Abstract
Cardiopteris (Cardiopteridaceae), a twining herb of two or three species distributed from Southeast Asia to Northern Australia, requires an embryological study for better understanding of its reproductive features. The present study of C. quinqueloba showed that the ovule and seed development involves a number of unusual structures, most of which are unknown elsewhere in angiosperms. The ovule pendant from the apical placenta is straight (not orthotropous), ategmic, and tenuinucellate, developing a monosporic seven-celled/eight-nucleate female gametophyte with an egg apparatus on the funicular side. Fertilization occurs by a pollen tube entering from the funicular side, resulting in a zygote on the funicular side. The endosperm is formed by the cell on the funicular side in the two endosperm cell stage. While retaining a (pro)embryo/endosperm as it is, the raphe (differentiating late in pre-fertilization stages) elongates toward the antiraphal side during post-fertilization stages, resulting in an anatropous seed. The two-cell-layered nucellar epidermis (belatedly forming by periclinal divisions), along with the raphe, envelops the embryo/endosperm entirely as the seed coat. The possibility was discussed that the arrested integument development triggers a series of the subsequent unusual structures of ovule and seed development. The fertilization mode in Cardiopteris underpins the hypothesis that the Polygonum‒type female gametophyte comprises two four-celled archegonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiopteris is a twining eudicot herb of two or three species distributed from Southeast Asia to New Guinea and Queensland in Australia (Cronquist 1981; Takhtajan 2009). Previously the genus was assigned to Olacaceae (Bentham and Hooker 1862‒1867), Icacinaceae (Engler 1897), or its own family Cardiopteridaceae (e.g. Blume 1847; Cronquist 1981; Hutchinson 1973; Mabberley 2008; Scholz 1964; Sleumer 1942, 1971; Takhtajan 1997, 2009; Thorne 1992). However, based on molecular evidence (Kårehed 2001; Soltis et al. 2011; Tank and Donoghue 2010), the Angiosperm Phylogeny Group (2003, 2009) has accepted the placement of Cardiopteris, along with Citronella, Gonocaryum, and Leptaulus, in a broadly circumscribed family Cardiopteridaceae that is sister to Stemonuraceae in Aquifoliales (see also Reveal 2012; Stevens 2001 onwards).
Despite its clear familial position, Cardiopteris has been poorly understood morphologically (Stevens 2001 onwards). Until recently, our knowledge on reproductive characters has been limited to fragmentary data published by Sleumer (1971). Sleumer (1971, p. 93) described two pendant anatropous ovules with a dorsal raphe in an ovarian cavity, a linear, sulcate seed with a thin testa, and a minute, conical embryo in the top of the granular fleshy albumen. Approximately 30 years later, some descriptions were provided by Doweld (2000) of ovule and seed structure, by Kong et al. (2002, 2014) on the development of the female gametophyte and flowers, by Tobe (2012) of floral structure, and by Schori and Furness (2014) on pollen morphology. Nevertheless, a few essential questions have remained unresolved, particularly with respect to ovule/seed structure and development.
Doweld (2000), based on examination of the seeds of Cardiopteris moluccana Blume, described its ovule and seed characteristics as follows. The ovule is anatropous, bitegmic, and crassinucellate; the seed coat has a vascular bundle running from the placenta to the tip of the integument through the raphe/chalaza; the integument(s) form a micropyle; the seed coat is composed of an endotegmen (i.e., an inner epidermis of a developed inner integument). Most of Doweld’s descriptions were reiterated by Takhtajan (2009). However, Kong et al. (2002) showed with a few micrographs that the ovules of C. platycarya Gagnep. are orthotropous, ategmic, and tenuinucellate. They observed a monosporic, seven-celled/eight-nucleate female gametophyte with an egg apparatus on the “chalazal” side, so that the zygote (or embryo) is formed on the “chalazal” side (see also Kong et al. 2014). Thus, the ovule structure and development of C. platycarya is very different from C. moluccana. This ovule type is also different from what has been known fragmentarily from other Cardiopteridaceae (Citronella, Gonocaryum, and Leptaurus) (for data of the other Cardiopteridaceae see Fagerlind 1945; Mauritzon 1936). Ovules of Citronella, Gonocaryum, and Leptaurus are similar in structure and development to those of C. moluccana, rather than to those of C. platycarya. Thus, development of the ovules and female gametophytes in Cardiopteris needs confirmation (Stevens 2001 onwards). Recently I have published the embryology of other families of the Aquifoliales, i.e., Helwingiaceae (Ao and Tobe 2015) and Phyllonomaceae (Tobe 2015), but the aforementioned uncertainties of Cardiopteris species have hindered critical comparisons of these families with Cardiopteridaceae (Ao and Tobe 2015; Tobe 2015).
The purpose of this paper is primarily to document the development of the anther, ovule, and seed in Cardiopteris. Previous reports of ovule structure and development require confirmation as explained above and there is little information on anther and seed development. For the present study I have examined C. quinqueloba (Hassk.) Hassk. using the material that I previously examined for a study of floral structure (Tobe 2012). According to The Plant List (2013), Cardiopteris consists of two species, C. moluccana and C. quinqueloba. The species “C. platycarya,” which was examined by Kong et al. (2002, 2014), is treated as a synonym of C. quinqueloba. However, C. quinqueloba differs from C. platycarya in inflorescence morphology, with a simple cyme in C. quinqueloba and a compound cyme in C. platycarya (Kong et al. 2014) and in ovule size (310‒320 μm long in C. quinqueloba and 680‒700 μm long in C. platycarya [measured from Figures 1‒3 in Kong et al. 2002]). Based on such morphological differences, C. platycarya can be treated as a distinct species from C. quinqueloba. In this paper, I will first compare C. quinqueloba with C. moluccana (Doweld 2000) and C. platycarya (Kong et al. 2002, 2014) to summarize embryological features of the whole genus and subsequently compare them with other Cardiopteridaceae and other Aquifoliales. As will be documented later, Cardiopteris has a number of unusual patterns of ovule and seed development. I will discuss possible causes of the unusual ovule and seed development and further explore the evolutionary implications of the female gametophyte that involves an unusual fertilization mode.
