Abstract
Salinity stress reduces plant productivity, but low levels of salinity often increase plant growth rates in some species. We herein describe the effects of salinity on plant growth while focusing on nitrogen use. We treated Trifolium alexandrinum with two nitrogen concentrations and salinity levels and determined growth rates, mineral concentrations, nitrogen use efficiency, photosynthesis rates, and nitrate reductase (NR, E.C. 1.6.6.1) and glutamine synthetase (GS, EC 6.3.1.2) activities. The T. alexandrinum growth rate increased following treatment with 100 mM NaCl in low nitrogen (LN) and high nitrogen (HN) conditions. Salt treatment also increased root volume, intrinsic water use efficiency, and nitrogen use efficiency in LN and HN conditions. These changes likely contributed to higher biomass production. Salinity also increased accumulations of sodium, chloride, and phosphate, but decreased potassium and calcium levels and total nitrogen concentrations in all plant organs independently of the available nitrogen level. However, the effect of salt treatment on magnesium and nitrate concentrations in photosynthetic organs depended on nitrogen levels. Salt treatment reduced photosynthesis rates in LN and HN conditions because of inhibited stomatal conductance. The effects of salinity on leaf NR and GS activities depended on nitrogen levels, with activities increasing in LN conditions. In saline conditions, LN availability resulted in optimal growth because of low chloride accumulation and increases in total nitrogen concentrations, nitrogen use efficiency, and NR and GS activities in photosynthetic organs. Therefore, T. alexandrinum is a legume forage crop that can be cultivated in low-saline soils where nitrogen availability is limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses, especially salinity and nutrient deficiency, are important factors that reduce crop yields worldwide. Salinity, in particular, is an increasing problem affecting 20 % of the world’s cultivated land and nearly half of the irrigated area (FAO 2002; Sosa et al. 2005). Moreover, salinity-affected areas are rapidly expanding because of faulty irrigation systems and poor water quality. Salinity stress affects plant productivity and agricultural sustainability in many areas of the world, especially in arid and semi-arid regions (Endris and Mohammed 2007; Feng et al. 2005). Its effects on plants are complex and may result in problems associated with water deficits, ionic imbalance, mineral nutrition, stomatal behavior, and photosynthetic activity (Bohnert and Jensen 1996; Moghaieb et al. 2001). However, plant species differ in their sensitivity or tolerance to salts (Brady and Weil 1996).
In saline soils, the concentrations of Na+ and Cl− may exceed those of essential macronutrients by an order of magnitude. The resulting changes to soil ion activities and ratios of Na+ to specific macronutrients may alter nutrient uptake by roots and nutrient translocation within the plant. Consequently, plants may become susceptible to nutritional disorders (Munns 2005; Niu et al. 1995; Parida and Das 2005). Thus, physiological mechanisms underlying interactions between salinity and mineral deficiency can only be studied in controlled environments. Additionally, plant responses to multiple stresses are highly complex and can differ from responses to individual stresses. Moreover, salt-affected soils are usually deficient in nitrogen (N) (Ashraf and McNeilly 1994). Therefore, N deficiency has been suggested as a major factor responsible for reducing plant growth in saline habitats (Chen 1998; Kao and Chang 1998).
Nitrogen is an essential nutrient for plants (Marschner 1995), and it constitutes 1.5–2 % of plant dry matter. It promotes rapid growth, increases leaf size and quality, hastens crop maturity, and promotes fruit and seed development. Nitrogen is a component of amino acids, which are required to synthesize proteins and other related compounds. It also has important roles in almost all plant metabolic processes. Nitrogen deficiency alters many morphological, physiological, and biochemical parameters. For example, it causes decreases in growth, leaf number, leaf area (Radin and Boyer 1982; Radin and Parker 1979), net photosynthetic assimilation (Huang et al. 2004; Shangguan et al. 2000; Terashima and Evans 1988; Wong et al. 1985; Zhu et al. 2014), chlorophyll content, intrinsic water use efficiency (iWUE), and concentrations of phosphorus and potassium (Malavolta et al. 2004; Zhu et al. 2014). Nitrogen deficiency also causes decreased concentrations of different N forms (Rubio-Wilhelmi et al. 2011). The main symptoms of N deficiency in plants are leaf senescence caused by lipid peroxidation and pigment loss as well as protein degradation, leading to reduced photosynthetic capacity (Casano et al. 1994). Therefore, considerable research has focused on plant responses to N deficiency, which is an abiotic stress that plants may experience several times during their growth and development (Scheible et al. 2004; Wang et al. 2000).
Nitrogen is required by plants as NH4 + or NO3 −, which are the main available forms of N in soils. These compounds are usually taken up from the soil and then assimilated, transformed, and mobilized within plants (Romero et al. 2004). Nitrogen assimilation is catalyzed by enzymes, including nitrate reductase (NR) and glutamine synthetase (GS), which are the first enzymes in the NO3 − and NH4 + assimilation pathways, respectively. Nitrate reductase activity is a limiting factor of plant growth and development (Solomonson and Barber 1990) and is influenced by several environmental conditions (Crawford 1995), including salinity. Salt-induced modification of NR activity depends on many factors, such as plant species, N availability, and salt concentration. Glutamine synthetase catalyzes the ATP-dependent condensation of ammonium with glutamate to yield glutamine, which then provides N groups, either directly or via glutamate, for the biosynthesis of all plant nitrogenous compounds (Forde and Cullimore 1989). Glutamine synthetase activity increases in response to saline conditions in several species such as ryegrass (Sagi et al. 1998), tomato (Cramer et al. 1999), cowpea (Silveira et al. 2001), and barley (Kant et al. 2007).
