Abstract
AMD from the Anna S coal mine in Pennsylvania (USA) has been treated successfully since 2004 in the Anna and Hunters Drift (HD) passive systems. The systems, which consist of vertical flow ponds and constructed wetlands, are the largest and most costly mine water treatment project installed by a non-profit group in the USA to date. 15 years of monitoring data show that the systems effectively treated 1910 L/min of flow with pH 2.8–3.1 containing 121–330 mg/L acidity (as CaCO3), 11–31 mg/L Al, 6–33 mg/L Fe, and 6 mg/L Mn. The systems produced effluents with pH 7.5, 134–140 mg/L alkalinity (as CaCO3), < 1 mg/L Al, 1 mg/L Fe, 2–3 mg/L Mn, and never discharged water with less than 60 mg/L alkalinity (106 samples). In 15 years of operation, the systems generated a combined 5600 tonnes (t) of net alkalinity. Unit treatment costs were converted to 2018 U.S. $ and compared to active treatment systems. Over a 20 year period, passive systems generate alkalinity at a cost of $1168/t of CaCO3, which is 50% less than unit costs for lime treatment plants currently operated in Pennsylvania.
Zusammenfassung
Saures Grubenabwasser (AMD) der Anne S. Kohlegrube in Pennsylvania (USA) wird seit 2004 erfolgreich durch passive Systeme (Anna und Hunters Drift (HD)) aufbereitet. Das passive Grubenwasserreinigungssystem besteht aus vertikal durchströmten Teichen sowie Feuchtgebieten und ist das größte und teuerste Grubenwasseraufbereitungsprojekt, das bisher von einer gemeinnützigen Gruppe in den USA installiert wurde. 15 Jahre Überwachungsdaten zeigen, dass die Systeme effektiv bei Durchflussmengen von 1.910 L/min und arbeiten. Das einströmende Grubenwasser weist pH-Werte von 2,8 bis 3,1 auf und enthält 121–330 mg/L Acidität (als CaCO3), 11–31 mg/L Al, 6–33 mg/L Fe und 6 mg/L Mn. Nach der Passage durch das System werden pH-Werte von 7,5, eine Alkalität von 134–140 mg/L, Al-Werte < 1 mg/L sowie Fe- und Mn-Werte von 1 bzw. 2–3 mg/L gemessen. Über den gesamten Zeitraum strömte niemals Wasser mit Alkalitäten kleiner 60 mg/L aus (106 Proben). In 15 Betriebsjahren wurden durch die passive Reinigung insgesamt 5.600 Tonnen (t) Nettoalkalität erzeugt. Die Behandlungskosten wurden 2018 in US-Dollar (USD) umgerechnet und mit aktiven Wasserbehandlungssystemen verglichen. Über einen Zeitraum von 20 Jahren wurde durch das passive Behandlungssystem Alkalinitäten von 1.168 USD/t CaCO3 erzeugt. Somit fallen durch die passive Grubenwasserreinigung 50% weniger Kosten pro Einheit an als bei derzeit in Pennsylvania in Betrieb befindlichen Kalkbehandlungsanlagen.
Resumen
El AMD de la mina de carbón Anna S en Pensilvania (EEUU) ha sido tratada con éxito desde 2004 en los sistemas pasivos Anna y Hunters Drift (HD). Los sistemas, que consisten en estanques de flujo vertical y humedales construidos, son el proyecto de tratamiento de aguas de mina más grande y costoso instalado por un grupo sin fines de lucro en los Estados Unidos hasta la fecha. 15 años de datos de monitoreo muestran que los sistemas trataron efectivamente 1.910 L/min de flujo con pH 2.8–3.1 que contenía 121-330 mg/L de acidez (como CaCO3), 11–31 mg/L Al, 6–33 mg/L Fe y 6 mg/L Mn. Los sistemas produjeron efluentes con pH 7,5, 134–140 mg/L de alcalinidad, <1 mg/L Al, 1 mg/L Fe y 2–3 mg/L Mn, y nunca se descargaron con menos de 60 mg/L de alcalinidad (106 muestras). En 15 años de operación, los sistemas generaron un total combinado de 5.600 toneladas (t) de alcalinidad neta. Los costos de tratamiento unitario se convirtieron a US $ 2018 y se compararon con los sistemas de tratamiento activos. Durante un período de 20 años, los sistemas pasivos generan alcalinidad a un costo de $ 1.168/t de CaCO3, que es un 50% menos que los costos unitarios de las plantas de tratamiento de cal actualmente operadas en Pennsylvania.