Materials and methods
Flowers and fruits of Cardiopteris quinqueloba (Hassk.) Hassk. in various stages of development were collected from Doi Sutep-Pui National Park, Chiang Mai Province, Thailand (voucher: J. F. Maxwell 08‒236, CMU Herbarium, Chiang Mai). They were fixed in FAA (five parts stock formalin; five parts glacial acetic acid; 90 parts 50 % ethanol). Flower buds and seeds were dehydrated through an ethanol series and then embedded in Technovit 7100 (Kulzer, Wehrheim, Germany) for microtomy. Serial resin sections cut at a thickness of 6–7 µm were stained with Heidenhain’s hematoxylin and mounted in Entellan new (Merck, Darmstadt, Germany). They were observed with an Olympus BX-51 microscope.
To observe the pollen tube pathway for fertilization, some pistils at the time of fertilization and in post-fertilization stages were examined. An excess part of the fruit wall was removed in a solution of 50 % ethanol, and ovules/seeds together with surrounding tissue were macerated in 1 N sodium hydroxide (NaOH) at 60 °C for 4–6 h. After rinsing two to three times in distilled water (DW), they were decolorized in a diluted solution (1.0 %) of sodium hypochlorite (NaClO) at 37 °C for 1–3 h until no more dark areas persist. After being rinsed two to three times with DW, they were treated in a clearing solution (8 g chloral hydrate, 1 mL glycerol, and 2 mL DW) at 37 °C for 12–24 h. Subsequently, the ovules/seeds were soaked twice in DW at room temperature for 15 min each and kept in DW overnight to remove the chloral hydrate. Thereafter, they were stained with 0.01 % aniline blue in 0.1 M PO4 buffer pH 8.0 for 24–48 h and observed under a fluorescence microscope (Olympus BX‒51) using a UV filter set (model no. 01) with an excitation filter (365 nm, band pass 12 nm), dichroic mirror (FT395), and barrier filter (LP397).
Because of the lack of the integument and because of the delayed differentiation of the raphe in ovules, several standard terms concerning the micropyle or chalaza could not be employed in the following descriptions. Of the terms indicating various positions of the ovule, the “funicular side” will be used instead of the “chalazal side,” and the “apical side” instead of the “micropylar side.” The apical side is opposite to the funicular side throughout ovule development.
Results
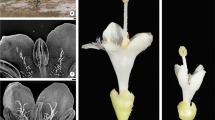
Plants are gynomonoecious, bearing sessile bisexual and pistillate flowers on an inflorescence axis (Fig. 1a). The bisexual flowers are 5-merous, consisting of five sepals, five petals, five stamens, and a unilocular gynoecium with a superior ovary and two dissimilar styles (Fig. 1b, c). The gynoecium is bicarpellate and pseudomonomerous, bearing two ovules in a fertile carpel (Tobe 2012).
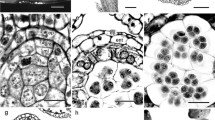
Development of anthers and microspores in Cardiopteris quinqueloba. a Part of inflorescence, bearing a bisexual flower, two pistillate flowers, and a young fruit. b Longitudinal section of bisexual flower bud. c Transverse section (TS) of bisexual flower bud. d TS of a theca of young anther. Note that the middle layer has a common histogenetic origin with the endothecium. e TS of young anther showing binucleate tapetal cells. f TS of young anther showing simultaneous cytokinesis in meiosis of microspore mother cells. g TS of mature anther. Arrowheads indicate positions of longitudinal slit. h TS of mature anther wall enlarged. i Two-celled mature pollen grains. Only the generative cell is stained. ax inflorescence axis, bf bisexual flower, ent endothecium, ep epidermis, fr fruit, gc generative cell, ml middle layer, mmc microspore mother cell, ov ovule primordium, pe petal, pf pistillate flower, se sepal, sl style, st stamen, t tapetum. Scale bars are 2 mm in a, 200 µm in b and c, 100 µm in g, and 20 µm in d, e, f, h, and i
Anthers and microspores
The anther is tetrasporangiate (Fig. 1c). The wall prior to maturation essentially comprises four cell layers: an epidermis, an endothecium, one middle layer, and a tapetum (Fig. 1d). The middle layer has a common histogenetic origin with the endothecium (Fig. 1d); the wall formation therefore conforms to the Dicotyledonous type (Davis 1966, p. 10). The tapetum is glandular and its cells are binucleate (Fig. 1e). During maturation, the middle layer degenerates and the epidermal cells are somewhat enlarged, while the cells of the endothecium become as greatly enlarged as those of the epidermis (Fig. 1e, f). By the time of anther wall dehiscence, the cells of the endothecium develop fibrous thickenings (Fig. 1g, h). The epidermis is persistent (Fig. 1h). Anther dehiscence takes place by longitudinal slits, with each slit common to two microsporangia of the theca (Fig. 1g).
Meiosis in a microspore mother cell is accompanied by simultaneous cytokinesis (Fig. 1f), and the resultant microspore tetrads are predominantly tetrahedral. Pollen grains are two-celled at the time they are shed (Fig. 1i).
Ovules and female gametophytes
The ovule is pendant from the apical placenta with a short, thick funicle and develops straight downward in the locule (Fig. 2a). The archesporium is one-celled, differentiating beneath the apical dermal layer of the ovule primordium (Fig. 2b). It enlarges to differentiate directly into a megaspore mother cell. No parietal cells are formed, and therefore the ovule is tenuinucellate as in Cardiopteris platycarya (Kong et al. 2002). The megaspore mother cell undergoes meiotic division and gives rise to a dyad of megaspores (Fig. 2c) and subsequently a linear or T-shaped tetrad of megaspores (Fig. 2d). In the tetrad, the megaspore deepest on the funicular side is functional, and the three megaspores on the apical side degenerate (Fig. 2e). The functional megaspore develops successively into a two- (Fig. 2f) and four-nucleate female gametophyte (Fig. 2g). After the four-nucleate stage, the female gametophyte rapidly elongates towards the funicular side (Fig. 2h, i). The mature female gametophyte is narrowly ellipsoid, occupying the whole length from the apical to the funicular side of the nucellus (Fig. 3a–c). It comprises eight nuclei in seven cells. Two polar nuclei are present in the center of the female gametophyte. However, an egg apparatus consisting of the egg cell together with two synergids positioned on the funicular, rather than the apical side, whereas the three antipodal cells are positioned on the apical, rather than the funicular side (Fig. 3a–c), as in C. platycarya (Kong et al. 2002). The egg cell is enlarged, and the two synergids have rich cytoplasm (Fig. 3d). This unusual position of the egg apparatus is verified by the fact that a zygote (Fig. 5a), proembryo (Fig. 5g, j), or embryo (Fig. 6b, c) is always found on the funicular side. The three antipodal cells on the apical side are little specialized (Fig. 3e) and disappear soon after fertilization. Figure 4 illustrates the whole developmental process of megasporogenesis and megagametogenesis and shows how the monosporic seven-celled/eight-nucleate female gametophyte develops with the egg apparatus on the funicular side.