In the present study, we investigated the combined effects of salinity and N deficiency on berseem (Trifolium alexandrinum), an annual legume forage crop known for its high yield and protein content, with the ultimate goal of improving the productivity of forage species in salinized soils.
Materials and methods
Plant materials, growth conditions, and treatments
Berseem seeds were surface sterilized with 3 % (w/v) calcium hypochlorite solution for 5 min, rinsed several times with distilled water, sown in Petri dishes, and incubated at 25 °C in the dark. Five uniform germinated seedlings were grown in 5-L pots filled with 1:20 diluted nutrient solution (Hewitt 1966) for 14 days. Seedlings were then grown in the same complete nutrient solution containing 1.6 mM KH2PO4, 0.6 mM K2HPO4, 1.5 mM MgSO4, 3 mM KCl, 3.5 mM CaCl2, and 3.0 µM Fe-K-EDTA. Trace elements were supplied as follows (µM): 0.05 Zn, 0.5 Mn, 0.04 Cu, 0.02 Mo, and 0.05 B. Nitrogen was supplied as NH4NO3 at 0.5 mM (Low N; LN) or 5.0 mM (High N; HN) concentrations. Salt treatments consisted of the addition of 100 mM NaCl to the nutrient solution (0 mM NaCl for controls). Plants were grown in a controlled environment room with a 16-h photoperiod (photosynthetically active radiation at plant level of 800–900 µmol m−2 s−1) and day/night conditions of 27/25 °C and 60/75 % relative humidity. The nutrient solution was continuously aerated and renewed every 7 days. The pH was adjusted to 6.5 ± 0.2.
Plants were harvested at 0 and 45 days after salt treatment, and samples were divided into leaves, stems, and roots. The harvested materials were quickly washed with distilled water and dried with towels before their fresh weights were determined. Dry weight was measured after samples were dried for 72 h in a thermo-ventilated oven at 65 °C. The relative growth rate (RGR) was determined according to the method of Khan et al. (2000).
Mineral analysis
Leaf, stem, and root samples were ground to a fine powder. Cations were extracted from homogenized powder with 0.5 % HNO3. Sodium and potassium concentrations in plant tissues were determined using flame spectrophotometry (Corning 410, UK), and calcium and magnesium contents were measured using atomic absorption spectrophotometry (Varian 06). Anions were extracted with boiling Milli-Q water (Drihem and Pilbeam 2002) and assayed using a Metrohm Model 761 ion chromatograph equipped with a Metrosep anion dual 2 column (6.1006.100) with 2.0 mM NaHCO3/1.3 mM Na2CO3 as the eluent. Total N was estimated as the sum of nitrate and reduced N. The latter was determined using the Kjeldahl method (Bremner 1965). Nitrogen use efficiency (NUE) was calculated as shoot dry weight divided by total shoot N content according to an established procedure (Maranville et al. 1980).
Photosynthetic measurements
Photosynthetic rate (A), stomatal conductance (g s), and transpiration rate (E) were measured in situ from 10 to 12 a.m. 1 d before harvest using a portable LCpro+ system. The measurement conditions were as follows: photosynthetically active radiation incident on leaf surface, 850 µmol m−2 s−1; CO2 reference, 430 ppm; leaf chamber temperature, 28 °C; and boundary resistance to H2O, 0.3 m2 s mol−1. According to Mediavilla et al. (2002), the A/g s ratio was considered an estimate of iWUE. Photosynthetic pigment content (carotenoids and chlorophylls a and b) was determined spectrophotometrically in 80 % acetone according to the method of Torrecillas et al. (1984).
Nitrate reductase and glutamine synthetase extraction and assays
To measure NR activity, frozen leaf and root samples were homogenized at 4 °C in an extraction solution containing 0.1 M potassium phosphate buffer (pH 7.4), 2.5 % (w/v) casein, 7.5 mM cysteine, and 1 mM EDTA. After filtration, the homogenate was centrifuged at 30,000g for 15 min at 4 °C. Nitrate reductase activity was determined according to the method of Wray and Filner (1970). The extract was incubated in 0.1 M potassium buffer phosphate (pH 7.4), 332 mM EDTA, 1 M KOH, and 0.15 mM NADH at 30 °C for 30 min. The reaction was stopped by the addition of 1 M zinc acetate. The absorbance of the supernatant was measured at 540 nm after diazotization of nitrite ions with 5.8 mM sulfanilamide and 0.8 mM N-(1-naphthyl)-ethylenediamine dihydrochloride.
For GS activity measurements, frozen samples were homogenized at 4 °C in grinding medium containing 50 mM Tris–HCl buffer (pH 7.6), 1 mM EDTA, 1 mM MgCl2, and 1 % (w/v) polyvinylpyrrolidone. The homogenate was centrifuged at 15,000g for 30 min at 4 °C. Glutamine synthetase activity was determined using hydroxylamine as the substrate. The formation of α-glutamylhydroxamate was determined using acidified ferric chloride (Wallsgrove et al. 1979). The α-glutamylhydroxamate was quantified using commercial glutamine as a standard after determining the absorbance of the incubation medium at 540 nm.
Statistical analysis
All data were subjected to two-way ANOVA using salinity and N as factors for each parameter. The data are presented as the mean ± standard error. Statistical analyses were performed using SPSS 16.0 software.
