Abstract
Zn deficiency is one of the major soil constraints currently limiting rice production. Although recent studies demonstrated that higher antioxidant activity in leaf tissue effectively protects against Zn deficiency stress, little is known about whether similar tolerance mechanisms operate in root tissue. In this study we explored root-specific responses of different rice genotypes to Zn deficiency. Root solute leakage and biomass reduction, antioxidant activity, and metabolic changes were measured using plants grown in Zn-deficient soil and hydroponics. Solute leakage from roots was higher in sensitive genotypes and linked to membrane damage caused by Zn deficiency-induced oxidative stress. However, total root antioxidant activity was four-fold lower than in leaves and did not differ between sensitive and tolerant genotypes. Root metabolite analysis using gas chromatography–mass spectrometry and high performance liquid chromatography indicated that Zn deficiency triggered the accumulation of glycerol-3-phosphate and acetate in sensitive genotypes, while less or no accumulation was seen in tolerant genotypes. We suggest that these metabolites may serve as biochemical indicators of root damage under Zn deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) deficiency, a major soil constraint in crop production, is prevalent in around 50% of lowland rice soils, especially in inland alkaline/sodic and costal saline soils of Asia (Alloway 2008; White and Zasoski 1999; Yoshida and Tanaka 1969). Soil physico-chemical factors linked with Zn deficiency are high organic matter, high bicarbonate content, high pH (>7.0), high exchangeable Mg+2: Ca+2 ratio, and high phosphorus availability (Neue and Lantin 1994; Quijano-Guetra et al. 2002). Bicarbonate toxicity is a stress that frequently occurs together with Zn deficiency, especially in sodic or calcareous soils or in perennially wet soils where organic matter decomposition under anaerobic conditions leads to bicarbonate accumulation (Mengel et al. 1984; Ponnamperuma 1972). Zn deficiency causes leaf bronzing in rice, and within 2–3 weeks after transplanting, brown blotches and streaks develop that eventually cover older leaves (Yoshida and Tanaka 1969). Plants experiencing Zn deficiency stress remain stunted and, under severe Zn deficiency, plant mortality can be high. If deficiency is less severe, plants may begin to recover 4–5 weeks after transplanting, however, delayed maturity and reductions in grain yield are common (Neue and Lantin 1994).
Oxidative stress has been considered a major factor inducing Zn deficiency symptoms. Zinc deficiency interferes with membrane-bound NADPH oxidase and internal iron concentration producing reactive oxygen species (ROS). Thus, Zn-deficient leaves are damaged by photooxidation in the chloroplast and rapidly become chlorotic and necrotic when exposed to high light intensity (Cakmak 2000). This hypothesis was proved by Frei et al. (2010), showing that higher antioxidant accumulation in leaves lowered leaf bronzing symptoms. Recently Mori et al. (2016) observed that maintaining root growth and activity is key to overcoming Zn deficiency. While Rose et al. (2012) reported that Zn deficiency caused cellular damage in roots as well. To what extent cellular damages and defense mechanisms are similar in roots and shoots is not known. The objective of this study is to explore root-specific physiological responses to Zn deficiency stress in both Zn-deficient soils and nutrient solution experiments, including evaluation of antioxidant defense mechanisms and characterization of metabolite patterns.
Materials and methods
Plant materials
Four rice (Oryza sativa L.) genotypes comprising two Zn deficiency-tolerant (A69-1, IR55179), and two Zn deficiency-sensitive genotypes [‘Kinandang Patong’ (KP), IR26] were used. The extent of tolerance to Zn deficiency in these genotypes was determined in preliminary experiments.
