Abstract
Low temperature inhibits photosynthesis and negatively affects plant growth. Cucumber (Cucumis sativus L.) is a chilling-sensitive plant, and its greenhouse production requires considerable energy during the winter. Therefore, a useful stress marker for selecting chilling-tolerant cucumber cultivars is desirable. In this study, we evaluated chilling-stress damage in different cucumber cultivars by measuring photosynthetic parameters. The majority of cultivars showed decreases in the quantum yield of photosystem (PS) II [Fv/Fm and Y(II)] and the quantity of active PS I (Pm) after chilling stress. In contrast, Y(ND)—the ratio of the oxidized state of PSI reaction center chlorophyll P700 (P700+)—differed among cultivars and was perfectly inversely correlated with Y(NA)—the ratio of the non-photooxidizable P700. It has been known that P700+ accumulates under stress conditions and protects plants to suppress the generation of reactive oxygen species. In fact, cultivars unable to induce Y(ND) after chilling stress showed growth retardation with reductions in chlorophyll content and leaf area. Therefore, Y(ND) can be a useful marker to evaluate chilling-stress tolerance in cucumber.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are continuously exposed to abiotic stresses, which are key factors that limit their growth. Chilling stress is one of the main abiotic stresses in middle to high latitudes that significantly influences crops (Allen and Ort 2001). Crop plants originating from tropical regions, such as rice, maize, and soybean, as well as those from temperate climate zones, such as cucumber and tomato, are chilling-sensitive plants, and yield loss due to chilling stress has been a serious problem. In addition, the winter cultivation of chilling-sensitive crops incurs large energy costs to maintain a desirable temperature in greenhouses, which is not suitable for a sustainable society. However, the factors that discriminate chilling-sensitive from chilling-tolerant plants remain unclear; therefore, the effective breeding of chilling-tolerant plants is challenging.
At low temperatures, plant membrane fluidity is reduced, enzyme activity is decreased, and the stomatal response is inhibited (Ding et al. 2019; Siddiqui and Cavicchioli 2006; Upchurch 2008; Wilkinson et al. 2001). Consequently, photosynthesis is often the first process to be inhibited in plants by chilling stress (Berry and Bjorkman 1980). Under chilling stress, electron transfer reactions driven by light energy become overdriven because of the reduced activity of the Calvin-Benson cycle (Bi et al. 2019; Heidarvand and Amiri 2010; Mittler 2006; Murchie and Niyogi 2011; Sage and Kubien 2007; Suzuki and Mittler 2006). Excess electrons downstream of the electron transfer chain leak into the surrounding O2 to produce toxic molecules, reactive oxygen species (ROS) (Asada and Takahashi 1987; Asada 1999, 2006). The generated ROS directly attack nearby photosystems or thylakoid membranes, resulting in a dramatic decrease in photosynthetic activity and inhibition of plant growth (Garstka et al. 2005, 2007; Takagi et al. 2016).
Cucumber (Cucumis sativus L.), which originated from the subtropical valleys of Southern Asia, has been studied as a typical chilling-sensitive crops. In particular, the inhibition characteristic of photosystem I (PSI) by chilling stress was first observed in cucumbers in vivo (Terashima et al. 1994). This phenomenon was called PSI photoinhibition, and has been proposed to be mediated by ROS (Sejima et al. 2014; Sonoike and Terashima 1994; Sonoike et al. 1995; Takagi et al. 2016). Excess energy accumulates on the electron acceptor side of PSI, and excess electrons reduce oxygen, generating ROS such as the superoxide anion radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (⋅OH) (Asada 2006; Mehler 1951; Rutherford et al. 2012). These ROS are highly reactive and can quickly inactivate PSI (Sonoike 1996a). Unlike the photoinhibition of PSII, which has a rapid recovery (Melis 1999), PSI photoinhibition takes several days to weeks to recover, so its impact on the plant growth is significant (Kudoh and Sonoike 2002; Zhang et al. 2016; Zhou et al. 2004). Therefore, protecting PSI from chilling stress is important in agriculture.
Plants have evolved protective mechanisms against PSI photoinhibition by regulating the redox state of the PSI reaction center chlorophyll P700. Chlorophyll P700 has three different states: a steady state (P700), a photo-excited state (P700*), and an oxidized state (P700+). The oxidized state, P700+, is commonly accumulated from land plants to cyanobacteria under abiotic stress conditions, such as high light, drought, and low CO2 levels (Harbinson and Foyer 1991; Heber and Walker 1992; Heber et al. 1992; Klughammer and Schreiber 1994). The quenching efficiency of P700+ has been known for a long time (Trissl 1997) and the physiological significance of P700 oxidation under stress conditions has been proposed (Karapetyan 2008; Sejima et al. 2014; Shimakawa et al. 2016, 2017; Takagi et al. 2017b). Namely, the accumulation of P700+ suppresses the risk of ROS generation in downstream of PSI, and the mechanism to induce P700+—P700 oxidation system—is regulated on both the electron acceptor and electron donor side of PSI (Miyake 2020; Shimakawa and Miyake 2018).
