Abstract
Multiple myeloma (MM) is the second most common hematologic malignancy. In spite of the development of new therapeutic agents, MM remains incurable due to multidrug resistance (MDR) and the 5-year survival rate is approximately 50%. Thus, further study is needed to investigate the mechanism of MDR and improve MM prognosis. Heat shock protein 90 (HSP90) is a molecular chaperone that is responsible for the stability of a number of client proteins, most of which are involved in tumor progression. Therefore, HSP90 inhibitors represent potential new therapeutic agents for cancer. Furthermore, inhibition of HSP90 leads to degradation of client proteins, overcoming acquired anti-cancer drug resistance. In this study, we assessed the role of HSP90 in MDR using established melphalan-resistant MM cells. We found that expression of HSP90 was higher in melphalan-resistant MM cells than in parent cells and that HSP90 inhibitors KW-2478 and NUV-AUY922 restored drug sensitivity to the level observed in parent cells. Activation of the unfolded protein response is a hallmark of MM, and expression of endoplasmic reticulum stress signaling molecules is reduced in melphalan-resistant cells; however, KW-2478 did not affect endoplasmic reticulum stress signaling. We demonstrated that treatment with KW-2478 decreased expression of Src, a client of HSP90, and suppressed the activity of ERK, Akt, and NF-κB. Our findings indicate that inhibition of HSP90 results in suppression of Src and its downstream effectors, including ERK, Akt, and NF-κB, and therefore that HSP90 inhibitors could be useful for treatment of MDR MM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is characterized by uncontrolled proliferation of monoclonal plasma cells in the bone marrow and accounts for approximately 10% of all hematologic malignancies [1, 2]. Prognosis can be improved by autologous stem cell transplant, high-dose chemotherapy, or new therapeutic agents, such as proteasome inhibitors and immunomodulators; however, MM remains an incurable disease owing to relapse [3,4,5].
Melphalan is an alkylating agent currently used in first-line treatment for transplant-ineligible MM patients; it is administered as a combination therapy in melphalan–prednisolone (MP), melphalan–prednisolone–thalidomide (MPT), or melphalan–prednisolone–bortezomib (VMP). MPT and VMP were reported to improve progression-free survival and overall survival compared to MP [5,6,7,8,9,10]. However, after continual treatment with a chemotherapeutic agent, MM cells acquire multidrug resistance (MDR), leading to tumor relapse [4, 11, 12]. While some mechanism of MDR have been determined, such as overexpression of drug transporters and reduction of apoptosis-related proteins, a therapeutic strategy remains to be established [13, 14]. Thus, further study is required to define the mechanisms of MDR in MM.
Heat shock protein 90 (HSP90) is a molecular chaperone that is responsible for the stabilization of a number of client proteins under normal cell condition. Most of these client proteins play a crucial role in proliferation or survival of tumor cells via regulation of the cell cycle, apoptosis, and signal transduction; therefore, inhibition of HSP90 leads to degradation of these proteins and tumor cell death [15, 16]. HSP90 inhibitors were reported to be effective against tumors, including those which have tolerance to specific anti-cancer drugs, in phase 2 studies. In addition, HSP90 inhibitors can overcome acquired anti-cancer drug resistance in gastric cancer, mantle cell lymphoma, and breast cancer [17,18,19,20,21]. Inhibition of HSP90 leads to anti-myeloma activity [22, 23] and enhances the anti-myeloma effect of bortezomib, both in vitro and in vivo [24]. These findings suggest that HSP90 inhibitors could be effective in overcoming MDR in MM; however, to date no studies have investigated this.
The aim of this study was to investigate a potential association between HSP90 with the mechanism of melphalan resistance, using an established melphalan-resistant MM cell line.
Materials and methods
Materials
Melphalan was obtained from Wako (Tokyo, Japan). KW-2478 and NVP-AUY922 were purchased from SelleckChem (Houston, TX, USA). These reagents were dissolved in dimethyl sulfoxide and diluted in phosphate-buffed saline (PBS; 0.05 M, pH 7.4).