Field experiment
Field experiments were conducted at the International Rice Research Institute (IRRI), Los Baños, Philippines during the dry seasons (DS) of 2013 and 2016. Experiments were conducted in a Zn-deficient plot with soil collected from a Zn-deficient hotspot in Tiaong, Quezon province, Philippines. Soil from this site had been brought to IRRI and placed in large concrete tanks (16 × 8 m, 0.3 m depth) that served as field plots. The soil has low Zn availability (0.05 mg kg− ), high pH (8.0), and high organic matter (3.4%) (Supplementary Table S1). For soil-Zn analysis, air-dried (at 27–32 °C and 60–80% RH) soil samples were ground to pass a 2-mm sieve and then analyzed using atomic absorption spectrometry (AAS 3300, Perkin-Elmer; Massachusetts, USA) at the Analytical Service Laboratory of IRRI. The difference in zinc availability between Zn-deficient and control plots (0.05 vs. 0.15 mg kg−1) was highly significant. During the plot preparation, NPK fertilizer (14–14–14) was applied at 20 kg ha−1 as basal dose. Nitrogen was applied in split doses at 24 and 42 days after transplanting (DAT) at 35 and 45 kg ha−1, respectively. Insect, disease, and weed control were accomplished following the standard procedures used for rice production at IRRI. Seeds were sown in seedling plots in the field, and 20 days-old seedlings were transplanted at a spacing of 15 × 18 cm (within and between rows) in experimental plots with six replicates arranged in a RCBD.
Nutrient solution experiment
The nutrient solution experiment was conducted in a greenhouse (controlled temperature and humidity, and insect-free) at IRRI during the 2013 wet season using the two contrasting genotypes, IR55179 (most tolerant in the field) and IR26 (sensitive but similar in plant type to IR55179). Germinated seeds were placed in holes made on a Styrofoam sheets floating on a solution containing 0.1 mM Ca and 36 µM Fe. After 8 days, seedlings were pre-cultured in 10 L black plastic boxes containing half strength Yoshida solution modified to contain full strength Fe (36 µM) but without zinc, to avoid build up of Zn in seedlings before treatment (Frei et al. 2010). After 7 days, treatments were imposed with full strength Yoshida solution. Treatments were two levels each of Zn (0 or 1 µM, as ZnSO4·7H2O) and bicarbonate (0 or 5 mM, as NaHCO3) in full strength Yoshida solutions (Rose et al. 2012; Yoshida et al. 1971). The pH was adjusted daily to 6.5 during the pre-treatment period, and to 8.0 for the treatment phase, which represents the natural pH of the bicarbonate treatment, similar to the field experiment.
Analysis of zinc concentration
Zinc concentration was determined following the method of Yoshida et al. (1971). Plants were collected at 4 weeks after transplanting (WAT) and carefully washed 3 times by reverse osmosis water to remove soil particles attached to the roots. Oven-dried samples (at 70 °C for 4 days) were cut into small pieces using scissors and then ground. Sample (100 mg) was digested by 5 mL 1 N HCl solution for 24 h and zinc concentration was determined using atomic absorption spectrometry (A Analyst 400, PerkinElmer; Massachusetts, USA).
Assessment of root damage
Seedlings of four genotypes (tolerant A69-1 and IR55179; sensitive KP and IR26) were gently pulled out from the field plots at 4 WAT. This was only possible because the texture of the soil used is extremely ‘loose’. Plant samples were carefully collected with minimum damage to the roots and washed three times in deionized water; the last was for 20 min to remove all solutes due to physical root damage. Root solute leakage was measured following Rose et al. (2011) and Ismail and Hall (1999) with slight modifications. Individual roots were placed in 50 mL Falcon tubes filled with 40 mL DI water and wrapped in aluminum foil. Electrical conductivity (EC) of the solution was measured every 1 h. After 6 h, the solutions containing roots were boiled for 1 h and the EC was measured again. Relative electrolyte leakage was calculated as percentage of maximum leakage after boiling.
