Abstract
Plant photoreceptors link environmental light cues with physiological responses, determining how individual plants complete their life cycles. Structural and functional evolution of photoreceptors has co-occurred as plants diversified and faced the challenge of new light environments, during the transition of plants to land and as substantial plant canopies evolved. Large-scale comparative sequencing projects allow us for the first time to document photoreceptor evolution in understudied clades, revealing some surprises. Here we review recent progress in evolutionary studies of three photoreceptor families: phytochromes, phototropins and neochromes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light exerts a powerful influence on most vegetable tissues, and there can be no doubt that it generally tends to check their growth—Charles Darwin, 1880 (in The Power of Movement in Plants)
Light provides the energy for plant growth but it also is one of the most important informational cues for plants. Light influences the direction in which plants grow, how they array their leaves and reproductive structures in space (Galvão and Fankhauser 2015), and the nature of their response to pathogens and herbivores (Ballaré 2009). Light signals also interact with gravity- and temperature-sensing pathways (Kami et al. 2010) and thus influence plant responses to gravity and temperature. At the interface between light and plant responses are the photoreceptors, which measure the intensity and wavelength of light, and use the information to modulate physiological responses.
Photoreceptors not only govern plant survival on the short term, but also influence long-term evolutionary trajectories and have a role in species diversification. When plants colonized the land, bringing their photoreceptors with them, it was largely bare. With respect to light, surface environments were uniformly well lit during the day. It is likely that plants at this time used photoreceptors to control the synthesis of beneficial biochemicals such as antioxidants and UV-protectants that would help them adapt to harsh environments, as well as to control reproductive timing and chloroplast movement (Mathews 2006). When wood and the arborescent habit later evolved, contrasting light environments came to exist in open and shaded habitats. Light gradients developed under canopies, and absorption by plant pigments drastically altered the light spectrum. From that time forward, plants diversified to occupy both shaded and open habitats, and they needed an array of photoreceptors that would enhance their success in a greater variety of environments (Mathews 2006). Indeed, ferns and bryophytes diversified under the canopy of seed plants (Laenen et al. 2014; Schneider et al. 2004; Schuettpelz and Pryer 2009), and photoreceptor families also have diversified. In particular, the phototropin and phytochrome families have evolved through gene duplication, resulting in divergent paralogs that require different amounts and/or quality of light to trigger a physiological response (Galvão and Fankhauser 2015; Li et al. 2015a, b).
Plants use diverse photoreceptors to monitor the light environment. Phytochromes (phy) measure the ratio of red to far-red light; phototropins (phot), cryptochromes (cry) and ZEITLUPE/FLAVIN-BINDING, KELCH REPEAT, F-BOX 1/LOV KELCH PROTEIN 2 (ZTL/FKF1/LKP2) measure blue/UV-A; UV RESISTANCE LOCUS 8 (UVR8) measures UV-B (Galvão and Fankhauser 2015); neochrome (neo) is a fusion of PHY and PHOT and measures both blue and red light (Kawai et al. 2003; Kanegae et al. 2006; Nozue et al. 1998; Suetsugu et al. 2005). The biochemistry, structure, and function of these photoreceptors have been characterized in considerable detail (Chaves et al. 2011; Christie 2007; Kami et al. 2010; Mathews 2010; Möglich et al. 2010; Rockwell et al. 2006; Suetsugu and Wada 2013), with the advances coming largely from studies of Arabidopsis thaliana and a handful of additional model systems. Only limited data have existed to address the molecular evolution of photoreceptors in land plants, except for seed plant PHY (Mathews et al. 2003; Mathews 2010), and thus a solid foundation for understanding their functional evolution has not been available.
The recent surge of genome and transcriptome sequencing projects on non-model organisms (e.g., the 1KP transcriptomes; Matasci et al. 2014) has opened an unprecedented opportunity to systemically survey photoreceptors from across all algal and land plant lineages. Data from these and from more focused projects are revealing fascinating evolutionary aspects of photoreceptors (Duanmu et al. 2014; Li et al. 2014, 2015a, b; Rockwell et al. 2014). We provide in this review updates on the evolution of three photoreceptor families, phytochromes, phototropins and neochromes, summarizing new insights into their origin, diversity, and evolution. We then highlight questions that should be priorities for future research projects.