Cell culture
The multiple myeloma cell line RPMI8226 was obtained from Japanese Cancer Research Resources Bank (Osaka, Japan). ARH-77 cells were obtained from DS Pharma Biomedical (Osaka, Japan). Melphalan-resistant variant (RPMI8226/L-PAM or ARH-77/L-PAM) was established in our laboratory, and this variant was resistance for adriamycin, vincristine, and dexamethasone [13]. These cells were maintained in RPMI1640 medium (Sigma) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 100 μg/ml penicillin (Gibco), 100 U/ml streptomycin (Gibco), and 25 mM HEPES (pH 7.4; Wako) in an atmosphere containing 5% CO2.
Trypan blue dye exclusion assay
The effect of various drugs on cell survival was determined using the trypan blue dye exclusion assay as previously described methods [13, 14].
Western blotting
Cells were cultured and collected by centrifugation and lysed in lysis buffer containing protease inhibitor and phosphatase inhibitor. The extracts (40 μg of protein) were fractionated on sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to polyvinyl difluoride (PVDF) membranes (GE Healthcare, Buckinghamshire, UK). The membranes were blocked with 5% skim milk and incubated overnight at 4 °C with each of the following antibodies: anti-phospho-IRE1 (Ser724) (Merck Millipore, Nottingham, UK), anti-phospho-p44/42 mitogen-activated protein kinase (MAPK, ERK1/2) (Thr202/Tyr204) (#9101), anti-phospho-Akt (Ser473) (#9271), anti-phospho-eukaryotic translation initiation factor 2α (eIF2α) (Ser51) (#9721), anti-phospho-Src (Tyr527) (#2105), anti-NF-κB p65 (#3034), anti-ERK1/2 (#9102), anti-Akt (#9272), anti-eIF2α (#9722), anti-Src (#2109), anti-IRE1α (#3294), anti-CHOP (#2895) (Cell Signaling Technology, Beverly, MA), anti-phospho-Protein kinase-like endoplasmic reticulum kinase (PERK) (Thr981) (sc-32577), anti-PERK (sc-13073), anti-Hsp90α/β (sc-7947), anti-lamin A/C (sc-20681) (Santa Cruz Biotechnologies, CA, USA), and anti-β-actin antibody (Sigma). Subsequently, the membranes were incubated with horseradish peroxidase-coupled anti-rabbit IgG sheep antibodies (GE Healthcare) at room temperature. The reactive proteins were visualized using Luminata Forte (Merck Millipore, Nottingham, UK) according to the manufacturer’s instructions.
Statistical analysis
All results are expressed as means and standard deviations of several independent experiments. Multiple comparisons of the data were done by ANOVA with Dennett’s test. P values less than 5% were considered as significant.
Results
HSP90 expression was higher in melphalan-resistant MM cells than in melphalan-sensitive MM cells
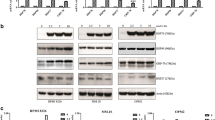
We first investigated HSP90 expression levels in RPMI8226, RPMI8226/L-PAM, ATH-77, and ARH-77/L-PAM cells by immunoblotting. HSP90 expression was higher in RPMI8226/L-PAM or ARH-77/L-PAM cells than in RPMI8226 or ARH-77 cells (Fig. 1).
Expression of HSP90 is overexpressed in RPMI8226/L-PAM and ARH-77/L-PAM cells. a The cell lysate was extracted and then subjected to SDS-PAGE/immunoblotting with antibody against HSP90. Anti-β-actin antibody was used as internal standards. b Quantification of the amount of HSP90, normalized to the amounts of β-actin. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test)
HSP90 inhibitors restored melphalan sensitivity in RPMI8226/L-PAM cells
To define whether HSP90 overexpression could be involved in melphalan resistance, we assessed cell viability after incubation with melphalan and/or HSP90 inhibitors. Melphalan (10 μM) induced cell death in RPMI8226 and ARH-77 cells, but not in RPMI8226/L-PAM and ARH-77/L-PAM cells. Combination of 0.1–0.5 µM KW-2478 with melphalan restored melphalan sensitivity in RPMI8226/L-PAM and ARH-77/L-PAM cells (Fig. 2a–d), while 0.5 μM KW-2478 plus melphalan reduced cell viability to approximately 20%. NVP-AUY922 overcame melphalan resistance in a similar manner (Fig. 2e–h). Treatment with KW-2478 or NVP-AUY922 alone did not affect cell viability in either RPMI8226 or RPMI8226/L-PAM cells. These results suggest that overexpressed HSP90 is critical to melphalan resistance, and that its inhibitor is effective for overcoming resistance in RPMI8226/L-PAM cells.