Results
Growth and morphological parameters
To characterize T. alexandrinum responses to salinity and N availability, plant growth parameters were evaluated following treatment with NaCl in LN and HN conditions. Salinity had a highly significant effect on whole plant growth (P < 0.001; F = 8.82), with 100 mM NaCl treatment increasing the total dry weight by about 56 % and 60 % in LN and HN conditions, respectively (Table 1). Salinity (100 mM NaCl) also had a highly significant effect on all organ dry weights (P < 0.001; F = 4.02, F = 17.10, and F = 0.89 for leaves, stems, and roots, respectively), but had no effect on the ratio of above-ground plant dry weight and root dry weight (P < 0.05; F = 3.05). Additionally, salinity had a highly significant effect on RGR (P < 0.001; F = 25.04) with increases of 42 % and 41 % in LN and HN conditions, respectively (Table 1). Nitrogen treatment significantly decreased whole plant dry weights (P < 0.01; F = 3.54) and leaf and root dry weights (P < 0.01; F = 4.23 and F = 2.25, respectively; Table 1). However, N had no significant effects on stem dry weight and the ratio of above-ground plant dry weight and root dry weight. Furthermore, N significantly decreased RGR (P < 0.001; F = 96.43). However, a combined treatment of salinity and N seemed to have a significant effect only on leaf and root dry weights (P < 0.05; F = 0.9 and F = 0.22, respectively; Table 1).
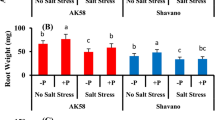
The effects of salinity, N, and their combined treatment on some morphological parameters are summarized in Table 2. Salinity significantly affected stem and root lengths and leaf number (P < 0.01; F = 11.37, F = 0.37, and F = 3.95 for stem length, root length, and leaf number (LfN), respectively). Additionally, root volume (RV) was significantly affected by salinity (P < 0.001, F = 14.35), as evidenced by RV increases of 130 % and 67 % in LN and HN conditions, respectively (Table 2). Nitrogen treatment significantly affected only root length and RV (Table 2). Similarly, a combined salinity and N treatment only affected RV (P < 0.01; F = 1.40).
Effects of salinity stress on mineral concentrations
Salinity stress substantially increased Na+ concentrations in leaves, stems, and roots (F = 97.4, F = 94.2, F = 372.66, respectively) regardless of N level (Table 3), with increases of about 473, 440, and 420 % in leaves, stems, and roots, respectively, in LN conditions. These increases were slightly lower than those in HN conditions (Table 3). Treatments of N or salinity and N combined had no significant effects on Na+ concentrations in leaves and roots, while a highly significant effect was observed for stems (P < 0.001; F = 18.25 and F = 16.31 for N and salinity + N, respectively; Table 3).
Salinity had a highly significant effect on K+ concentrations in different organs (P < 0.001; F = 87.19, F = 84.87, and F = 99.75 for leaves, stems, and roots, respectively). Salinity reduced K+ concentrations in leaves by 31 % and 41 %, in stems by 41 % and 47 %, and in roots by 32 and 37 % in LN and HN conditions, respectively (Table 3). However, N had a significant effect only on roots (P < 0.001; F = 25.11). The K+ concentration decreased by about 20 and 29 % in non-saline and saline conditions, respectively (Table 3). The combined salinity and N treatment had no significant effect on K+ concentrations in different plant organs (Table 3).
Exposure to saline conditions reduced Ca2+ acquisition in leaves by about 20 and 27 %, in stems by 62 and 22 %, and in roots by 81 and 49 % in LN and HN conditions, respectively (Table 3). Nitrogen treatments also had a highly significant effect on Ca2+ concentrations in all plant organs (P < 0.001; F = 22.93, F = 29.98, and F = 18.47 for leaves, stems, and roots, respectively). Increases in N availability resulted in decreased Ca2+ concentrations in leaves and stems, but increased concentrations in roots. Additionally, the combined salinity and N treatment significantly affected Ca2+ acquisition in stems and roots, but had no effect on leaf Ca2+ concentrations (Table 3).
Salinity had a significant effect on Mg2+ concentrations in leaves, stems, and roots (P < 0.01; F = 1.54, F = 2.56, and F = 13.80 for leaves, stems, and roots, respectively). Following salinity treatments, Mg2+ concentrations increased in leaves and stems in LN conditions, but decreased in HN conditions (Table 3). In roots, salinity stress increased Mg2+ accumulation in LN and HN conditions. Treatments with N significantly affected Mg2+ concentrations in leaves (P < 0.01; F = 11.96), but had no effect on stems. For roots, increasing N availability led to decreased Mg2+ acquisition in saline and non-saline conditions (Table 3). The combined salinity and N treatment had a highly significant effect (P < 0.001; F = 16.11) only on leaf Mg2+ concentrations.
Salinity increased Cl− concentrations in leaves, stems, and roots in LN and HN conditions. The increases were greater in HN conditions regardless of plant organ. The highest increase relative to the controls was observed in leaves (107 %) (Table 3). Nitrogen treatment had a highly significant effect on Cl− concentrations in leaves, stems and roots (P < 0.001; F = 22.30, F = 26.28, and F = 1.18, respectively). In HN conditions, the leaf and root Cl− concentrations increased following exposure to salinity stress, but decreased in control plants. The combined salinity and N treatment had a highly significant effect on Cl− concentrations only in leaves and stems (P < 0.001; F = 58.41 and F = 19.40, respectively).
