Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease with complex genetic predisposing factors involved. PU.1 is an important member of the ETS transcription factors family which has diverse functions such as regulating the proliferation, differentiation of immune cells and multiple inflammatory cytokines. Previous studies preliminary explored the relation between PU.1 and SLE. To further explain the potential role of PU.1 in the pathogenesis of SLE, 40 SLE patients and 20 age-sex matched healthy controls (HC) were recruited in this study. Flow cytometry was used to test the percentages of CD4+PU.1+T cells in peripheral blood mononuclear cells (PBMCs) from patients with SLE and HC. Expression levels of PU.1 mRNA in CD4+T cells from SLE patients and HC were analyzed by real-time transcription-polymerase chain reaction. Expression levels of plasma IL-1β, IL-9, IL-18, IL-6, IFN-α, TNF-α, IL-10 and TGF-β1 were measured by enzyme-linked immunosorbent assay. The percentage of CD4+PU.1+T cells in PBMCs from patients with SLE was significantly higher than that from HC (P < 0.001). In addition, the PU.1 mRNA expression in CD4+T cells from SLE patients was increased than that from HC (P = 0.002). In SLE patients, no significant correlation was found between the percentage of CD4+PU.1+T cells and the expression of PU.1 mRNA in CD4+T cells (P > 0.05). Associations of PU.1 mRNA expression in CD4+T cells with major clinical and laboratory parameters of SLE patients were also analyzed, but no significant correlations were found. Consistent with previous studies, SLE patients had increased IL-1β, IL-18, IL-6, IFN-α, TNF-α and IL-10 plasma concentrations than HC (P < 0.01). The expression level of plasma TGF-β1 was significantly decreased in SLE patients than in HC (P < 0.001). In SLE patients, the expression level of IL-1β was positive correlated with PU.1 mRNA expression in CD4+T cells (P = 0.001). Our study first time evaluated the expression profile of PU.1 in CD4+T cells from SLE patients confirming that PU.1 may participate in the pathogenesis of SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic, systemic autoimmune disease caused by genetic factors, sex hormones and environment factors. Currently, the prevalence of SLE ranges from approximately 0 ~ 241cases per 100,000 persons worldwide, while the prevalence of SLE in China ranges from 30 to 70 cases per 100,000 persons [1, 2]. The etiology of SLE has not been fully elucidated. Several mechanisms lead to a loss of self-tolerance and organ dysfunction. The action of pathogenic factors results in the generation of auto antibodies, immune complexes and inflammatory cytokines that may initiate and amplify inflammation [3]. By so far, the disease has no effective treatment. Further exploration of the etiology is essential to discover new therapeutic targets for SLE.
The Ets (E26 transformation specific) family is a diverse group of transcription factors that control the expressions of genes that participate in an array of intracellular processes and development, proliferation, differentiation of immune cells [4]. PU.1 (Purine Rich Box-1), also named SPI-1, is one of the important members of the ETS family, which was found as the product of the gene targeted by recurrent insertions of the Spleen Focus Forming Virus in Friend’s erythroleukemia. The SPI1 gene is located on human chromosome 11p11 and is regulated through the proximal promoter and upstream regulatory element located 17 kb upstream of the transcription start site [5]. PU.1 mainly expresses in hematopoietic cells and plays an important role in the development of essential for lymphoid and myeloid [6]. PU.1 has a large impact on immunity. PU.1-deficient mice exhibit defective development of macrophages, T and B cells. In addition, more than 110 direct target genes of PU.1 were found since it has been discovered. Previous studies have identified that 20% of PU.1 target gene encode intracellular proteins, 19% encode cytoplasmic protein and other 61% encode extracellular proteins or transmembrane proteins [7]. Based on the functions of genes that activated by PU.1, it can regulate the differentiation of immune cells, expression of antibodies, inflammation cytokines and complement. In summary, PU.1 is a critical regulator of cellular communication in immune system.