KW-2478 overcomes melphalan resistance in RPMI8226/L-PAM and ARH-77/L-PAM cells. a, e RPMI8226, b, f RPMI8226/L-PAM, c, g ARH-77, and d, h ARH-77/L-PAM cells were exposed to melphalan or/and KW-2478 or NVP-AUY922 at the indicated concentrations. After incubation for 72 h, the number of cells was counted by trypan blue dye exclusion assay. *p < 0.01 versus untreated cells (one-way ANOVA with Dunnett’s test)
Endoplasmic reticulum stress signaling in RPMI8226/L-PAM cells
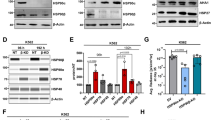
Accumulation of misfolded or unfolded proteins causes endoplasmic reticulum (ER) stress, and excessive ER stress induces cell death [25]. Since HSP90 is essential for protein folding, inhibition of this molecule results in cell death via ER stress [26]. To evaluate the involvement of ER stress in melphalan resistance, we compared expression levels of ER stress-related proteins in RPMI8226/L-PAM cells and RPMI8226 cells. IRE1 and CHOP expression was lower in RPMI8226/L-PAM cells, while PERK and eIF2α expression levels were similar in both cell types (Fig. 3a, b). Next, we assessed whether KW-2478 and NVP-AUY922 affects ER stress signaling in RPMI8226/L-PAM cells. However, there were no substantial changes in the activity and expression of these proteins (Fig. 3c–f). These findings indicated that ER stress is suppressed in RPMI8226/L-PAM cells; however, KE-2478 and NVP-AUY922 may overcome drug resistance in a mechanism independent of ER stress.
Activation and expression level of ER stress signaling in RPMI8226 and RPMI8226/L-PAM cells. a The cell lysates were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-PERK, PERK, phospho-eIF2α, eIF2α, phospho-IRE1, IRE1, and CHOP. Anti-β-actin antibody was used as internal standards. b Quantification of the amount of phospho-PERK, phospho-eIF2α, phospho-IRE1, and CHOP, normalized to the amounts of corresponding protein. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test). c RPMI8226/L-PAM cells were exposed to KW-2478 at the indicated concentrations. After incubation with KW-2478 for 72 h, the cell lysates were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-PERK, PERK, phospho-eIF2α, eIF2α, phospho-IRE1, IRE1, and CHOP. Anti-β-actin antibody was used as internal standards. d Quantification of the amount of phospho-PERK, phospho-eIF2α, phospho-IRE1, and CHOP, normalized to the amounts of corresponding protein. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test). e RPMI8226/L-PAM cells were exposed to NVP-AUY922 at the indicated concentrations. After incubation with NVP-AUY922 for 72 h, the cell lysates were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-PERK, PERK, phospho-eIF2α, eIF2α, phospho-IRE1, IRE1, and CHOP. Anti-β-actin antibody was used as internal standards. f Quantification of the amount of phospho-PERK, phospho-eIF2α, phospho-IRE1, and CHOP, normalized to the amounts of corresponding protein. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test)
KW-2478 suppressed activity of ERK, Akt, and NF-κB via reduction of Src
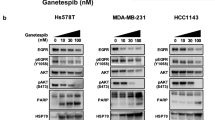
We previously showed that high levels of ERK, Akt, and NF-κB activation result in MDR via overexpression of survivin and reduction of Bim expression [13, 14]. We assessed the activity of these effectors in RPMI8226/L-PAM cells treated with KW-2478 and NVP-AUY922. KW-2478 and NVP-AUY922 reduced expression of phosphorylated ERK, Akt, and nuclear localization of NF-κB in a dose-dependent manner (Fig. 4a–d).