Following salt treatment, leaf nitrate concentrations increased in LN conditions and decreased in HN conditions. Additionally, stem and root nitrate concentrations decreased in LN and HN conditions (Table 3). Nitrogen treatment significantly affected nitrate concentrations in leaves and roots (P < 0.001; F = 93.70 and F = 33.34, respectively). Higher N contents increased leaf nitrate concentrations by almost 310 and 113 % in control and salinity-treated plants, respectively. In contrast, increased N contents decreased root nitrate concentrations by 33 and 89 % in control plants and salinity-treated plants, respectively. However, N levels did not influence stem nitrate concentrations (Table 3). The combined salinity and N treatment significantly affected nitrate concentrations only in photosynthetic organs (P < 0.001; F = 69.11; Table 3).
Salinity had a significant effect on PO4 3− concentrations in leaves and roots (P < 0.01; F = 15.77 and F = 16.58, respectively), but had no effect on stems (P < 0.05; F = 2.99; Table 3). In HN conditions, PO4 3− concentrations decreased in roots by 76 and 23 % in salinity-treated and control plants, respectively, while there were no significant changes to leaf and stem PO4 3− concentrations (P < 0.05; F = 1.60 and F = 4.27, respectively; Table 3). The combined salinity and N treatment significantly affected PO4 3− concentrations only in roots (P < 0.01) (Table 3).
Salinity stress had no significant effect on SO4 2− concentrations in leaves (P < 0.05; F = 0.97). However, it decreased SO4 2− concentrations in stems by 42 and 40 %, and in roots by 68 and 36 % in LN and HN conditions, respectively (Table 3). Increasing N availability decreased stem and root SO4 2− concentrations in saline and non-saline conditions, while no significant effect was observed for leaves (Table 3). The combined salinity and N treatment had no significant effect on SO4 2− concentrations in leaves and stems, but significantly affected roots (P < 0.01; F = 13.32; Table 3).
Effects of salinity stress on nitrogen status
Salinity had a highly significant effect on total nitrogen concentration (TNC) in leaves, stems, and roots (P < 0.001; F = 91.0, F = 127.0, and F = 27.31, respectively; Table 4). Treatment with 100 mM NaCl decreased TNC in leaves by 28 and 55 %, in stems by 49 and 50 %, and in roots by 35 and 51 %, in LN and HN conditions, respectively (Table 4). Nitrogen levels also significantly affected TNC in leaves, stems, and roots (P < 0.001; F = 2.24, F = 18.09, and F = 128.61, respectively; Table 4). Increasing N availability decreased TNC in leaves of salinity-treated plants, increased it in leaves of control plants, and increased it in stems and roots in saline and non-saline conditions. The combined salinity and N treatment had a highly significant effect on leaf and root TNC (P < 0.001; F = 24.66 and F = 27.34, respectively), while no significant effect was observed for stems (P < 0.05; F = 2.96; Table 4). Treatment with 100 mM NaCl increased NUE in LN and HN conditions. Conversely, NUE decreased in saline and non-saline conditions with increasing N availability (Table 4). The combined salinity and N treatment significantly affected NUE (P < 0.001; F = 112.81; Table 4).
Effects of salinity stress on photosynthetic parameters
Photosynthetic parameters were used to investigate plant responses to individual or multiple abiotic stresses. Salt treatment (100 mM NaCl) had a highly significant effect on net photosynthetic assimilation rate, transpiration, and stomatal conductance (P < 0.001; F = 45.66, F = 46.75, and F = 231.26, respectively; Table 5). Salinity decreased the net photosynthetic assimilation rate by 55 and 53 %, transpiration by 41 and 37 %, and stomatal conductance by 80 and 65 % in LN and HN conditions, respectively (Table 5). Nitrogen treatments had a highly significant effect on stomatal conductance (P < 0.001; F = 44.26), but had no effect on net photosynthetic rate or transpiration. Increasing N availability decreased stomatal conductance by 49 and 10 % in non-saline and saline conditions, respectively. The combined salinity and N treatment had a highly significant effect on stomatal conductance (P < 0.001; F = 208.78), but no effect on net photosynthetic assimilation rate and transpiration (Table 5).
The iWUE was significantly influenced by salinity and N (P < 0.05; F = 66.59 and F = 32.12, respectively). In fact, treatment with 100 mM NaCl increased iWUE by 125 and 33 % in LN and HN conditions, respectively. Increasing N availability increased iWUE by 96 and 16 % in non-saline and saline conditions, respectively (Table 5). Similarly, the combined salinity and N treatment significantly affected iWUE (P < 0.05; F = 6.55; Table 5).
Concerning photosynthetic pigments, salinity had no significant effect on carotenoids and chlorophylls a or b (Table 5). Increasing N availability had no significant effects on chlorophyll a or b contents, but increased carotenoid contents by 20 % in saline and non-saline conditions (Table 5). The combined salinity and N treatment had no significant effect on leaf pigment contents (Table 5).
Nitrate reductase and glutamine synthetase assays
To clarify the T. alexandrinum responses to N deficiency, especially in saline conditions, activities associated with N assimilation were investigated. Salinity increased leaf NR activity in LN conditions and decreased it in HN conditions. Salt stress increased root NR activity in LN and HN conditions (Table 6). Greater N concentrations resulted in increases in leaf NR activity by almost 300 % in non-saline conditions and decreases in activity by 68 % in saline conditions. In contrast, higher N concentrations had no effects on root NR activity (Table 6). The combined salinity and N treatment significantly affected NR activity in leaves (P < 0.001; F = 78.00), but not in roots (Table 6).