The origin and diversity of phytochromes
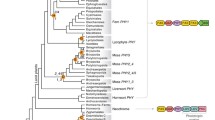
Phytochromes are red/far-red photoreceptors that regulate seed germination, seedling de-etiolation, shade avoidance, and flowering time, and that modulate responses to pathogens, herbivores, gravity, and temperature (Franklin and Quail 2010; Kami et al. 2010). Phytochromes are present in all land plant and most green algal lineages, except in the chlorophytes (Fig. 1b). Phytochromes also have been detected in a brown algae, diatoms, fungi, but not in red algae (Rhodophytes) (Li et al. 2015a); bacteriophytochromes are widespread in prokaryotes (Karniol et al. 2005). Phytochromes comprise two modules—the photosensory module at the N-terminus, and the regulatory module at the C-terminus. The domain composition of the photosensory module is largely conserved across the phytochrome superfamily as PAS-GAF-PHY, whereas the regulatory module is much more variable (Auldridge and Forest 2011; Li et al. 2015a) (Fig. 1b)
a Domain composition of the canonical plant phytochromes, neochromes and phototropins. The length of the photoreceptors and domains are not to scale. b The origin and evolution of plant phytochromes and phototropins. Blue and red backgrounds mark the distribution of phototropins and canonical plant phytochromes, respectively. Phytochrome domain diversity is shown on the right. Zygnematales and Coleochaetales have both canonical and non-canonical plant phytochromes, although the origin of the latter is unclear. Cryptophytes have two types of phytochromes, with different C-terminal regulatory modules (marked by “/”). Domains that are not always present are indicated by dashed outlines. The blue and red stars mark the origin of phototropins and canonical plant phytochromes, respectively. GAF cGMP phosphodiesterase/adenylate cyclase/FhlA, H/KD histidine phosphorylation site (H) in the histidine kinase domain (KD), LOV Light/Oxygen/Voltage, PAS Per/Arnt/Sim, PHY phytochrome, PKC Protein Kinase C, REC response regulator, RING really interesting new gene (color figure online)
Land plants and charophyte algae (together called streptophytes) have the canonical plant phytochrome structure, characterized by having a PAS–PAS repeat and a histidine-kinase related domain (HKRD) in the C-terminal regulatory module (Li et al. 2015a) (Fig. 1b). HKRD has high sequence similarity to histidine kinase, but it lacks the conserved histidine residue that is necessary for kinase activity (Yeh and Lagarias 1998). The origin of this canonical structure has until recently remained obscure. Herdman et al. (2000) sought to understand relationships of the different PHY being discovered in cyanobacteria at the time and used PCR to obtain PHY from a greater diversity of cyanobacterial species. Their data established that CPH1 from Synechocystis to its homologs in other cyanobacteria had N-terminal modules more closely related to canonical plant PHY than did other PHY-like sequences in cyanobacterial genomes. Lamparter and colleagues (Buchberger and Lamparter 2015; Lamparter 2004) revisited the question as more data became available. Buchberger and Lamparter (2015) separately analyzed sequences from the N- and C-terminal modules and established that they had different evolutionary origins. They further suggested that the photosensory module was inherited from cyanobacteria (likely through endosymbiotic transfer), and the regulatory module from proteobacteria. Their data, however, could not rule out other explanations for the origins of these modules because key taxa had not been sampled.
It is possible that the ancestor of the archaeplastid lineage (Fig. 1b) obtained a phytochrome during or after the primary endosymbiosis that gave rise to plastids (Gould et al. 2008), as has often been suggested (e.g., Herdman et al. 2000; Karniol et al. 2005; Buchberger and Lamparter 2015). This would be supported if cyanobacterial phytochromes were paraphyletic to, or formed the sister clade of, archaeplastid phytochromes in trees from analyses of sequences from all the additional phytochrome-bearing lineages: bacteriophytochromes, fungi, stramenopiles (diatoms and brown algae), glaucophytes, cryptophytes. The first study to include all the relevant lineages found that cyanobacterial sequences were nested within bacteriophytochromes rather than on the branch leading to the Archaeplastida (Duanmu et al. 2014) (Fig. 1b). A later study including fewer bacterial sequences but a much more extensive sampling of phytochromes from the green algal lineages obtained a similar result (Li et al. 2015a). Although the trees are unrooted, they cannot be rooted in a way that would place cyanobacterial and archaeplastidal sequences together. This suggests Archaeplastida could as easily have acquired a phytochrome from a bacterial donor via horizontal gene transfer (HGT) as from a cyanobacterium via endosymbiotic gene transfer. Regardless of the prokaryotic source of an ancestral archaeplastidal phytochrome, the additional data obtained from Viridiplantae by Li et al. (2015a) provided compelling confirmation of the origin of canonical phytochrome from within the green algae, and allowed them to reconstruct the steps by which its structure evolved, through assembly of modules and the loss of a conserved histidine.