Development of ovules and female gametophytes in Cardiopteris quinqueloba. a Longitudinal section (LS) of gynoecium with ovule primordium (rectangle). b Magnified view of LS enclosed by a rectangle in b, showing ovule primordium with an archesporial cell. c LS of ovule with a dyad of megaspores. d LS of ovule with a tetrad of megaspores. e LS of ovule with a functioning megaspore on funicular side. Note degenerating megaspores (arrowhead) on the apical side. f LS of ovule with two-nucleate female gametophyte and degenerating megaspores (arrowhead). g LS of ovule with four-nucleate female gametophyte and degenerating megaspores (arrowhead). h and i Two successive LSs of ovule with four-nucleate female gametophyte. Note that the female gametophyte elongates towards the funicular side. arc archesporial cell, fm functioning megaspore, fu funicle, m megaspore, n nucleus in a female gametophyte, nep nucellar epidermis, ov ovule primordium. Scale bars are 100 µm in a, 50 µm in h and i, and 20 µm in b‒g
Structure of organized female gametophytes in Cardiopteris quinqueloba. a‒c Three successive longitudinal sections (LSs) of mature ovule. Note that an egg apparatus is positioned on the funicular side, two polar nuclei in the middle of the central cell, and antipodals on apical side. Periclinal division occurs in nucellar epidermis (arrowheads). d and e LSs of ovule (different from the one indicated in a–c), indicating an egg apparatus on funicular side (d) and antipodal on apical side (e). Two other antipodals appear in the adjacent sections. f Transverse section of ovule with mature female gametophyte, showing that periclinal division occurs in nucellar epidermis (arrowheads). Note that the raphe is differentiated. ant antipodal cell, e.g. egg cell, fg female gametophyte, fu funicle, po polar nucleus, rap raphe, sy synergid. Scale bars are 50 µm in a‒c, 20 µm in f, and 10 µm in d and e
Some notes should be given on the ovule curvature and differentiation of the raphe. The mature ovule, which occupies the whole space of the ovarian locule, is straight or even looks “orthotropous,” as Kong et al. (2002, 2014) described for the ovule of Cardiopteris platycarya. However, it has a raphe (Fig. 3b, c, f), which usually appears in a more or less anatropous ovule (Jackson 1928). The raphe differentiates late in ovule development and is recognized by the presence of its (procambial) vascular strand extended from the placenta (Fig. 3b, c, f). The terminal of the raphe reaches approximately 1/3 of the whole length of the straight mature ovule (Fig. 3b, c). Because of delayed differentiation of the raphe (and because of the lack of the integument), the tissue of the ovule is mostly that of the nucellus. The nucellus is massive throughout ovule development and some amount of tissue remains around the mature female gametophyte (Fig. 3a–c, f).
In the ovular apex, which is considered to correspond to the nucellar apex because it is destroyed later by a growing female gametophyte as in other Aquifoliales (Ao and Tobe 2015; Tobe 2015), epidermal cells do not divide periclinally to form a nucellar cap (Fig. 2d‒g, i). As the ovule reaches maturity, the apical epidermal cells are destroyed by the enlarging female gametophyte (Fig. 3a–c, e) and epidermal cells on the lateral side divide periclinally except for the raphal region, so that the nucellar epidermis becomes two-cell-layered (Fig. 3f, arrowheads). The funicle is massive and as thick as the ovule itself (but is distinguishable in seeds). No obturator develops from the funicle.
The ovule is ategmic during its development (Figs. 2a–i, 3a‒c, f), as in Cardiopteris platycarya (Kong et al. 2002, 2014). No micropyle is formed for the entry of a pollen tube. Due to the lack of an integument, not only the boundary between the nucellus and raphe but also the position of the chalaza (the part of the ovule where the nucellus joins the integument) is uncertain. Accordingly, the presence of the hypostase, which would appear at the chalaza, is also uncertain. At least the mature ovule has no tissue that looks like the hypostase.
Endosperm and embryo
A few seeds in early post-fertilization stages are presented in Fig. 5a‒h and j. A pollen tube enters the female gametophyte through the funicular tissue. Fertilization occurs with the egg apparatus on the funicular side of the ovule as well as with polar nuclei in the center of the central cell. Figure 5e is a micrograph of fluorescent microscopy, showing a single pollen tube reaching the female gametophyte from the funicular side, not from the apical side of the ovule. As already noted, a zygote or proembryo is always observed on the funicular side (Fig. 5a, g, j).
Development of seeds in Cardiopteris quinqueloba. a Longitudinal section (LS) of young seed with a zygote and a two-celled endosperm. Note that the zygote is on the funicular side and that the endosperm cell on the apical side is enlarged and extruded from the nucellus. b LS of young seed with multicellular endosperm. Note that endosperm cell on apical side occupies the whole space of a locule. c Magnified view of b. Note periclinal divisions in nucellar epidermis (arrowhead). d Transverse section (TS) of young seed, showing periclinal divisions in nucellar epidermis (arrowhead). e Fluorescence micrograph of young seed Note that pollen tube reaches female gametophyte directly from the funicular side. f LS of young seed that has initiated curvature. An arrowhead indicates two cell-layered nucellar epidermis. g LS of young seed. Note that the raphe reaches near the funicle. A proembryo present on funicular side (arrow) is indicated within a rectangle. h TS of immature seed. Note that the raphe and a layer of two to three nucellar epidermal cells enclose the endosperm as seed coat. i Magnified view of TS enclosed by a rectangle in h, showing the seed coat of nucellar epidermal origin. j LS of immature seed. A proembryo present on funicular side (arrow) is indicated within a rectangle. Asterisks in e–g and j indicate apical opening of the endosperm that was left by bursting of the apical endosperm cell. ae endosperm cell on apical side, en endosperm, fe endosperm cell on funicular side, fu funicle, pt pollen tube, rap raphe, so sterile ovule, z zygote. Scale bars are 1 mm in j, 200 µm in b and f‒h, 100 µm in c and e, 50 µm in a, d and i, and 20 µm in rectangles of g and j
Division of the primary endosperm nucleus occurs at nearly the center of the central cell, resulting in two endosperm cells: one on the apical side and the other on the funicular side (Fig. 5a). Since the division of the primary endosperm nucleus as well as the subsequent divisions of the two daughter endosperm cells are accompanied by cell wall formation, endosperm formation can be referred to as being of the ab initio cellular type. However, after the two-celled endosperm stage, the endosperm cell on the apical side enlarges and is extruded from the seed (Fig. 5a). Thereafter, it further enlarges to occupy the whole space of an ovary locule (Fig. 5b), eventually bursting within the locule. Consequently, the apical cell in the two-celled endosperm stage remains only as a remnant on the seed surface and it continues to remain as the apical opening of the endosperm in later stages of seed development (Fig. 5e‒g, j). On the other hand, the endosperm cell on the funicular side repeats ordinary cell divisions to form a massive endosperm (Fig. 5b, c, f, g, h, j). Thus, the endosperm is formed only by the cell on the funicular side at the two-celled endosperm stage.