On the electron acceptor side of PSI, photorespiration functions as an important electron sink for P700 oxidation in C3 plants (Hanawa et al. 2017; Sejima et al. 2016). In addition, many photosynthetic organisms, excluding vascular plants, have flavodiiron proteins (FLV) that mediate electron flow from PSI to oxygen and safely remove excessive electrons (Helman et al. 2003, 2005). Easing acceptor side limitation of PSI by far-red light illumination also contributes to the high oxidation state of P700 (Kono et al. 2017). On the electron donor side of PSI, acidification of the thylakoid lumen (ΔpH) regulates electron flux and the size of the ΔpH reflects the balance between the rate of photosynthetic electron transport and that of ATP consumption in subsequent assimilation processes. Under stressful conditions, various assimilation processes are downregulated, and the resulting ΔpH suppresses electron transport in Cytb6f (Anderson 1992; Hope 2000; Nishio and Whitmarsh 1993), as well as the oxidation of H2O in PSII. These processes are known as “photosynthetic control” (Baker et al. 2007; Foyer et al. 1990; Schöttler and Tóth 2014; Schöttler et al. 2015; West and Wiskish 1968). ΔpH is also regulated by the activity of alternative electron flow (AEF) (Miyake 2010; Shikanai 2007) and chloroplast ATPase (Kanazawa et al. 2017; Kohzuma et al. 2009; Miyake 2010; Takagi et al. 2017a). Furthermore, over-reduction of PQ inhibits the Q-cycle of the Cytb6f complex and contributes to the oxidation of P700, which is named as the reduction-induced suppression of electron transport (RISE) (Shaku et al. 2016; Shimakawa et al. 2018; Wada et al. 2020). These diverse mechanisms constitute a robust P700 oxidation system; however, they appear to be ineffective in cucumbers under chilling stress.
In this study, we evaluated chilling stress tolerance by measuring photosynthetic activity of different cucumber cultivars. Differences in chilling sensitivity among cucumber cultivars have been reported (Shen et al. 1999; Yu et al. 2002; Zhou et al. 2004); however, an effective method for evaluating these differences has not yet been established. We propose that the measurement of P700 oxidation ability is a useful indicator for evaluating chilling stress sensitivity/tolerance of cucumber plants, facilitating the early diagnosis of chilling injury level and selection of chilling-tolerant cultivars.
Materials and methods
Plant materials and growth conditions
Ten cucumber (Cucumis sativus) cultivars, cv. High Green 21, Homi 1-gou, SHARP-1 (Saitama Genshu. Ikuseikai, Japan), Shinhokusei (Tokiwa cucumber, Japan), Natsusuzumi, V-archi, V-road, Nanshin (Takii Shubyo, Japan), Shimoshirazu-Jibai, and Riru (Sakata Seed Corp., Japan) were purchased and used as the plant materials. The following abbreviations were used to indicate the above cultivars, respectively: HG, HM, sharp, shin, natsu, archi, rodo, nan, shimo, and riru. Cucumber plants were grown in plastic pots with soil under a photoperiod of 16 h light (250–300 µmol photons m−2 s−1)/8 h darkness at 27 °C in a growth chamber. Two to three weeks after germination, the second or third fully expanded leaves (from the bottom) were used for analyses.
Light-chilling treatment
The 2–3-week-old cucumber plants were exposed to short-term (3 h or 5 h) chilling stress at 4 °C in a cold room under the light illumination of 180–250 µmol photons m−2 s−1. After the light-chilling treatment, the plants were returned to normal temperature for evaluation of their recovery. Plants without chilling treatment were used as the controls.
Measurement of chlorophyll fluorescence and measurement of P700
Chlorophyll fluorescence parameters were measured using a Dual-PAM 100 (Heinz Walz GmbH, Effeltrich, Germany) (Klughammer and Schreiber 2008a). The maximum quantum yield of PSII in the dark was calculated as Fv/Fm; the effective quantum yield of PSII as Y(II) = (Fm′ − Fs)/Fm′; the quantum yield of nonregulated energy dissipation in PSII as Y(NO) = Fs/Fm; and the quantum yield of regulated energy dissipation in PSII as Y(NPQ) = Fs/Fm′ − Y(NO). Fo and Fm levels, the minimum and maximum fluorescence, respectively, were measured after 15 min of dark adaptation. The light-chilling-treated plants were adapted to darkness for 15 min in the cold room, whereas the control plants were adapted at room temperature (23 °C). After the onset of light illumination (325 µmol photons m−2 s−1), saturation pulses (SP, 300 ms and 10,000 µmol photons m−2 s−1) were applied every 30 s for 5 min to monitor the Fm´ level (the maximum fluorescence under light illumination) and the Fs level (the steady-state fluorescence under light illumination).
The redox change of P700 was analyzed by monitoring the absorbance changes of the transmitted light at 830 and 875 nm (Klughammer and Schreiber 2008b). The quantum yield of Y(I) was calculated as Y(I) = (Pm − P)/Pm; the fraction of P700 that could not be oxidized by SP to the overall P700 as Y(NA) = (Pm − Pm′)/Pm; and the P700 oxidation ratio in a given actinic light as Y(ND) = P/Pm (Klughammer and Schreiber 1994). Pm was determined by the application of SP in the presence of far-red light. Pm′, which represents the maximal level of P700+ during actinic light, and P, which represents the steady-state P700+ level, were recorded immediately before the application of SP.
Chlorophyll content and leaf area
Leaves (20–30 mg fresh weight) were harvested from the second expanded leaves (from the bottom to top), immediately frozen, and powdered by grinding in liquid nitrogen. Chlorophyll was extracted with 80% (v/v) acetone and the chlorophyll content was determined by spectrophotometry, as described by Porra et al., (1989). The leaf area was calculated using ImageJ software (ver. 1.53 k; National Institutes of Health, Bethesda, MD, USA) on scanned freshly harvested leaves.