Canonical plant phytochromes are present in all streptophyte lineages, including the earliest-diverging mesostigmatalean algae (Fig. 1a; Li et al. 2015a). These phytochromes have experienced multiple rounds of gene duplications, both in the charophyte algae and in land plants (Fig. 1b). The earliest one appeared to take place after Mesostigmatales diverged, resulting in charophyte PHY1 and PHY2 (Li et al. 2015a); the latter includes the previously known Mougeotia and Mesotaenium phytochromes (Lagarias et al. 1995; Winands and Wagner 1996). Land plant phytochromes were derived from the charophyte PHY2 lineage, and then independently duplicated in mosses, homosporous lycophytes, ferns and seed plants, while remaining single-copy in heterosporous lycophytes, liverworts and hornworts (Li et al. 2015a). Comparison of gene and species trees, allows one to establish the orthology and the timing of gene duplications for land plant phytochromes. At least ten phytochrome duplications occurred on the major branches of the land plant species tree (Fig. 1; Li et al. 2015a).
The origin and diversity of phototropins
Phototropins are blue-light photoreceptors that regulate shoot phototropism, chloroplast movement, stomatal opening, and leaf expansion; they also contribute to rapid de-etiolation (Christie 2007; Fankhauser and Christie 2015). Phototropins comprise two Light/Oxygen/Voltage (LOV) domains (LOV1 and LOV2), and a Serine/Threonine Kinase domain (STK; Fig. 1a). Much less was known about the evolutionary history of phototropins than phytochromes, since even for flowering plants and other seed plants, detailed gene phylogenies did not exist. In a second study, Li et al. (2015b) found that phototropins were ubiquitous in Viridiplantae (land plants + green algae; Li et al. 2015b), but no phototropin homologs were found in glaucophytes or in red algae, suggesting that phototropins originated in an ancestor of Viridiplantae (Li et al. 2015b) (Fig. 1b). The use of LOV domains in blue light sensing is found in structurally diverse proteins from plants, animals, fungi, and bacteria (Crosson et al. 2003; Krauss et al. 2009; Suetsugu and Wada 2013). The uniqueness of phototropins to plants is thus not surprising.
In contrast to the phytochrome diversity observed in algae, algal phototropin apparently exists as a single-copy gene, except in Zygnematales where two copies are present (PHOTA and PHOTB). Previous studies indicated that phototropins are multi-copy in angiosperms, ferns, and mosses, although relationships among these phototropin homologs remained unclear. Based on limited taxonomic samplings, Lariguet and Dunand (2005) and Galván-Ampudia and Offringa (2007) both found that angiosperm phototropins fell into two groups, PHOT1 and PHOT2, and that fern phototropins clustered with PHOT2. This relationship, if true, would suggest that the known fern phototropins are orthologous to Arabidopsis PHOT2.
As in the studies of phytochrome evolution, greatly expanding the taxonomic sampling in a study of phototropins provided clear insights into the question of homology of PHOT genes. Li et al. (2015b) found that phototropins independently duplicated in mosses, ferns, and seed plants (Li et al. 2015b) (Fig. 1b). The independence of the duplications was strongly supported in bootstrapping analyses. Thus, contra (Lariguet and Dunand 2005) and (Galván-Ampudia and Offringa 2007), seed plant PHOT1 and PHOT2 do not have orthologs outside of seed plants. Rather, separate duplications have resulted in three distinct pairs of paralogous PHOT1 and PHOT2 in land plants. Phototropins likely are single-copy in hornworts and liverworts, based on genomic and transcriptomic evidence (Komatsu et al. 2014; Li et al. 2015b). Interestingly, all the hornwort phototropins known to date lack introns, and were likely derived from a retrotransposition event (Li et al. 2014). These intronless phototropins are the close relative of the phototropin module of neochromes (see below).
