locus inhibitor (Rodriguez et al. 2019; Ye et al. 2018). Gene silencing through RNAi is a cellular mechanism in which double-stranded RNA (dsRNA) leads to the cleavage of complementary endogenous mRNA leading to gene silencing (Geley and Muller 2004). A breakdown in self-incompatibility would result from mutations in genes being unrelated to the S locus that encode downstream components of the mechanism or affects the self-recognition responses which are broadly called modifier genes (Nasrallah 2017). In perennial grasses, mutations in the Z and S loci along with other loci (e.g., T and SF loci), primarily influence pollen (Do Canto et al. 2018).
Sporophytic self-incompatibility breakdown can also occur when two different pollen S-haplotypes expresses in the same pollen grain that results in the down regulation of multiple genes leading to loss of pollen gene function (Ascher 1976; Chantha et al. 2013). The loss of pistils’ ability to reject their own pollen can be achieved by adding an additional S factor or SLF (Qiao et al. 2004; Sijacic et al. 2004). In Petunia species, 16–20 SLF genes were identified and grouped into 18 types. Each of these SLF genes could repress a subset of S-RNases (Kubo et al. 2015). Depending on the mechanism governing self-incompatibility, pollen part mutations are broadly classified into two types. In the first type, the segmental duplication of a chromosome or the competition between two unlike pollen S factors (e.g., SLF or SFB) occurs within an individual pollen grain during polyploidization leading to the breakdown of pollen S function while in the second type, the insertion of a transposon into an S-related gene that encodes an F-box protein or a genetic deletion leads to a loss of pollen S function (Mase et al. 2014). Similarly, the duplication of pollen S (S-locus F-box) also led to breakdown of SI in Petunia axillaris and antirrhinum (Golz et al. 2001; Tsukamoto et al. 2005).
Self-incompatibility: a boon as well
In spite of acting as a pre-fertilization barrier, self-incompatibility plays a dichotomous role serving both positive and negative purpose in the case of Asteraceae crops. Self-incompatibility offers an alternative to tedious hand emasculation and subsequent hand pollination in the production of ornamentals because many of these species are non-domesticated where natural self-incompatibility systems are fully functional as reported in dahlia (Munoz et al 2015) and chrysanthemum (Wang et al. 2018). It also promotes outcrossing which increases heterozygosity and variability that creates new gene recombination resulting in the evolution of new crops. Self-incompatible lines can be directly used for forward and reverse crossings as well as to create double and three-way hybrids. A key domestication trait selected by early farmers and modern plant breeders in many crops is the ability to self-pollinate that allows for unmasking of recessive traits and enforcement of favorable gene interactions, for example, silphium, a genus selected for domestication in Europe where degree of self-pollination is genotype dependent allowing it to be a target for selection (DeHaan et al. 2016; Reinert et al. 2020). Inbreeding in these species facilitate fixation of domestication trait genes in the germplasm pool, enhancement of the efficiency of selection in breeding and production of uniform cultivars/inbred lines that can be incorporated into functionally diverse landscapes. The flexibility of reproductive biology to allow for maximal use of genetic pools, efficient selection and curation of the genetic diversity, will enhance the probability to add biodiversity and restore ecosystem services to the landscape. However, regardless of the breeding methods used, it is critical to select a strong self-incompatible reaction without pseudo self-compatibility (PSC) in seed production to continue the selection for self-incompatibility throughout the development of the cultivar (Ascher 1976).
Conclusion
The concept of self-incompatibility (SI) has profound implications in optimizing plant breeding methods for the understanding of mating systems in Asteraceae, an underutilized family of plants with great potential for additional domesticated species. This mechanism limits the seed setting in species causing a redundancy in further research on inbred lines. Therefore, it is peremptory to explore the mechanism of SI and screen self-compatible (SC) mutants for effective breeding. As seed setting and seed yield are the primary goals in any crop cultivation, it is crucial to gather advance genetic knowledge on SI traits. There are numerous methods involved where compatibility of selfed plants could be assessed for further examinations like pollen germination, pollen tube growth, pollen viability and embryo development by employing numerous techniques such as physical, chemical, pollination time and tissue culture. In addition, studying the cell-to-cell interactions underlying pollen-pistil recognition is of potential importance. Even though efforts to elucidate the molecular features of SI were initiated only during the past thirty years, the progress is steady and promising. Advances in omics and genome editing technologies are increasing the pace of identification of new SI factors. Besides, analyzing the role of individual genes is increasingly adopting a more quantitative approach with respect to environmental influences, phenotypic plasticity, and epigenetics. Full molecular dissection of known SI systems may provide additional gene targets for creating SC lines as seen in canola (Glyoxylase I) and apricot (ParMDO). Nonetheless, our current knowledge of SI environment interactions at the molecular level is very sparse and it remains to be explored. Linking molecular approaches (Genome Wide Association Studies-GWAS, transcriptomic analysis) with phenotypic traits and applying genetic manipulations (gene silencing, CRISPR-Cas9) to break effectiveness of SI which otherwise could hinder seed setting rate, require further studies. Nevertheless, at the same time, this floral trait is a useful breeding strategy in hybridization to alleviate the tedious process of hand emasculation and pollination in the F1 hybrid seed production. This mechanism also increases heterozygosity and variability that result in the evolution of new crops. Although, a lot of work related to self-incompatibility is carried out in diverse species of Asteraceae, still, many loopholes need to be fixed to assess and study this mechanism in commercially promising ornamental crops.
Data availability
No new data has been generated.