KW-2478 affect activity of Src and its downstream effectors. a RPMI8226/L-PAM cells were exposed to KW-2478 at the indicated concentrations. After incubation with KW-2478 for 72 h, the cytoplasmic and nuclear fractions were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-ERK1/2, ERK1/2, phospho-Akt, Akt, and NF-κB p65. Anti-β-actin and anti-lamin antibody was used as internal standard. b Quantification of the amount of phospho-ERK1/2, phospho-Akt, and NF-κB, normalized to the amounts of corresponding protein. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test). c RPMI8226/L-PAM cells were exposed to NVP-AUY922 at the indicated concentrations. After incubation with NVP-AUY922 for 72 h, the cytoplasmic and nuclear fractions were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-ERK1/2, ERK1/2, phospho-Akt, Akt, and NF-κB p65. Anti-β-actin and anti-lamin antibody was used as internal standard. d Quantification of the amount of phospho-ERK1/2, phospho-Akt, and NF-κB, normalized to the amounts of corresponding protein. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test). e RPMI8226/L-PAM cells were exposed to KW-2478 at the indicated concentrations. After incubation with KW-2478 for 72 h, the cytoplasmic fractions were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-Src and Src. Anti-β-actin antibody was used as internal standard. f Quantification of the amount of phospho-Src and Src, normalized to the amounts of β-actin. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test). g RPMI8226/L-PAM cells were exposed to NVP-AUY922 at the indicated concentrations. After incubation with NVP-AUY922 for 72 h, the cytoplasmic fractions were extracted and then subjected to SDS-PAGE/immunoblotting with antibodies against phospho-Src and Src. Anti-β-actin antibody was used as internal standard. h Quantification of the amount of phospho-Src and Src, normalized to the amounts of β-actin. The results are representative of 5 independent experiments. *p < 0.01, compared to controls (one-way ANOVA with Dunnett’s test)
Src, a client of HSP90, regulates downstream effectors including ERK, Akt, and NF-κB [27,28,29]. Our previous study indicated that levels of phosphorylated Src were higher in RPMI8226/L-PAM cells than in RPMI8226 cells. Incubation with KW-2478 and NVP-AUY922 led to a reduction in Src and phosphorylated Src in RPMI8226/L-PAM cells, in a dose-dependent manner (Fig. 4e–h). These results indicate that overexpression of HSP90 mediates melphalan resistance via activation of Src and its downstream signaling effectors, including ERK, Akt, and NFκB. Furthermore, HSP90 inhibitors have restored melphalan sensitivity by suppressing the activity of these effectors. Our findings show that HSP90 inhibitors could be useful for the treatment of melphalan-resistant MM.
We also investigated whether KW-2478 and NVP-AUY922 suppresses the MM cell migration. KW-2478 and NVP-AUY922 inhibited the SDF-1-induced cell migration in RPMI8226 and RPMI8226/L-PAM cells (Suppl. Fig. 1). This finding indicated that HSP90 inhibitors suppress the MM cell migration.
Discussion
HSP90 maintains the stability of numerous client proteins, many of which are associated with tumor pathogenesis, and thus HSP90 represents an important therapeutic target for cancer [15, 16]. HSP90 overexpression has been observed in B cell lymphoma patients [30] and plays a role in Burkett’s lymphoma progression via activation of B cell receptor signaling [31]. In addition, high-level expression of HSP90 is correlated with malignancy, poor prognosis, and chemoresistance in a number of contexts [32,33,34,35,36,37]. Increased nuclear localization of HSP90 is also associated with metastasis in non-small cell lung cancer [38]. In the present study, expression of HSP90 was higher in RPMI8226/L-PAM or ARH-77/L-PAM cells than RPMI8226 or ARH-77 cells, indicating that enhanced HSP90 expression plays an important role in survival of melphalan-resistant MM cells.