Neochromes
Neochromes are unique chimeric photoreceptors, having a phytochrome photosensory module at the N-terminus and a full set of phototropin domains at the C-terminus (Nozue et al. 1998; Suetsugu et al. 2005) (Fig. 1a). They are found in zygnematalean algae, hornworts, and ferns (Li et al. 2015b). All algal neochromes known to date lack the conserved cysteine residue in LOV2 domain, which is essential for flavin mononucleotide (FMN) adduct formation and blue light perception (Christie 2007). It is thus possible that algal neochromes function only as a red light photoreceptor. Kanegae and Kimura (2015) recently showed, however, that in Adiantum capillus-veneris (a fern) neochromes, FMN-cysteinyl adduct formation is not required for blue light-induced phototropism, suggesting that algal neochromes might still be able to relay both blue and red/far-red signals (Suetsugu et al. 2005; Kagawa and Suetsugu 2007).
Neochromes have a remarkable evolutionary history, having originated twice, once in zygnematalean algae and once in hornworts (Li et al. 2014). Furthermore, after their origin, hornwort neochromes apparently were transferred horizontally to ferns (Li et al. 2014). Hornwort and fern neochromes completely lack introns, and the fern sequences are nested within the hornwort neochrome clade. Algal neochromes, in contrast, have introns in both the PHY and PHOT modules, and their PHOT module is not closely related to those in land plants. These data, together with the apparent lack of neochromes from all other land plant clades and from early-diverging ferns, suggest that fern neochromes were derived from hornworts via horizontal transfer. Molecular dating placed the divergence time between fern and hornwort neochromes to be around 178 million years ago, much later than the separation of ferns and hornworts (at least 400 million years ago), further supporting the HGT scenario.
In ferns, neochromes can sense both blue and red/far-red light to regulate chloroplast movement and phototropism (Kanegae et al. 2006; Kanegae and Kimura 2015; Kawai et al. 2003), and confer a significantly higher sensitivity to light. Consequently, neochromes may have played a critical role in facilitating the diversification under low-light angiosperm canopies (Kawai et al. 2003; Schneider et al. 2004; Schuettpelz and Pryer 2009). The vehicle and mechanism of neochrome HGT is unknown, but the sequencing of whole fern genomes will likely help address this question (Li and Pryer 2014; Sessa et al. 2014).
Interplay of phytochromes and phototropins
The integration of blue and red/far-red light sensory systems is widespread in land plants and it may be particularly useful for plants facing light environments under canopies. Integration occurs in diverse ways: these include merging the receptors (i.e. neochromes), forming protein complexes (Purschwitz et al. 2008; Kasahara et al. 2004; Jaedicke et al. 2012), and sharing signaling pathways (Hughes 2013; Galvão and Fankhauser 2015). We found that phytochromes and phototropins exhibit a similar pattern of gene family expansion and stasis (Fig. 1b). They both duplicated in seed plants, ferns and mosses, while remaining single-copy in liverworts and hornworts. A shared whole genome duplication history may explain this pattern in part, but the exact branches where duplication took place differ in some cases. For example, in ferns, phototropin duplicated once after Ophioglossales diverged (Li et al. 2015b), whereas phytochromes duplicated three times, once in an ancestor of all extant ferns, once after Gleicheniales diverged and the other after Schizaeales diverged (Li et al. 2015a).
Li et al. (2015b) hypothesized that phytochromes and phototropins might have been co-evolving. This is consistent with their physical interaction (Jaedicke et al. 2012). It may be also likely, however, that coincidence or near coincidence of duplications in the PHY and PHOT families indicates that in both red and blue light signaling systems, having separate copies for responses in high and low light environments was adaptive. For example, lineages in which there has been functional diversification in both phytochrome and phototropin families appear to be more species rich. The highest diversity in the fern and moss lineages is in Polypodiales and Bryopsida, both of which experienced the most phytochrome and phototropin gene duplication events compared to other fern and moss clades. The extent to which these extra duplications are due to whole genome duplication remains to be thoroughly tested, and future studies should also examine the adaptive effect of many other genes that have duplicated on the branches to these clades.
