Abstract
The function of microRNA-27a (miR-27a) expression in cholangiocarcinoma (CCA) remains largely unclear; therefore, this study aimed to investigate the clinical significance and functional role of miR-27a in CCA. This study included 117 paired CCA tissues and adjacent normal tissues from CCA patients who received surgical resection. Reverse transcription-quantitative polymerase chain reaction was used to measure the expression levels of miR-27a in CCA tissues and cell lines. A Kaplan–Meier curve and Cox regression analysis were used to determine overall prognostic performance. The effects of miR-27a on cell proliferation, migration, and invasion were measured by CCK-8 and Transwell assays. The expression levels of miR-27a in patients with CCA and cell lines were higher than those in adjacent normal tissues and normal cells, respectively. Additionally, miR-27a levels were found to be associated with lymph node metastasis and TNM stages. The overall survival time of CCA patients with high miR-27a expression was poorer than that of those with low miR-27a expression. Furthermore, miR-27a overexpression promoted CCA cell proliferation, migration, and invasion, whereas knockdown of miR-27a suppressed cell proliferation, migration, and invasion. Taken together, these results suggest the potential usefulness of miR-27a in the prognosis and progression of CCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CCA) is a highly aggressive malignant tumor that is difficult to diagnose and has a high mortality rate; it originates from the ductular epithelium of the biliary tree [1]. CCA is still common in Asia, but the incidence of CCA has significantly increased globally in recent years in countries, such as in Europe and North America [2]. Radical surgery is the only curative treatment for CCA; however, the survival rate of CCA patients is still limited due to early invasion and metastasis [1, 3]. Although CCA is considered a rare tumor, it is still a refractory disease with a low 5-year survival rate [4, 5]. Thus, finding novel prognostic and therapeutic approaches for CCA is urgently needed.
In recent years, microRNAs (miRNAs) have been demonstrated to have important functions in numerous biological and pathological processes, such as cell cycle progression, cell proliferation, migration, and invasion [6]. MiRNAs, a group of small non-coding RNAs of 20–22 nucleotides, are important regulators of gene expression at the posttranscriptional level that directly bind to the 3′-UTR of target mRNAs [7, 8]. Increasing evidence has confirmed that miRNAs, such as miR-545 [9], miR-433 [10], and miR-300 [11], are aberrantly expressed in various diseases, including cancers, and function as oncogenes or suppressor gene. miR-27a has been indicated to participate in tumor biology and act as a promising biomarker and potential therapeutic target in various tumors [12]. In a miRNA profiling study, Collins AL and colleagues identified miR-27a as one of the overexpressed miRNAs in CCA tissues [13]. However, the clinical significance and functional role of miR-27a in CCA remain largely unclear.
In this study, we first explored the expression levels of miR-27a in CCA tissues and cell lines. Next, the clinical significance of miR-27a was analyzed. Finally, the potential functional role of miR-27a in CCA was further investigated. The results of this study suggest that miR-27a may function in an oncogenic role in CCA through promoting cell proliferation, migration, and invasion, and it may be a prognostic marker and therapeutic strategy for CCA.
Materials and methods
Patient and tissue specimens
All the experiments in this study were approved by the Ethics Committee of the Affiliated Hospital of Weifang Medical University. In total, 117 CCA patients who underwent surgical treatment were enrolled in this study between January 2010 and December 2013 at the Affiliated Hospital of Weifang Medical University. Paired CCA tissues and matched adjacent normal tissues were obtained from these patients and immediately place in liquid nitrogen and stored at − 80 °C. According to the ethical and legal standards, all specimens were made anonymous. All patients included in this study did not receive radiotherapy, chemotherapy, or immunotherapy before surgical treatment. All the patients received the standardized treatments (gemcitabine plus cisplatin as first-line treatment) according to the Chinese Society of Clinical Oncology (CSCO) guideline after surgical resection. The clinicopathological characteristics of CCA patients were collected and are listed in Table 1. All patients included had undergone surgical resection, and at least 5-year follow-up information was available for analysis. Follow-up data were collected every 3 months through the first 2 years, every 6 months during 3 and 4 years, and 12 months in the last year.