Statistical analysis
Statistical analyses of the data were based on the Tukey–Kramer’s multiple comparison test after the one-way ANOVA or Dunnett’s test. All calculations were performed with at least three independent biological replicates.
Results
Varietal differences in photosynthetic activities after short-term light-chilling treatment
Cucumber is a chilling-sensitive plant with suggested varietal differences. To determine sensitivity, we investigated the chilling-stress responses of 10 cucumber cultivars. After the short-term light-chilling treatment at 4 °C for 3 h, all cucumber cultivars showed wilted leaves; however, there were no visible differences among the cultivars. We then measured the photosynthetic parameters at room temperature (23 °C) immediately after the short-term light-chilling treatment. Fv/Fm (the maximum quantum yield of PSII) decreased after the chilling treatment in all cultivars. Y(II) (the effective quantum yield of PSII under light illumination) also decreased in the majority of cultivars, indicating that PSII photoinhibition was induced by chilling stress (Fig. 1). Y(NPQ) (the yield of non-photochemical quenching) tended to increase in most cultivars, but decreased in shimo and HG, although no statistical difference was detected. Y(NO) (the yield of non-regulated energy quenching) increased significantly in HG. Y(NPQ) is a fraction of the light energy that is safely dissipated as heat, which is normally induced during stress, while Y(NO) reflects a fraction of the excess light energy that can be damaging (Klughammer and Schreiber 2008a). Therefore, the photoprotective mechanism would be less efficient in cultivars with decreased Y(NPQ) and/or enhanced Y(NO) (Fig. 1).
Effect of chilling stress on photosystem II (PSII) parameters. The steady state of chlorophyll fluorescence of the 10 cucumber cultivars was analyzed using Dual-PAM. Plants were grown in a growth chamber (27 °C) for 2–3 weeks, and intact leaves were analyzed before chilling treatment (open bars) and after chilling treatment at 4 °C for 3 h (shaded bars). The leaves were illuminated with actinic light of 325 µmol photons m−2 s−1 under ambient conditions (23 °C). Fifteen minutes of dark adaptation were performed before the Dual-PAM analysis. Cucumber cultivars are indicated by the following abbreviations: Homi 1-gou (HM), SHARP-1 (sharp), Riru (riru), V-road (rodo), Natsusuzumi (natsu), V-archi (archi), Shinhokusei (shin), Shimoshirazu-Jibai (shimo), Nanshin (nan) and High Green 21 (HG). Values are the mean ± SE, n = 3–4, biological replicates. Different letters indicate statistically significant differences among cultivars/treatments by Tukey–Kramer’s multiple comparisons test after the one-way ANOVA (p < 0.05)
Pm, the total amount of PSI reaction center chlorophyll P700, tended to decrease in all cultivars after the chilling treatment, indicating a decrease in the active PSI (Fig. 2). Thus, both PSII and PSI were inhibited to some extent by the short-chilling treatment in all cultivars. However, the distribution of light energy use in PSI differed greatly among the cultivars. In particular, Y(ND)—the non-photochemical loss due to oxidized primary donor representing the ratio of the oxidized state of the PSI reaction center chlorophyll P700 (P700+) (Klughammer and Schreiber 2008b)—was considerably different among the 10 cultivars after the chilling treatment (Fig. 2). HM, sharp, and riru maintained Y(ND) near 0.4, while other cultivars showed lower Y(ND); in particular, nan and HG showed severely reduced Y(ND) after the chilling treatment (< 0.1). Accumulation of P700+ in HM, sharp, and riru protects the plant body by suppressing excessive electron flow under chilling stress. Conversely, cultivars (such as nan and HG) with less P700+ would have greater electron influx to PSI under steady state photosynthesis, which increases the risk of ROS. In fact, the P700+ after SP illumination reduced fully within 50–60 ms in HM and sharp (t1/2 = 6.59 ± 0.05 and 7.51 ± 0.81, respectively), whereas that in HG and nan returned to the reduced state more swiftly within 30–40 ms after SP illumination (t1/2 = 3.64 ± 0.62 and 4.26 ± 0.17, respectively) (Fig. 3). This rapid P700+ decay after SP illumination in the cultivars with small Y(ND) indicates the accumulation of electron donors for P700+ under steady state photosynthesis after chilling stress. Furthermore, in the cultivar with a large decrease in Y(ND), Y(NA)—the non-photochemical loss due to reduced acceptor representing the acceptor side limitation of electron flow in PSI (Klughammer and Schreiber 2008b)—increased strongly, suggesting the accumulation of electrons downstream of PSI and the concomitant risk of ROS production (Fig. 2).