The ROS level (hydrogen peroxide (H2O2) accumulation) in the roots was visualized for several plants collected from the field at the same time, using DAB (3, 3′-diaminobenzidine 4 HCl) staining following Schraudner et al. (1998). Samples were photographed using fluorescence microscopy. Antioxidant activity was tested following Lee and Lee (2004) and Dewanto et al. (2002). Ground samples of roots and leaf tissues (0.3 g) were separately extracted with 6 mL of 80% MeOH at 40 °C for 3 h. After chemical reaction with 1, 1-diphenyl-2-picrylhydrazyl (DPPH; Sigma Co. Singapore) at 25 °C for 30 min, free radical scavenging activity was determined by maximum absorption at 518 nm. After chemical reactions with 50% Folin–Ciocalteu’s phenol reagents, 2 N (Sigma Aldrich, USA) plus 2% Na2CO3 solutions, total phenol concentration was determined by maximum absorption at 725 nm.
Root metabolite analyses
Metabolites in root extracts were analyzed following Roessner et al. (2001) and Ripperger (1993) with modifications. Approximately 100 mg of fresh root tissue was incubated in 1.4 mL 95% methanol at 70 °C for 3 h and the extracts filtered through a 0.45-μm membrane filter (Millex-FH, PTFE). Organic acids were measured by high performance liquid chromatography (Agilent 1260 HPLC System; DKSH Technology, Philippines Inc.) at 210 nm with an organic acid column (Agilent Hi-Plex H), and mobile phase (0.005 M H2SO4 at 25 °C, 0.8 mL min−1 flow rate). For the qualitative analysis of root metabolites, 100 μL of extract was vacuum-evaporated and the dry residue was derivatised with 50 μL of MSTFA at 50 °C for 24 h. The final sample was analyzed using gas chromatography–mass spectrometry (GC–MS) (QP2010 Plus, Shimadzu; Tokyo, Japan) with SLB-5MS column (15 mL × 0.25 mm ID × 0.25 μm df) and helium and nitrogen as carrier gases. Detected chemical components were identified using the library of mass spectrometry.
Statistical analysis
The field experiments were arranged in a RCBD with six replications in both Zn-deficient and + Zn control plots. Relative values were calculated on treatment mean values. The nutrient solution experiment was arranged in a completely randomized design with Zn and Bicarbonate as main treatment factors and genotype as subfactor. Data were analyzed by (ANOVA) and within each Zn treatment and genotype, means were tested for significant differences based on LSD (P ≤ 0.05) using STAR v2.0.1 (International Rice Research Institute).
Results and discussion
Root solute leakage under Zn deficiency stress
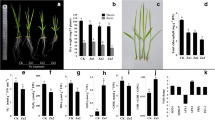
Data from the field trial showed that Zn deficiency stress caused membrane damage, which resulted in roots becoming increasingly ‘leaky’ (Fig. 1a). Sensitive genotypes were more severely affected than the tolerant genotypes. Solute leakage was most severe in IR26, increasing to 43% after 6 h immersion in a collection solution. This compares with around 30% solute leakage in tolerant genotypes. Staining of collected roots in DAB indicated the level of ROS present in roots was limited to the cell elongation zone in roots supplied with adequate Zn, but that ROS was present along more mature sections of the roots under Zn deficiency (Fig. 1b). This increase in ROS due to Zn deficiency was more pronounced in sensitive genotypes.
Comparison of root damages of 4 rice genotypes grown in the Zn-deficient plot. a Root solute leakage (%) of A69-1 (tolerant), IR55179 (tolerant), Kinandang Patong (sensitive), IR26 (sensitive). Plants had been grown for 4 weeks under Zn deficiency before being gently removed from the loose soil, washed, and leakage determined. Vertical bars represent ± SE. b DAB staining for histochemical detection of hydrogen peroxide (H2O2) accumulation in roots. From top to bottom IR26, Kinandang Patong, IR55179, A69-1
Effects of Zn deficiency versus bicarbonate stress
To further examine whether root leakage was directly due to Zn deficiency or may have been caused by the presence of high bicarbonate concentrations in the soil solution, a nutrient solution experiment was conducted under Zn deficiency or high bicarbonate stress. Both stresses led to reduced root growth but bicarbonate toxicity had a far more severe effect (Fig. 2; Supplementary Fig. S1). Genotypes did not differ in solute leakage at the onset of the experiment and in the +Zn control treatment, and leakage gradually decreased with time, possibly because the pre-treatment in 0-Zn solution induced some root damage that was reversed by the addition of Zn. The comparison between the –Zn and bicarbonate stress revealed that bicarbonate had a far more damaging effect on the sensitive genotype, IR26. Data by Rose et al. (2012) indicated that membrane damage and subsequent root leakage were more severely affected by elevated bicarbonate concentration than by Zn deficiency per se and the present data confirms this observation in a different set of genotypes. Since Zn deficiency in the field typically coincides with excess bicarbonate (Kirk 2004), bicarbonate sensitivity will be of practical relevance.