In recent years, a lot of studies were conducted to explore the potential role of PU.1 in the pathogenesis of autoimmune diseases including SLE. First, it is well known that type I interferon (IFN) pathway plays an important role in participate and development of SLE, the majority of patients with SLE display an increased expression of IFN in both serum and mRNA level. PU.1 can interact with interferon regulatory factor 2 (IRF2), IRF4 and IRF8 to induce the expression of a variety of genes, including pro-inflammatory cytokines [8]. IRF8 has also been shown involved in both the development and the activation of distinct cells of the immune system by directing the expression and activity of the type I interferon system [9]. Thus, PU.1 may contribute to the production of inflammatory cytokines through interaction with IRF family transcription factors. Second, in terms of epigenetic, a genome-wide DNA methylation analysis was carried out in monozygotic twins discordant for SLE, in which they compared methylation profiles of white blood cells from affected twin members with those from respective unaffected members. PU.1 was found as one of the 49 hypomethylated genes in the affected twin members, suggesting that PU.1 epigenetically upregulated in SLE [10]. Furthermore, a follow-up GWAS confirmed PU.1 gene polymorphism was related to the genetic susceptibility of SLE, especially in patients with nephritis [11]. Third, PU.1 has also been proved to regulate the production of several important inflammatory cytokines such as IL-6, IL-10 and TNF-α which are involved in SLE [12, 13]. IL-9 is likely to contribute to the development of autoimmune diseases; an increased expression of IL-9 and CD4+IL-9+T cells were found in patients with SLE [14, 15]. Chang et al. have identified PU.1 as a factor that promotes the Th9 phenotype by both repressing Th2 cytokine production and increasing IL-9 production [16]. In addition, PU.1 has been identified as a critical factor for maximal activity of the downstream IL-18 promoter and then to enhance the activity of NK and Th1 cells [17]. As a target gene of PU.1, it was found that the full activity of the IL-1β promoter was dependent on PU.1-binding of two sites [18].
In conclusion, all these studies indicated that PU.1 may participate in the occurrence and development of SLE through multiple mechanisms. High BAFF expression was associated with active disease in systemic lupus erythematosus [19]. Previous studies have showed that PU.1 expression is remarkably increased in both peripheral blood mononuclear cells (PBMCs) and B cells from pediatric SLE patients and MiR-155 can suppress autoimmunity through transcriptional repression of PU.1 and TNF-α, which in turn suppresses BAFF and CD19 protein expression [20]. But the expression of PU.1 mRNA in CD4+T cells and the mechanisms in T cells were still unknown. In this study, we evaluated the percentage of CD4+PU.1+T cells in PBMCs, the expression of PU.1 mRNA in CD4+T cells and several inflammation cytokines levels to further explore the role of PU.1 in the pathogenesis of SLE.
Materials and methods
Subjects
Peripheral blood samples were obtained from forty patients (mean age 41.38 ± 12.89, 36 females and 4 male) with SLE according to the criteria of the American College of Rheumatology [21] from Department of Rheumatology and Immunology, The First Affiliated Hospital of USTC. Nine patients were new onset. Samples of healthy controls were obtained from twenty sex-and age-matched healthy volunteers (mean age 39.55 ± 5.71, 18 females and 2 male), none of them suffered from any rheumatologic diseases. Individual disease activity was quantified by Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) 2000 score [22]. Inactive SLE patients were defined as a SLEDAI score ≤ 4, while patients with SLEDAI > 4 were evaluated as disease active at the time of the study. The median and interquartile range of SLEDAI was 4.50 (1.50, 7.50). Organs involvement were defined by clinical and laboratory parameters. The patients with renal involvement were defined by persistent proteinuria (> 0.5 g/24 h), hematuria or the presence of cellular casts by microscopic examination of urinary sediment. Leucopenia was defined as white blood cell count < 4,000/mm3, while thrombocytopenia was defined as platelet count < 100,000/mm3. Non-erosive arthritis involving two or more peripheral joints, characterized by tenderness, swelling or effusion was defined as arthritis. The processes involved in the present study as well as informed consent forms were conformed to the provisions of the World Medical Association Declaration of Helsinki and approved by the respective institutions. Clinical, demographic characteristics and laboratory findings of all SLE patients were reviewed during their routine evaluation and presented in Table 1.
Sample preparation and flow cytometric analysis
10 ml whole blood samples were collected in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) from each SLE patient and control subject. Plasma was obtained by centrifugation of blood samples at 4,000 rpm for 5 min, divided into aliquots and frozen at −80 °C until use. PBMCs were isolated from peripheral blood by Ficoll density-gradient centrifugation. Part of the PBMCs was labeled with fluorescent antibodies specific for CD4-FITC, PU.1-PE (Biolegend, USA) accordance to the operating instructions. Intracellular proteins were also labeled using the permeabilization kit (eBioscience, USA). The percentages of CD4+PU.1+T cells were determined by flow assay using FacsCalibur flow cytometry instrument. The remaining cells were used for separate CD4+T cells by Human CD4 T Cell Isolation Kit(Biolegend, USA). Isolated CD4+T cells from SLE patients and healthy controls were used for the analysis of the mRNA expressions of PU.1. Blood samples from the same subject were used for flow cytometric analysis, RT-PCR analysis and ELISA test at different stages of the experiment.