波兰立井开拓水害防治经验
自2004年,Anna and Hunters Drift(HD)被动处理系统成功地处理美国宾夕法尼亚州安娜硫矿的酸性矿井废水(AMD)。系统由垂向流水池和人工湿地组成,至今仍是规模最大、费用最高的矿井废水处理项目,项目由一家美国非盈利组织承担。15年的运行数据表明,该系统能够对pH值2.8–3.1、酸度121–330 mg/L(以CaCO3计)、铝11–31 mg/L、铁6-33 mg/L和锰6 mg/L的矿山污水有效处理,处理能力1910 L/min。处理之后,废水pH值7.5、碱度134–140 mg/L、铝<1 mg/L、铁1 mg/L和锰2–3 mg/L,系统未排放过碱度低于60 mg/L的废水 (106个检测水样)。15年运行净产碱5,600吨。单位处理成本折合2018美元,可与主动处理系统对比。20年之后,被动系统产碱成本为1,168美元/(吨CaCO 3),仍比目前宾夕法尼亚州石灰处理法的单位成本低50%。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Passive mine drainage treatment systems use natural materials and biogeochemical processes to generate alkalinity, neutralize acidity, and remove metal contaminants while making full use of gravity to transfer water to and through the systems (Hedin et al. 1994; Younger et al. 2002). Passive treatment technologies are a primary tool for the restoration of streams polluted by legacy coal mines in Pennsylvania (USA). As of 2015, ≈ 275 passive mine water treatment systems have been installed in Pennsylvania at a total cost of ≈ $93 Mio. (Stream Restoration Inc. 2019). Eighty percent of the systems were installed by non-profit citizen groups, while the balance were installed by the PA Department of Environmental Protection.
The passive treatment approach is often preferred over conventional active treatment due to cost savings arising from the avoidance of routine maintenance activities and reduced energy requirements. Conventional systems require the continuous addition of chemical reagents, the management of large volumes of low-solids sludge, and the perpetual input of electricity (Younger et al. 2002). Through its use of natural substrates as a source of chemical modification and gravity as a source of energy, passive treatment avoids these routine costs. The neutralization of acidity is achieved through limestone dissolution or through a biologically active organic substrate. The limited solubility and kinetics of these processes allow the initial installation of enough reactive substrates to supply years of treatment. Passive treatment processes produce a low volume of high-solids sludge, and it is feasible to design systems with years of storage capacity.
The sustained effective treatment of passive systems requires long-term maintenance, which can be divided into minor and major categories. Minor maintenance events generally occur quarterly or semi-annually and include tasks that can be performed by hand and do not involve the management or replacement of treatment components. Minor maintenance also includes inspections and monitoring efforts to identify developing problems.
Major maintenance tasks are scheduled activities that are too large to be accomplished as a routine action. Examples include the removal of metal sludge deposits and the replacement of reactive substrates. These actions are typically performed on multi-year intervals and involve heavy equipment to replace treatment system components and/or replenish treatment materials. Though infrequent, major maintenance tasks can be costly because they deal with years of sludge accumulation or large-scale substrate replacement. The need for major maintenance actions must be recognized in the operation of passive treatment systems and included in cost comparisons of treatment approaches. Because passive systems are often designed with 10–20 years of substrate and sludge storage capacity, major maintenance considerations are typically theoretical (Hedin 2008). A more meaningful analysis is based on realized maintenance costs from installed functional passive treatment systems.
This paper presents performance and cost data for two passive systems that were installed in 2004 to treat acidic mine water discharging from the Anna S Mine in Tioga County, Pennsylvania. The systems are among the largest and most costly passive treatment projects undertaken by a nonprofit citizen group in the U.S to date. The installation and first five years of treatment performance were described in Hedin et al. (2010). This paper provides 10 more years of monitoring information and an accounting of the realized minor and major maintenance costs. The data are used to develop unit treatment costs that are compared to similar calculations made for three systems using conventional chemical treatment technologies.
Background
Mining History and Pollution History
Table 1 shows a timeline of the mining, monitoring, and remediation activities at the site. The Anna S underground mine is in the Bloss coal seam and was operated by the Fall Brook Coal Company between the 1890 s and the 1930s. The mine is above drainage, so the workings are largely unflooded. The coal and associated strata are acidic, while the overlying sandstone geology is largely inert. Mining in these geologic conditions without alkaline addition results in acidic metal-contaminated drainage (AMD). The Anna S mine was also subjected to surface mining activities that focused on the extraction of shallow crop coal and the overlying Cushing coal. Between 1977 and 1986, surface mining methods known as “daylighting” were used to remove stumps and pillars from the previously abandoned deep mine as well as the Cushing coal seam. The surface mining avoided disturbance to the primary mine portal and a drainage tunnel, which together produced most of the mine drainage flow. Areas surface mined after 1977 were regraded and successfully revegetated with standard herbaceous reclamation species.
The mine discharges flow to Wilson Creek, a tributary of Babb Creek, which is a major tributary to Pine Creek, a world-renowned cold-water trout fishery. Prior to the daylighting activities in the watershed, Pine Creek was able to assimilate pollution from Babb Creek without degradation. In the 1980s, the quality of Pine Creek below Babb Creek deteriorated substantially, causing the stream to be placed on the EPA’s 303(d) list of degraded streams. The cause was attributed to increased contamination from mines in the Babb Creek watershed. In 1990, the Pennsylvania Fish and Boat Commission surveyed Babb Creek and found native trout in its headwaters but no fish downstream of Wilson Creek. Soon after the survey, the Babb Creek Watershed Association (BCWA) was formed to promote the restoration of Babb Creek. Over the next 30 years, the BCWA implemented three reclamation projects and installed 10 treatment systems. The two largest treatment projects are a lime treatment plant and the Anna S Mine passive treatment systems. The BCWA supports a small staff that operates the lime plant and maintains its passive treatment systems.