Effect of chilling stress on photosystem I (PSI) parameters. The steady state of chlorophyll absorption of the 10 cucumber cultivars was analyzed using Dual-PAM under the same conditions as described in Fig. 1. Values are the mean ± SE, n = 3–4, biological replicates. Different letters indicate statistically significant differences among cultivars/treatments by Tukey–Kramer’s multiple comparisons test after the one-way ANOVA (p < 0.05)
The reduction kinetics of oxidized P700 after exposure to short pulse (SP) illumination in the leaves of cucumber cultivars after chilling stress. The decay of P700+ after chilling stress was extracted from one SP illumination (300 ms, 10,000 µmol photons m−2 s−1) at the time point when a steady state of photosynthetic activity was obtained (5 min after the onset of actinic light illumination) in Fig. 1 and Fig. 2, using the same plants: HM, sharp, HG, and nan, and the same chilling treatment as described in Figs. 1 and 2. SP illumination was terminated at 0 ms in the graph, and the P700+ signal was monitored for 60 ms. The maximum and minimum levels of P700+ during and after SP were used to normalize each signal. Values are the mean ± SE, n = 3, biological replicates. The half-life (t1/2, ms) of P700+ was calculated from the single-exponential decay kinetics of P700+ after the SP illumination. Values are the mean ± SE, n = 3, biological replicates. Different letters indicate statistically significant differences among cultivars by Tukey–Kramer’s multiple comparisons test after the one-way ANOVA (p < 0.05)
Short-term light-chilling treatment altered correlations among the photosynthetic parameters
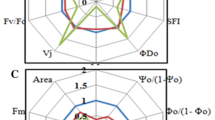
The PSI reaction center chlorophyll P700 exists as P700+ after passing electrons to the downstream reactions and then returns to the steady state P700 by receiving electrons from PSII. Therefore, the redox state of P700 depends on the balance between the efflux and influx of electrons around PSI. An inverse correlation between Y(II) and Y(ND) was consistently observed before chilling stress treatment (Fig. 4a). However, after the chilling treatment, this proper correlation was broken, and the different values of Y(ND) were observed regardless of the values of Y(II) (Fig. 4a). In all cultivars except riru, Y(II) was originally approximated 0.3–0.4 under the light illumination of 325 µmol photons m−2 s−1 at ambient conditions (23 °C), and decreased below 0.3 after the chilling treatment, reflecting the suppression of the PSII activity under chilling stress. Meanwhile, electron influx to PSI was reduced, which should promote the accumulation of P700+. However, Y(ND) decreased in all cultivars after the chilling treatment, regardless of the decrease in Y(II). Seven of the ten cultivars showed Y(ND) below 0.3, which were much lower than that before the chilling treatment. In particular, Y(ND) was less than 0.1 in HG and nan. This reduced Y(ND) after short-term light-chilling stress suggested that the PSI protection mechanism—the P700 oxidation system—did not work properly after short-term light-chilling stress. The rapid reduction kinetics of P700+ after SP illumination suggests greater electron influx to PSI in the low Y(ND) induction cultivars after chilling stress (Fig. 3). In contrast, three cultivars (HM, sharp, and riru) maintained relatively high Y(ND) (approximately 0.4) even after the chilling treatment, which should protect PSI to some degree.
Correlations between photosynthetic parameters before and after the chilling treatment. Correlations between a Y(ND) and Y(II); b Y(NA) and Y(ND); c Y(NPQ) and Y(ND) at steady state; d Y(NPQ) and Y(ND) at induction state; and e Y(I) and Y(II) are shown. The data in each panel were obtained from the analysis shown in Figs. 1 and 2, except those for the panel d, in which the induction phase of Y(NPQ) was measured 2 min after turning on the actinic light after 15 min of dark adaptation. Values are the mean ± SE, n = 3–4, biological replicates
A negative correlation between Y(ND) and Y(NA) occurred after the chilling treatment (Fig. 4b), whereas Y(NA) remained low regardless of the values of Y(ND) in all cultivars before the stress treatment. For example, HG and nan showed low Y(ND) and high Y(NA), while HM, sharp, and riru showed relatively high Y(ND) and low Y(NA) after chilling treatment. The other cultivars showed intermediate values for both parameters. These results suggested that the ability to suppress electron influx to PSI, indicated by Y(ND), differed among cultivars and determines the risk of ROS production after stress treatment (Fig. 4b).
Acidification of the thylakoid lumen (ΔpH) is a main factor limiting the electron influx to PSI, resulting in P700 oxidation. As ΔpH also regulates the thermal dissipation of excess light energy around PSII, we investigated the relationship between Y(ND) and Y(NPQ). Although the correlation between Y(ND) and Y(NPQ) after the chilling treatment was not clear (Fig. 4c), a large variation among cultivars was observed during the induction phase of photosynthesis after dark adaptation (Fig. 4d): The cultivars HG and shimo had lower Y(ND) and Y(NPQ), whereas HM showed higher Y(ND) and Y(NPQ). This suggested that the cultivars with lower Y(NPQ) after chilling stress could not maintain the ΔpH across the thylakoid membrane, resulting in a lower ability to induce Y(ND). However, the relationship between Y(ND) and the induction of Y(NPQ) cannot be simply explained because some cultivars, such as nan and shin, were able to induce Y(NPQ) at lower values of Y(ND) (Fig. 4d).
Y(II) and Y(I) showed a positive linear correlation before chilling stress treatment (Fig. 4e). After stress treatment, this correlation became unclear: Y(I) had a relatively higher value regardless of Y(II). Before the chilling treatment, Y(I) ranged from 0.2 to 0.5 among cultivars, but after the chilling treatment, Y(I) ranged from 0.5 to 0.6 for the majority of cultivars. However, Y(I) depends on the redox status of P700 upon SP illumination, making it difficult to estimate the exact quantum yield of PSI, especially under stress conditions. Since the photo-reduction/oxidation cycle of P700 is driving even within a few milliseconds during SP illumination, the determination of Pm′ by SP application may not reflect the state of P700 under steady state photosynthesis (Furutani et al. 2021). Therefore, quantitative comparisons of the difference in quantum yields between the two photosystems are refrained here.