Comparison of root dry weight (mg plant−1) and root solute leakage (%) between IR55179 (tolerant) and IR26 (sensitive) genotypes grown in Yoshida nutrient solution between 0 and 14 days after transplanting. Vertical bars represent ± SE. ANOVA significant levels at 14 DAT: ns not significant; **P ≤ 0.01
Does oxidative stress defense plays a role in roots?
Antioxidant activity as measured by free radical scavenging was less than 20% in roots compared with around 80% in leaf tissue (Fig. 3a). Genotypic differences were not significant in roots, whereas the tolerant genotype had significantly higher antioxidant activity in leaves. In leaves, detoxification of ROS would involve antioxidants such as ascorbate and phenolics (Frei et al. 2010) and experimental evidence (Fig. 3a, b) showed that these processes operated in leaves as expected. Higher levels of free radical scavenging and higher phenolic concentrations in leaves of the tolerant genotype indicated that ROS were more effectively suppressed. However, in roots, antioxidant activity was at least four-fold lower than in leaves and did not differ between genotypes. One possible reason for this is the higher concentration of Zn in roots than in shoots, with similar concentrations in both tolerant and sensitive genotypes (Table 1). Moreover, accumulation of glycerol-3-phosphate and acetate was higher in sensitive genotypes, with less to no accumulation in tolerant genotypes (Fig. 4), suggesting the sensitive genotypes are experiencing low oxygen stress (Miro and Ismail 2013). Thus it appears that antioxidant defense mechanisms are different between roots and shoots, and more effective in leaves. ROS are formed in leaves as reactive intermediates of photosynthesis and are known to cause leaf damage (Asada 2006). Less is known about ROS formation in roots but causes must be fundamentally different as excess light energy cannot be a factor. Our qualitative data nevertheless indicated the presence of high ROS concentrations in the roots of field-grown plants, particularly of sensitive genotypes (Fig. 1b). Having established that membrane damage occurred to a larger extent in roots of sensitive genotypes (Fig. 1a, b), which also had higher ROS concentration in roots, the question remains whether this difference is due to excess ROS production or other causes.