Here, we observed that KW-2478 and NVP-AUY922 restored melphalan sensitivity in RPMI8226/L-PAM and ARH-77/L-PAM cells. Inhibition of HSP90 has been shown to demonstrate anti-lymphoma activity by inhibition of PI3 K/Akt/mTOR signaling pathway [39]. Other studies have shown that KW-2478 can suppress MM proliferation and enhance the anti-tumor activity of bortezomib in MM cell lines, samples derived from patients, and xenograft models [22,23,24]. The efficiency of KW-2478 in relapsed or refractory MM was established using combination regimens in clinical trials [40, 41]. In addition, NVP-AUY922 enhanced the cytotoxic effect of melphalan, doxorubicin, suberoyl anilid hydroxamate, NVP-LBH589 in MM cells and primary myeloma cells from patients [42]. These results indicate that HSP90 inhibitors are useful to overcome melphalan resistance in MM.
We further investigated details of melphalan resistance mechanism. ER stress is continually activated in MM cells, due to excessive protein production. Therefore, the unfolded protein response (UPR), which is the mechanism that prevents ER stress, is crucial for MM cell survival [12, 43, 44]. Proteasome inhibitors have been shown to target UPR and suppress myeloma progression, via accumulation of unfolded proteins beyond UPR capacity [45, 46]. A reduction in ER stress signaling molecules is correlated with bortezomib resistance in MM cell lines and in patients [47, 48]. Thus, we assessed ER stress-related proteins and found that IRE1 and CHOP expression was reduced in RPMI8226/L-PAM cells compared to RPMI8226 cells, while PERK and eIF2α expression levels were equal in both cell types. Since HSP90 is essential for proper protein folding, inhibition of this molecule results in ER stress-dependent apoptosis [26, 49]. We therefore assessed the activity of ER stress signaling in RPMI8226/L-PAM cells treated with KW-2478 and NVP-AUY922; however, KW-2478 and NVP-AUY922 did not affect these proteins. These results indicate that ER stress is lower in RPMI8226/L-PAM cells, but that inhibition of HSP90 does not overcome melphalan resistance via this mechanism.
Several intracellular signaling pathways are reported to participate in pathogenesis and drug resistance in MM [50,51,52]. Co-treatment with MEK and Akt inhibitors overcomes cell adhesion-mediated drug resistance, and an Akt inhibitor alone enhances the anti-myeloma activity of dexamethasone, doxorubicin, melphalan, and bortezomib [53]. Our previous study indicated that ERK, Akt, and NF-κB activity is higher in established melphalan-resistant MM cells than in parent cells, and that inhibitors of each can successfully restore drug sensitivity [13]. In the present study, KW-2478 and NVP-AUY922 reduced levels of phosphorylated ERK and Akt and nuclear localization of NF-κB in a dose-dependent manner.
HSP90 inhibitor NVP-AUY922 was shown to effectively overcome anti-cancer drug resistance via degradation of client proteins [18,19,20,21]. Src is a HSP90 client protein and plays a role in cell survival by regulating downstream effectors, including ERK, Akt, and NF-κB [27,28,29]. Constitutive activation of Src has been observed in MM cells from initial/recurrent MM patients; furthermore, inhibition of Src with dasatinib showed synergistic cytotoxic activity when combined with anti-myeloma agents such as melphalan, prednisolone, thalidomide, and bortezomib [54]. In addition, activation of Src by stimulation of vascular endothelial growth factor (VEGF) induces the cell migration, and dasatinib suppressed the VEGF-induced cell migration in MM cells [54]. Moreover, Dasatinib overcame MDR via reduction of MDR protein 1 and survivin, and via enhancement of Bim in drug-resistant MM cells [55]. In the present study, higher Src activity was observed in RPMI8226/L-PAM cells than in RPMI8226 cells. In addition, we found that KW-2478 and NVP-AUY922 reduced Src expression at concentrations that also restored melphalan sensitivity. Furthermore, KW-2478 and NVP-AUY922 suppressed the SDF-1-induced cell migration in RPMI8226 and RPMI8226/L-PAM cells.
In conclusion, our results suggest that high-level expression of HSP90 plays an important role in melphalan resistance by activating Src and its downstream effectors, including ERK, Akt, and NF-κB. Furthermore, an HSP90 inhibitor overcomes melphalan resistance via reduction of Src expression. These findings indicate that HSP90 inhibitors could be useful for the treatment of MM patients with tolerance to melphalan.