References
Abbott RJ, Forbes DG (1993) Outcrossing rate and self-incompatibility in the colonizing species Senecio squalidus. Heredity 71:155–159. https://doi.org/10.1038/hdy.1993.119
Allen AM, Thorogood CJ, Hegarty MJ, Lexer C, Hiscock SJ (2011) Pollen-pistil interactions and self-incompatibility in the Asteraceae: new insights from studies of Senecio squalidus (Oxford ragwort). Ann Bot 108:687–698. https://doi.org/10.1093/aob/mcr147
Aloisi I, Cai G, Tumiatti V, Minarini A, Duca SD (2015) Natural polyamines and synthetic analogs modify the growth and the morphology of Pyrus communis pollen tubes affecting ROS levels and causing cell death. Plant Sci 239:92–105. https://doi.org/10.1016/j.plantsci.2015.07.008
Aloisi I, Cai G, Serafini-Fracassini D, Duca SD (2016) Polyamines in pollen: from microsporogenesis to fertilization. Front Plant Sci 7:155. https://doi.org/10.3389/fpls.2016.00155
Anderson NO (2007) Chrysanthemum. Dendranthema grandiflora Tzvelv. In: Anderson NO (ed) Flower breeding and genetics. Springer, Amsterdam, pp 389–437
Anderson NO, Ascher PDA (1996) Inheritance of pseudo-self-compatibility in self-incompatible garden and greenhouse chrysanthemums (Dendranthema grandiflora Tzvelv Neil). Euphytica 87:153–164. https://doi.org/10.1007/BF00021888
Anderson NO, Ascher PDA (2000) Fertility changes in inbred families of self-incompatible chrysanthemums (Dendranthema grandiflora). J Am Soc Hortic Sci 125:619–625
Anderson NO, Ascher PD, Widmer RE (1992) Inbreeding depression in garden and glasshouse chrysanthemums: germination and survivorship. Euphytica 62:155–169. https://doi.org/10.1007/BF00041750
Antognoni F, Bagni N (2008) Bis (guanylhydrazones) negatively affect in vitro germination of kiwi fruit pollen and alter the endogenous polyamine pool. Plant Biol 10:334–341. https://doi.org/10.1111/j.1438-8677.2007.00016.x
Ascher ED (1976) Self-incompatibility systems in floriculture crops. Acta Hortic 63:205–215. https://doi.org/10.17660/ActaHortic.1976.63.26
Avila GSV, Mena-Al JI, Stephenson AG (2008) Genetic and environmental causes and evolutionary consequences of variations in self-fertility in self-incompatible species. In: Franklin-Tong VE (ed) Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Springer, Berlin/Heidelberg, pp 33–51
Barrett SCH (1992) Heterostylous genetic polymorphisms: model systems for evolutionary analysis. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer Verlag, Heidelberg, pp 1–29
Bateman AJ (1954) Self-incompatibility systems in angiosperms II. Iberis Amara Heredity 8:305–332. https://doi.org/10.1038/hdy.1954.36
Bateman AJ (1955) Self-incompatibility systems in angiosperms III. Cruciferae Heredity 9:52–68. https://doi.org/10.1038/hdy.1955.2
Beardsell DV, Knox RB, Williams EG (1993) Breeding system and reproductive success of Thryptomene calycina (Myrtaceae). Aust J Bot 41:333–353. https://doi.org/10.1071/BT9930333
Bharadwaj DN (2015) Polyploidy in crop improvement and evolution. Plant biology and biotechnology. Springer, New Delhi, pp 619–638. https://doi.org/10.1007/978-81-322-2286-6_24
Bittencourt NS, Gibbs PE, Semir J (2003) Histological study of post-pollination events in Spathodea campanulata Beauv. (Bignoniaceae), a species with late-acting self-incompatibility. Ann Bot 91:827–834. https://doi.org/10.1093/aob/mcg088
Bower MS, Matias DD, Fernandes-Carvalho E, Mazzurco M et al (1996) Two members of the thioredoxin-h family interact with the kinase domain of a Brassica S locus receptor kinase. Plant Cell 8:1641–1650. https://doi.org/10.1105/tpc.8.9.1641
Boyle TH, Stimart DP (1986) Self-incompatibility and interspecific incompatibility: relationships in intra-and interspecific crosses of Zinnia elegans Jacq. and Z. angustifolia HBK (Compositae). Theor Appl Genet 73:305–315. https://doi.org/10.1007/978-1-4613-8622-3_44
Brauner S, Gottlieb LD (1987) A self-compatible plant of Stephanomeria exigua subsp. coronaria (Asteraceae) and its relevance to the origin of its self-pollinating derivative S. malheurensis. Syst Bot 2:299–304. https://doi.org/10.2307/2419325
Brennan AC, Harris SA, Tabah DA, Hiscock SJ (2002) The population genetics of sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae). I. S allele diversity in a natural population. Heredity 89:430–438. https://doi.org/10.1038/sj.hdy.6800159
Brennan AC, Tabah DA, Harris SA, Hiscock SJ (2011) Sporophytic self-incompatibility in Senecio squalidus (Asteraceae): S allele dominance interactions and modifiers of cross-compatibility and selfing rates. Heredity 106:113–123. https://doi.org/10.1038/hdy.2010.29
Brewbaker JL (1957) Pollen cytology and incompatibility mechanisms in plants. Heredity 48:271–277. https://doi.org/10.1093/oxfordjournals.jhered.a106742
Brewer JG, Parlevliet JE (1969) Incompatibility as a new method for identification of Pyrethrum clones. Euphytica 18:320–325. https://doi.org/10.1007/BF00397778
Broertjes C, Ballego JM (1967) Mutation breeding of Dahlia variabilis. Euphytica 16:171–176. https://doi.org/10.1007/BF00043451
Cabin RJ, Evans AS, Jennings DL, Marshall DL, Mitchell RJ, Sher AA (1996) Using bud pollinations to avoid self-incompatibility: implications from studies of three mustards. Canad J Bot 74:285–289. https://doi.org/10.1139/b96-034
Campbell LG, Husband BC (2007) Small populations are mate-poor but pollinator-rich in a rare, self-incompatible plant, Hymenoxys herbacea (Asteraceae). New Phytol 174:915–925. https://doi.org/10.1111/j.1469-8137.2007.02045.x
Caser M (2017) Pollen grains and tubes University of Torino, Turin, Italy. Elsevier, New York. https://doi.org/10.1016/B978-0-12-809633-8.05077-9
Chahal GS, Gosal SS (2002) Principles and procedures of plant breeding: biotechnological and conventional approaches. Alpha Sci. International Ltd, United Kingdom, pp 605
Chantha SC, Herman AC, Platts AE, Vekemans X, Schoen DJ (2013) Secondary evolution of a self-incompatibility locus in the Brassicaceae genus Leavenworthia. Plos Biol 11:e1001560. https://doi.org/10.1371/journal.pbio.1001560
Charlesworth B (1980) The cost of sex in relation to mating system. J Theor Biol 84:655–671. https://doi.org/10.1016/S0022-5193(80)80026-9
Charlesworth D (1985) Distribution of dioecy and self-incompatibility in angiosperms. In: Greenwoog PJ, Harvey PH, Slatkin M (eds) Evolution: essays in honour of John Maynard Smith. Cambridge University Press, Cambridge, pp 237–268
Charlesworth D (2010) Self-incompatibility. F1000 Biol Rep 2:68. https://doi.org/10.3410/B2-68
Charlesworth D, Charlesworth B (1979) The evolutionary genetics of sexual systems in flowering plants. Proc R Soc London b Biol Sci 205:513–530. https://doi.org/10.1098/rspb.1979.0082
Chen RD, Zhang QX (2004) Primary study on the effect of GA on fruit setting rate in hybridization of Prunus mume. J Beijing for Univ 26:57–63
Chen T, Xie M, Jiang Y, Yuan T (2022) Abortion occurs during double fertilization and ovule development in Paeonia ludlowii. J Plant Res 135:295–310. https://doi.org/10.1007/s10265-021-01366-5
Cheptou PO, Lepart J, Escarre J (2002) Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae). J Evol Biol 15:753–762. https://doi.org/10.1046/j.1420-9101.2002.00443.x
Christ B (1959) Entwicklungsgeschichtliche undphysiologische Untersuchungen uber die Selbststerilitat von Cardomine pratensis L. J Bot 47:88112
Clure MB (2006) New views of S-RNase-based self-incompatibility. Curr Opin Plant Biol 9:639–646. https://doi.org/10.1016/j.pbi.2006.09.004
Cope FW (1962) The mechanism of self-incompatibility in Theobroma cacao. Heredity 17:157–182. https://doi.org/10.1038/hdy.1962.14
Crawford DJ, Moura M, Silva LB, Mort ME, Kerbs B, Schaefer H et al (2019) The transition to selfng in Azorean Tolpis (Asteraceae). Plant Syst Evol 305:305–317. https://doi.org/10.1007/s00606-019-01573-7
Crowe LK (1954) Incompatibility in Cosmos bipinnatus. Heredity 8:1–11. https://doi.org/10.1038/hdy.1954.1
Dana MN, Ascher ED (1985) Pseudo-self-compatibility (PSC) in Petunia integrifolia. Heredity 76:468–470. https://doi.org/10.1093/oxfordjournals.jhered.a110147
Darwin CR (1876) The effect of cross and self-fertilization in the vegetable kingdom. John Murray, London, p 393
DeHaan LR, Van Tassel DL, Anderson JA, Asselin SR, Barnes R, Baute GJ et al (2016) A pipeline strategy for grain crop domestication. Crop Sci 56:917–930. https://doi.org/10.2135/cropsci2015.06.0356
Deng YM, Chen SM, Lu AM, Chen FD, Guan ZY, Teng NJ (2010) Production and characterization of the intergeneric hybrids between Dendranthema morifolium and Artemisia vulgaris exhibiting enhanced resistance to chrysanthemum aphid (Macrosiphoniella sanbourni). Planta 231:693–703. https://doi.org/10.1007/s00425-009-1081-5
Desrochers AM, Rieseberg LH (1998) Mentor effects in wild species of Helianthus (Asteraceae). Am J Bot 85:770–775. https://doi.org/10.2307/2446411
Dickinson HG (1995) Dry stigmas, water and self-incompatibility in Brassica. Sex Plant Reprod 8:1–10. https://doi.org/10.1007/BF00228756
Dickinson H, Elleman C, Doughty J (2000) Pollen coatings-chimaeric genetics and new functions. Sex Plant Reprod 12:302–309. https://doi.org/10.1007/s004970050199
Dickinson HG, Lewis D (1975) Interaction between the pollen grain coating and the stigmatic surface during compatible and incompatible interspecific pollinations in Raphanus. In: Duckett JC, Racey PA (eds) The biology of the male gamete. Biol J Linn Soc 7: 165–175.