Quantitative RT-PCR analysis
Total RNA was extracted from CD4+T cells using by HiPure Total RNA Mini Kit (Magen Co., Ltd), followed by reverse transcription using an BioRT Master HiSensi cDNA First Strand Synthesis kit (Hangzhou Bioer Technology Co., Ltd) according to the manufacturer’s instructions. The resulting cDNA was used as a template and GAPDH was used as an internal reference. 2 × SYBR Green PCR Master Mix was used for qPCR amplification. Each 20 μl reaction mixture consisted of 10 μl of 2 × SYBR Green PCR Master Mix, template DNA 1 μl, ROX Reference Dye 0.4 μl, the Primer 0.4 μl and 8.2ul ddH2O. The reaction mixture was preheated at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 34 s. Real-time quantitative PCR was performed in duplicate using an Applied Biosystems Prism 7500 Sequence Detection System quantitative PCR instrument (Applied Biosystems, USA). The PCR for PU.1 and GAPDH was duplicated twice in each sample. Gene expression was normalized to GAPDH, and the value of 2–ΔΔCt was calculated to quantify the expression of the target gene in each group. Primers were as follows: PU.1 forward primer: CACAGCGAGTTCGAGAGCTT; PU.1 reverse primer: GGTATCGAGAACGTGCATCT; GAPDH forward: GTCTCCTCTGACTTCAACAGCG; GAPDH reverse: ACCACCCTGTTGCTGTAGCCAA.
Plasma cytokines quantification
Expression levels of cytokine IL-9(MultiSciences, China), IL-1β(R&D Systems, USA), IL-18(R&D Systems, USA), IL-6 (MultiSciences, China), IFN-α (MultiSciences, China), TNF-α (MultiSciences, China), IL-10 (MultiSciences, China) and TGF-β1 (MultiSciences, China) were detected using the enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturer. The results were expressed as pg/ml. Samples with readings below the detection limit were assigned a value of 0.5 times the minimum detection value (IL-18: 1.25 pg/ml; IL-1β: 0.033 pg/ml; IL-9: 0.06 pg/ml; IFN-α: 0.23 pg/ml; TNF-α: 0.16 pg/ml; IL-6: 0.02 pg/ml; IL-10: 0.05 pg/ml; TGF-β1: 3.36 pg/ml). Given the low concentrations of IL-9 in plasma, IL-9 levels were categorized as detectable or undetectable.
Statistical analysis
Statistical analysis of the data was performed by SPSS18.0 (SPSS, Inc., Chicago, IL), and statistical graph was mapped by GraphPad Prism 5.0 (GraphPad Prism Inc., USA). Chi-square test was applied to examine differences of qualitative variables between patients and healthy controls. Normal distribution quantitative variables were described using mean ± standard deviation (SD), and the differences were analyzed by t test. Nonparametric distribution data were expressed as median value and interquartile range (IQR), and the differences were analyzed by the nonparametric Mann–Whitney U test. Correlations between the expression PU.1 mRNA and several cytokines levels, as well as the SLEDAI and laboratory parameters were analyzed by Spearman’s rank test. Two-sided P values < 0.05 were considered to indicate a statistically significant difference.
Results
The percentages of CD4+PU.1+T cells in PBMCs from patients with SLE and HC
The percentages of CD4+PU.1+T cells from 40 SLE and 20 healthy controls were analyzed using flow cytometry. The percentage of CD4+PU.1+T cells in PBMCs from SLE patients (1.018 ± 0.661)% was higher than that from HC (0.413 ± 0.296)%; the difference was statistically significant (t = 4.892, P < 0.001). SLE patients were separated into active (n = 20) and inactive group (n = 20) according to the SLEDAI score. As shown in Fig. 1, the percentages of CD4+PU.1+T cells in PBMCs from active group (0.929 ± 0.670) % and inactive group (1.108 ± 0.657)% were higher than that from HC, respectively (active SLE group vs. HC, t = 3.151, P = 0.004; inactive SLE group vs. HC, t = 4.309, P < 0.001). No significant difference of CD4+PU.1+T cells percentage was found between active SLE group and inactive SLE group (t = −0.851, P = 0.400).