References
Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197–208.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60.
Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20.
Mitsiades CS, Hayden PJ, Anderson KC, Richardson PG. From the bench to the bedside: emerging new treatments in multiple myeloma. Best Pract Res Clin Haematol. 2007;20(4):797–816.
Merchionne F, Perosa F, Dammacco F. New therapies in multiple myeloma. Clin Exp Med. 2007;7(3):83–97.
San Miguel JF, Schlag R, Khuageva NK, et al. VISTA trial investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17.
Facon T, Mary JY, Hulin C, et al. Intergroupe Francophone du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–18.
Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70.
Wijermans P, Schaafsma M, Termorshuizen F, et al. Dutch-Belgium Cooperative Group HOVON. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–6.
Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117(11):3025–31.
Krishnan SR, Jaiswal R, Brown RD, Luk F, Bebawy M. Multiple myeloma and persistence of drug resistance in the age of novel drugs. Int J Oncol. 2016;49(1):33–50.
Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43(8):732–41.
Tsubaki M, Satou T, Itoh T, et al. Overexpression of MDR1 and survivin, and decreased Bim expression mediate multidrug-resistance in multiple myeloma cells. Leuk Res. 2012;36(10):1315–22.
Tsubaki M, Takeda T, Tomonari Y, et al. Overexpression of HIF-1α contributes to melphalan resistance in multiple myeloma cells by activation of ERK1/2, Akt, and NF-κB. Lab Investig. 2019;99(1):72–84.
Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2(1):3–24.
Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–49.
Wang H, Lu M, Yao M, Zhu W. Effects of treatment with an Hsp90 inhibitor in tumors based on 15 phase II clinical trials. Mol Clin Oncol. 2016;5(3):326–34.
Jacobson C, Kopp N, Layer JV, et al. HSP90 inhibition overcomes ibrutinib resistance in mantle cell lymphoma. Blood. 2016;128(21):2517–26.
Park KS, Hong YS, Choi J, et al. HSP90 inhibitor, AUY922, debilitates intrinsic and acquired lapatinib-resistant HER2-positive gastric cancer cells. BMB Rep. 2018;51(12):660–5.
Canonici A, Qadir Z, Conlon NT, et al. The HSP90 inhibitor NVP-AUY922 inhibits growth of HER2 positive and trastuzumab-resistant breast cancer cells. Investig New Drugs. 2018;36(4):581–9.
Wainberg ZA, Anghel A, Rogers AM, et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol Cancer Ther. 2013;12(4):509–19.
Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107(3):1092–100.
Nakashima T, Ishii T, Tagaya H, et al. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin Cancer Res. 2010;16(10):2792–802.
Ishii T, Seike T, Nakashima T, et al. Anti-tumor activity against multiple myeloma by combination of KW-2478, an Hsp90 inhibitor, with bortezomib. Blood Cancer J. 2012;2(4):e68.
Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Investig. 2005;115(10):2656–64.
Taiyab A, Sreedhar AS, Rao ChM. Hsp90 inhibitors, GA and 17AAG, lead to ER stress-induced apoptosis in rat histiocytoma. Biochem Pharmacol. 2009;78(2):142–52.
Corey SJ, Anderson SM. Src-related protein tyrosine kinases in hematopoiesis. Blood. 1999;93(1):1–14.
Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediat Inflamm. 2012;2012:512926.
Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96(1):109–14.
Valbuena JR, Rassidakis GZ, Lin P, et al. Expression of heat-shock protein-90 in non-Hodgkin’s lymphomas. Mod Pathol. 2005;18(10):1343–9.
Walter R, Pan KT, Doebele C, et al. HSP90 promotes Burkitt lymphoma cell survival by maintaining tonic B-cell receptor signaling. Blood. 2017;129(5):598–608.
Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67(7):2932–7.
Cheng Q, Chang JT, Geradts J, et al. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012;14(2):R62.
Gallegos Ruiz MI, Floor K, Roepman P, et al. Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS ONE. 2008;3(3):e0001722.
McCarthy MM, Pick E, Kluger Y, et al. HSP90 as a marker of progression in melanoma. Ann Oncol. 2008;19(3):590–4.