Some of the nucellar tissue remains around the endosperm in early stages of seed development (Fig. 5a‒d, f). However, as the seed develops, the whole nucellar tissue, except for a thin layer of epidermal cells that forms the seed coat (as described later), is replaced by a developing cellular endosperm (Fig. 5g‒j). The mature seed has a copious endosperm, whose cells accumulate abundant lipids (Fig. 6b‒d).
Structure of mature seed and seed coat in Cardiopteris quinqueloba. a Mature fruit. b Magnified view of longitudinal section (LS) enclosed by a rectangle in c, showing a small dicotyledonous embryo present on the funicular side. c LS of mature seed. d Transverse section (TS) of mature fruit obtained through a line d in a. e Magnified view of TS of mature seed indicated by an arrowhead in d, showing a collapsed nucellar epidermis. f Magnified view of LS of mature seed indicated by an arrow in c, showing a collapsed nucellar epidermis. g Magnified view of TS of mature seed indicated by an arrow in d. em embryo, en endosperm, enc endocarp, fu funicle, ne nucellar epidermis, rap raphe. Scale bars are 5 mm in a, 2 mm in d, 1 mm in c, 500 µm in b, and 50 µm in e‒g
Embryogenesis proceeds very slowly compared to the development of endosperm. I did not examine embryogenesis in detail. However, fragmentary data on early and late embryogenesis indicated that it proceeded normally to form a globular proembryo with a long, slender suspensor (Fig. 5g, j). The embryo in mature seeds is small, approximately 1/50 of the length of the endosperm (Fig. 6b, c). It has two slightly developed cotyledons. A similar embryo is found in mature seeds of C. moluccana (Doweld 2000).
Seed and seed coat
The mature fruit is a bilaterally flattened samara with an appendage on the top and two wings on both sides (Fig. 6a, d) and is 39.0‒40.0 mm long (including the appendage that is 10.0‒12.0 mm long), 16.0‒17.0 mm wide, and 3.2‒4.0 mm thick. Although the gynoecium has two pendant ovules in the ovary locule, the fruit is one-seeded. The mature seed is pendant with a thick funicle (Fig. 6b), exarillate, linear, and approximately 10.0 mm long (Fig. 6c). It looks straight but, as will be described below, the seed is anatropous owing to its developmental change in post-fertilization stages.
Soon after fertilization, the seed is nearly straight with a short raphe (Fig. 5a‒c). However, as the seed develops, the raphe, along with an adjacent layer of the nucellar epidermis, develops so as to enclose the embryo/endosperm. The raphe first elongates downward (Fig. 5c), then toward the horizontal direction (Fig. 5e, f), and finally upwards (or towards the funicular side) (Fig. 5g). On a few micrographs of median longitudinal sections of seeds presented in Fig. 5e‒g and j, I placed symbols (asterisks) on the apical opening of the endosperm to trace how its position moves as the seed develops. In a young seed the apical opening of the endosperm is positioned at the point most distant from the funicle (Fig. 5e). In contrast, the apical opening of the endosperm in older seeds is positioned near the funicle (Fig. 5j). Figure 7 illustrates the entire developmental process of seeds, showing how the raphe (or its vascular strand) develops and how the apical opening of the endosperm changes its position. Observing an older seed without knowing such developmental changes of the seed in post-fertilization stages could lead to the misunderstanding that the ovule is anatropous and that the seed coat possesses a vascular bundle.
Diagrams illustrating the development of seeds in Cardiopteris quinqueloba when viewed from lateral side. Note that while the raphe develops, the seed curves from straight to anatropous. Asterisks indicate the apical opening of the endosperm, which was left by bursting of the apical endosperm cell formed in the two-celled stage. ae endosperm cell on apical side, em embryo, en endosperm, fe endosperm cell on funicular side, pe proembryo, rap raphe, z zygote
The seed coat, i.e., an envelope of the endosperm/embryo, is formed by a thin two-cell-layered nucellar epidermis and the raphe in young seeds (Fig. 5h, i). As already documented, the mature ovules have the two-cell-layered nucellar epidermis, which is formed by periclinal divisions of the epidermis late in ovule development (Fig. 3f). This layer is found at the antiraphal side of the nucellus (Figs. 3f, 5d). As the seed develops, the nucellar epidermis, along with the raphe, is peeled from a developing endosperm/proembryo because the nucellar tissue disappears except for the epidermis. The nucellar epidermis and the raphe entirely wrap the endosperm/embryo in older seeds. In a transverse section of an older seed (Fig. 5h, i), the two-cell-layered nucellar epidermis is observed on both the lateral (upper and lower) sides, and the raphe on both (right and left) sides. In mature seeds, the nucellar epidermis is almost collapsed on the lateral sides and remains as a thin layer, i.e., seed coat, outside of the endosperm (Fig. 6e, f). The raphal tissue remains as it was (Fig. 6g).