Do Canto J, Studer B, Frei U, Lubberstedt T (2018) Fine mapping a self-fertility locus in perennial ryegrass. Theor Appl Genet 131:817–827. https://doi.org/10.1007/s00122-017-3038-6
Dorken ME, Husband BC (1999) Self-sterility in the understory herb Clintonia borealis (Liliaceae). Int J Plant Sci 160:577–584. https://doi.org/10.1086/314141
Dowrick GJ (1953) The chromosomes of chrysanthemum II Garden varieties. Heredity 7:59–72. https://doi.org/10.1038/hdy.1953.5
Drewlow LW, Ascher ED, Widmer RE (1973) Genetic studies of self-incompatibility in the garden chrysanthemum (Chrysanthemum morifolium Ramat.). Theor Appl Genet 43:1–5. https://doi.org/10.1007/BF00277824
Drewlow LW (1971) Incompatibility in the garden chrysanthemum (Chrysanthemum morifolium Ramat). Dissertation, University of Minnesota.
East EM, Park JB (1917) Studies on self-sterility I. The behaviour of self-sterile plants. Genetics 2:505–609. https://doi.org/10.1093/genetics/2.6.505
Eenink AH (1981) Compatibility and incompatibility in witloof-chicory (Cichorium intybus L.). The incompatibility system. Euphytica 30:77–85. https://doi.org/10.1007/BF00033662
Elleman CJ, Franklin-Tong VE, Dickinson HG (1992) Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol 121:413–424. https://doi.org/10.1111/j.1469-8137.1992.tb02941.x
Endo S, Shinohara H, Matsubayashi Y, Fukuda H (2013) A novel pollen-pistil interaction conferring high-temperature tolerance during reproduction via CLE45 signaling. Curr Biol 23:1670–1676. https://doi.org/10.1016/j.cub.2013.06.060
Ferrer MM, Avila GSV (2007) Macro phylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytol 173:401–414. https://doi.org/10.1111/j.1469-8137.2006.01905.x
Ferrer MM, Good-Avila SV, Montana C, Domínguez CA, Eguiarte LE (2009) Effect of variation in self-incompatibility on pollen limitation and inbreeding depression in Flourensia cernua (Asteraceae) scrubs of contrasting density. Ann Bot 103:1077–1089. https://doi.org/10.1093/aob/mcp033
Geley S, Muller C (2004) RNAi: ancient mechanism with a promising future. Exp Gerontol 39:985–998. https://doi.org/10.1016/j.exger.2004.03.040
Gerstel DU (1950) Self-incompatibility studies in guayule II Inheritance. Genetics 35:482–506. https://doi.org/10.1093/genetics/35.4.482
Gerstel DU, Riner ME (1950) Self-incompatibility studies in guayule; pollen-tube behavior. J Hered 41:49–55. https://doi.org/10.1093/oxfordjournals.jhered.a106087
Gibbs PE (2014) Late-acting self-incompatibility-the pariah breeding system in flowering plants. New Phytol 203:717–734. https://doi.org/10.1111/nph.12874
Gibbs PE, Talavera S (2001) Breeding system studies with three species of Anagallis (Primulaceae): self-incompatibility and reduced female fertility in A. monelli L. Ann Bot 88:139–144. https://doi.org/10.1006/anbo.2001.1439
Gibbs PE, Milne C, Carrillo MV (1975) Correlation between the breeding system and recombination index in five species of Senecio. New Phytol 75:619–626. https://doi.org/10.1111/j.1469-8137.1975.tb01428.x
Giles LK, Prakash PJ (1987) Pollen cytology and development. Int Rev Cytol 5:107–151. https://doi.org/10.1007/BF02854789
Golz JF, Clarke AE, Newbigin E (2000) Mutational approaches to the study of self-incompatibility: revisiting the pollen-part mutants. Ann Bot 85:95–103. https://doi.org/10.1006/anbo.1999.1060
Golz JF, Oh HY, Su V, Kusaba M, Newbigin E (2001) Genetic analysis of Nicotiana pollen-part mutants is consistent with the presence of an S-ribonuclease inhibitor at the S-locus. Proc Natl Acad Sci, USA 98:15372–15376. https://doi.org/10.1073/pnas.261571598
Gonthier L, Blassiau C, Meorchen M, Cadalen T, Poiret M, Hendriks T et al (2013) High-density genetic maps for loci involved in nuclear male sterility (NMS1) and sporophytic self-incompatibility (S-locus) in chicory (Cichorium intybus L., Asteraceae). Theor Appl Genet 126:2103–2121. https://doi.org/10.1007/s00122-013-2122-9
Goodwillie C (1999) Multiple origins of self-compatibility in Linanthus section Leptosiphon (Polemoniaceae): phylogenetic evidence from internal-transcribed-spacer sequence data. Evol 53:1387–1395. https://doi.org/10.1111/j.1558-5646.1999.tb05403.x
Habura E (1957) Ch Parasterilitatt Bei Sonnenblumen. Z Pflanzenziicht 37:280–298
Haffani Y, Gaude T, Cock J, Goring D (2004) Antisense suppression of thioredoxin h mRNA in Brassica napus cv Westar pistils causes a low-level constitutive pollen rejection response. Plant Mol Biol 55:619–630. https://doi.org/10.1007/s11103-004-1126-x
Hancock CN, Kondo K, Beecher B, McClure B (2003) The S-locus and unilateral incompatibility. Philos Trans Royal Soc B Biol Sci 358:133–1140. https://doi.org/10.1098/rstb.2003.1284
Hao JH, Qiang S, Chrobock T, Kleunen V, Liu QQ (2011) A test of baker’s law: breeding systems of invasive species of Asteraceae in China. Biol Invasions 13:571–580. https://doi.org/10.1007/s10530-010-9850-4
Hao YQ, Zhao XF, She DY, Xu B, Zhang DY, Liao WJ (2012) The role of late-acting self-incompatibility and early-acting inbreeding depression in governing female fertility in monkshood, Aconitum kusnezoffii. PLoS ONE 7:1–7. https://doi.org/10.1371/journal.pone.0047034
Harrison HJ (1975) Incompatibility and the pollen-stigma interaction. Annu Rev Plant Physiol 26:403–425
Harrison HY (1981) Stigma characteristics and angiosperm taxonomy. Nord J Bot 1:401–420. https://doi.org/10.1111/j.1756-1051.1981.tb00707.x
Harrison HY, Shivanna KR (1977) The receptive surface of the angiosperm stigma. Ann Bot 41:1233–1258. https://doi.org/10.1093/oxfordjournals.aob.a085414
Hatakeyama K, Takasaki T, Suzuki G, Nishio T et al (2001) The S receptor kinase gene determines dominance relationships of stigma expression in self-incompatibility in Brassica. Plant J 26:69–76. https://doi.org/10.1046/j.1365-313x.2001.01009.x