Future directions
The recent findings on photoreceptor origin, diversity and orthology suggest many interesting research questions, and below we highlight some that we believe are feasible and worthwhile to pursue in the future.
What happens when both canonical and non-canonical phytochromes are present in the same organism?
Some streptophyte algae (Zygnematales and Coleochaetales) possess both canonical and non-canonical phytochromes (Fig. 1). The non-canonical ones (PHYX1 and PHYX2) have the PAS-PAS repeat, but the histidine residue is conserved in the C-terminal kinase domain and sometimes a response regulator is also present (Li et al. 2015a) (Fig. 1b). It would be of particular interests to examine the functions and interactions between the canonical and non-canonical phytochromes within the same organism. Do they form heterodimers, have different spectral properties, modulate different functions, and/or use different signaling transduction pathways?
Which photoreceptors control hornwort chloroplast contraction and rotation?
One of the key functions of phototropin is to regulate chloroplast movement, which has been shown in seed plants (Kagawa et al. 2001), ferns (Kagawa et al. 2004), mosses (Kasahara et al. 2004) and liverworts (Komatsu et al. 2014). In hornworts (a bryophyte lineage), chloroplasts are usually large and occupy most of the cellular space. It is unknown if such chloroplasts move (if there is much space to move), but an early study found that they can significantly contract/expand, or rotate according to light intensity (Burr 1968). We recently found that the contraction response can be elicited by high intensity blue light (Li et al. 2014), which suggests phototropins and/or neochromes might be involved. With Anthoceros agrestis being developed as a model hornwort (Szövényi et al. 2015), it will be possible in the near future to carry out genetic studies dissecting the roles of phototropins and neochromes in chloroplast contraction and rotation.
What are the functions of phytochromes and phototropins in algae?
Algae have markedly different body plans and habitats compared to land plants; therefore, their photoreceptors likely modulate different physiological responses than those that have been characterized in land plants (Hegemann 2008). A recent study found a fascinating spectral diversity in algal phytochromes—in addition to the conventional red/far-red light absorption spectrum, some of them have shifted to blue, green, or orange light (Rockwell et al. 2014). This very diverse spectral sensitivity likely results from adaption to aquatic environments. Red and far-red light does not penetrate far into water, although red light signals may be generated in marine environments by processes such as Raman scattering and chlorophyll a fluorescence (Ragni and d’Alcalá, 2004). Relying on other regions of the light spectrum would therefore have adaptive advantages (Rockwell et al. 2014). To date, only a fraction of algal phytochromes have been characterized, and further exploration will likely find additional spectral and biochemical variants.
Similarly, only a few studies have examined phototropin functions in algae. In Chlamydomonas reinhardtii (a chlorophyte alga), phototropin (Crphot) regulates sex determination, eyespot size, phototactic behavior, and expression of photosynthesis genes (Huang and Beck 2003; Im et al. 2006; Trippens et al. 2012), and it can restore most of the phototropin functions in the Arabidopsis phot1 phot2 double mutant (Onodera and Kong 2005). Sullivan et al. (2015) recently characterized the phototropin from Ostreococcus tauri (Otphot), a prasinophyte alga that diverged earlier than C. reinhardtii. Otphot exhibits several typical phototropin properties. For example, Otphot is plasma membrane-bound, and partially internalized upon blue-light irradiation. On the other hand, it cannot rescue chloroplast avoidance or phototropic responses in the Arabidopsis phot1 phot2 double mutant. The function of Otphot within O. tauri, however, was not examined. More studies focusing on phototropins in algal lineages will be essential to understand not only the early events in phototropin functional evolution, but also how phototropins were later co-opted to modulate multi-cellular (e.g., shoot phototropism) and cell-autonomous (e.g., stomatal opening; Doi et al. 2015) physiological responses in land plants.
Conclusion
Past research on model organisms has greatly advanced our understanding of photoreceptor functions and their molecular underpinnings. These studies can now be augmented with data from all of plant biodiversity. The great diversity of phytochromes and phototropins from across the plant kingdom that has recently been revealed (Duanmu et al. 2014; Li et al. 2015a, b; Rockwell et al. 2014), and the resolution of relationships among them sets the stage for studies that take advantage of such diversity to gain a holistic view on photoreceptor molecular biology and its role in plant evolution.