Salinity caused GS activity to increase in leaves by 87 % in LN conditions. However, salt treatment resulted in a 6 % decrease in leaf GS activity in HN conditions. Additionally, leaf GS activity increased with increasing N availability in non-saline conditions, but decreased in saline conditions. These effects were reversed in roots (Table 6). The combined salinity and N treatment had highly significant effects on GS activity in roots (P < 0.001; F = 28.42) and leaves (P < 0.05; F = 7.41; Table 6).
Discussion
Previous studies have reported that salt treatments markedly reduce growth of forage species even at low levels (Cordovilla et al. 1996; Delgado et al. 1994). However, our findings indicate that T. alexandrinum productivity increased when culture media were supplemented with 100 mM NaCl in LN or HN conditions (Table 1). Our results are consistent with those for another legume (Alhagi pseudoalhagi) exposed to low salinity environments (Kurban et al. 1999). Based on our data, it is difficult to identify the main factor that promoted biomass production in response to 100 mM NaCl treatment. However, it is likely that increased NUE, iWUE, and RV contributed to the higher biomass production. The RGRs of plants exposed to salinity stress were about 42 and 41 % higher than those of controls in LN and HN conditions, respectively (Table 1). The application of 100 mM NaCl increased the RV in both conditions. The RVs in LN and HN conditions were 130 and 67 % higher than those of the corresponding controls (Table 1).
Mineral nutrients are crucial for plant growth and development because the majority of minerals are involved in vital plant processes. At least 40 mineral elements are necessary for adequate plant nutrition (Marschner 1995; Mengel et al. 2001), with six mineral elements (N, P, K, Ca, Mg, and S) being required in larger amounts. Optimal growth is rarely achieved in non-agricultural settings because most soils are deficient in one or more essential minerals, leading to nutrient stress. This may be particularly true for saline environments. Salinity can differentially affect the mineral nutritional status of plants. Nutrient imbalances induced by salinity decrease plant growth by affecting the availability, transport, and partitioning of mineral nutrients. These imbalances result from the competition of Na+ and Cl− with other nutrients such as K+, Ca2+, Mg2+, and NO3 − (Hasegawa et al. 2000; Hu and Schmidhalter 2005; Munns 2002; Netondo et al. 2004). Our investigation showed that the addition of 100 mM NaCl to the culture media increased the accumulation of Na+ and Cl− and decreased the abundance of K+ and Ca2+ in all plant organs. These effects occurred independently of available N levels and were in agreement with observations from other studies (Barhoumi et al. 2010; Tabatabaei 2006). Our results showed that the effects of salinity on Mg2+ accumulation in photosynthetic organs depended on N level while previous research suggested that Mg2+ accumulation is largely reduced by salinity (Khan et al. 2000). Similarly, the effects of 100 mM NaCl on NO3 − accumulation in leaves was dependent on the abundance of available N. In HN conditions, 100 mM NaCl reduced NO3 − accumulation in photosynthetic organs, while the opposite effect was observed in LN conditions (Table 3). Sulfate accumulation in photosynthetic organs was insensitive to 100 mM NaCl treatment, while salinity increased PO4 3− accumulation independently of available N.
In our study, increasing available N led to higher NO3 − accumulation in photosynthetic organs in both saline and non-saline conditions. This result was consistent with those of a previous study (Santamaria et al. 2002). Nitrogen availability had no significant effects on Na+, K+, PO4 3−, and SO4 2− accumulation in leaves (Table 3). Interestingly, increasing N availability alleviated the adverse effects of salinity on Ca2+ accumulation, especially in stems and roots. However, increasing N availability increased Cl− accumulation in photosynthetic organs and in stems and roots in saline conditions (Table 3). These results are inconsistent with those of a previous study involving Poaceae species, Aeluropus littoralis and Catapodium rigidum, in which HN conditions enhanced Cl− accumulation in photosynthetic organs exposed to salt stress (Barhoumi et al. 2010).
To date, adaptations to steady-state LN conditions in a saline environment have been poorly described. Some studies have indicated that N applications to saline soils increase N concentrations in salt-tolerant plants, which may alleviate the negative impact of salinity (Barhoumi et al. 2010; Wang and Tian 2011). However, in some salt-sensitive plants, applying N to saline soils may aggravate the deleterious effects of salinity stress and decrease N content and dry matter accumulation (Beltrao et al. 2002). Our results showed that increasing N availability in saline conditions increased TNC in stems and roots, but caused a decrease in TNC in photosynthetic organs (Table 4). The TNC decreased following 100 mM NaCl treatment in LN or HN conditions (Table 4). This result is in agreement with previous findings that indicated high salinity inhibits the accumulation of N in plants (Garg et al. 1993; Van Hoorn et al. 2001) by influencing assimilation pathways (Gouia et al. 1994; Rao and Gnanam 1990). Moreover, some plant nutrition experts have shown that the effects of the interactions between salinity and N stresses on plants is complex because they depend not only on plant type, growth stages, organs, and salt composition, but also on N source type and amount (Ding et al. 2010). The application of N using commercial fertilizers is expensive and represents the main cost during plant production (Singh 2005). Therefore, reducing fertilizer input and breeding plants with better NUE are major goals of current agricultural research (Hirel et al. 2007; Lea and Azevedo 2006). According to the NUE data, T. alexandrinum can use available N more efficiently under saline conditions than under control conditions (Table 4). This result is inconsistent with those observed for A. littoralis, Brachypodium distachyum (Barhomi et al. 2010), and Capsicum annuum (Huez-Lopez et al. 2011). However, T. alexandrinum used the available N less efficiently in HN conditions than in LN conditions. The NUE decreased by about 64 and 24 % in non-saline and saline conditions, respectively (Table 4). Additionally, NUE was significantly influenced by the interactions between salinity and N stresses.