He M, Gao W, Gao Y, Liu Y, Yang X (2016) Polyploidy induced by colchicine in Dendranthema indicum var. Aromaticum, a scented chrysanthemum. Eur J Hortic Sci 81:219–226. https://doi.org/10.17660/eJHS.2016/81.4.5
Heywood JS (1993) Biparental inbreeding depression in the self-incompatible annual plant Gaillardia pulchella (Asteraceae). Am J Bot 80:545–550. https://doi.org/10.1002/j.1537-2197.1993.tb13838.x
Hiscock SJ (2000) Genetic control of self-incompatibility in Senecio squalidus L. (Asteraceae): a successful colonizing species. Heredity 85:10–19. https://doi.org/10.1046/j.1365-2540.2000.00692.x
Hiscock SJ, Allen AM (2008) Diverse cell signalling pathways regulate pollen-stigma interactions: the search for consensus. New Phytol 179:286–317. https://doi.org/10.1111/j.1469-8137.2008.02457.x
Hiscock SJ, Kues U (1999) Cellular and molecular mechanisms of sexual incompatibility in plants and fungi. Int Rev Cytol 193:165–295. https://doi.org/10.1016/S0074-7696(08)61781-7
Hiscock SJ, Tabah DA (2003) The different methods of sporophytic self-incompatibility. Philos Trans R Soc 358:1037–1045. https://doi.org/10.1098/rstb.2003.1297
Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG (2002) The stigma surface and pollen-stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. Int J Plant Sci 163:1–16. https://doi.org/10.1086/324530
Hiscock SJ, McInnis SM, Tabah DA, Henderson CA, Brennan AC (2003) Sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae)-the search for S. J Exp Bot 54:169–174. https://doi.org/10.1093/jxb/erg005
Hormazo JI, Herrero M (1992) Pollen selection. Theor Appl Genet 83:663–672. https://doi.org/10.1006/anbo.1998.0697
Hou S, Zhao T, Yang Z, Liang L, Ma W, Wang G et al (2022) Stigmatic transcriptome analysis of self-incompatible and compatible pollination in Corylus heterophylla Fisch. × Corylus avellana L. Front Plant Sci. https://doi.org/10.3389/2Ffpls.2022.800768
Howlett BJ, Knox RB, Harrison HJ (1973) Pollen-wall proteins: release of the allergen Antigen E from intine and exine sites in pollen grains of ragweed and Cosmos. J Cell Sci 3:603–619. https://doi.org/10.1242/jcs.13.2.603
Howlett BJ, Knox RB, Paxton JD (1975) Pollen-wall proteins: physicochemical characterization and role in self-incompatibility in Cosmos bipinnatus. Proceed Royal Soc B 188:167–182. https://doi.org/10.1098/rspb.1975.0010
Hu SY (2005) Reproductive biology of angiosperms. Higher Education Press, Beijing, pp 1–307. https://doi.org/10.1007/978-3-642-50133-3_1
Hughes MB, Babcock EB (1950) Self-incompatibility in Crepis foetida L. subsp. Rhoea difolia Bieb. Schinz Et Keller Genetics 35:570–588
Igic B, Bohs L, Kohn JR (2004) Historical inferences from the self-incompatibility locus. New Phytol 161:97–105. https://doi.org/10.1046/j.1469-8137.2003.00952.x
Imrie BC, Knowles PF (1971) Genetic studies of self-incompatibility in Carthamus flavescens Spreng. Crop Sci 11:6–9. https://doi.org/10.2135/cropsci1971.0011183X001100010002x
Iwano M, Takayama S (2012) Self/non-self discrimination in angiosperm self-incompatibility. Curr Opin Plant Biol 15:78–83. https://doi.org/10.1016/j.pbi.2011.09.003
Jaime A, Silva TD (2014) Organogenesis from chrysanthemum (Dendranthema grandiflora Ramat.) Kitamura petals (disc and ray florets) induced by plant growth regulators. Asia Pac J Mol Biol Biotechnol 22:145–151
Janas AB, Szelag Z, Musial K (2021) In search of female sterility causes in the tetraploid and pentaploid cytotype of Pilosella brzovecensis (Asteraceae). J Plant Res 134:803–810. https://doi.org/10.1007/s10265-021-01290-8
Jeong HJ, Uddin AN, Jong P, Kumar TS, Hye-Ran K, Yong GC (2014) Analysis of S-locus and expression of S-alleles of self-compatible rapid-cycling Brassica oleracea ‘TO1000DH3.’ Mol Biol Rep 41:263514879. https://doi.org/10.1007/s11033-014-3526-6
Jing SX (2000) Breeding science of horticulture. China Agriculture Press, Beijing
Johnson AG (1971) Factors affecting the degree of self-incompatibility in inbred lines of Brussels sprouts. Euphytica 20:561–573. https://doi.org/10.1007/BF00034212
Johnson SD, Collin CL, Wissman HJ, Halvarsson E, Agren J (2004) Factors contributing to variation in seed production among remnant populations of the endangered Daisy Gerbera aurantiaca. Biotropica 36:148–155. https://doi.org/10.1111/j.1744-7429.2004.tb00307.x
Johnson IM, Edwards TJ, Johnson SD (2021) Geographical variation in flower color in the grassland Daisy Gerbera aurantiaca: testing for associations with pollinators and abiotic factors. Front Ecol Evol. 9:676520. https://doi.org/10.3389/fevo.2021.676520
Joshi M, Verma LR, Masu MM (2010) Performance of different varieties of chrysanthemum in respect of growth, flowering and flower yield under north Gujarat condition. Asian J Hort 4:292–294
Kakita M, Murase K, Iwano M, Matsumoto T, Watanabe M, Shiba H et al (2007) Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. Plant Cell. 19:3961–3973. https://doi.org/10.1105/2Ftpc.106.049999
Kanta K, Rangaswamy NS, Maheshwari P (1962) Test-tube fertilization in a flowering plant. Nature 194:1214–1217. https://doi.org/10.1038/1941214a0
Kim MH, Shin DI, Park HS, Chung IK (2001) In vitro function of S-RNases in Lycopersicon peruvianum. Mol Cells 12:329–335
Kitashiba H, Nasrallah JB (2014) Self-incompatibility in Brassicaceae crops: lessons for interspecific incompatibility. Breed Sci 64:23–37. https://doi.org/10.1270/jsbbs.64.23
Knox RB (1973) Pollen-wall proteins: cytochemical observations of pollen-stigma interactions in ragweed and cosmos (Compositae). J Cell Sci 12:421–443. https://doi.org/10.1242/jcs.12.2.421
Knox RB, Willing RR, Ashford AE (1972) Role of pollen wall proteins as recognition substances in interspecific hybridization in poplars. Nature 237:381–383. https://doi.org/10.1038/237381a0