References
Auldridge ME, Forest KT (2011) Bacterial phytochromes: more than meets the light. Crit Rev Biochem Mol Biol 46:67–88. doi:10.3109/10409238.2010.546389
Ballaré CL (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32:713–725. doi:10.1111/j.1365-3040.2009.01958.x
Buchberger T, Lamparter T (2015) Streptophyte phytochromes exhibit an N-terminus of cyanobacterial origin and a C-terminus of proteobacterial origin. BMC Res Notes 8:144. doi:10.1186/s13104-015-1082-3
Burr FA (1968) Chloroplast Structure and Division in Megaceros Species, PhD dissertation, University of California at Berkeley. University of California at Berkeley
Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen LO, van der Horst GT, Batschauer A, Ahmad M (2011) The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol 62:335–364
Christie JM (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58:21–45. doi:10.1146/annurev.arplant.58.032806.103951
Crosson S, Rajagopal S, Moffat K (2003) The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry 42:2–10. doi:10.1021/bi026978l
Doi M, Kitagawa Y, Shimazaki K-I (2015) Stomatal blue light response is present in early vascular plants. Plant Physiol 169:1205–1213. doi:10.1104/pp.15.00134
Duanmu D, Bachy C, Sudek S, Wong CH, Jiménez V, Rockwell NC, Martin SS, Ngan CY, Reistetter EN, van Baren MJ, Price DC, Wei CL, Reyes-Prieto A, Lagarias JC, Worden AZ (2014) Marine algae and land plants share conserved phytochrome signaling systems. Proc Natl Acad Sci USA 111:15827–15832. doi:10.1073/pnas.1416751111
Fankhauser C, Christie JM (2015) Plant Phototropic Growth. Curr Biol 25:R384–R389. doi:10.1016/j.cub.2015.03.020
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61:11–24. doi:10.1093/jxb/erp304
Galván-Ampudia CS, Offringa R (2007) Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci 12:541–547. doi:10.1016/j.tplants.2007.10.004
Galvão VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34:46–53. doi:10.1016/j.conb.2015.01.013
Gould SB, Waller RF, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59:491–517. doi:10.1146/annurev.arplant.59.032607.092915
Hegemann P (2008) Algal sensory photoreceptors. Annu Rev Plant Biol 59:167–189. doi:10.1146/annurev.arplant.59.032607.092847
Herdman M, Coursin T, Rippka R, Houmard J, Tandeau de Marsac N (2000) A new appraisal of the prokaryotic origin of eukaryotic phytochromes. J Mol Evol 51:205–213. doi:10.1007/s002390010082
Huang K, Beck C (2003) Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 100:6269
Hughes J (2013) Phytochrome cytoplasmic signaling. Annu Rev Plant Biol. doi:10.1146/annurev-arplant-050312-120045
Im CS, Eberhard S, Huang K, Beck CF, Grossman AR (2006) Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J 48:1–16. doi:10.1111/j.1365-313X.2006.02852.x
Jaedicke K, Lichtenthäler AL, Meyberg R, Zeidler M, Hughes J (2012) A phytochrome-phototropin light signaling complex at the plasma membrane. Proc Natl Acad Sci USA 109:12231–12236. doi:10.1073/pnas.1120203109
Kagawa T, Suetsugu N (2007) Photometrical analysis with photosensory domains of photoreceptors in green algae. FEBS Lett 581:368–374. doi:10.1016/j.febslet.2006.12.041
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141. doi:10.1126/science.291.5511.2138
Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M (2004) Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol 45:416–426
Kami C, Lorrain S, Hornitschek P, Fankhauser C (2010) Light-regulated plant growth and development. Curr Top Dev Biol 91:29–66
Kanegae T, Kimura I (2015) A phytochrome/phototropin chimeric photoreceptor of fern functions as a blue/far-red light-dependent photoreceptor for phototropism in Arabidopsis. Plant J. doi:10.1111/tpj.12903