Cell culture and transfection
The CCA cell lines HuCCT1, RBE, HuH28, and QBC939, and a human intrahepatic bile duct epithelial cell line (HIBEpic) were purchased from the cell bank of the Institute of Biochemistry and Cell Biology (Shanghai, China). All cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) medium supplemented with 10% FBS (Gibco, USA) at 37 °C in a 5% CO2 incubator.
The miR-27a mimic, inhibitor, and corresponding negative controls (NCs; mimic NC and inhibitor NC) were purchased from Gene Pharma (Shanghai, China) and transfected into CCA cells using Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, Inc.).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from CCA tissues and cell lines following the manufacture’s instruction. Purified total RNA was reverse-transcribed into complementary DNA (cDNA) using M-MLV Reverse Transcriptase (Promega, Madison, Wisconsin). The qRT-PCR was run using SYBR Premix Ex Taq II (Takara, Dalian, China) on an ABI 7500 system (Applied Biosystems, Foster City, California, USA). The relative expression levels of miR-27a were calculated using 2−ΔΔCt methods. U6 was an endogenous control for normalization.
CCK-8 assay
The effect of miR-27a on cell proliferation was calculated using the Cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Transfected cells were suspended with 5000 cells/well in 96-well plates and incubated at 37 °C in a humidified chamber with 5% CO2. At 0, 24, 48, and 72 h after the cell-seeded, the CCK-8 kit was added into each well and the cells were incubated for 2 h. Absorbance was measured at 450 nm using a microplate spectrophotometer (Thermo Fisher Scientific, Inc.).
Transwell migration and invasion assays
The effect of miR-27a on cell migration and invasion was investigated using Transwell migration and invasion assays. For the Transwell invasion assays, the top chamber was precoated Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Meanwhile, no Matrigel was added for the Transwell migration assays. A total of 5 × 104 cells in serum-free medium were placed in the upper chambers at 37 °C with 5% CO2. Medium containing 10% FBS was used as the attractant and added to the bottom chamber. After incubation for 24 h, the migrated/invaded cells on the lower sides of the membrane were fixed, stained, and counted with a microscope with at least five random fields.
Statistical analysis
Data from at least three independent experiments are presented as the mean ± SD. The statistical analysis was performed using SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software, Inc., La, Jolla, CA, USA). The χ2 tests, Student’s t test, and one-way ANOVA were used for comparisons. Kaplan–Meier methods were used to calculate survival curves, and a multivariate Cox regression analysis was performed using the Cox proportional hazards model with enter version to assess the prognostic risk factors for prognosis. Differences were considered significant when P < 0.05.
Results
Expression of miR-27a in CCA tissues and cell lines
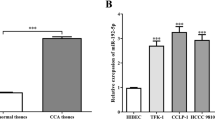
From the qRT-PCR analysis, the expression levels of miR-27a were found to be increased in tumor tissue specimens of CCA patients compared with adjacent normal tissues (P < 0.001, Fig. 1a). Moreover, the levels of miR-27a were higher in all CCA cells than in the HiBEC cells (P < 0.001, Fig. 1b).
The relative miR-27a expression in CCA tissues and cells was determined using a qRT-PCR assay. a The relative miR-27a expression was upregulated in CCA tissues compared with the normal tissues. ***P < 0.001 versus normal tissues. b The expression level of miR-27a in four CCA cell lines was higher than that in normal cells. ***P < 0.001 versus HIBEpics
Relationship of the expression level of miR-27a with clinicopathological features of patients with CCA
The relationship of the expression of miR-27a with the clinicopathological characteristics of CCA patients was analyzed. According to the relative mean expression level (1.915) of miR-27a in CCA tissue samples as a cutoff value, the CCA patients were divided into two groups, including the low miR-27a expression group (n = 57) and the high miR-27a expression group (n = 60). As shown in Table 1, the expression level of miR-27a was significantly associated with lymph node metastasis (P = 0.032) and clinical stage (P = 0.019). However, no correlations were found between the expression of miR-27a and other clinical features of CCA patients, including age, sex, and differentiation (P > 0.05).