Koseva B, Crawford DJ, Brown KE, Mort ME, Kelly JK (2017) The genetic breakdown of sporophytic self-incompatibility in Tolpis coronopifolia (Asteraceae). New Phytol 216:1256–1267. https://doi.org/10.1111/nph.14759
Kovaleva L, Zakharova E (2003) Hormonal status of the pollen-pistil system at the progamic phase of fertilization after compatible and incompatible pollination in Petunia hybrida L. Sex Plant Rep 16:191–196. https://doi.org/10.1007/s00497-003-0189-1
Krahulcova A, Krahulec F (1999) Chromosome numbers and reproductive systems in selected representatives of Hieracium subgen. Pilosella in the Krkonose Mts (the Sudeten Mts). Preslia 71:217–234
Kubo K, Paape T, Hatakeyama M, Entani T, Takara A, Kajihara K et al (2015) Gene duplication and genetic exchange drive the evolution of S-RNase-based self-incompatibility in Petunia. Nat Plants 1:14005. https://doi.org/10.1038/nplants.2014.5
Kucera V, Chytilova V, Vyvadilova M, Klima M (2006) Hybrid breeding of cauliflower using self-incompatibility and cytoplasmic male sterility. Hort Sci 33:148–152. https://doi.org/10.17221/3754-HORTSCI
Kundu M, Dubey A, Srivastav M, Mallik SK (2017) Induction of haploid plants in citrus through gamma irradiated pollen and ascertainment of ovule age for maximum recovery of haploid plantlets. Turk J Biol 41:469–483. https://doi.org/10.3906/biy-1606-28
Lafuma L, Maurice S (2007) Increase in mate availability without loss of self-incompatibility in the invasive species Senecio inaequidens (Asteraceae). Oikos 116:201–208. https://doi.org/10.1111/j.0030-1299.2007.15220.x
Lai Z, Ma W, Han B, Liang L, Zhang Y, Hong G et al (2002) An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol 50:29–42. https://doi.org/10.1023/a:1016050018779
Laitinen RA, Broholm S, Albert VA, Teeri TH, Elomaa P (2006) Patterns of MADS-box gene expression mark flower-type development in Gerbera hybrida (Asteraceae). BMC Plant Biol 6:1–18. https://doi.org/10.1186/1471-2229-6-11
Lande R, Schemske DW (1985) The evolution of self-fertilization and inbreeding depression in plants I Genetic models. Evolution 39:24–40. https://doi.org/10.1111/j.1558-5646.1985.tb04077.x
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linn Soc 69:503–520. https://doi.org/10.1111/j.1095-8312.2000.tb01221.x
Lawrence WJC (1931) The genetics and cytology of Dahlia variabilis. J Genet 24:257–306. https://doi.org/10.1007/BF02985563
Les DH, Reinartz JA, Esselman EJ (1991) Genetic consequences of rarity in Aster furcatus (Asteraceae), a threatened self-incompatible plant. Evolution 45:1641–1650. https://doi.org/10.1111/j.1558-5646.1991.tb02669.x
Levin DA (1996) The evolutionary significance of pseudo self-fertility. Am Nat 148:321–332. https://doi.org/10.1086/285927
Lewis D (1951) Structure of the incompatibility gene. III. Types of spontaneous and induced mutations. Heredity 5:399–411. https://doi.org/10.1038/hdy.1951.39
Lewis D (1954) Comparative incompatibility in angiosperms. Adv Genet 6:235–285. https://doi.org/10.1016/s0065-2660(08)60131-5
Ling LRE, Widmer MR (1966) Influence of temperature, nutrition and combining ability on seed production in chrysanthemum. Proc Amer Soc Hort Sci 88:621–626
Little TM, Kantor JH, Robinson BA (1940) Incompatibility studies in Cosmos bipinnatus. Genetics 25:150–156. https://doi.org/10.1093/genetics/25.2.150
Litzow ME, Ascher PD (1983) The inheritance of pseudo-self-compatibility (PSC) in Raphanus sativus L. Euphytica 32:9–15. https://doi.org/10.1007/BF00036859
Lundqvist A (1990) One-locus sporophytic S-gene system with traces of gametophytic pollen control in Cerastium arvense ssp. strictum (Caryophyllaceae). Hereditas 113:203–215. https://doi.org/10.1111/j.1601-5223.1990.tb00085.x
Ma R, Han Z, Hu Z, Lin G, Gong X, Zhang H et al (2016) Structural basis for specific self-incompatibility response in Brassica. Cell Res 26:1320–1329. https://doi.org/10.1038/cr.2016.129
Martin FW (1968) The system of self-incompatibility in Ipomea. Heredity 59:263–267. https://doi.org/10.1093/oxfordjournals.jhered.a107713
Mase N, Sawamura Y, Yamamoto T, Takada N, Nishio S, Saito TH et al (2014) A segmental duplication encompassing S-haplotype triggers pollen- part self-compatibility in Japanese pear (Pyrus pyrifolia). Mol Breed 33:117–128. https://doi.org/10.1007/s11032-013-9938-5
Mattson O, Knox RB, Harrison HJ, Harrison HY (1974) Protein pellicle of stigmatic papillae as a probable recognition site in incompatibility reactions. Nature 247:298–300. https://doi.org/10.1038/247298a0
Mayfield JA, Fiebig A, Johnstone SE, Preuss D (2001) Gene families from the Arabidopsis thaliana pollen coat proteome. Science 292:2482–2485. https://doi.org/10.1126/science.1060972
Mehlenbacher SA (1997) Revised dominance hierarchy for S-alleles in Corylus avellana L. Theor Appl Genet 94:360–366. https://doi.org/10.1007/s001220050424
Miler N, Kulus D (2022) Effect of parental components and pollination frequency on the setting and germination of chrysanthemum seeds. Horticulturae 8:827. https://doi.org/10.3390/horticulturae8090827
Miler N, Wozny A (2021) Effect of pollen genotype, temperature and period of storage on in vitro germinability and in vivo seed set in chrysanthemum-preliminary study. Agronomy 11:2395. https://doi.org/10.3390/agronomy11122395
Mraz P (2003) Mentor effects in the genus Hieracium S. STR (Compositae, Lactuceae). Folia Geobot 38:345–350. https://doi.org/10.1007/BF02803204
Munoz M, Merced J, Flores-Espinosa C, Pena G (2015) Interspecific hybridization between Dahlia dibecta and D. Rupicola. Acta Hortic 1087:321–324. https://doi.org/10.17660/ActaHortic.2015.1087.41
Nakano M, Taniguchi K, Masuda Y, Kozuka T, Aruga Y, Han J et al (2019) A pure line derived from a self-compatible Chrysanthemum seticuspe mutant as a model strain in the genus Chrysanthemum. Plant Sci 287:110174. https://doi.org/10.1016/j.plantsci.2019.110174