Discussion
Comparisons with other species of Cardiopteris, and summary of embryological features of the genus
Prior to the present study, the information on the development of the anther, ovule, and seed of Cardiopteris was fragmentary and limited to descriptions by Sleumer (1971) of a few ovule and seed characters, as well as to observations by Doweld (2000) mainly on the seed structure of C. moluccana and Kong et al. (2002, 2014) on the development of the ovule and female gametophyte in C. platycarya. My work based on C. quinqueloba provided most of the missing information on the development of the anther and seed. It also updated concepts of a few characters relating to the development of the ovule. Before summarizing the embryological features of the genus, we need some explanation for a few characters of the ovule and seed that have been uncertain in previous reports.
First, the ovules of Cardiopteris quinqueloba are neither anatropous as Sleumer (1971) and Doweld (2000) described for all Cardiopteris species and C. moluccana, respectively, nor orthotropous as Kong et al. (2002, 2014) described for C. platycarya. The ovules of C. quinqueloba, like those of C. platycarya (Kong et al. 2002, 2014), grow downward from the apical placenta and appear straight during the pre-fertilization stages. The present study of C. quinqueloba showed that the raphe (or raphal vascular strand extended through the funicle from the placenta), which is prevalent in anatropous ovules, differentiates late in ovule development and that the seed gradually changes its form from straight to anatropous in the post-fertilization stages. If Sleumer (1971) and Doweld (2000) observed older (anatropous) seeds alone, they must have misunderstood the ovules of Cardiopteris as anatropous. This also seems to be the case for C. platycarya. Kong et al. (2002, 2014) may have failed to recognize the raphe in the ovules of C. platycarya, which differentiate late in ovule development and can only be distinguished by the differentiation of its procambial strand in C. quinqueloba. Because C. platycarya has a similar ovule morphology and structure to that of C. quinqueloba in the pre-fertilization stages, C. platycarya also likely exhibits the delayed differentiation of the raphe (whose post-fertilization development results in the anatropous seed), as in C. quinqueloba. Since such ovules of Cardiopteris are not characterized by any category of ovule curvature, I simply described them as straight.
Second, the ovules of Cardiopteris quinqueloba were not bitegmic as Doweld (2000) described for C. moluccana, but were ategmic as Kong et al. (2002, 2014) observed in C. platycarya. According to Doweld (2000), the ovules of C. moluccana are bitegmic; the inner integument is 3‒4 cells thick, and the outer integument is 5‒6 cells thick; a micropyle is formed by both integuments; and a vascular bundle reaches the tip of one of the two integuments through the chalaza. A line drawing of an old seed of C. moluccana, which looks similar to that of C. quinqueloba, was presented as evidence for the presence of the vascular bundle in the integument (Doweld 2000, Fig. 2b on p. 120). However, we now know that in C. quinqueloba, the vascular bundle running the periphery of the seed is not that of the seed coat but that of the raphe. Thus, because of the lack of explicit evidence for bitegmy in C. moluccana, we should regard the ovules of Cardiopteris as ategmic.
Third, the ovules of Cardiopteris quinqueloba were not crassinucellate as Doweld (2000) described for C. moluccana, but tenuinucellate as Kong et al. (2002, 2014) observed in C. platycarya. Since Doweld (2000) did not provide any figure illustrating the crassinucellate ovules, we should regard the ovules of Cardiopteris as tenuinucellate.
Fourth, the mature seed and seed coat structure of Cardiopteris quinqueloba are both very different from those of C. moluccana reported by Doweld (2000). As stated previously, Doweld (2000) described the ovule of C. moluccana as bitegmic. The seed coat of C. moluccana was described as consisting of the testa and tegmen and at maturity as consisting only of the endotegmen (i.e., an inner epidermis of a developed inner integument). Because the descriptions by Doweld (2000) of the ovule and seed structure were not based on observations of their development, they need confirmation. Therefore, I will regard the data on the seed and seed coat from C. quinqueloba as the only data available for the genus.
The overall information on the embryological features of Cardiopteris can be summarized as follows:
Anther tetrasporangiate; anther wall four-cell-layers thick; formation of the Dicotyledonous type; anther epidermis persistent; endothecium fibrous; middle layer degenerating; tapetum glandular, and its cells binucleate. Cytokinesis in the microspore mother cell simultaneous; microspore tetrads predominantly tetrahedral; pollen grains two-celled when shed.
Ovule pendant from apical placenta with a short thick funicle, growing straight with its apex downward (but not orthotropous); archesporium hypodermal (i.e., differentiating beneath apical dermal cell-layer of the ovule primordium) and one-celled. No parietal cells formed, and thus, ovule tenuinucellate. Archesporial cell developing directly into a megaspore mother cell, which undergoes meiosis resulting in a linear or T-shaped tetrad of megaspores; megaspore most deeply positioned on the funicular side functional, developing into a seven-celled/eight-nucleate female gametophyte with an egg apparatus on the funicular side, two polar nuclei in the middle, and three antipodals on the opposite (i.e., apical) side. Antipodals ephemeral. Apical nucellar epidermal cells destroyed by an enlarging female gametophyte; hypostase not differentiating on the funicular side; obturator absent. Ovule ategmic; a raphe differentiating late in ovule development. Periclinal divisions occurring in the nucellar epidermis on the antiraphal side late in ovule development.
Fertilization occurring by a pollen tube entering from the funicular side (neither porogamous, nor chalazogamous), resulting in a zygote on the funicular side and the primary endosperm nucleus in the middle of the central cell; endosperm formation of ab initio cellular type; division of the primary endosperm cell resulting in two cells, one on the funicular side and the other on the apical side; thereafter, while the former repeating ordinary cell divisions to form a massive endosperm, the latter enlarging and extruding from the seed and eventually bursting on apical side; its remnant remaining as an apical opening of the endosperm during seed development. Endosperm thus formed by a single cell on the funicular side at the two endosperm cell stage. Mature seeds having lipids in copious endosperm.
Periclinal division of the nucellar epidermis initiated late in ovule development, resulting in a two-cell-layered epidermis on the antiraphal side of the seed. All other nucellar tissue disappearing.
Embryogenesis uncertain; embryo in mature seed small, approximately 1/50 of length of endosperm. Mature seed exarillate and anatropous (with embryo/endosperm at the original positions). While retaining the embryo and endosperm as they are, the seed gradually changing its form from straight to anatropous due to the distinctive development of the raphe and the nucellar epidermis. Raphe and adjacent nucellar epidermis growing around the embryo and endosperm and wrapping it entirely; apical opening of the endosperm moving from the apical to the funicular side.