Upregulation of miR-27a is associated with poor prognosis in CCA patients
The results of the Kaplan–Meier survival curve analysis using the overall survival information of CCA patients indicated that CCA patients with high expression of miR-27a had a lower overall survival rate than those with low expression of miR-27a (log-rank test P = 0.029, Fig. 2). The prognostic significance of miR-27a was evaluated using multivariate Cox regression analyses. First, the univariate Cox regression analysis results showed that miR-27a, age, lymph node metastasis, and TNM stage were risk factors for CCA patients (all P < 0.05, Table 2). Furthermore, all variables with a P-value of less than 0.1 in the univariate analysis were included in a final multivariate Cox regression analysis to identify independent risk factors. The results revealed that miR-27a expression (HR = 2.099, 95% CI 1.089–4.048, P = 0.027), lymph node metastasis (HR = 1.997, 95% CI 1.037–3.847, P = 0.039), and TNM stage (HR = 2.244, 95% CI 1.063–4.738, P = 0.034) were independently associated with the overall survival (Table 2).
Overexpression of miR-27a promotes proliferation, migration, and invasion of CCA cells
The confirmation of the clinical significance of miR-27a led us to investigate the biological role of miR-27a in CCA progression. We overexpressed miR-27a using miR-27a mimic and downregulated miR-27a by miR-27a inhibitor in Huh28 and QBC939 cells (P < 0.001, Fig. 3a). Both cell lines had relatively high miR-27a expression levels and were chosen for subsequent analysis. The CCK-8 assays indicated that overexpression of miR-27a promoted the proliferation of CCA cells, while downregulation of miR-27a inhibited the proliferation of CCA cells at 48 h and 72 h (P < 0.05, Fig. 3b). The Transwell migration and invasion assays clarified that overexpression of miR-27a enhanced CCA cell migration and invasion, while miR-27a knockdown suppressed CCA cell migration and invasion (P < 0.01, Fig. 4a, b).
Overexpression of miR-27a promoted cell proliferation in CCA cells (Huh28 and QBC939), while the downregulation of miR-27a inhibited cell proliferation. a The expression of miR-27a was significantly higher following transfection with a miR-27a mimic but significantly lower following transfection with a miR-27a inhibitor. ***P < 0.001 versus untreated cells. b Effects of miR-27a on cell proliferation. *P < 0.05 versus untreated cells
Discussion
CCA is a rare form type of cancer; however, patients with CCA are usually in advanced stages at the time of initial diagnosis, which makes their tumors unsuitable for surgical resection and leads to a high mortality rate with a poor prognosis [14]. In recent years, CCA research has received a more specific focus on the treatment of this disease [15, 16]. For example, a heat shock protein 90 (HSP90) inhibitor 17-AAG inhibits CCA cell growth and induces apoptosis in human CCA cells, providing a promising therapeutic strategy for the treatment of CCA [17]. An increasing number of studies have revealed a close connection between the aberrant expression of miRNAs and the progression of cancers [18]. MiRNAs can function as oncogenes or tumor suppressor genes in the development and progression of cancers, including CCA [19]. Considering the important role of miRNAs in cancer, finding novel effective biomarkers associated with the progression of CCA is still essential.
Multiple miRNAs have been identified as potential diagnostic/prognostic biomarkers in various types of cancers [20,21,22]. As reported, numerous miRNAs are aberrantly expressed in CCA and involved in the development and progression of CCA [23, 24]. For example, miR-29a is significantly upregulated in tissues and associated with the progression of CCA, and it may be a prognostic biomarker in CCA [25]. A previous study reported miR-27a as one of the dysregulated miRNAs in CCA [13]; however, the potential role of miR-27a in CCA is still unclear. In the current study, we revealed that miR-27a expression was significantly upregulated in CCA tissues and cells. Besides, the upregulation of miR-27a was associated with the poor overall survival of CCA patients. Furthermore, miR-27a may play an oncogenic role in the progression of CCA.