Tian WL, He F, Fu X, et al. High expression of heat shock protein 90 alpha and its significance in human acute leukemia cells. Gene. 2014;542(2):122–8.
Záčková M, Moučková D, Lopotová T, Ondráčková Z, Klamová H, Moravcová J. Hsp90—a potential prognostic marker in CML. Blood Cells Mol Dis. 2013;50(3):184–9.
Su JM, Hsu YY, Lin P, Chang H. Nuclear accumulation of heat-shock protein 90 is associated with poor survival and metastasis in patients with non-small cell lung cancer. Anticancer Res. 2016;36(5):2197–203.
Giulino-Roth L, van Besien HJ, Dalton T, et al. Inhibition of Hsp90 suppresses PI3K/AKT/mTOR signaling and has antitumor activity in Burkitt lymphoma. Mol Cancer Ther. 2017;16(9):1779–90.
Yong K, Cavet J, Johnson P, et al. Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. Br J Cancer. 2016;114(1):7–13.
Cavenagh J, Oakervee H, Baetiong-Caguioa P, et al. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br J Cancer. 2017;117(9):1295–302.
Kaiser M, Lamottke B, Mieth M, et al. Synergistic action of the novel HSP90 inhibitor NVP-AUY922 with histone deacetylase inhibitors, melphalan, or doxorubicin in multiple myeloma. Eur J Haematol. 2010;84:337–44.
Fu YF, Liu X, Gao M, Zhang YN, Liu J. Endoplasmic reticulum stress induces autophagy and apoptosis while inhibiting proliferation and drug resistance in multiple myeloma through the PI3K/Akt/mTOR signaling pathway. Oncotarget. 2017;8(37):61093–106.
Nikesitch N, Lee JM, Ling S, Roberts TL. Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clin Transl Immunol. 2018;7(1):e1007.
Selimovic D, Porzig BB, El-Khattouti A, et al. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal. 2013;25(1):308–18.
Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16.
Nikesitch N, Tao C, Lai K, et al. Predicting the response of multiple myeloma to the proteasome inhibitor Bortezomib by evaluation of the unfolded protein response. Blood Cancer J. 2016;6:e432.
Ling SC, Lau EK, Al-Shabeeb A, et al. Response of myeloma to the proteasome inhibitor bortezomib is correlated with the unfolded protein response regulator XBP-1. Haematologica. 2012;97(1):64–72.
Wang X, Wang S, Liu Y, et al. The Hsp90 inhibitor SNX-2112 induces apoptosis of human hepatocellular carcinoma cells: the role of ER stress. Biochem Biophys Res Commun. 2014;446(1):160–6.
Hu J, Hu WX. Targeting signaling pathways in multiple myeloma: pathogenesis and implication for treatments. Cancer Lett. 2018;414:214–21.
Mashimo K, Tsubaki M, Takeda T, et al. RANKL-induced c-Src activation contributes to conventional anti-cancer drug resistance and dasatinib overcomes this resistance in RANK-expressing multiple myeloma cells. Clin Exp Med. 2019;19(1):133–41.
Tsubaki M, Takeda T, Yoshizumi M, et al. RANK–RANKL interactions are involved in cell adhesion-mediated drug resistance in multiple myeloma cell lines. Tumour Biol. 2016;37(7):9099–110.
Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107(10):4053–62.
Coluccia AM, Cirulli T, Neri P, et al. Validation of PDGFRbeta and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood. 2008;112(4):1346–56.
Tsubaki M, Komai M, Itoh T, et al. By inhibiting Src, verapamil and dasatinib overcome multidrug resistance via increased expression of Bim and decreased expressions of MDR1 and survivin in human multidrug-resistant myeloma cells. Leuk Res. 2014;38(1):121–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All experiment in studies was not a clinical study by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tabata, M., Tsubaki, M., Takeda, T. et al. Inhibition of HSP90 overcomes melphalan resistance through downregulation of Src in multiple myeloma cells. Clin Exp Med 20, 63–71 (2020). https://doi.org/10.1007/s10238-019-00587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-019-00587-2