Flow cytometry analysis of CD4+PU.1+T cells in patients with SLE and healthy controls. a The CD4+PU.1+T cells were measured by flow cytometry using FITC-CD4 antibody and PE-PU.1 antibody, flow cytometry plots of healthy control, active SLE and inactive SLE patients were presented representatively. b Statistical analysis of CD4+PU.1+T cells percentage was summarized. Bars show the mean ± SEM. The two independent samples T test for the difference between each two groups was conducted, and the p values of the significant differences were indicated
The expression levels of PU.1 mRNA in CD4+T cells from patients with SLE and HC
In order to examine whether the expression of PU.1 mRNA in CD4+T cells from SLE patients was altered, the levels of PU.1 mRNA in CD4+T cells from SLE patients (n = 40) and HC (n = 20) were measured by real-time PCR. Our results found that the expression of PU.1 mRNA in CD4+T cells from SLE patients (1.715 ± 1.197) was significantly increased than that from HC (1.047 ± 0.230) (t = 3.330, P = 0.002). As shown in Fig. 2, the expression of PU.1 mRNA in CD4+T cells from active SLE group (1.679 ± 1.012) was significantly increased than HC (t = 2.676, P = 0.014); similar result was found when inactive SLE group (1.752 ± 1.383) compared with HC (t = 2.228, P = 0.037). However, no significant difference was found between active SLE group and inactive group (t = −0.191, P = 0.850). Spearman correlation analysis was used to evaluate the relation between CD4+PU.1+T cells percentage and PU.1 mRNA expression in CD4+T cells from all SLE patients. As shown in Fig. 3, no significant correlation was found (r = 0.164, P = 0.312).
Expressions of transcription factors PU.1 mRNA in CD4+T cells of SLE patients and healthy controls. Peripheral blood mononuclear cells were isolated from healthy controls, active SLE and inactive SLE. RNA was isolated for measuring mRNA expression of PU.1 by quantitative real-time PCR. Bars show the mean ± SEM. The two independent samples T test for the difference between each two groups was conducted and the p values of the significant differences are indicated
Associations of PU.1 mRNA expression in CD4+T cells and the percentage of CD4+PU.1+T cells with clinical features and laboratory parameters of SLE
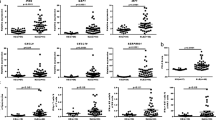
To determine whether PU.1 mRNA expression in CD4+T cells and the percentage of CD4+PU.1+T cells were associated with disease activity, Spearman correlation analysis was conducted. As shown in Fig. 4, no significant correlation was found between the percentage of CD4+PU.1+T cells and SLEDAI (r = −0.174, P = 0.283), the same result was found between PU.1 mRNA expression in CD4+T cells and SLEDAI (r = 0.001, P = 0.996). In the view of that SLE is an extremely heterogeneous disease with highly variable manifestations, we compared PU.1 mRNA expression in CD4+T cells as well as the percentage of CD4+PU.1+T cells between SLE patients with or without some major clinical features including lupus nephritis, serositis, butterfly erythema, leucopenia, thrombocytopenia, anti-ds DNA, decreased C3, decreased C4, increased IgA, increased IgG and decreased Hb. However, no significant associations were found in all comparisons, and the results are summarized in Fig. 5.
Association of PU.1 mRNA level and the percentage of CD4+PU.1+ T cells with clinical features and laboratory parameters. Bars show the mean ± SEM. The association of PU.1 mRNA and the percentage of CD4+PU.1+T cells with major clinical features of SLE patients were analyzed by two independent samples T test, where no correlation was found among them (P > 0.05). Decreased C3: C3 < 0.85 g/L; decreased C4: C4 < 0.12 g/L; increased IgA: IgA > 3.82 g/L; increased IgG: IgG > 16.85 g/L; decreased Hb: Hb < 110 g/L
The levels of IL-1β, IL-9, IL-18, IL-6, IFN-α, TNF-α, IL-10 and TGF-β1 cytokines from patients with SLE and HC
Plasma IL-1β, IL-9, IL-18, IL-6, IFN-α, TNF-α, IL-10 and TGF-β1 expression levels were detected in SLE patients and HC by ELISA. However, plasma IL-9 levels were undetectable in the majority of SLE and HC samples. As shown in Table 2 and Fig. 6, SLE patients had statistically significantly higher expression levels of IL-1β, IL-18, IL-6, IFN-α, IL-10 and TNF-α than HC (P < 0.01). The expression level of plasma TGF-β1 in SLE patients was significantly decreased than in HC. Spearman correlation analyses were conducted to evaluate the associations of PU.1mRNA level with several cytokines. As shown in Fig. 7, the plasma IL-1β level was significantly correlated with PU.1 mRNA expression in CD4+T cells (r = 0.486, P = 0.001).
Discussion
Systemic lupus erythematosus is a chronic autoimmune disease characterized by immunologic abnormalities and multiorgan injury. The etiology of SLE is very complex and has been verified to be related to multiple factors such as genetic factors, sex hormones and environment. Dysregulation of both innate and adaptive immune responses has been implicated in the pathogenesis of SLE. Among them, the imbalance of helper T cell subsets such as Th1, Th2, Th17 cells and regulatory Treg cells, which is followed by production of various autoantibodies, may be responsible for multiple organ systems involvement.