Seed coat (envelope of the endosperm/embryo) not testal but formed by a thin layer that is two to three nucellar epidermal cells thick and the raphe. Nucellar epidermis collapsed at maturity, remaining as a permanent layer.
Comparisons with other Cardiopteridaceae and other families of the Aquifoliales
On the basis of the aforementioned embryological features, Cardiopteris was compared with four other genera of Cardioperidaceae (Citronella [=Villarestia], Gonocaryum, Leptaulus, and Pseudobotrys), as well as with four other families of Aquifoliales (Aquifoliaceae, Helwingiaceae, Phyllonomaceae, and Stemonuraceae) in Supplementary Table S1. Except for Helwingiaceae and Phyllonomaceae, whose embryological features have recently been published by Ao and Tobe (2015) and Tobe (2015), the embryology of the other Cardiopteridaceae, Aquifoliaceae, and Stemonuraceae remain poorly understood. As in the papers on Helwingiaceae and Phyllonomaceae (Ao and Tobe 2015; Tobe 2015), I obtained data from Fagerlind (1945) and Mauritzon (1936) for three genera of Cardiopteridaceae (Citronella, Gonocaryum, and Leptaulus); from Brewbaker (1967), Copeland (1963), Corner (1976), Herr (1959, 1961), Schürhoff (1921), and van Tieghem (1898) for Aquifoliaceae; and from Fagerlind (1945), Mauritzon (1936), and Padmanabhan (1961) for Stemonuraceae. Comparisons based on the available information (Table S1) showed that, Cardiopteris agrees with the other families of Aquifoliales (data not available for the other Cardiopteridaceae) in characters of the anther and microspore development. Features common to Cardiopteris and the other Aquifoliales include the following: anther tetrasporangiate; anther wall four-cell-layers thick, formation of the Dicotyledonous type; anther epidermis persistent; endothecium fibrous; middle layer(s) degenerating; tapetum glandular, and binucleate (multinucleate in Stemonuraceae). Cytokinesis in the microspore mother cell simultaneous; microspore tetrads predominantly tetrahedral; pollen grains two-celled when shed. Very little diversity exists in the anther and microspore characters within the Aquifoliales.
Rather, Cardiopteris clearly differs from the other Aquifoliales (including the other Cardiopteridaceae) in many of the ovule and seed characters. Cardiopteris has a straight, ategmic ovule, while the other Aquifoliales have anatropous, unitegmic ovules. In addition, Cardiopteris has some unusual developmental features that are not found in the other Aquifoliales. They include: (1) delayed differentiation of the raphe; (2) periclinal divisions, which occur in the nucellar epidermis on the antiraphal side late in ovule development, resulting in a two-celled layer of dermal origin; (3) female gametophyte development forming the egg apparatus on the funicular side; (4) fertilization by a pollen tube entering from the funicle directly to the female gametophyte; (5) endosperm formed by the cell on the funicular side in two endosperm-cell stage (another cell on the apical side extruding from the seed and bursting); (6) a distinctive post-fertilization development of the raphe resulting in the anatropous seed; and (7) the two-cell-layered structure of nucellar dermal origin (formed late in ovule development) developing into a seed coat to wrap the embryo and endosperm. Among these features, only the female gametophyte with the egg apparatus on the funicular side is reported in C. platycarya (Kong et al. 2002, 2014).
All of the aforementioned features (1‒7) are unknown in other angiosperms. If Cardiopteris requires a suitable taxonomic rank based on such distinctive features, we may have to provide it with an ordinal or even higher rank. That is unacceptable of course, because molecular evidence shows that Cardiopteris, Citronella, Gonocaryum, Leptaulus, and Pseudobotrys, constitute a broadly circumscribed family Cardiopteridaceae that is sister to Stemonuraceae in Aquifoliales (Kårehed 2001; Soltis et al. 2011; Tank and Donoghue 2010). Floral anatomy further shows that the pseudomonomerous gynoecium is very likely a synapomorphy to support the sister-group relationship between Cardiopteridaceae and Stemonuraceae (Tobe 2012). Why are so many unusual features found in Cardiopteris and nowhere else? Possibly, this is because all or most of them are mutually related to one another, since the unusual features mostly appear to occur one by one according to the development of the ovule and seed.
Another characteristic of the unusual embryological features of Cardiopteris is that most involve the delay or suppression of the ordinary development of the ovule and seed that is observed in the other angiosperms (including other Aquifoliales). For instance, the differentiation of the raphe is weak and delayed in Cardiopteris (Feature 1) compared with the other Aquifoliales. In fact, in Aquifoliaceae and Helwingiaceae the raphe develops on the ventral side of the ovule, whereas in Cardiopteris it is recognized only by its procambial vascular strand that differentiates late in ovule development. We may regard the differentiation of the raphe as being suppressed in Cardiopteris until late in ovule development. Ovule curvature also is initiated belatedly in Cardiopteris. The ovule primordium of Cardiopteris is similar to those of Aquifoliaceae (Herr 1959) and Helwingiaceae (Ao and Tobe 2015) in that it is pendant straight downward from the apical placenta. However, in Cardiopteris it is curved from straight to anatropous in the post-fertilization stages (Feature 6), rather than in pre-fertilization stages as in Aquifoliaceae (Herr 1959) and Helwingiaceae (Ao and Tobe 2015). In other words, the initiation of the ovule curvature in Cardiopteris is suppressed until post-fertilization stages. Such a delayed ovule curvature might have caused the differentiation of an egg apparatus on the funicular side in Cardiopteris (Feature 3), as well as the pollen tube entry from the funicular side to the female gametophyte (Feature 4).
In addition, the two-cell-layered structure is formed by periclinal divisions of the epidermis on the antiraphal side of the ovule late in ovule development (Feature 2), and later it wraps the embryo and endosperm as the seed coat (Feature 7). This two-cell-layered structure may represent the single integument common to Aquifoliales, but its development is suppressed in Cardiopteris. In the unitegmic ovules of the other angiosperms, the integument is generally initiated by periclinal divisions in the epidermis of the ovule primordium (see Bouman 1984 for review). Indeed, in Phyllonoma the integument is initiated by perclinal divisions in the epidermis of an ovule primordium (Fig. 2b in Tobe 2015). Additionally, the integument does develop into the seed coat as in the other angiosperms (including other Aquifoliales, Table S1). The two-cell-layered epidermis of Cardiopteris also develops into the seed coat. I do not know whether and how the unusual endosperm development (Feature 5) is related causally to the other unusual features of ovule and seed development. The delayed ovule curvature, or a reversed polarity of the female gametophyte in terms of the position of the egg apparatus, might be related to the phenomenon that the endosperm cell on the apical side is extruded from the seed and bursts.