Nakano M, Hirakawa H, Fukai E, Toyoda A, Kajitani R, Minakuchi Y et al (2021) A chromosome-level genome sequence of a model chrysanthemum: evolution and reference for hexaploid cultivated chrysanthemum. 1167:1-11. https://doi.org/10.1101/2021.06.28.450068
Nasrallah JB (2017) Plant mating systems: self-incompatibility and evolutionary transitions to self-fertility in the mustard family. Curr Opin Genet Dev 47:54–60. https://doi.org/10.1016/j.gde.2017.08.005
Nasrallah ME, Wallace DH (1967) Immunochemical detection of antigens in self-incompatibility genotypes of cabbage. Nature 213:700–701. https://doi.org/10.1038/213700a0
Negi R, Dhiman SR, Gupta YC (2019) Studies on growth and flowering behavior of newly evolved genotypes of chrysanthemum (Dendranthema grandiflora Tzvelev) for loose flower production. Int J Curr Microbiol App Sci 8:341–346
Nemaz JP, Ruzicka J, Miller J (2019) Self-incompatibility in Matricaria chamomilla L. (Asteraceae) is linked to differential esterase activity. Int J Plant Sci 180:366–373. https://doi.org/10.1086/702850
Nettancourt D (1997) Incompatibility in Angiosperms Sex. Plant Rep 10:185–199. https://doi.org/10.1007/s004970050087
Niwa T (1931) Pollination and self-incompatibility in chrysanthemum (Outlines). Jap Assoc for the Adv of Sci Report 6:479–487
Ockendon DJ (1978) Effect of hexane and humidity on self-incompatibility in Brassica oleracea. Theor Appl Genet 52:113–117. https://doi.org/10.1007/BF00264743
Ohmiya A (2018) Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers. Breed Sci 68:17075. https://doi.org/10.1270/jsbbs.17075
Oliveira PE, Gibbs PE, Barbosa AA, Talavera S (1992) Contrasting breeding systems in two Eriotheca (Bombacaceae) species of the Brazilian Cerrado. Plant Syst Evol 179:207–219. https://doi.org/10.1007/BF00937597
Ortiz M, Talavera S, Garcia-castan O, Tremetsberger K, Stuessy T, Balao F et al (2006) Self-incompatibility and floral parameters in Hypochaeris sect. Hypochaeris (Asteraceae). Am J Bot 93:234–244. https://doi.org/10.3732/ajb.93.2.234
Owen FV (1942) Male sterility in sugar beets produced by complementary effects of cytoplasmic and Mendelian inheritance. Am J Bot 29:692. https://doi.org/10.1016/j.scienta.2021.109931
Palumbo F, Draga S, Magon G, Gabelli G, Vannozzi A, Farinati S et al (2023) MIK2 is a candidate gene of the S-locus for sporophytic self-incompatibility (SSI) in chicory (Cichorium intybus Asteraceae). Front Plant Sci 14:1–13. https://doi.org/10.3389/fpls.2023.1204538
Pandey KK (1977) Mentor pollen: possible role of wall-held pollen growth promoting substances in overcoming intra and interspecific incompatibility. Genetica 47:219–229. https://doi.org/10.1007/BF00123243
Parker J (1975) Aneuploidy and isolation in two Hypochoeris species. Chromosoma 52:89–101. https://doi.org/10.1007/BF00285792
Porcher E, Lande R (2005) Loss of gametophytic self-incompatibility with evolution of inbreeding depression. Evol 59:46–60. https://doi.org/10.1111/j.0014-3820.2005.tb00893.x
Price JH, Van Tassel DL, Picasso VD, Smith KP (2022) Assessing phenotypic diversity in Silflower (Silphium integrifolium Michx.) to identify traits of interest for domestication selection. Crop Sci 62:1443–1460. https://doi.org/10.1002/csc2.20748
Prigoda NL, Nassuth A, Mable BK (2005) Phenotypic and genotypic expression of self-incompatibility haplotypes in Arabidopsis lyrata suggests unique origin of alleles in different dominance classes. Mol Biol Evol 22:1609–1620. https://doi.org/10.1093/molbev/msi153
Pu Y, Huo R, Lin Q, Wang F, Chun X, Huang H et al (2021) Investigation and screening of chrysanthemum resources to identify self-compatible mutants. Sci Hortic 281:109931. https://doi.org/10.1016/j.scienta.2021.109931
Qiao H, Wang F, Zhao L, Zhou J, Lai Z, Zhang Y et al (2004) The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16:2307–2322. https://doi.org/10.1105/tpc.104.024919
Rehana S, Bala M (2021) Self-incompatibility in chrysanthemum: mechanisms governing, breeding potential and overcoming methods. 4th Global Meet on Science and Technology (GMST-2020). pp 129–135.
Reinartz JA, Les DH (1994) Bottleneck-induced dissolution of self-incompatibility and breeding system consequences in Aster furcatus (Asteraceae). Am J Bot 81:446–455. https://doi.org/10.1002/j.1537-2197.1994.tb15469.x
Reinert S, John HP, Brian CS, Cloe SP, Nolan CK, David LVT et al (2020) Mating compatibility and fertility studies in an herbaceous perennial Aster undergoing de novo domestication to enhance agroecosystems. Agron Sustain Dev 40:27. https://doi.org/10.1007/s13593-020-00632-5
Richards AJ (1997) Plant breeding sytems edition 2. Chapman & Hall, London
Rodriguez EF, Manrique-Carpintero NC, Nadakuduti SS, Buell CR, Zarka D, Douches D (2019) Overcoming self-incompatibility in diploid potato using T cinerarifolium CRISPR-Cas9. Front Plant Sci. 10:376. https://doi.org/10.3389/fpls.2019.00376
Roggen HPJR, Van AJ (1973) Electric aided and bud pollination: which method to use for self-seed production in Cole crops (Brassica oleracea L.)? Euphytica 22:260–263. https://doi.org/10.1007/BF00022633
Ronald WG, Ascher PD (1975a) Effects of high temperature treatments on seed yield and self-incompatibility in chrysanthemum. Euphytica 24:317–322. https://doi.org/10.1007/BF00028196
Ronald WG, Ascher PD (1975b) Self-compatibility in garden chrysanthemum: occurrence, inheritance and breeding potential. Theor Appl Genet 46:45–54. https://doi.org/10.1007/BF00264754
Ronald WG, Ascher PD (1975) Transfer of self-compatibility from garden to greenhouse strains of Chrysanthemum morifolium Ramat. Jour Amer Soc Hort Sci. 100:351–353. https://doi.org/10.21273/JASHS.100.4.351
Ronald WG (1974) Genetic and high temperature control of self-compatibility in Chrysanthemum morifolium Ramat. Dissertation, University of Minnesota, St. Paul.