Photosynthesis is an indispensable process responsible for plant growth and productivity. Its activity is often considered an indicator of plant responses to environmental stress (Liu et al. 2008; Nandy et al. 2007). Several studies have shown a positive relationship between photosynthetic capacity and growth in numerous species (Ashraf 2001; Hamilton et al. 2001; Munns 2002; Naumann et al. 2007). However, our results revealed a negative relationship between growth and photosynthetic capacity. Other studies have reported that there is little to no association between growth and photosynthetic capacity (Loreto et al. 2003; Rogers and Noble 1992). Moreover, previous research has suggested that the photosynthetic rate is reduced by salinity in several plant species (Long and Baker 1986). Reduced photosynthesis in saline environments is generally due to limited stomatal conductance, uptake of carbon dioxide, carboxylase activity of Rubisco, regeneration of RubP, and chlorophyll content (Lambers et al. 2008; Lawlor and Cornic 2002), leading to inhibited plant growth (Naumann et al. 2007). Other researchers have attributed the decline in photosynthesis activity to Na+ and Cl− accumulation in leaves (Munns 1993; Tattini and Traversi 2009). In this study, the suppression of photosynthesis was mainly caused by decreases in stomatal conductance by nearly 80 and 65 % in LN and HN conditions, respectively (Table 5).
Nitrogen is a basic component of many compounds involved in photosynthesis and a large proportion of N in plants is localized in leaf chloroplasts. Thylakoid membranes contain about 20–25 % of the total N content in leaves. Additionally, N is also an important element in Rubisco photosynthetic complexes (Lambers et al. 1998), Calvin-Benson cycle enzymes, chlorophyll, and carotenoids (Correia et al. 2005). Its deficiency leads to reduced transpiration, stomatal conductance, and chlorophyll and carotenoid contents (Ciompi et al. 1996; Huang et al. 2004; Pompelli et al. 2010). However, our results indicated that increasing N availability had no significant effect on net photosynthetic assimilation rate and chlorophyll contents (Table 5). This observation is explained by the fact that T. alexandrinum NUE is 277 % (non-saline environment) and 131 % (saline environment) higher when cultivated in LN conditions than in HN conditions. In saline environments, T. alexandrinum can use the available water more efficiently than in non-saline environments. This increase in efficiency is nearly 125 and 33 % in LN and HN conditions (Table 5).
Nitrogen assimilation into carbon skeletons is one of the most important physiological processes in plant growth and development. Nitrate and ammonium are assimilated into amino acids that play a pivotal role as N-transport compounds (Lea and Miflin 2003). Reports on the effects of salinity on NR activity in plants have frequently produced contradictory results. For example, previous studies have concluded that NR activity can be decreased (Silveira et al. 2001), not affected (Ourry et al. 1992), or increased (Parida and Das 2004) by low salinity. Our results showed that salinity increased NR activity in roots in LN and HN conditions, while activity levels in leaves depended on N content. In LN conditions, salinity (100 mM NaCl) increased NR activity in leaves by nearly 740 % over that of the controls (Table 6). The effect of N on NR activity in leaves is salt dependent. Interestingly, in saline conditions, N deficiency increased NR activity by about 314 % over that of the controls, while no effect was observed for NR activity in roots (Table 6).
In T. alexandrinum, the effects of salinity on GS activity in leaves and roots depended on N content. Additionally, the effects of N availability on GS activity in leaves and roots depended on salt content (Table 6). Leaf GS activity was stimulated by LN and saline conditions (Table 6). The enhancement of NR and GS activities in leaves in LN conditions and in roots under HN conditions may at least partially explain why plant growth increased following salt treatment.
In summary, under saline and LN conditions, T. alexandrinum exhibited the highest growth rate, which is likely because of the high NUE and leaf NR and GS activities. Therefore, T. alexandrinum is an interesting legume forage crop that can be cultivated in low-saline soils where N is lacking.