In the present study, miR-27a was upregulated in CCA tissues and cells, which is consistent with previously published data [13] and the expression pattern in several types of cancer [26]. Previous studies have shown that miR-27a is upregulated in many cancers, including pancreatic ductal adenocarcinoma [26], ovarian cancer [27], and gastric cancer [28]. On the other hand, some studies have demonstrated that miR-27a is downregulated in esophageal squamous cell carcinoma [29], ovarian cancer [30], and non-small cell lung cancer [31]. Thus, miR-27a may play an oncogenic role or tumor suppressor role depending on the type of cancer. Besides, overexpression of miR-27a was significantly associated with positive lymph node metastasis and advanced TNM stages of CCA patients in the current study, suggesting that miR-27a expression may be involved in the development of CCA. Tang et al. [32] demonstrated that high miR-27a expression was associated with poor overall survival of breast cancer patients, suggesting that miR-27a could be a valuable marker of breast cancer progression. Interestingly, high miR-27a expression was also found to be correlated with shorter overall survival of CCA patients and miR-27a might be an independent prognostic predictor for patients with CCA. The prognostic significance of miR-27a in CCA was similar to that in several other cancers, such as colorectal cancer [33] and esophageal squamous cell carcinoma [34].
Moreover, we investigated the functional role of miR-27a in the progression of CCA through the upregulation or knockdown of miR-27a. The cell experiment results showed that overexpression of miR-27a promoted cell proliferation, migration, and invasion, whereas knockdown of miR-27a suppressed these biological behaviors, suggesting the oncogenic role of miR-27a in regulating the progression of CCA. These results are consistent with the role of miR-27a in several other cancers. For instance, miR-27a promoted proliferation, migration, invasion, and suppressed apoptosis of colorectal cancer cells by targeting RXRα [33]. In colon cancer, miR-27a could promote the proliferation and invasion of colon cancer cells by targeting SFRP1 through the Wnt/β-catenin signaling pathway [35]. In another study, miR-27a acted as an oncogene involved in the development and progression of ovarian cancer by targeting BTG1 [30]. Despite all of these studies, few clinical and functional studies on miR-27a in CCA exist. Our findings further demonstrate the cell- and tissue-specific functions of miR-27a in CCA. These findings in CCA suggest that miR-27a may be a novel prognostic marker and play an oncogenic role in CCA. In our future investigations, we will determine the detailed molecular mechanism of miR-27a in CCA.
Taken together, this study demonstrated that miR-27a expression was upregulated in CCA and associated with poor overall survival of CCA patients. Moreover, miR-27a may play an oncogenic role in CCA by promoting tumor cell proliferation, migration, and invasion. These results suggest that miR-27a might be a prognostic biomarker and a promising therapeutic target for CCA patients.
Abbreviations
- CCA:
-
Cholangiocarcinoma
- qRT-PCR:
-
Reverse transcription-quantitative polymerase chain reaction
- miRNAs:
-
MicroRNAs
- HIBEpic:
-
Human intrahepatic bile duct epithelial cell line
- NCs:
-
Negative controls
- cDNA:
-
Complementary DNA
- CCK-8:
-
Cell counting kit-8
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- TNM:
-
Tumor–node–metastasis
References
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–79.
Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver. 2017;11(1):13–26.
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–21.
Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11–31.
Zhang D, Li H, Xie J, et al. Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma. Int J Oncol. 2018;52(6):1777–866.
Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227.
Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–73.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Yuan G, Wu H, Du Y, He F. Tumor suppressor role of microRNA-545 in oral squamous cell carcinoma. Oncol Lett. 2019;17(2):2063–8.
Li J, Chen M, Yu B. miR-433 suppresses tumor progression via Smad2 in non-small cell lung cancer. Pathol Res Pract. 2019;215:152591.
He FY, Liu HJ, Guo Q, Sheng JL. Reduced miR-300 expression predicts poor prognosis in patients with laryngeal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2017;21(4):760–4.
Li X, Xu M, Ding L, Tang J. MiR-27a: a novel biomarker and potential therapeutic target in tumors. J Cancer. 2019;10(12):2836–48.
Collins AL, Wojcik S, Liu J, et al. A differential microRNA profile distinguishes cholangiocarcinoma from pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21(1):133–8.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111.
Puik JR, Meijer LL, Le Large TY, et al. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18(14):1343–58.