Root metabolic responses to Zn deficiency. a Glycerol-3-phosphate (tR 11.2) accumulation in IR55179 (tolerant) and IR26 (sensitive) between control (black peak/unfilled arrow) and Zn deficiency (red peak/filled arrow) at 14 days after transplanting in the Yoshida nutrient solution. b Acetate accumulation (tR 11.5) of A69-1 (tolerant) and Kinandang Patong (sensitive) grown in the Zn-deficient plot
Metabolic changes in roots triggered by Zn deficiency
12 major root metabolites with peak intensities similar to or above the internal standard were identified by gas chromatography-mass spectrometry (Fig. 4; Supplementary Fig. S2). Among these metabolites, glycerol-3-phosphate, a major component of glycerol-based phospholipids in cellular membranes (Berg et al. 2002) was most relevant to root damage in Zn-deficient conditions (Fig. 4a). Zn deficiency increased the accumulation of glycerol-3-phosphate in both genotypes but more significantly in IR26. In a recent study, Bandyopadhyay et al. (2017) reported 77 genes to be differentially expressed in response to Zn deficiency in a rice genotype, some of which (e.g. Os03g58290; indole-3-glycerol phosphate lyase) could be associated with this respones. Increased levels of glycerol-3-phosphate further confirmed that membrane breakdown occurred, and this may explain the higher root solute leakage in sensitive genotypes. A completely contrasting pattern between genotypes was observed for acetate, which only appeared in sensitive genotype KP under Zn deficiency (Fig. 4b). Acetate, the result of tissue anoxia imposed by a reduced availability of oxygen in rice paddy (El et al. 2003), has been known to increase in plants due to wounding or environmental stresses (Dat et al. 2000; Kimmerer and Kozlowski 1982). Acetate is the product of acetaldehyde oxidation through a reaction catalyzed by aldehyde dehydrogenase (ALDH), and ALDH upregulation under low oxygen stress or hypoxia caused by flooding in rice, was reported in several studies (Estioko et al. 2014; Nakazono et al. 2000). This process has been linked to detoxification of acetaldehyde that accumulates during low oxygen stress and to maintenance of energy production for survival and growth (Miro and Ismail 2013). The question still remains whether the high ROS (Fig. 1b) and acetate (Fig. 4b) detected in roots of the sensitive genotypes is associated with hindered root aeration, possibly through Zn-deficiency induced suppression of aerenchyma formation. There had not yet been a cross-study of Zn deficiency and submergence tolerance mechanisms.
With more Zn being taken up, one may argue that tolerant genotypes experienced less stress compared with sensitive genotypes, but this is not supported by data on tissue Zn concentrations, which were found to not differ between genotype groups in the present study (Table 1) and elsewhere (Mori et al. 2016; Wissuwa et al. 2006).
Our study has shown that Zn deficiency causes cellular damage in leaves and roots, that damage is less pronounced in genotypes considered to be tolerant to Zn deficiency, and that the degree of damage observed in genotypes was not associated with differences in tissue Zn concentrations that were equally low in all genotypes. We confirm the results of Frei et al. (2010) that maintaining a higher ROS defense level was associated with reduced cellular damage in leaf tissue. However, in roots, different tolerance mechanisms must operate, as the antioxidant level was similarly low in all genotypes. Accumulation of high acetate concentration in roots of sensitive genotype KP is indicative of low oxygen stress, which is probably associated with Zn deficiency-induced reduction in root aeration. Further biochemical screening on a broad range of metabolites across more diverse genotypes together with studies on cross-tolerance of Zn deficiency and submergence are needed to completely understand the Zn deficiency-tolerance mechanism.
Conclusions
We have shown that the defense against ROS-induced cell membrane damage is an important component of tolerance to Zn deficiency. In leaf tissue, this process is mediated by antioxidants such as ascorbate and phenolics but in root tissue, these defense mechanisms do not seem to be important. Tolerance mechanisms operating in roots need to be identified but in the meantime, two metabolites we discovered in roots, glycerol-3-phosphate and acetate, appear to indicate root damage and could be used as biochemical indicators for Zn deficiency and related oxidative stress.