PU.1 is an important member of the ETS transcription factors family and is critical for proliferation and differentiation of hematopoietic stem cell. Currently, more than 110 direct target genes of PU.1 have been found, whose function mainly acting on regulating the expression of antibodies, proliferation and differentiation of immune cells, and the expression of inflammation cytokines. It has been found that PU.1-deficient mice exhibit defective development of macrophages, T and B cells [23]. Chang et al. found PU.1 expression affects the heterogeneity of Th2 phenotypes by antagonizing GATA-3-DNA-binding activity, and therefore regulates the level of Th2 cytokine expression [24]. Further study in mice indicates that PU.1 modulates the levels of TCR expression in CD4+T cells by regulating the DNA-binding activity of GATA-3 to affect the activation of Th2 cells [25]. Recently, several genome-wide studies were performed to identify PU.1 binding site in B cells and macrophages [26, 27]. Based on all these findings, PU.1 was confirmed to play key roles in several steps of the inflammatory pathway. Therefore, a lot of studies were performed to explore its role in the pathogenesis of several autoimmune diseases, such as rheumatoid arthritis (RA), autoimmune uveoretinitis and autoimmune encephalomyelitis [28,29,30]. It is believed that multiple genes likely play roles in the etiology of SLE [31]; a genome-wide association study (GWAS) found a related, functional polymorphism in the 3'-untranslated region of PU.1 [11]. Another study reported that PU.1 was overrepresented in the promoters of genes linked to SLE susceptibility [32]. In addition, it was found that PU.1 expression was upregulated in PBMCs and B cells of pediatric SLE patients and correlated with SLEDAI. It is all known that CD4+T cells including Th1, Th2, Th17 and Treg cells play an essential role in SLE; the expression of PU.1 in CD4+T cells and mechanism in CD4+T cells is still unknown.
Therefore, the current study first time analyzed the PU.1 expression profile in CD4+T cell from SLE patients and HC using flow cytometric and RT-PCR. Our results showed that the percentage of CD4+PU.1+T cells from SLE patients was remarkably higher than that from HC. Similar result was also found in the measurement of PU.1 mRNA expression in CD4+T cells. These findings provide further support for the previous study and suggest that PU.1 up-regulation not only appear in B cells but also in CD4+T cells of SLE patients. Subgroup analysis results show that neither the percentage of CD4+PU.1+T cells nor the PU.1 mRNA expression in CD4+T cells between active and inactive SLE patients was significantly different. In addition, no correlation was found between PU.1 expression and SLEDAI, which was different from previous study that conducted in B cells of pediatric SLE patients. First it may due to the inconsistent expression level of PU.1 in T cells and B cells, and the effect of glucocorticoid and immunosuppressive therapies on not new-onset patients should also take into consider. In this study, the median SLEDAI score of SLE patients was 4.5. Low degree of overall disease activity may not reveal the true differences between the active and inactive SLE patients. On another aspect, SLE patients are usually accompanied by anemia [33]; the function of PU.1 on the proliferation and differentiation of hematopoietic stem cell was initially clarify, but in this study PU.1 expression profile in CD4+T cell from SLE patients with leucopenia thrombocytopenia and decreased hemoglobin was not significantly different with patients who without the clinical features. It might be the limited number of SLE patients with positive specific clinical features. SLE is an autoimmune disease with highly heterogeneity. Small sample size may not accurately represent the large group of patients.