Cultivars unable to accumulate P700+ were delayed in growth after the chilling treatment
The observed differences in photosynthetic parameters suggested that there were varietal differences in cucumber chilling-stress tolerance. In particular, since the inducibility of Y(ND) is important for the robustness of PSI, we predicted that HM, sharp, and riru, which maintained Y(ND) properly even after the chilling treatment (Fig. 4a), should be chilling-tolerant cultivars. In contrast, HG, nan, and shimo, which had small Y(II) and small Y(ND) (Fig. 4a) after the chilling treatment, are likely chilling-sensitive cultivars. We then investigated whether these chilling-tolerant and chilling-sensitive cultivars, predicted from the photosynthetic parameters, reflected differences in plant growth.
Cultivars HM, sharp, riru, shimo, nan, and HG were exposed to chilling stress at 4 °C for 5 h and then returned to the original growth conditions (27 °C) for 3 days. Fv/Fm decreased to approximately 0.6 in all the cultivars immediately after the chilling treatment (Fig. 5a). Thereafter, it gradually recovered on the first and second days after the chilling treatment, and reached approximately 0.8 in all cultivars on the third day. Therefore, the PSII recovery system appeared not to be damaged by low temperatures. Pm also decreased significantly to approximately 1.0 right after the chilling treatment, and continued to decrease 1 day after the chilling treatment, except for in HG (Fig. 5b). However, the recovery level 2 days after the chilling treatment differed among the cultivars: the Pm levels of the Y(ND)-inducible cultivars (HM, sharp, and riru) on the third day were similar to those of the pre-treatment plants. In contrast, the Y(ND)-non-inducible cultivars (shimo, nan, and HG) had significantly lower Pm values even 3 days after the chilling treatment, indicating a delayed recovery of PSI. Therefore, in Y(ND)-non-inducible cultivars, PSI, but not PSII, remained damaged after chilling stress treatment.
Effect of chilling stress on the recovery of PSII and PSI. The 2–3-week-old plants of different cultivars as indicated in the figure were treated at 4 ºC for 5 h and then returned to their original growth chamber (27 ºC). HM, sharp, and riru were used as Y(ND)-inducible cultivars, and shimo, nan, and HG as Y(ND)-non-inducible cultivars. a Fv/Fm and b Pm were measured using the Dual-PAM after 15 min of dark adaptation. Measurements were performed at five time-points: before chilling, right after the 5 h chilling treatment, 1 day, 2 days, and 3 days after the chilling treatment. Values are the mean ± SE, n = 3, biological replicates. * and ** indicate significant differences compared with the non-chilling plants (open bar, before chilling) using the Dunnett's test at p < 0.01 and p < 0.001, respectively, and “ns” signifies no significant difference
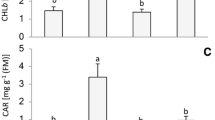
The growth of the Y(ND)-non-inducible cultivars (shimo, nan, and HG) after the chilling treatment was delayed compared to that of the Y(ND)-inducible cultivars (HM, sharp, and riru) (Fig. 6), reflecting the delayed Pm recovery in shimo, nan, and HG (Fig. 5b). In fact, Y(ND)-inducible cultivars maintained approximately 75% of their relative shoot fresh weight, whereas Y(ND)-non-inducible cultivars only maintained approximately 60–70% (Fig. 7a). The total chlorophyll content and leaf area also showed a larger decrease in Y(ND)-non-inducible cultivars than that of Y(ND)-inducible cultivars (Fig. 7b, c). Therefore, we conclude that the growth inhibition by chilling stress was more severe in cultivars that could not induce Y(ND).
Growth inhibition by chilling stress in cucumber cultivars with different Y(ND) inducing abilities. Pictures of untreated (control) and chilling-treated plants of Y(ND)-inducible cultivars (HM, sharp, and riru) and Y(ND)-non-inducible cultivars (shimo, nan, and HG). Control plants were grown for 16 days at 27 °C; chilled plants were grown for 14 days at 27 °C, then exposed to the chilling stress (4 °C, 5 h), and returned to the original growth chamber (27 °C) for 2 days. Each image shows a typical leaf from three biological replicates
Growth of Y(ND)-inducible and Y(ND)-non-inducible cultivars after chilling stress. Plants at 3 days after the chilling treatment (4 °C, 5 h) were compared with the plants without chilling treatment. Measurements of the same age and the same cultivar without chilling treatment were set to 1 in each figure. HM, sharp, and riru were used as Y(ND)-inducible cultivars, and shimo, nan, and HG as Y(ND)-non-inducible cultivars. a Relative shoot fresh weight of chilling-treated plants compared to untreated plants. Values are the mean ± SE, n = 3. No significant difference was observed using Tukey–Kramer’s multiple comparisons test after one-way ANOVA (p < 0.05). b Relative chlorophyll content of chilling-treated plants compared with untreated plants. Chlorophyll was extracted using 80% acetone. Values are the mean ± SE, n = 8–12. Different letters indicate significant differences by Tukey–Kramer’s multiple comparisons test after one-way ANOVA (p < 0.01). c Relative leaf area of chilling-treated plants compared to that of untreated plants. The second fully expanded leaves (from the bottom) were scanned, and their areas were calculated using the ImageJ software. Values are the mean ± SE, n = 3–4. Different letters indicate significant differences by Tukey–Kramer’s multiple comparisons test after one-way ANOVA (p < 0.05)
Discussion
Chilling stress causes an over-reduction of the photosynthetic electron chain and leads to a high risk of ROS generation, especially in PSI (Sonoike 1996a, b; Sonoike and Terashima 1994; Takagi et al. 2016). It has been shown that the oxidation of P700 is necessary to hinder PSI photoinhibition (Heber and Walker 1992; Karapetyan 2008; Kono et al. 2017; Sejima et al. 2014; Takagi et al. 2017b; Miyake 2020). Therefore, the evaluation of the P700 oxidation level as a stress marker is a promising method for selecting stress tolerance in plants. In this study, we suggest that P700 oxidation, represented by the parameter Y(ND), can be used as a stress marker to discriminate chilling stress tolerance among cucumber cultivars. Comparison of 10 cucumber cultivars showed differences in Y(ND) after chilling stress (Fig. 2), and the Y(ND)-non-inducible cultivars showed delayed growth after chilling stress (Figs. 5,6,7).