Roxas NJL, Yosuke T, Sadami M, Shiro I, Akito T (1995) Meiosis and pollen fertility in Higo Chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitam.). J Japan Soc Hort Sci 64:161–168. https://doi.org/10.2503/jjshs.64.161
Sachiko M (1985) Overcoming the self-incompatibility of Raphaanus sativus by application of amino acids, vitamins and phytohormones. Jpn Soc Hortic Sci 54:46–57. https://doi.org/10.2503/jjshs.54.46
Safavian D, Zayed Y, Indriolo E, Chapman L, Ahmed A, Goring DR (2015) RNA silencing of exocyst genes in the stigma impairs the acceptance of compatible pollen in Arabidopsis. Plant Physiol 169:2526–2538. https://doi.org/10.1104/pp.15.00635
Sage TL, Bertin R, Williams EG (1994) Ovarian and other late-acting self-incompatibility. In: Williams EG, Clark AE, Knox RB (eds) Genetic control of self-incompatibility and reproductive development in flowering plants, vol 2. Kluwer, Dordrecht, pp 116–140
Samaha RR, Boyle TH, Mulcahy DL (1989) Self-incompatibility of Zinnia angustifolia HBK (Compositae) application of visible light and fluorescence microscopy for assessment of self-incompatibility. Sex Plant Reprod 2:18–26. https://doi.org/10.1007/BF00190115
Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR (2009) Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at EXO70A1, a putative component of the exocyst complex. Plant Cell 21:2655–2671. https://doi.org/10.1105/tpc.109.069740
Sanz JVM, Zuriaga E, Cruz-Garcia F, McClure B, Romero C (2020) Self-(In)compatibility systems: target traits for crop-production, plant breeding, and biotechnology. Front Plant Sci 11:195. https://doi.org/10.3389/fpls.2020.00195
Sastri DC, Shivanna KR (1976) Attempts to overcome interspecific incompatibility in Sesamum by using recognition pollen. Ann Bot 40:891–893. https://www.jstor.org/stable/42759473. Accessed 20 Jan 2023
Sawamura Y, Mase N, Takada N, Sato A, Nishitani C, Abe K et al (2013) A self-compatible pollen-part mutant of Japanese pear produced by crossing ‘Kosui’ with pollen from gamma-irradiated ‘Kosui.’ Jpn Soc Hortic Sci 82:222–226. https://doi.org/10.2503/JJSHS1.82.222
Scandola S, Samuel MA (2019) A flower-specific phospholipase D is a stigmatic compatibility factor targeted by the self-incompatibility response in Brassica napus. Curr Biol 29:506–512. https://doi.org/10.1016/j.cub.2018.12.037
Schierup MH, Bechsgaard JS, Nielson LH, Christiansen FB (2006) Selection at work in self-incompatible Arabidopsis lyrata: mating patterns in a natural population. Genetics 172:477–484. https://doi.org/10.1534/genetics.105.045682
Schopfer CR, Nasrallah ME, Nasrallah JB (1990) The male determinant of self-incompatibility in Brassica. Science 286:1697–1700. https://doi.org/10.1126/science.286.5445.1697
Sears ER (1937) Cytological phenomena connected with self-sterility in the flowering plants. Genetics 22:130–181. https://doi.org/10.1093/genetics/22.1.130
Seavey SR, Bawa KS (1986) Late-acting self-incompatibility in Angiosperms. Bot Rev 52:195–219. https://doi.org/10.1007/BF02861001
Shiba H, Iwano M, Entani T, Ishimoto K, Shimosato H, Che FS et al (2002) The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level. Plant Cell 14:491–504. https://doi.org/10.1105/tpc.010378
Shim MS, Shin HK, Kim KS (2007) Microscopic observations of incompatibility in crossing of chrysanthemum cultivars. Hortic Environ Biotechnol 48(1):73–80
Sicard A, Lenhard M (2011) The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann Bot 107:1433–1443. https://doi.org/10.1093/aob/mcr023
Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, McCubbin AG et al (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429:302–305. https://doi.org/10.3389/fpls.2020.00195
Silva NF, Goring DR (2001) Mechanisms of self-incompatibility in flowering plants. Cell Mol Life Sci 58:1988–2007. https://doi.org/10.1007/PL00000832
Smith ED (1913) Chrysanthemum manual. Elmer D. Smith Co., Adrian
Sonneveld T, Tobutt KR, Vaughan SP, Robbins TP (2005) Loss of pollen-S function in two self-compatible selections of Prunus avium is associated with deletion/mutation of an S haplotype-specific F-Box gene. Plant Cell 17:37–51. https://doi.org/10.1105/tpc.104.026963
Sparrow FK, Pearson NL (1948) Pollen compatibility in Asclepias syriaca. J Agric Res 77:187–199
Steinbachs JE, Holsinger KE (2002) S-RNase-mediated gametophytic self-incompatibility is ancestral in Eudicots. Mol Biol Evol 19:825–829. https://doi.org/10.1093/oxfordjournals.molbev.a004139
Stephens LC, Ascher PD, Widmer RE (1982) Genetics of self-incompatibility in diploid Ageratum houstonianum Mill. Theor Appl Genet 63:387–394. https://doi.org/10.1007/BF00303913
Stephenson AG, Good SV, Vogler DW (2000) Interrelationships among inbreeding depression, plasticity in the self-incompatibility system and the breeding system of Campanula rapunculoides L. (Campanulaceae). Ann Bot 85:211–219. https://doi.org/10.1006/anbo.1999.1033
Stone SL, Anderson EM, Mullen RT, Goring DR (2003) ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell 15:885–898. https://doi.org/10.1105/tpc.009845
Stout AB (1917) Fertility in Cichorium intybus: the sporadic occurrence of self-fertile plants among the progeny of self-sterile plants. Am J Bot 4:375–395. https://doi.org/10.2307/2434970
Sun C, Chen F, Fang W, Liu Z, Hou X, Teng N (2009a) Investigation on the factors leading to infertility in the cross between Dendranthema lavandulifolium and D. grandiflorum “Jinling Huangyu.” Acta Hortic Sin 36:1333–1338. https://doi.org/10.3390/ijms19030832
Sun C, Chen F, Fang W, Liu Z, Ma J, Teng N et al (2009b) Cellular mechanism of reproductive barrier during cross breeding between Dendranthema grandiflorum cv. Aoyuntianshi and D. japonense. Sci Agric Sin 42:2085–2091
Sun C, Chen F, Teng N, Liu Z, Fang W, Hou X (2010) Factors affecting seed set in the crosses between Dendranthema grandiflorum (Ramat.) Kitamura and its wild species. Euphytica 171:181–192. https://doi.org/10.1007/s10681-009-0005-6
Sun CQ, Huang ZZ, Wang YL, Chen FD, Teng NJ, Fang WM et al (2011) Overcoming pre-fertilization barriers in the wide cross between Chrysanthemum grandiflorum Ramat. Kitamura and Chrysanthemum nankingense (Nakai) Tzvel by using special pollination techniques. Euphytica 178:195–202. https://doi.org/10.1007/s10681-010-0297-6
Takahashi K, Shimada T, Kondo M, Tamai A (2010) Ectopic expression of an esterase, which is a candidate for the unidentified plant cutinase, causes cuticular defects in Arabidopsis thaliana. Plant Cell Physiol 51:123–131. https://doi.org/10.1093/pcp/pcp173
Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K (2000) The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403:913–916. https://doi.org/10.1038/35002628
Takayama S, Isogai A (2005) Self-incompatibility in plants. Annu Rev Plant Biol 56:467–489. https://doi.org/10.1146/annurev.arplant.56.032604.144249
Tang FP, Chen FD, Chen SM, Teng NJ, Fang WM (2009) Intergeneric hybridization and relationship of genera within the tribe Anthemideae Cass. (I. Dendranthema crassum (kitam.) kitam. x Crossostephium chinense (L.) Makino). Euphytica 169:133–140. https://doi.org/10.1007/s10681-009-9956-x
Tantikanjana T, Nasrallah ME, Nasrallah JB (2010) Complex networks of self-incompatibility signaling in the Brassicaceae. Curr Opin Plant Biol 13:520–526. https://doi.org/10.1016/j.pbi.2010.06.004
Teng NJ, Chen FD, Jiang ZC, Fang WM, Chen TT (2008) Detection of genetic variation by RAPD among chrysanthemum plantlets regenerated from irradiated calli. Acta Hortic 766:413–419. https://doi.org/10.17660/ActaHortic.2008.766.54
Teynor TM, Ascher PD, Widmer RE, Luby JJ (1989) Inheritance of flower color in Dendranthema grandiflora Tzvelev (Chrysanthemum morifolium Ramat.) using cultivars and in-breds I. Plastid Pigmentation Euphytica 42:199–207. https://doi.org/10.1007/BF00034455
Thompson MM (1979) Genetics of self-incompatibility in Corylus avellana L. Theor Appl Genet 54:113–116. https://doi.org/10.1007/bf01159464
Thorpe HC (1940) Pyrethrum breeding. East Afr Agric J 5:364–368
Townsend CE (1965) Seasonal and temperature effects on self-compatibility in tetraploid alsike clover (Triyblium hybridum L.). Crop Sci 5:329–332
Townsend CE (1966) Self-compatibility response to temperature and the inheritance of the response in tetraploid alsike clover (Trifolium hybridum L.). Crop Sci 6:409–414. https://doi.org/10.2135/cropsci1966.0011183X000600050006x
Townsend CE (1971) Further studies on the inheritance of a self-compatibility response to temperature and the segregation of S alleles in diploid alsike clover. Crop Sci 11:860–863. https://doi.org/10.2135/cropsci1971.0011183X001100060028x
Tsukamoto T, Ando T, Watanabe H, Marchesi E, Kao TH (2005) Duplication of the S-locus F-box gene is associated with breakdown of pollen function in an S-haplotype identified in a natural population of self-incompatible Petunia axillaris. Plant Mol Biol 57:141–153. https://doi.org/10.1007/s11103-004-6852-6
Ushijima K, Yamane H, Watari A, Kakehi E, Ikeda K, Hauck NR et al (2004) The S haplotype-specific F-box protein gene, SFB, is defective in self-compatible haplotypes of Prunus avium and Prunus mume. Plant J 39:573–586. https://doi.org/10.1111/j.1365-313X.2004.02154.x
Vaughton G, Ramsey M, Johnson SD (2010) Pollination and late-acting self-incompatibility in Cyrtanthus breviflorus (Amaryllidaceae): implications for seed production. Ann Bot 106:547–555. https://doi.org/10.1093/aob/mcq149
Vijayakumar BN, Suma B, Minimol JS (2018) Self-incompatibility: a pollination control mechanism in plants. Int J Plant Sci 13:201–212. https://doi.org/10.15740/HAS/IJPS/13.1/201-212
Vithanage HIMV, Knox RB (1977) Development and cytochemistry of stigma surface and response to self and foreign pollination in Helianthus annuus. Phytomorphology 27:168–179
Vu HS, Tamura P, Galeva NA, Chaturvedi R, Roth MR, Williams TD et al (2012) Direct infusion mass spectrometry of oxylipin-containing Arabidopsis membrane lipids reveals varied patterns in different stress responses. Plant Physiol 158:324–339. https://doi.org/10.1104/pp.111.190280
Wang F, Zhang F, Chen F, Fang W, Teng NJ (2014) Identification of chrysanthemum (Chrysanthemum morifolium) self-incompatibility. Sci World J. https://doi.org/10.1155/2014/625658
Wang F, Zhong X, Wang H, Song A, Chen F, Fang W et al (2018) Investigation of differences in fertility among progenies from self-pollinated chrysanthemum. Int J Mol Sci 19:832. https://doi.org/10.3390/ijms19030832
Waser NM, Price MV (1991) Reproductive costs of self-pollination in Ipomopsis aggregata (Polemoniaceae). Are ovules usurped? Am J Bot 78:1036–1043. https://doi.org/10.2307/2444892
Weller SG, Donoghue MJ, Charlesworth D (1995) The evolution of self-incompatibility in flowering plants: a phylogenetic approach. In: Hoch PC, Stephenson AG (eds) Experimental and molecular approaches to plant biosystematics. Missouri Botanical Garden, St. Louis, pp 355–82. https://doi.org/10.1007/s00497-009-0122-3
Wollenweber TE, Van DN, Roelfs KU, Prufer D, Gronover CS (2021) Microscopic and transcriptomic analysis of pollination processes in self-incompatible Taraxacum koksaghyz. Plants 10:555. https://doi.org/10.3390/plants10030555
Xu L, Liu CL, Wang HD, Chen KL (2012) Study on the pollen viability and stigma receptivity of Chrysanthemum morifolium “Fubaiju.” Zhong Yao Cai 35:1546–1550
Yang Y, Liu Z, Zhou T, Duan Z, Li B, Ma C (2018a) Mechanism of salt-induced self-compatibility dissected by comparative proteomic analysis in Brassica napus L. Int J Mol Sci 19:1652. https://doi.org/10.3390/ijms19061652
Yang ZY, Shang JQ, Zhang CL, Lujin H (2018b) Stigma receptivity in Chrysanthemum morifolium Ramat L. J Northeast for Univ 46:50–53
Ye M, Peng Z, Tang D, Yang Z, Li D, Xu Y et al (2018) Generation of self-compatible diploid potato by knockout of S-RNase. Nat Plants 4:651–654. https://doi.org/10.1038/s41477-018-0218-6
Young AC, Gregory ME, Langston A (2000) Sporophytic self-incompatibility in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Aust J Bot 48:667–672. https://doi.org/10.1071/BT99024
Zagorski JS, Ascher PD, Widmer RE (1983) Multigenic self-incompatibility in hexaploid Chrysanthemum. Euphytica 32:1–7. https://doi.org/10.1007/BF00036858
Zhao H, Chen F, Wang Y, Chen S, Fang W, Guo W et al (2008) Study on pollen viability, longevity and pistil receptivity of self-compatible chrysanthemum with small inflorescences. Acta Hort 766:405–412. https://doi.org/10.17660/ActaHortic.2008.766.53
Zinkl GM, Preuss D (2000) Dissecting Arabidopsis pollen-stigma interactions reveals novel mechanisms that confer mating specificity. Ann Bot 85:15–21. https://doi.org/10.1006/anbo.1999.1066
Zinkl GM, Zwiebel BI, Grier DG, Preuss D (1999) Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126:5431–5440. https://doi.org/10.1242/dev.126.23.5431
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest has been declared by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bala, M., Rehana, S. & Singh, M.P. Self-incompatibility: a targeted, unexplored pre-fertilization barrier in flower crops of Asteraceae. J Plant Res 136, 587–612 (2023). https://doi.org/10.1007/s10265-023-01480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-023-01480-6