Furthermore, another mechanism that PU.1 may participate in the pathogenesis of SLE is through regulating the differentiation of Th9 cells and expression of inflammation cytokines. Th9 cells, a new subset of Th cells which mainly secret IL-9, have been discovered recently and were confirmed to play an important role in autoimmune diseases and allergic diseases [34]. It has been found that the mRNA and protein level of serum IL-9 in patients with SLE were significantly higher than those in HC [35]. Inconsistent with previous study, in our study plasma IL-9 levels were undetectable in the majority of SLE and almost entirely HC samples. It may be due to the low expression of IL-9 in human at the protein level. Chang et al. [16] found that PU.1-deficient T cells had less IL-9 production, and ectopic expression of PU.1 resulted in more IL-9 production by Th2 and Th9 cell cultures. PU.1 was proved as a critical regulator of the IL-9-secreting T cell phenotype. In this study, the correlation between PU.1 mRNA and plasma IL-9 level cannot be observed due to the undetectable plasma IL-9. The exact mechanism needs to further research. IL-9 is also known to induce Th17 differentiation and IL-17 production, and these cytokines may work together synergistically in promoting SLE pathogenesis [36]. It may be another regulatory pathway that PU.1 participates in the pathogenesis SLE. It has been proved that PU.1 can activate the transcription of a number of important cytokines and cytokine receptors in lymphocytes including IL-18 and IL-1β, two members of the IL-1 superfamily. In addition, Marecki et al. have shown that transcription of the endogenous IL-1β gene can be activated by ectopic expression of PU.1 and the IRF proteins [37]. Studies using the MRL/lpr mouse model of lupus-like disease reported that increased IL-1β gene expression was associated with disease severity and accelerated disease progression [38]. In human study, it has been found serum IL-1β expression in SLE patients was significantly higher than HC, and a positive association between the level of serum IL-1β and disease activity has been observed [39, 40]. As shown in results, our findings were consisted with previous studies. Furthermore, we observed a positive correlation between PU.1 mRNA and plasma IL-1β level. It may be because of that full activity of the IL-1β promoter is dependent on PU.1-binding of two sites [18]. These findings indicate that PU.1 may regulate IL-1β expression level in SLE patients. For IL-18, Mende R et al. [41] found that SLE patients have significantly higher levels of serum IL-18 than HC, and associations of IL-18 with active disease and damage were reported. Our study corroborates previous studies showing that IL-18 expression was increased in SLE patients. Koyama N et al. found that PU.1 can bind to the —36 to —22 region of the human IL-18 promoter and could regulate the IL-18 promoter activity through this region [42]. But in our study, significant correlation was not observed between PU.1 mRNA and IL-18 expression. The expression profile of IFN-α, IL-6, IL-10 and TNF-α which have been identified as important players in SLE was also evaluated in this study. Consistent with previous studies [43,44,45], we found SLE patients had significantly increased plasma IFN-α, IL-6, IL-10 and TNF-α expression. The correlation or regulatory mechanism of PU.1 with these cytokines has been studied more or less. It was proposed that TNF-α promoter is directly activated by PU.1 [12]. Using siRNAs to knock down PU.1 revealed a suppressive effect on TNF-α expression in both PBMCs and B cells of pSLE patients. Hence, Aboelenein et al. found miR-155 may be an indirect regulator of TNF-α mediating its action through PU.1 [20]. Another study found that overexpression of PU.1 markedly upregulated IL-6, IL-13 and TNF-α levels generated from mast cell in response to LPS-stimulation [46]. PU.1 can interact with IRF2, IRF4 and IRF8 to induce the expression of a variety of genes and pro-inflammatory cytokines. Contribution of IRF-4/8-PU.1 is one of many mechanisms by which IFN signaling is modulated in immune cells [47]. Larsson et al. [13] found Sp1 binds to the G allele of the −1087 polymorphism in the IL-10 promoter and promotes IL-10 mRNA transcription and protein production. TGF-β1 can inhibit T and B cell proliferation. Previous meta-analysis study [48] revealed a significantly lower circulating TGF-β1 level in SLE patients which was similar with our findings. PU.1 was reported as a downstream signals of TGF-β [49]. The correlation of PU.1 expression with these cytokines in SLE was evaluated in our study, but we did not find any significant association. Little is known about the exact mechanisms that PU.1 regulating the expression of inflammatory cytokines and signal pathway in CD4+T cells. These issues await much future work.
Another point to note was that in our study although both PU.1 mRNA and CD4+PU.1+T cells percentage were elevated, no significant correlation was found between PU.1 mRNA and CD4+PU.1+T cell as well as CD4+PU.1+T cell and plasma IL-1β level (P > 0.05). The results indicate that PU.1 mRNA expression may have no effect on peripheral CD4+PU.1+T cells in SLE patients. It was known that the protein expression level is affected by multiple mechanisms such as transcriptional, translational and post-translational processes. The transcript levels by themselves are not sufficient to predict protein levels in many scenarios due to complexity of gene expression regulation [50]. The spatial and temporal variations of mRNAs, as well as the local availability of resources for protein biosynthesis, strongly influence the relationship between protein levels and their coding transcripts [51]. Another reason may be the limited sample size of this study; the real statistical association was not appeared. Further studies such as siRNA-mediated gene silencing experiment need to be conducted in SLE to clarify the regulatory mechanisms in the expression of PU.1 protein.
Aberrant T lymphocyte activation and altered cytokines production are important contributors to SLE pathogenesis. However, so far the exact mechanisms that lead to the pathogenesis and development of SLE remain undefined. In consideration of the regulatory function of PU.1 in immune cells and inflammation cytokines, we first time evaluate the expression profile of PU.1 in CD4+T cells from SLE patients, as well as the correlations between PU.1 and several inflammation cytokines. In conclusion, increased CD4+PU.1+T cells percentage as well as PU.1 mRNA expression in CD4+T cells is observed in SLE patients, and further researches are needed to explore the specific regulatory mechanism.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
05 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10238-021-00731-x
References
Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–61.