Given that the unusual features in ovule and seed development in Cardiopteris are correlated to one another, rather than being independent phenomena, they may occur like a cascade of events. Considering the developmental orders of diverse ovule and seed characters, the suppression of the integument or the delayed ovule curvature seems to be a trigger of subsequent unusual ovule and seed development, including the formation of the two-cell-layered nucellar epidermis, the delayed differentiation of the raphe, the differentiation of the egg apparatus on the funicular side, endosperm formation only by the cell on the funicular side in the two endosperm celled stage, and seed coat formation by the two-cell-layered nucellar epidermis and the raphe. This scenario is speculative, but it is likely because, otherwise, the many unusual features of ovules and seeds of Cardiopteris do not facilitate comparisons even with closely related genera. However, because none of the other cardioperidaceous genera have ever been investigated fully (Table S1), it is still uncertain whether any of the four other genera of Cardiopteridaceae have the similar features to those of Cardiopteris. We need thorough studies of embryological characters in any one of the four other cardioperidaceous genera in the future.
Evolutionary implications of the fertilization mode in Cardiopteris
Irrespective of whether the aforementioned unusual ovule and seed development are correlated to one another, the female gametophyte of Cardiopteris requires some explanation because of its unusual polarity. The ovule of Cardiopteris grows straight downwards and produces the megaspore mother cell beneath the epidermis on the apical side of the ovule. Both the position of the megaspore mother cell and its subsequent development into the seven-celled/eight-nucleate female gametophyte agree with what we know as the mode of the Polygonum type. In addition, the apical nucellar epidermal cells are destroyed by the developing female gametophyte in Cardiopteris, a phenomenon observed at the micropylar side in the anatropous ovules of the other Aquifoliales (Ao and Tobe 2015; Tobe 2015). Accordingly, we can regard the apical side of the female gametophyte of Cardiopteris as equivalent to the micropylar side of the Polygonum type female gametophyte of the other Aquifoliales and the funicular side of the former as equivalent to the chalazal side of the latter (hereafter, the apical and funicular side of the Cardiopteris female gametophyte is referred to as the micropylar and chalazal side). In other words, the female gametophyte of Cardiopteris has the egg apparatus on the chalazal, rather than the micropylar side as in the Polygonum type female gametophyte. The pollen tube for fertilization enters the female gametophyte directly from the chalazal, rather than the micropylar side. Nevertheless, this is not a case of chalazogamy because, in chalazogamy, the pollen tube, passing through the lateral side of the female gametophyte, extends from the chalazal to the micropylar side as in Betulaceae and Casuarinaceae (Sogo et al. 2004; Sogo and Tobe 2005).
Thus, the fertilization mode in Cardiopteris is neither porogamy nor chalazogamy. This is not as striking of a phenomenon if we accept the hypothesis that the seven-celled/eight-nucleate Polygonum type female gametophyte consists of two, reduced, four-celled archegonia that are positioned oppositely (Porsch 1907). The reduced, four-celled archegonium is found in the premature stages of female gametophyte development in some gymnosperms, such as Cycadaceae and Ginkgoaceae (Chamberlain 1935) and even in the basal angiosperms Austrobaileyales and Nymphaelaes (Friedman and Williams 2003; Tobe et al. 2007; Williams and Friedman 2004). Friedman and Williams (2003) and Williams and Friedman (2004), using the term “module” for a developmental unit resulting in a nuclear quartet (or the four-celled archegonium), discussed that the first angiosperm female gametophyte was composed of a single developmental module on the micropylar end and that the seven-celled/eight-nucleate Polygonum type female gametophyte (consisting of two modules) evolved by duplication of the single ancestral module. Given their hypothesis, the seven-celled/eight-nucleate Polygonum type female gametophyte has two egg cells, one at the micropylar and the other at the chalazal end. In all angiosperms but Cardiopteris with the Polygonum type female gametophyte, the egg cell at the micropylar end is fertilized. However, in Cardiopteris the egg cell at the chalazal end is fertilized. The fertilization mode in Cardiopteris allows us to confirm for the first time that the Polygonum type female gametophyte is composed of two archegonia.
The mode of the female gametophyte development in Cardiopteris does not differ from the mode known as Polygonum type until the four-nucleate stage. The difference merely exists in which egg cell at the micropylar or chalazal end is fertilized. When is such polarity determined? That is, when is it determined which egg cell at the micropylar or chalazal end is fertilized? A recent study of the genome-wide gene expression in female gametophytes of rice shows that gene expression profiles of an egg cell and synergids are already specified at the micropylar end during the period of megagametogenesis (Ohnishi et al. 2011). As we have seen, in Cardiopteris the female gametophyte rapidly elongates toward the funicle during the four-nucleate stage, so that the four-nucleate female gametophyte becomes two to three times longer than the one prior to the extension, and its chalazal end reaches the funicle (Fig. 2g‒i). Considering that fertilization occurs by the pollen tube extending down through the funicle, the rapid extension of the chalazal end of the four-nucleate female gametophyte toward the funicle may be closely associated with the fertilization of the egg cell formed at the chalalzal side at the subsequent, seven-celled/eight-nucleate stage and the polarity of the female gametophyte, that is, which egg cell at the micropylar or chalazal end is fertilized is determined by the four-nucleate stage.