Terashima et al. (1994) first reported PSI photoinhibition in leaves, and the temperature threshold causing the damage to PSI is below 10 °C. Treatment of cucumber at 4–5 °C for several hours degrades PsaA and PsaB proteins, which are the heterodimer subunits of the PSI reaction center, as a result of PSI photoinhibition (Ivanov et al. 1998; Oh et al. 2009; Sonoike 1996a; Sonoike et al. 1997). The recovery from PSI photoinhibition is slow and has a significant impact on plant growth. The recovery speed of Pm was consistently slow after the chilling treatment, especially in Y(ND)-non-inducible (chilling-sensitive) cultivars (shimo, nan, and HG) (Fig. 5b). The active PSI complex appeared not to fully reconstruct until at least 24 h after the treatment. However, there was a varietal difference in the speed of PSI recovery after 24 h, leading to phenotypic differences among the cultivars (Figs. 6, 7). The cultivar “nan,” which has been used to investigate PSI photoinhibition by chilling stress (Sonoike 2011), belongs to the chilling-sensitive cultivar in our study.
Our results suggest that measurement of P700 oxidation, Y(ND), should be practically useful for evaluating the damage caused by chilling stress in cucumber plants. It can be applied to the breeding of tolerant cultivars and the management of cultivation conditions in greenhouses. However, other well-known parameters, such as Fv/Fm, Y(II), and Y(I), did not show varietal differences and would not be suitable as indicators of chilling damage. Y(NO) and Y(NPQ), parameters indicating dissipation of excess light energy, appeared to have limited correlations with chilling stress tolerance, presumably because they are unstable and can change rapidly during sample preparation for measurements. Y(NA) is inversely correlated with Y(ND); therefore, Y(NA) can also be a useful stress marker; however, it is difficult to estimate the exact Pm′ level with SP illumination (Furutani et al. 2021). Other studies have also suggested that Y(ND) provides a good overview of plants stress tolerance. Y(ND) has been used to diagnose drought stress thresholds in rice and soybean (Suzuki et al. 2021; Wada et al. 2019), and the combined use of Y(ND) with other photosynthetic parameters can be a physiological diagnostic marker for poor plant fertility (Ohnishi et al. 2021).
The electron influx into PSI was mainly limited by “photosynthetic control” in the Cyt b6f complex, induced by proton accumulation in the thylakoid lumen (ΔpH) (Miyake 2020). It has been reported that degradation of the subunit of chloroplast ATPase causes the decoupling of thylakoid membranes during cucumber chilling stress (Terashima et al. 1989, 1991), which results in the loss of photosynthetic control and a decrease in Y(ND). This may explain the relative decrease in Y(NPQ), which reflects the reduction in proton accumulation in the thylakoid lumen (ΔpH), in the chilling-sensitive cultivars HG and shimo after chilling stress (Fig. 4c, d). In contrast, another report rejects the uncoupling of thylakoid membranes during cucumber chilling damage (Oxborough and Ort 1995) and it is possible that the use of different cucumber cultivars may cause differences in the chilling stress response.
P700 oxidation is also regulated by a variety of mechanisms of both the electron acceptor and donor sides of PSI (Miyake 2020). In fact, there were exceptional chilling-sensitive cultivars with lower Y(ND) and higher Y(NPQ) (Fig. 4c, for example, nan or shin). Therefore, acceptor-side regulation of PSI may affect the level of Y(ND) after chilling stress. It has been reported that the introduction of the plastid terminal oxidase (PTOX) from Chlamydomonas into Arabidopsis pgr5 mutant results in low Y(ND) with substantial Y(NPQ), indicating the importance of the acceptor-side regulation of PSI for P700 oxidation (Zhou et al. 2021). It is possible that some AEF pathways, such as photorespiratory and PSI-CEF activity, may be downregulated under chilling stress in those cultivars.
Previous studies have suggested that ROS accumulation is lower in chilling-tolerant cucumber cultivars (Shen et al. 1999; Zhou et al. 2004). In the present study, an increase of the electron influx to PSI was observed in the chilling-sensitive cultivars (Figs. 2, 3), indicating the accumulation of electrons downstream of PSI, which would result in the ROS generation. Therefore, the ability of P700 oxidation would determine differences in the initial ROS levels, which dictates the subsequent ROS production that affects stress tolerance. Further studies on the mechanism regulating ROS levels under chilling stress are necessary to elucidate the factors that discriminate the Y(ND)-inducible and Y(ND)-non-inducible cultivars after short-chilling stress under light illumination.