Zhang S, Ye Z, Li C, et al. Chinese systemic lupus erythematosus treatment and research group (CSTAR) registry XI: gender impact on long-term outcome. Lupus. 2018;28(5):635–41.
Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21.
Gallant S, Gilkeson G. ETS transcription factors and regulation of immunity. Arch Immunol Ther Exp (Warsz). 2006;54(3):149–63.
Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988;331(6153):277–80.
Dakic A, Wu L, Nutt SL. Is PU.1 a dosage-sensitive regulator of haemopoietic lineage commitment and leukaemogenesis? Trends Immunol. 2007;28(3):108–14.
Turkistany SA, DeKoter RP. The transcription factor PU.1 is a critical regulator of cellular communication in the immune system. Arch Immunol Ther Exp (Warsz). 2011;59(6):431–40.
Huang W, Horvath E, Eklund EA. PU.1, interferon regulatory factor (IRF) 2, and the interferon consensus sequence-binding protein (ICSBP/IRF8) cooperate to activate NF1 transcription in differentiating myeloid cells. J Biol Chem. 2007;282(9):6629–43.
Cheng J, Wu R, Long L, et al. miRNA-451 a targets IFN regulatory factor 8 for the progression of systemic lupus erythematosus. Inflammation. 2017;40(2):676–87.
Javierre BM, Fernandez AF, Richter J, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20(2):170–9.
Hikami K, Kawasaki A, Ito I, et al. Association of a functional polymorphism in the 3’-untranslated region of SPI1 with systemic lupus erythematosus. Arthritis Rheum. 2011;63(3):755–63.
Fukai T, Nishiyama C, Kanada S, et al. Involvement of PU.1 in the transcriptional regulation of TNF-alpha. Biochem Biophys Res Commun. 2009;388(1):102–6.
Larsson L, Rymo L, Berglundh T. Sp1 binds to the G allele of the-1087 polymorphism in the IL-10 promoter and promotes IL-10 mRNA transcription and protein production. Genes Immun. 2010;11(2):181–7.
Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127(4):450–8.
Ouyang H, Shi Y, Liu Z, et al. Increased interleukin-9 and CD4+IL-9+ T cells in patients with systemic lupus erythematosus. Mol Med Rep. 2013;7(3):1031–7.
Chang HC, Sehra S, Goswami R, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11(6):527–34.
Kim YM, Kang HS, Paik SG, et al. Roles of IFN consensus sequence binding protein and PU.1 in regulating IL-18 gene expression. J Immunol. 1999;163(4):2000–7.
Kominato Y, Galson D, Waterman WR, et al. Monocyte expression of the human prointerleukin 1 beta gene (IL1B) is dependent on promoter sequences which bind the hematopoietic transcription factor Spi-1/PU.1. Mol Cell Biol. 1995;163(4):58–68.
Marín-Rosales M, Cruz A, Salazar-Camarena DC, et al. High BAFF expression associated with active disease in systemic lupus erythematosus and relationship with rs9514828C>T polymorphism in TNFSF13B gene. Clin Exp Med. 2019;19(2):183–90.
Aboelenein HR, Hamza MT, Marzouk H, et al. Reduction of CD19 autoimmunity marker on B cells of paediatric SLE patients through repressing PU.1/TNF-α/BAFF axis pathway by miR-155. Growth Factors. 2017;35(2–3):49–60.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
Gladman DD, Iban~ez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Spain LM, Guerriero A, Kunjibettu S, et al. T cell development in PU.1-deficient mice. J Immunol. 1999;163(5):2681–7.
Chang HC, Zhang S, Thieu VT, et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22(6):693–703.
Chang HC, Han L, Jabeen R, et al. PU.1 regulates TCR expression by modulating GATA-3 activity. J Immunol. 2009;183(8):4887–94.
Ghisletti S, Barozzi I, Mietton F, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32(3):317–28.
Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89.
Xie Z, Qu Y, Shen P, et al. PU.1 attenuates TNF-α-induced proliferation and cytokine release of rheumatoid arthritis fibroblast-like synoviocytes by regulating miR-155 activity. Mol Med Rep. 2018;17(6):8349–56.
Umazume A, Kezuka T, Matsuda R, et al. Role of PU1 expression as an inflammatory marker in experimental autoimmune uveoretinitis. Ocul Immunol Inflamm. 2018;26(6):951–63.
Shakerian L, Ghorbani S, Talebi F, et al. MicroRNA-150 targets PU.1 and regulates macrophage differentiation and function in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2018;323:167–74.
Wu Z, Zhang S, Li P, et al. Association between complement 4 copy number variation and systemic lupus erythematosus: a meta-analysis. Clin Exp Med. 2020;20(4):627–34.