References
Ashraf M (2001) Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ Exp Bot 45:155–163
Ashraf M, McNeilly T (1994) Responses of three arid zone grasses to N deficiency: a greenhouse study. Arid Soil Res Rehab 6:125–136
Barhoumi Z, Atia A, Rabhi M, Djebali W, Abdelly C, Smaoui A (2010) Nitrogen and NaCl salinity effects on the growth and nutrient acquisition of the grasses Aeluropus littoralis, Catapodium rigidum, and Brachypodium distachyum. J Plant Nutr Soil Sci 173:149–157
Beltrao J, Jesus SB, Panagopoulos T, Ben Asher J (2002) Combined effects of salts and nitrogen on the yield function of lettuce. In: Aksoy U, Anac S (eds) Proceedings of the International Symposium on Techniques to Control Salination for Horticultural Productivity. International Society of Horticultural Science, Leuven, pp 363–368
Bohnert HJ, Jensen RG (1996) Metabolic engineering for increased salt tolerance: the next step. Aust J Plant Physiol 23:661–667
Brady NC, Weil RR (1996) The nature and properties of soils, 11th edn. Prentice-Hall, Englewood Cliffs
Bremner JM (1965) Total nitrogen. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison, pp 1149–1178
Casano LM, Martin M, Sabater B (1994) Sensitivity of superoxide dismutase transcript levels and activities of oxidative stress is lower in mature-senescent than in young barley leaves. Plant Physiol 106:1033–1039
Chen MJ (1998) Study on the relationship between mineral nutrients and the growth of Kandelia candel (L.) Druce in Chuwei mangrove swamp. Dissertation, National Dong Hwa University (in Chinese)
Ciompi S, Gentili E, Guidi L, Soldatini GF (1996) The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci 118:177–184
Correia CM, Moutinho-Pereira JM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a Mediterranean field study. Eur J Agron 22:337–347
Cramer MD, Gao ZF, Lips SH (1999) The influence of dissolved inorganic carbon in the rhizosphere on carbon and nitrogen metabolism in salinity-treated tomato plants. New Phytol 142:441–450
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7:859–868
Ding X, Tian C, Zhang S, Song J, Zhang F, Mi G, Feng G (2010) Effects of NO3 −-N on the growth and salinity tolerance of Tamarix laxa Willd. Plant Soil 331:57–67
Drihem K, Pilbeam DJ (2002) Effects of salinity on accumulation of mineral nutrients in wheat grown with nitrate-nitrogen or mixed ammonium:nitrate-nitrogen. J Plant Nutr 25:2091–2113
Endris S, Mohammed MJ (2007) Nutrient acquisition and yield response of barley exposed to salt stress under different levels of potassium nutrition. Int J Environ Sci Tech 4:323–330
FAO (2002) World agriculture towards 2015/2030. Summary Report. Food and Agriculture Organization of the United Nations http://www.fao.org/docrep/004/y3557e/y3557e08.htm. 2005
Feng ZZ, Wang X, Feng ZW (2005) Soil N and salinity leaching after the autumn irrigation and its impact on groundwater in Hetao Irrigation District, China. Agric Water Manage 71:131–143
Forde BG, Cullimore JV (1989) The molecular biology of glutamine synthetase in higher plants. Oxford Surv Plant Mol Cell Biol 6:247–296
Garg BK, Vyas SP, Kathju S, Lahiri AN, Mali PC, Sharma PC (1993) Salinity-fertility interaction on growth, mineral composition and nitrogen metabolism of Indian mustard. J Plant Nutr 161:1637–1650
Gouia H, Ghorbal MH, Touraine B (1994) Effects of NaCl on flows of N and mineral ions and NO3 − reduction rate within whole plants of salt-sensitive bean and salt-tolerant cotton. Plant Physiol 105:1409–1418
Hamilton EW, McNaugthon SJ, Coleman JS (2001) Soil Na stress: molecular, physiological and growth responses in Serengeti C4 grasses. Am J Bot 88:1069–1076
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonwealth Bureau of Horticulture Plantation Crops, Maidstone
Hirel B, Le Gouis J, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58:2369–2387
Huang ZA, Jiang DA, Yang Y, Sun JW, Jin SH (2004) Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 42:357–364
Huez-Lopez MA, Ulery AL, Samani Z, Picchioni G, Flynn RP (2011) Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: I. growth and yield. Trop Subtrop Agroecosyst 14:137–147
Kant S, Kant P, Lips H, Barak S (2007) Partial substitution of NO3 − by NH4 + fertilization increases ammonium assimilating enzyme activities and reduces the deleterious effects of salinity on the growth of barley. J Plant Physiol 164:303–311
Kao WY, Chang WY (1998) Stable carbon isotope ratio and nutrient contents of the Kandelia candel mangrove populations of different growth forms. Bot Bull Acad Sinica 39:39–45
Khan MA, Ungar IA, Showalter AM (2000) Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte. Atriplex griffithii var. stocksii. Ann Bot 85:225–232
Kurban H, Saneoka H, Nehira K, Gnanasiri R, Premachandra S, Fujita K (1999) Effect of salinity on growth, photosynthesis and mineral composition in leguminous plant Alhagi pseudoalhagi (Bieb.). Soil Sci Plant Nutr 45:851–862
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology, 3rd edn. Springer Verlag, New York
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Lea PJ, Azevedo RA (2006) Nitrogen use efficiency. 1. Uptake of nitrogen from the soil. Ann Appl Biol 149:243–247
Lea PJ, Miflin BJ (2003) Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol Biochem 41:555–564
Liu RX, Guo WQ, Chen BL, Zhou ZG (2008) Physiological responses of cotton plant to fertilizer nitrogen at flowering and boll-forming stages under soil drought. Chin J Appl Ecol 19:1475–1482 (in Chinese)
Long SP, Baker NR (1986) Saline terrestrial environments. In: Baker NR, Long SP (eds) Photosynthesis in contrasting environments. Elsevier, Amsterdam, pp 63–102
Loreto F, Centritto M, Chartzoulakis K (2003) Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell Environ 26:595–601
Malavolta E, Nogueira NGL, Heinrichs R, Higashi EN, Rodrıguez V, Guerra E, de Oliveira SC, Cabral CP (2004) Evaluation of nutritional status of the cotton plant with respect to nitrogen. Commun Soil Sci Plant Anal 35:1007–1019