Kanegae T, Hayashida E, Kuramoto C, Wada M (2006) A single chromoprotein with triple chromophores acts as both a phytochrome and a phototropin. Proc Natl Acad Sci USA 103:17997–18001. doi:10.1073/pnas.0603569103
Karniol B, Wagner JR, Walker JM, Vierstra RD (2005) Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J 392:103–116. doi:10.1042/BJ20050826
Kasahara M, Kagawa T, Sato Y, Kiyosue T, Wada M (2004) Phototropins Mediate Blue and Red Light-Induced Chloroplast Movements in Physcomitrella patens. Plant Physiol 135:1388–1397. doi:10.1104/pp.104.042705
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290. doi:10.1038/nature01310
Komatsu A, Terai M, Ishizaki K, Suetsugu N, Tsuboi H, Nishihama R, Yamato KT, Wada M, Kohchi T (2014) Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol 166:411–427. doi:10.1104/pp.114.245100
Krauss U, Minh BQ, Losi A, Gärtner W, Eggert T, von Haeseler A, Jaeger KE (2009) Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J Bacteriol 191:7234–7242. doi:10.1128/JB.00923-09
Laenen B, Shaw B, Schneider H, Goffinet B, Paradis E, Désamoré A, Heinrichs J, Villarreal JC, Gradstein SR, McDaniel SF, Long DG, Forrest LL, Hollingsworth ML, Crandall-Stotler B, Davis EC, Engel J, Von Konrat M, Cooper ED, Patiño J, Cox CJ, Vanderpoorten A, Shaw AJ (2014) Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts. Nat Commun 5:5134. doi:10.1038/ncomms6134
Lagarias DM, Wu S-H, Lagarias JC (1995) Atypical phytochrome gene structure in the green alga Mesotaenium caldariorum. Plant Mol Biol 29:1127–1142. doi:10.1007/BF00020457
Lamparter T (2004) Evolution of cyanobacterial and plant phytochromes. FEBS Lett 573:1–5. doi:10.1016/j.febslet.2004.07.050
Lariguet P, Dunand C (2005) Plant photoreceptors: phylogenetic overview. J Mol Evol 61:559–569. doi:10.1007/s00239-004-0294-2
Li F-W, Pryer KM (2014) Crowdfunding the Azolla fern genome project: a grassroots approach. GigaScience 3:16. doi:10.1186/2047-217X-3-16
Li FW, Villarreal JC, Kelly S, Rothfels CJ, Melkonian M, Frangedakis E, Ruhsam M, Sigel EM, Der JP, Pittermann J, Burge DO, Pokorny L, Larsson A, Chen T, Weststrand S, Thomas P, Carpenter E, Zhang Y, Tian Z, Chen L, Yan Z, Zhu Y, Sun X, Wang J, Stevenson DW, Crandall-Stotler BJ, Shaw AJ, Deyholos MK, Soltis DE, Graham SW, Windham MD, Langdale JA, Wong GK, Mathews S, Pryer KM (2014) Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc Natl Acad Sci USA 111:6672–6677. doi:10.1073/pnas.1319929111
Li FW, Melkonian M, Rothfels CJ, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Pryer KM, Mathews S (2015a) Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun 6:1–12. doi:10.1038/ncomms8852
Li FW, Rothfels CJ, Melkonian M, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Mathews S, Pryer KM (2015b) The origin and evolution of phototropins. Front Plant Sci 6:1–11. doi:10.3389/fpls.2015.00637
Matasci N, Hung LH, Yan Z, Carpenter EJ, Wickett NJ, Mirarab S, Nguyen N, Warnow T, Ayyampalayam S, Barker M, Burleigh JG, Gitzendanner MA, Wafula E, Der JP, dePamphilis CW, Roure B, Philippe H, Ruhfel BR, Miles NW, Graham SW, Mathews S, Surek B, Melkonian M, Soltis DE, Soltis PS, Rothfels C, Pokorny L, Shaw JA, DeGironimo L, Stevenson DW, Villarreal JC, Chen T, Kutchan TM, Rolf M, Baucom RS, Deyholos MK, Samudrala R, Tian Z, Wu X, Sun X, Zhang Y, Wang J, Leebens-Mack J, Wong GK (2014) Data access for the 1,000 Plants (1KP) project. GigaScience 3:1–10. doi:10.1186/2047-217X-3-17
Mathews S (2006) Phytochrome-mediated development in land plants: red light sensing evolves to meet the challenges of changing light environments. Mol Ecol 15:3483–3503. doi:10.1111/j.1365-294X.2006.03051.x
Mathews S (2010) Evolutionary studies illuminate the structural-functional model of plant phytochromes. Plant Cell 22:4–16. doi:10.1105/tpc.109.072280