Dozmorov MG, Wren JD, Alarcón-Riquelme ME. Epigenomic elements enriched in the promoters of autoimmunity susceptibility genes. Epigenetics. 2014;9(2):276–85.
Huang Y, Chen L, Zhu B, et al. Evaluation of systemic lupus erythematosus disease activity using anti-α-enolase antibody and RDW. Clin Exp Med. 2020. https://doi.org/10.1007/s10238-020-00657-w.
Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–6.
Ouyang H, Shi Y, Liu Z, et al. Increased interleukin-9 and CD4+IL-9+T cells in patients with systemic lupus erythematosus. Mol Med Rep. 2013;7(3):1031–7.
Elyaman W, Bradshaw EM, Uyttenhove C, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106(31):12885–90.
Marecki S, Riendeau CJ, Liang MD, et al. PU.1 and multiple IFN regulatory factor proteins synergize to mediate transcriptional activation of the human IL-1 beta gene. J Immunol. 2001;166(11):6829–38.
Lemay S, Mao C, Singh AK. Cytokine gene expression in the MRL/lpr model of lupus nephritis. Kidney Int. 1996;50(1):85–93.
McCarthy EM, Smith S, Lee RZ, et al. The association of cytokines with disease activity and damage scores in systemic lupus erythematosus patients. Rheumatol (Oxford). 2014;53(9):1586–94.
Umare V, Pradhan V, Nadkar M, et al. Effect of proinflammatory cytokines (IL-6, TNFα, and IL-1β) on clinical manifestations in Indian SLE patients. Mediators Inflamm. 2014;2014:385297.
Mende R, Vincent FB, Kandane-Rathnayake R, et al. Analysis of serum interleukin (IL)-1β and IL-18 in systemic lupus erythematosus. Front Immunol. 2018;9:1250.
Koyama N, Hoelzer D, Ottmann OG. Regulation of human IL-18 gene expression: interaction of PU.1 with GC-box binding protein is involved in human IL-18 expression in myeloid cells. Eur J Immunol. 2004;34(3):817–26.
Sabry A, Sheashaa H, El-Husseini A, et al. Proinflammatory cytokines (TNF-alpha and IL-6) in Egyptian patients with SLE: its correlation with disease activity. Cytokine. 2006;35(3–4):148–53.
Paradowska-Gorycka A, Wajda A, Stypinska B, et al. Variety of endosomal TLRs and interferons (IFN-alpha, IFN-beta, IFN-gamma) expression profiles in patients with SLE, SSc and MCTD. Clin Exp Immunol. 2020. https://doi.org/10.1111/cei.13566.
Uzrail AH, Assaf AM, Abdalla SS. Correlations of expression levels of a panel of genes (IRF5, STAT4, TNFSF4, MECP2, and TLR7) and cytokine levels (IL-2, IL-6, IL-10, IL-12, IFN-gamma, and TNF-alpha) with systemic lupus erythematosus outcomes in jordanian patients. Biomed Res Int. 2019;2019:1703842.
Niwa Y, Nishiyama C, Nakano N, et al. Opposite effects of PU.1 on mast cell stimulation. Biochem Biophys Res Commun. 2008;375(1):95–100.
Kanno Y, Levi BZ, Tamura T, et al. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25(12):770–9.
Lee YH, Bae SC. Association between circulating transforming growth factor-beta1 level and polymorphisms in systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Cell Mol Biol (Noisy-le-grand). 2017;63(1):53–9.
Sugimoto A, Kawakami R, Mikami N. Transcription factors downstream of IL-4 and TGF-beta signals: analysis by quantitative PCR, Western Blot, and Flow Cytometry. Methods Mol Biol. 2017;1585:141–53.
Jovanovic M, Rooney MS, Mertins P, et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens[J]. Science. 2015;347(6226):1259038.
Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance [J]. Cell. 2016;165(3):535–50.
Acknowledgements
We thank all the volunteers who generously participated in this study.
Funding
This article was supported by the National Natural Science Foundation of China (No. 81871271), Anhui Key Research and Development Program (Grant Number 1804b06020354) and the Fundamental Research Funds for the Central Universities (Grant Number WK 9110000148).
Author information
Authors and Affiliations
Contributions
NX conceived and designed the study, NX and XF performed the experiments, and XL reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
All authors read and approved the manuscript.
Ethical approval
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Anhui Provincial Hospital. Written informed consent was obtained from individual participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiang, N., Fang, X., Sun, XG. et al. Expression profile of PU.1 in CD4+T cells from patients with systemic lupus erythematosus. Clin Exp Med 21, 621–632 (2021). https://doi.org/10.1007/s10238-021-00717-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-021-00717-9