Maranville JW, Clark RB, Ross WM (1980) Nitrogen efficiency in grain sorghum. J Plant Nutr 2:577–589
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Mengel K, Kirkby EA, Kosegarten H, Appel T (2001) Principles of plant nutrition. Kluwer Academic, Dordrecht
Moghaieb REA, Saneokab H, Ito J, Fujita K (2001) Characterization of salt tolerance in tomato plant in terms of photosynthesis and water relations. Soil Sci Plant Nutr 47:377–385
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 67:645–663
Nandy P, Das S, Ghose M, Spooner-Hart R (2007) Effects of salinity on photosynthesis, leaf anatomy, ion accumulation and photosynthetic nitrogen use efficiency in five Indian mangroves. Wetlands Ecol Manage 15:347–357
Naumann B, Busch A, Allmer J, Ostendorf E, Zeller M, Kirchhoff H, Hippler M (2007) Comparative quantitative proteomics to investigate the remodeling of bioenergetics pathways under iron deficiency in Chlamydomonas reinhardtii. Proteomics 7:3964–3979
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Ourry A, Mesle S, Boucaud J (1992) Effects of osmotic stress (NaCl and polyethylene glycol) on nitrate uptake, translocation, storage and reduction in ryegrass (Lolium perenne L.). New Phytol 120:275–280
Parida AK, Das AB (2004) Effects of NaCl stress on nitrogen and phosphorous metabolism in a true mangrove Bruguiera parviflora grown under hydroponic culture. J Plant Physiol 161:921–928
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60:324–349
Pompelli MF, Barata-Luís R, Vitorino HS, Gonçalves ER, Rolim EV, Santos MG, Almeida-Cortez JS, Ferreira VM, Lemos EEP, Endres L (2010) Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenerg 34:1207–1215
Radin JW, Boyer JS (1982) Control of leaf expansion by nitrogen nutrition in sunflower plants. Plant Physiol 69:771–775
Radin JW, Parker LL (1979) Water relations of cotton plants under nitrogen deficiency. I. Dependence upon leaf structure. Plant Physiol 64:495–498
Rao KR, Gnanam A (1990) Inhibition of nitrate and nitrate reductase activity by salinity stress in Sorghum vulgare. Phytochem 29:1047–1049
Rogers ME, Noble CL (1992) Variation in growth and ion accumulation between two selected populations of Trifolium repens L. differing in salt tolerance. Plant Soil 146:131–136
Romero L, Sanchez E, Rivero RM, Ruiz JM (2004) Yield and biosynthesis of nitrogenous compounds in fruits of green bean (Phaseolus vulgaris L. cv Strike) in response to increasing N fertilization. J Sci Food Agric 84:575–580
Rubio-Wilhelmi MM, Sanchez-Rodriguez E, Rosales MA, Begona B, Rios JJ, Romero L, Blumwald E, Ruiz JM (2011) Effect of cytokinins on oxidative stress in tobacco plants under nitrogen deficiency. Environ Exp Bot 72:167–173
Sagi M, Dovrat A, Kipnis T, Lips SH (1998) Nitrate reductase, phosphoenolpyruvate carboxylase and glutamine synthetase in annual ryegrass as affected by salinity and nitrogen. J Plant Nutr 21:707–723
Santamaria P, Elia A, Serio F (2002) Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. J Plant Nutr 25:245–258
Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Shangguan Z, Shao M, Dyckmans J (2000) Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J Plant Physiol 156:46–51
Silveira JAG, Melo ARB, Viégas RA, Oliveira JTA (2001) Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ Exp Bot 46:171–179
Singh U (2005) Integrated nitrogen fertilization for intensive and sustainable agriculture. J Crop Improve 15:213–257
Solomonson LP, Barber MJ (1990) Assimilatory nitrate reductase: functional properties and regulation. Annu Rev Plant Physiol Plant Mol Biol 41:225–253
Sosa L, Lianes A, Reinoso H, Reginato M, Luna V (2005) Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann Bot 96:261–267
Tabatabaei SJ (2006) Effects of salinity and N on the growth, photosynthesis and N status of olive (Olea europaea L.) trees. Sci Hortic 108:432–438
Tattini M, Traversi ML (2009) On the mechanism of salt tolerance in olive (Olea europaea L.) under low- or high-Ca2+ supply. Environ Exp Bot 65:72–81
Terashima I, Evans JR (1988) Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol 29:143–155
Torrecillas A, León A, Del Amor F, Martinez-Mompeán MC (1984) Determinación rápida de clorofila en discos foliares de limonero. Fruits 39:617–622 (in Spanish)
Van Hoorn JW, Katerji N, Hamdy A, Mastrorilli M (2001) Effect of salinity on yield and nitrogen uptake of four grain legumes and on biological nitrogen contribution from the soil. Agr Water Manage 51:87–98
Wallsgrove RM, Lea PJ, Miflin BJ (1979) The distribution of the enzymes of nitrogen assimilation within the pea cell leaf. Plant Physiol 63:232–236
Wang JP, Tian CY (2011) Analysis of growth and salt accumulation features of Salicornia europaea under different nitrogen and phosphorus levels. Acta Pratacult Sin 20:234–243 (in Chinese)
Wang R, Guegler K, LeBrie ST, Crawford NM (2000) Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 12:1491–1509
Wong SC, Cowan IR, Farquhar GD (1985) Leaf conductance in relation to rate of CO2 assimilation. I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density, and ambient partial pressure of CO2 during ontogeny. Plant Physiol 78:821–825
Wray JL, Filner P (1970) Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J 119:715–725
Yi Zhu, Fan X, Hou X, Wu J, Wang T (2014) Effect of different levels of nitrogen deficiency on switch grass seedling growth. Crop J 2:223–234
Acknowledgments
This work was financially supported by the Tunisian Ministry of High Education, Scientific Research, and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zouhaier, B., Mariem, M., Mokded, R. et al. Physiological and biochemical responses of the forage legume Trifolium alexandrinum to different saline conditions and nitrogen levels. J Plant Res 129, 423–434 (2016). https://doi.org/10.1007/s10265-016-0791-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0791-6