Mathews S, Burleigh JG, Donoghue MJ (2003) Adaptive evolution in the photosensory domain of phytochrome A in early angiosperms. Mol Biol Evol 20:1087–1097. doi:10.1093/molbev/msg123
Möglich A, Yang X, Ayers RA, Moffat K (2010) Structure and function of plant photoreceptors. Annu Rev Plant Biol 61:21–47. doi:10.1146/annurev-arplant-042809-112259
Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh KC, Lagarias JC, Wada M (1998) A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc Natl Acad Sci USA 95:15826–15830
Onodera A, Kong SG, Doi M, Shimazaki K, Christie J, Mochizuki N, Nagatani A (2005) Phototropin from Chlamydomonas reinhardtii is functional in Arabidopsis thaliana. Plant Cell Physiol 46:367–374. doi:10.1093/pcp/pci037
Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA, Atoui A, Calvo AM, Fischer R (2008) Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr Biol 18:255–259. doi:10.1016/j.cub.2008.01.061
Ragni M, d’Alcalá MR (2004) Light as an information carrier underwater. J Plankton Res 26:433–443. doi:10.1093/plankt/fbh044
Rockwell NC, Su Y-S, Lagarias JC (2006) Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858. doi:10.1146/annurev.arplant.56.032604.144208
Rockwell NC, Duanmu D, Martin SS, Bachy C, Price DC, Bhattacharya D, Worden AZ, Lagarias JC (2014) Eukaryotic algal phytochromes span the visible spectrum. Proc Natl Acad Sci USA 111:3871–3876. doi:10.1073/pnas.1401871111
Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallón S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428:553–557. doi:10.1038/nature02361
Schuettpelz E, Pryer KM (2009) Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc Natl Acad Sci USA 106:11200–11205. doi:10.1073/pnas.0811136106
Sessa EB, Banks JA, Barker MS, Der JP, Duffy AM, Graham SW, Hasebe M, Langdale J, Li FW, Marchant DB, Pryer KM, Rothfels CJ, Roux SJ, Salmi ML, Sigel EM, Soltis DE, Soltis PS, Stevenson DW, Wolf PG (2014) Between two fern genomes. GigaScience 3:15. doi:10.1186/2047-217X-3-15
Suetsugu N, Wada M (2013) Evolution of Three LOV Blue Light Receptor Families in Green Plants and Photosynthetic Stramenopiles: Phototropin, ZTL/FKF1/LKP2 and Aureochrome. Plant Cell Physiol 54:8–23. doi:10.1093/pcp/pcs165
Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102:13705–13709. doi:10.1073/pnas.0504734102
Sullivan S, Petersen J, Blackwood L, Papanatsiou M, Christie JM (2015) Functional characterization of Ostreococcus tauri phototropin. New Phytol. doi:10.1111/nph.13640
Szövényi P, Frangedakis E, Ricca M, Quandt D, Wicke S, Langdale JA (2015) Establishment of Anthoceros agrestis as a model species for studying the biology of hornworts. BMC Plant Biol 15:98. doi:10.1186/s12870-015-0481-x
Trippens J, Greiner A, Schellwat J, Neukam M, Rottmann T, Lu Y, Kateriya S, Hegemann P, Kreimer G (2012) Phototropin influence on eyespot development and regulation of phototactic behavior in Chlamydomonas reinhardtii. Plant Cell 24:4687–4702. doi:10.1105/tpc.112.103523
Winands A, Wagner G (1996) Phytochrome of the green alga Mougeotia: cDNA sequence, autoregulation and phylogenetic position. Plant Mol Biol 32:589–597
Yeh KC, Lagarias JC (1998) Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA 95:13976–13981. doi:10.1073/pnas.95.23.13976
Author information
Authors and Affiliations
Corresponding author
Additional information
For the special issue “The cutting edge of photoresponse mechanisms: photoreceptor and signaling mechanism”.
Rights and permissions
About this article
Cite this article
Li, FW., Mathews, S. Evolutionary aspects of plant photoreceptors. J Plant Res 129, 115–122 (2016). https://doi.org/10.1007/s10265-016-0785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0785-4