References
Alloway BJ (2008) Zinc in soils and crop Nutrition, 2nd edn. International Zinc Association, Brussels and International Fertilizer Industry Association, Paris
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Bandyopadhyay T, Mehra P, Hairat S, Giri J (2017) Morpho-physiological and transcriptome profiling reveal novel zinc deficiency-responsive genes in rice. Funct Integr Genom. doi:10.1007/s10142-017-0556-x
Berg MJ, Tymoczko JL, Stryer L (2002) Biochemistry, 5th edn. W H Freeman, New York
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Dewanto V, Wu X, Liu RH (2002) Processed sweet corn has higher antioxidant activity. J Agric Food Chem 50:4959–4964
EI B, Ram PC, Jackson MB, Reuss J, Harren FJ (2003) Dynamic aspects of alcoholic fermentation of rice seedlings in response to anaerobiosis and to complete submergence: relationship to submergence tolerance. Ann Bot 91:279–290
Estioko LP, Miro B, Baltazar AM, Merca FE, Ismail AM, Johnson DE (2014) Differences in responses to flooding by germinating seeds of two contrasting rice cultivars and two species of economically important grass weeds. AoB Plants 6:plu064
Frei M, Wang Y, Ismail AM, Wissuwa M (2010) Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct Plant Biol 37:74–84
Ismail AM, Hall AE (1999) Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in Cowpea. Crop Sci 39:1762–1768
Kimmerer T, Kozlowski TT (1982) Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol 69:840–847
Kirk GJD (2004) The biogeochemistry of submerged soils. Wiley, Chichester
Lee DJ, Lee JY (2004) Antioxidant activity by DPPH assay. Kor J Crop Sci 49:187–194
Mengel K, Breininger MTh, Bübl W (1984) Bicarbonate, the most important factor inducing iron chlorosis in vine grapes on calcareous soil. Plant Soil 81:333–344
Miro B, Ismail AM (2013) Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front Plant Sci 4:269
Mori A, Kirk GJD, Lee JS, Morete MJ, Nanda AK, Johnson-Beebout SE, Wissuwa M (2016) Rice genotype differences in tolerance of zinc-deficient soils: evidence for the importance of root-induced changes in the rhizosphere. Front Plant Sci 6:1160
Nakazono M, Tsuji H, Li Y, Saisho D, Arimura S, Tsutsumi N, Hirai A (2000) Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiol 124:587–598
Neue HU, Lantin RS (1994) Micronutrient toxicities and deficiencies in rice. In: Yeo AR, Flowers TJ,(eds) Soil mineral stresses: approaches to crop improvement. Springer, Berlin, pp 175–200
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Quijano-Guerta C, Kirk GJD, Portugal AM, Bartolome VI, McLaren GC (2002) Tolerance of rice germplasm to zinc deficiency. Field Crops Res 76:123–130
Ripperger H (1993) Gas chromatography/mass spectrometry of Trimethylsilyl derivatives of Nicotianamine and related amino acids. Biol Mass Spect 22:163–169
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie A (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environ-mentally modified plant systems. Plant Cell 13:11–29
Rose MT, Rose TJ, Pariasca-Tanaka J, Widodo, Wissuwa M (2011) Revisiting the role of organic acids in the bicarbonate tolerance of zinc-efficient rice genotypes. Funct Plant Biol 38:493–504
Rose MT, Rose TJ, Pariasca-Tanaka J, Yoshihashi T, Neuweger H, Goesmann A, Frei M, Wissuwa M (2012) Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta 236:959–973
Schraudner M, Möder W, Wiese C, Van Camp W, Inzé D, Langebartels C, Sandermann H (1998) Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J 16:235–245
White JG, Zasoski RJ (1999) Mapping soil micronutrients. Field Crops Res 60:11–24
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142:731–741
Yoshida S, Tanaka A (1969) Zinc deficiency of the rice plant in calcareous soils. Soil Sci Plant Nutr 15:75–80
Yoshida S, Forno DA, Cock JH, Gomez KA (1971) Laboratory manual for physiological studies of rice, 3rd edn. IRRI, Los Baños
Acknowledgements
This research was part of the PhD dissertation of JSL at the College of Agriculture, University of the Philippines Los Baños. The study was partially funded by the SCPRID programme of BBSRC/DIFID (BB/J011584/1) and by IRRI. We wish to thank Sarah E. Johnson-Beebout and Glenn B. Gregorio (IRRI) and Yoshiaki Ueda and Michael Frei (University of Bonn) for their advice during the study; and James A. Egdane, Ricardo L. Eugenio, Myrish A. Pacleb and Francis H. Rubianes (IRRI) for technical support and Fiona R. Hay (IRRI) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, JS., Wissuwa, M., Zamora, O.B. et al. Biochemical indicators of root damage in rice (Oryza sativa) genotypes under zinc deficiency stress. J Plant Res 130, 1071–1077 (2017). https://doi.org/10.1007/s10265-017-0962-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-017-0962-0