References
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–42
Anderson JM (1992) Cytochrome b6f complex: dynamic molecular organization, function and acclimation. Photosynth Res 34:341–357
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–287
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Biol 50:601–639
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30:1107–1125
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Bi H, Li F, Wang H, Ai X (2019) Overexpression of transketolase gene promotes chilling tolerance by increasing the activities of photosynthetic enzymes, alleviating oxidative damage and stabilizing cell structure in Cucumis sativus L. Physiol Plant 167:502–515
Ding Y, Shi Y, Yang S (2019) Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol 222:1690–1704
Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25:83–100
Furutani R, Ohnishi M, Mori Y et al (2021) The difficulty of estimating the electron transport rate at photosystem I. J Plant Res. https://doi.org/10.1007/s10265-021-01357-6
Garstka M, Drożak A, Rosiak M et al (2005) Light-dependent reversal of dark-chilling induced changes in chloroplast structure and arrangement of chlorophyll–protein complexes in bean thylakoid membranes. Biochim Biophys Acta (BBA) 1710:13–23
Garstka M, Venema JH, Rumak I et al (2007) Contrasting effect of dark-chilling on chloroplast structure and arrangement of chlorophyll–protein complexes in pea and tomato: plants with a different susceptibility to non-freezing temperature. Planta 226:1165–1181
Hanawa H, Ishizaki K, Nohira K et al (2017) Land plants drive photorespiration as higher electron-sink: Comparative study of post-illumination transient O2-uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol Plant 161:138–149
Harbinson J, Foyer CH (1991) Relationships between the efficiencies of photosystems I and II and stromal redox state in CO2-free air: evidence for cyclic electron flow in vivo. Plant Physiol 97:41–49
Heber U, Walker D (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol 100:1621–1626
Heber U, Neimanis S, Siebke K et al (1992) Chloroplast energization and oxidation of P700/plastocyanin in illuminated leaves at reduced levels of CO2 or oxygen. Photosynth Res 34:433–447
Heidarvand L, Amiri RM (2010) What happens in plant molecular responses to cold stress? Acta Physiol Plant 32:419–431
Helman Y, Tchernov D, Reinhold L et al (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235
Helman Y, Barkan E, Eisenstadt D et al (2005) Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol 138:2292–2298
Hope AB (2000) Electron transfers amongst cytochrome f, plastocyanin and photosystem I: kinetics and mechanisms. Biochim Biophys Acta (BBA) 1456:5–26
Ivanov AG, Morgan RM, Gray GR et al (1998) Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Lett 430:288–292
Kanazawa A, Ostendorf E, Kohzuma K et al (2017) Chloroplast ATP synthase modulation of the thylakoid proton motive force: implications for photosystem I and photosystem II photoprotection. Front Plant Sci 8:719
Karapetyan NV (2008) Protective dessipation of excess absorbed energy by photosynthetic apparatus of cyanobacteria: role of antenna terminal emitters. Photosynth Res 97:195–204
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192:261–268
Klughammer C, Schreiber U (2008a) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:27–35
Klughammer C, Schreiber U (2008b) Saturation Pulse method for assessment of energy conversion in PS I. PAM Appl Notes 1:11–14
Kohzuma K, Cruz JA, Akashi K et al (2009) The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ 32:209–219
Kono M, Yamori W, Suzuki Y, Terashima I (2017) Photoprotection of PSI by Far-red light against thefFluctuating light-induced photoinhibition in Arabidopsis thaliana and field-grown plants. Plant Cell Physiol 58:35–45
Kudoh H, Sonoike K (2002) Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 215:541–548
Mehler AH (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch Biochem Biophys 33:65–77
Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Miyake C (2010) Alternative electron flows (water–water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Miyake C (2020) Molecular Mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to elctron-sink limitations in C3 Plants. Antioxidants 9:230
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155:86–92
Nishio JN, Whitmarsh J (1993) Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol 101:89–96
Oh M-H, Safarova RB, Eu Y-J et al (2009) Loss of peripheral polypeptides in the stromal side of photosystem I by light-chilling in cucumber leaves. Photochem Photobiol Sci 8:535–541
Ohnishi M, Furutani R, Sohtome T et al (2021) Photosynthetic parameters show specific responses to essential mineral deficiencies. Antioxidants 10:996
Oxborough K, Ort DR (1995) In situ evidence that chilling in the light does not cause uncoupling of photophosphorylation or detachment of coupling factor in chilling-sensitive plants. Photosynth Res 43:93–105
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta (BBA) 975:384–394
Rutherford AW, Osyczka A, Rappaport F (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett 586:603–616
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Schöttler MA, Tóth SZ (2014) Photosynthetic complex stoichiometry dynamics in higher plants: environmental acclimation and photosynthetic flux control. Front Plant Sci 5:188
Schöttler MA, Tóth SZ, Boulouis A, Kahlau S (2015) Photosynthetic complex stoichiometry dynamics in higher plants: biogenesis, function, and turnover of ATP synthase and the cytochrome b6f complex. J Exp Bot 66:2373–2400
Sejima T, Takagi D, Fukayama H et al (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55:1184–1193