Kim EY, Lee SS, Shin JH, Kim SH, Shin DH, Baek SY. Anticancer effect of arsenic trioxide on cholangiocarcinoma: in vitro experiments and in vivo xenograft mouse model. Clin Exp Med. 2014;14(2):215–24.
Zhang J, Zheng Z, Zhao Y, Zhang T, Gu X, Yang W. The heat shock protein 90 inhibitor 17-AAG suppresses growth and induces apoptosis in human cholangiocarcinoma cells. Clin Exp Med. 2013;13(4):323–8.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22.
Namwat N, Chusorn P, Loilome W, et al. Expression profiles of oncomir miR-21 and tumor suppressor let-7a in the progression of opisthorchiasis-associated cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):65–9.
Pan B, He B, Xu X, et al. MicroRNA-371-3 cluster as biomarkers for the diagnosis and prognosis of cancers. Cancer Manag Res. 2019;11:5437–57.
Alizadeh M, Safarzadeh A, Beyranvand F, et al. The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J Cell Physiol. 2019;234(11):19280–97.
An JX, Ma ZS, Ma MH, Shao S, Cao FL, Dai DQ. MiR-1236-3p serves as a new diagnostic and prognostic biomarker for gastric cancer. Cancer Biomark. 2019;25(2):127–32.
Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52(4):297–303.
Sun C, Zhu J, Wu B, et al. Diagnostic and prognostic value of microRNAs in cholangiocarcinoma: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:2125–39.
Deng Y, Chen Y. Increased expression of miR-29a and its prognostic significance in patients with cholangiocarcinoma. Oncol Res Treat. 2017;40(3):128–32.
Ling J, Dong X, Wang L, et al. MiR-27a-regulated FOXO1 promotes pancreatic ductal adenocarcinoma cell progression by enhancing Wnt/beta-catenin signaling activity. Am J Transl Res. 2019;11(5):3069–80.
Si L, Jia Y, Lin R, Jian W, Yu Q, Yang S. MicroRNA-27a regulates the proliferation, chemosensitivity and invasion of human ovarian cancer cell lines by targeting Cullin 5. Arch Biochem Biophys. 2019;668:9–15.
Zhou L, Liang X, Zhang L, et al. MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget. 2016;7(32):51943–54.
Wang X, An D, Liu X, Wang X, Li B. MicroRNA-27a downregulates the expression of Hsp90 and enhances the radiosensitivity in esophageal squamous cell carcinoma. Onco Targets Ther. 2019;12:5967–77.
Li E, Han K, Zhou X. MicroRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1. Open Med (Wars). 2019;14:577–85.
Yan X, Yu H, Liu Y, Hou J, Yang Q, Zhao Y. miR-27a-3p functions as a tumor suppressor and regulates non-small cell lung cancer cell proliferation via targeting HOXB8. Technol Cancer Res Treat. 2019;18:1533033819861971.
Tang W, Zhu J, Su S, et al. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS ONE. 2012;7(12):e51702.
Liang J, Tang J, Shi H, et al. miR-27a-3p targeting RXRalpha promotes colorectal cancer progression by activating Wnt/beta-catenin pathway. Oncotarget. 2017;8(47):82991–3008.
Maghsudlu M, Farashahi Yazd E, Amiriani T. Increased expression of MiR-27a and MiR-24-2 in esophageal squamous cell carcinoma. J Gastrointest Cancer. 2019;51:227–33.
Ba S, Xuan Y, Long ZW, Chen HY, Zheng SS. MicroRNA-27a promotes the proliferation and invasiveness of colon cancer cells by targeting SFRP1 through the Wnt/beta-catenin signaling pathway. Cell Physiol Biochem. 2017;42(5):1920–33.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YL made substantial contributions to the conception and design of the study and analysis and interpretation of the data. YL, XL, and YZ performed the experiments and completed the acquisition of data. TL and JL contributed to the patients' recruitments and the acquisition of tissue specimens and collected important background information. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The current study was approved by the Ethics Committee of the Affiliated Hospital of Weifang Medical University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Liu, X., Zhou, Y. et al. Overexpression of miR-27a predicts poor prognosis and promotes the progression in cholangiocarcinoma. Clin Exp Med 21, 121–128 (2021). https://doi.org/10.1007/s10238-020-00655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00655-y