References
Angiosperm Phylogeny Group (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141:399–436
Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APGIII. Bot J Linn Soc 161:105–121
Ao C, Tobe H (2015) Floral morphology and embryology of Helwingia (Helwingiaceae, Aquifoliales): systematic and evolutionary implications. J Plant Res 128:161–175
Bentham G, Hooker JD (1862‒1867) Genera plantarum, vol 9. Reeve Co., London
Blume CL (1847) De genere Cardiopteri. Rumphia 3:205–207
Bouman F (1984) The ovule. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 123–157
Brewbaker JL (1967) The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in angiosperms. Am J Bot 54:1069–1083
Chamberlain CJ (1935) Gymnosperms: structure and evolution. The University of Chicago Press, Chicago
Copeland HF (1963) Structural notes on hollies (Ilex aquifolium and I. cornuta, family Aquifoliaceae). Phytomorphology 13:455–464
Corner EJH (1976) The seeds of dicotyledons, vols 1, 2. Cambridge University Press, Cambridge
Cronquist S (1981) An integrated system of classification of flowering plants. Columbia University Press, New York
Davis GL (1966) Systematic embryology of flowering plants. Wiley, New York
Doweld AB (2000) Cardiopteridaceae. In: Takhtajan A (ed) Comparative seed anatomy, vol 6. Nauka, St. Petersburg, pp 120–121 (in Russian)
Engler A (1897) Icacinaceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, Teil 3(5). Wilhelm Engelmann, Leipzig, pp 233–257
Fagerlind F (1945) Bau des Gynöceums, der Samenanlage und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. Svensk Bot Tidskr 39:346–364
Friedman WE, Williams JH (2003) Modularity of the angiosperm female gametophyte and its bearing on the early revolution of endosperm in flowering plants. Evolution 57:216–230
Herr JM Jr (1959) The development of the ovule and the megagametophyte in the genus Ilex. J Elisha Mitchell Sci Soc 75:107–128
Herr JM Jr (1961) Endosperm development and associated ovule modifications in the genus Ilex. J Elisha Mitchell Sci Soc 77:26–32
Hutchinson J (1973) The Families of flowering plants, 3rd edn. Oxford University Press, Oxford
Jackson BD (1928) A glossary of botanic terms, 4th edn. Duckworth, London
Kårehed J (2001) Multiple origin of the tropical forest tree family Icacinaceae. Am J Bot 88:2259–2274
Kong D-R, Peng H, Liang H-X (2002) A new type of embryo sac in Cardiopteris and its systematic implications. Acta Bot Sin 44:496–498
Kong DR, Schori M, Lu SG, Li L, Peng H (2014) Floral development of Cardiopteris, with emphasis on gynoecial structure and ovular morphology. J Syst Evol 52:629–642
Mabberley DJ (2008) Mabberley’s plant book. A portable dictionary of plants, their classification and uses. Cambridge University Press, New York
Mauritzon J (1936) Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. Svensk Bot Tidskr 30:541–550
Ohnishi T, Takanashi H, Mogi M, Takahashi H, Kikuchi S, Yano K, Okamoto T, Fujita M, Kurata N, Tsutsumi N (2011) Distinct gene expression profiles in egg and synergid cells of rice as revealed by cell type-specific microarrays. Plant Physiol 155:881–891
Padmanabhan D (1961) A contribution to the embryology of Gomphandra polymorpha. Proc Natl Inst Sci India B 27:389–398
Porsch O (1907) Versuch einer phylogenetischen Erkärung des Embryosackes und der doppelten Befruchtung der Angiospermen. Gustav Fischer, Jena
Reveal JL (2012) An outline of a classification scheme for extant flowering plants. Phytoneuron 37:1–221
Scholz H (1964) Celastrales. In: Melchior H (ed) A Engler’s Syllabus der Pflanzenfamilien. II. Gebrüder Borntraeger, Berlin, pp 289–300
Schori M, Furness CA (2014) Pollen diversity in Aquifoliales. Bot J Linn Soc 175:169–190
Schürhoff PN (1921) Die Entwicklungsgeschichte von Ilex aquifolium. Ber Dtsch Bot Ges 39:377–379
Sleumer H (1942) Peripterygiaceae. In: Engler A, Prantl K (eds) Die Natürlichen Pflanzenfamilien, 20b. Duncker & Humblot, Berlin, pp 397–400
Sleumer H (1971) Cardiopteridaceae. In: van Steenis CGGJ (ed) Flora Malesiana, vol 7. Noordhoff International Publishing, Leyden, pp 93–96
Sogo A, Tobe H (2005) Intermittent pollen-tube growth in pistils of alders (Alnus). Proc Natl Acad Sci USA 102:8770–8775
Sogo A, Noguchi J, Jaffré T, Tobe H (2004) Pollen-tube growth pattern and chalazogamy in Casuarina equisetifolia (Casuarinaceae). J Plant Res 117:37–46
Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Matvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu Y-L, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Am J Bot 98:704–730
Stevens PF (2001 onwards) Angiosperm phylogeny Website. Version 12, July 2012. http://www.mobot.org/MOBOT/research/APweb/. Accessed 27 Feb 2015
Takhtajan A (1997) Diversity and classification of flowering plants. Columbia University Press, New York
Takhtajan A (2009) Flowering plants, 2nd edn. Springer, New York
Tank DC, Donoghue MJ (2010) Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Syst Bot 35:425–441
The Plant List (2013) Version 1.1. Published on the Internet; http://www.theplantlist.org/. Accessed 1st Jan
Thorne RF (1992) Classification and geography of the flowering plants. Bot Rev (Lancaster) 58:225–348
Tobe H (2012) Floral morphology and structure of Cardiopteris (Cardiopteridaceae) with special reference emphasis on the gynoecium: systematic and evolutionary implications. J Plant Res 125:361–369
Tobe H (2015) Embryology of Phyllonoma (Phyllonomaceae, Aquifoliales): characteristics and character evolution. J Plant Res 128:633–642
Tobe H, Kimoto Y, Prakash N (2007) Development of the female gametophyte in Austrobaileya scandens (Austrobaileyaceae). J Plant Res 120:431–436
van Tieghem P (1898) Structure de quelques ovules et patti qu’on en pent tirer am liorer la classification. J Bot (Morot) 12:197–220
Williams JH, Friedman WE (2004) The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): implications for understanding the origin and early evolution of monocots, eumagnoliids, and eudicots. Am J Bot 91:332–351
Acknowledgments
I dedicate this article to the late James F. Maxwell, who collected the materials used in the present study. I am grateful to Tomoki Kadokawa and Takenori Yamamoto for their assistance in preparing some figures used in the present article, and to Tomoko Fukuda for translating a Russian article into English. The study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25440208).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tobe, H. Embryology of Cardiopteris (Cardiopteridaceae, Aquifoliales), with emphasis on unusual ovule and seed development. J Plant Res 129, 883–897 (2016). https://doi.org/10.1007/s10265-016-0845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0845-9