Sejima T, Hanawa H, Shimakawa G et al (2016) Post-illumination transient O2-uptake is driven by photorespiration in tobacco leaves. Physiol Plant 156:227–238
Shaku K, Shimakawa G, Hashiguchi M, Miyake C (2016) Reduction-induced suppression of electron flow (RISE) in the photosynthetic electron transport system of Synechococcus elongatus PCC 7942. Plant Cell Physiol 57:1443–1453
Shen W, Nada K, Tachibana S (1999) Effect of cold treatment on enzymic and nonenzymic antioxidant activities in leaves of chilling-tolerant and chilling-sensitive cucumber (Cucumis sativus L.) cultivars. J Jpn Soc Hortic Sci 68:967–973
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199–217
Shimakawa G, Miyake C (2018) Oxidation of P700 ensures robust photosynthesis. Front Plant Sci 9:1617
Shimakawa G, Shaku K, Miyake C (2016) Oxidation of P700 in photosystem I is essential for the growth of cyanobacteria. Plant Physiol 172:1443–1450
Shimakawa G, Ishizaki K, Tsukamoto S et al (2017) The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiol 173:1636–1647
Shimakawa G, Shaku K, Miyake C (2018) Reduction-induced suppression of electron flow (RISE) is relieved by non-ATP-consuming electron flow in Synechococcus elongatus PCC 7942. Front Microbiol 9:886
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433
Sonoike K (1996a) Degradation of psaB gene product, the reaction center subunit of photosystem I, is caused during photoinhibition of photosystem I: possible involvement of active oxygen species. Plant Sci 115:157–164
Sonoike K (1996b) Photoinhibition of photosystem I: Its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol 37:239–247
Sonoike K (2011) Photoinhibition of photosystem I. Physiol Plant 142:56–64
Sonoike K, Terashima I (1994) Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 194:287–293
Sonoike K, Terashima I, Iwaki M, Itoh S (1995) Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett 362:235–238
Sonoike K, Kamo M, Hihara Y et al (1997) The mechanism of the degradation of psaB gene product, one of the photosynthetic reaction center subunits of photosystem I, upon photoinhibition. Photosynth Res 53:55–63
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Suzuki Y, Nagao K, Takahashi Y et al (2021) Oxidation of the reaction center chlorophyll of photosystem I is induced via close cooperation of photosystems II and I with progress of drought stress in soybean seedlings. Soil Sci Plant Nutr. https://doi.org/10.1080/00380768.2021.2002124
Takagi D, Takumi S, Hashiguchi M et al (2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171:1626–1634
Takagi D, Amako K, Hashiguchi M et al (2017a) Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant J 91:306–324
Takagi D, Ishizaki K, Hanawa H et al (2017b) Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol Plant 161:56–74
Terashima I, Huang L-K, Osmond CB (1989) Effects of leaf chilling on thylakoid functions, measured at room temperature, in Cucumis sativus L. and Oryza sativa L. Plant Cell Physiol 30:841–850
Terashima I, Kashino Y, Katoh S (1991) Exposure of leaves of Cucumis sativus L. to low temperatures in the light causes uncoupling of thylakoids I. Studies with isolated thylakoids. Plant Cell Physiol 32:1267–1274
Terashima I, Funayama S, Sonoike K (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193:300–306
Trissl H-W (1997) Determination of the quenching efficiency of the oxidized primary donor of Photosystem I, P700+: implications for the trapping mechanism. Photosynth Res 54:237–240
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977
Wada S, Takagi D, Miyake C et al (2019) Responses of the photosynthetic electron transport reactions stimulate the oxidation of the reaction center chlorophyll of photosystem I, P700, under drought and high temperatures in rice. Int J Mol Sci 20:2068
Wada S, Suzuki Y, Miyake C (2020) Photorespiration enhances acidification of the thylakoid lumen, reduces the plastoquinone pool, and contributes to the oxidation of P700 at a lower partial pressure of CO2 in wheat leaves. Plants 9:319
West KR, Wiskish JT (1968) Photosynthetic control by isolated pea chloroplasts. Biochem J 109:527–532
Wilkinson S, Clephan AL, Davies WJ (2001) Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol 126:1566–1578
Yu J-Q, Zhou Y-H, Huang L-F, Allen DJ (2002) Chill-induced inhibition of photosynthesis: genotypic variation within Cucumis sativus. Plant Cell Physiol 43:1182–1188
Zhang Z-S, Jin L-Q, Li Y-T et al (2016) Ultraviolet-B radiation (UV-B) relieves chilling-light-induced PSI photoinhibition and accelerates the recovery of CO2 assimilation in Cucumber (Cucumis sativus L.) leaves. Sci Rep 6:1–10
Zhou YH, Yu JQ, Huang LF, Nogués S (2004) The relationship between CO2 assimilation, photosynthetic electron transport and water–water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant Cell Environ 27:1503–1514
Zhou Q, Wang C, Yamamoto H, Shikanai T (2021) PTOX-dependent safety valve does not oxidize P700 during photosynthetic induction in the Arabidopsis pgr5 mutant. Plant Physiol 188:1264–1276
Acknowledgements
We would like to thank Mr. Shintaro Matsui, Takii Shubyo, Japan for his helpful suggestions.
Funding
This work was supported by JST, CREST, Japan, Grant Number JPMJCR15O3 and JPMJCR17O2 to Kentaro Ifuku and Chikahiro Miyake.
Author information
Authors and Affiliations
Contributions
KI, TN, and CM conceived the project; KT and YC performed the experiments; and KT and KI wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takeuchi, K., Che, Y., Nakano, T. et al. The ability of P700 oxidation in photosystem I reflects chilling stress tolerance in cucumber. J Plant Res 135, 681–692 (2022). https://doi.org/10.1007/s10265-022-01404-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-022-01404-w