Anna S Mine Passive Treatment Systems
Two passive treatment systems were installed in 2003–04 to treat three discharges from the Anna S Mine (Fig. 1). The Hunters Drift (HD) system treats water flowing from the HD drainage tunnel with four parallel vertical flow ponds (VFPs) followed by a series of three constructed wetlands. The Anna system treats water flowing from the S1 and S2 mine portals with four parallel VFPs, followed by a single polishing pond.
Due to topographical and geologic constraints, a limited area was available for construction of gravity-driven treatment systems. The available sites required long pipelines to transfer the discharges from their collection points. The pipelines for the HD, S1, and S2 discharges are 730, 267, and 318 m long, respectively. The pipelines flow into structures that distribute water into the VFPs. The structures facilitate maintenance activities by allowing restriction of flow to VFP units undergoing maintenance, while maintaining treatment through the other VFPs.
In both the HD and Anna systems, each individual VFP consists of 0.9 m of limestone aggregate overlain with 0.3 m of alkaline organic substrate overlain with 0.6–0.9 m of standing water (Fig. 2). An underdrain system constructed with perforated plastic pipe is located at the bottom of the limestone aggregate. Mine water flows into each VFP through a piped inlet at the surface, down through the organic substrate and limestone aggregate to the underdrain collection system. Water discharges through a structure that controls the water elevation in each VFP. In each system, the effluents from the VFPs are collected into a single flow that is discharged to a series of ponds and constructed wetlands for polishing via aerobic reactions. Details of the designs, including quantities, volumes, and surface areas are available in Hedin et al. (2010).
The alkaline organic substrate in each VFP is a mixture of spent mushroom compost and limestone fines that is intended to help treat the mine drainage and protect the integrity of the limestone underdrain. Calcite dissolution and microbial activity in the substrate generate alkalinity, which raises pH and promotes the hydrolysis of dissolved Al and Fe3+ to hydroxide solids. The fertile organic substrate supports microbial activity that removes dissolved oxygen, reduces ferric iron (Fe3+) to ferrous iron (Fe2+), and generates dissolved carbon dioxide. As water flows through the limestone aggregate, calcite dissolution generates additional bicarbonate alkalinity. Fe2+ and Mn2+, which are highly soluble at circumneutral pH, pass through the limestone aggregate and are discharged from the VFP. Both metals are subject to removal by oxidizing reactions in the aerobic ponds and wetlands.
The systems contain flow restriction mechanisms that limit the maximum treated flow and bypasses excess flow. The Anna system was designed to accept up to 1635 L/min, approximately the 90th percentile flow rate for the combined S1 and S2 discharges. The Hunters Drift was designed to accept up to 1211 L/min, approximately the 75th percentile flow rate.
Methods
Historic water chemistry and flow data for the discharges were obtained from various sources. In 1975–1976, discharges from the Anna S Mine were monitored through Pennsylvania’s Operation Scarlift program (Boyer Kantz and Associates 1976). Between 1977 and 1984, the discharges were monitored by the United States Geological Survey (USGS) as part of a project intended to measure the effect of daylighting operations on mine drainage quality (Reed 1980). During this period, weirs were installed and monitored continuously for flow, while water samples were collected monthly and analyzed for mine drainage parameters using procedures reported in Reed (1980). Between 1985 and 1989, the discharges were monitored by Antrim Mining Co. as part of their mining permit requirements. Data from this period was obtained from permit files available from the Pennsylvania Department of Environmental Protection (PADEP) District Mining Operations. Between 1996 and 2000, the discharges were monitored by PADEP and Babb Creek Watershed Association as a prelude to development of treatment plans.
The passive treatment systems were installed in 2004. Monitoring has occurred regularly at influent and effluent locations and irregularly at internal points. The pH and temperature were measured using a calibrated electronic pH meter and field alkalinity was measured within 4 h of sample collection by titration with 1.6 N sulfuric acid to pH 4.5 (American Public Health Association 1999). A raw sample was analyzed in a PADEP-certified laboratory for pH, alkalinity, hot hydrogen peroxide acidity, sulfate, and total suspended solids by standard methods. When available, field measurements of pH and alkalinity were used in preference to laboratory values. An acidified sample (pH < 2 with nitric acid) was analyzed for total concentrations of Fe, Al, and Mn by inductively coupled plasma spectrometry (American Public Health Association 1999). Metals and sulfate are reported as mg/L. Acidity and alkalinity are reported as mg/L CaCO3 equivalents. In 2004 and 2005, laboratory analyses were conducted by the Pennsylvania State Laboratory. Since 2006, laboratory analyses have been conducted by G&C Coal Analysis Laboratory (Summerville, PA).
The quality of field and laboratory analyses was evaluated by comparing measured and calculated acidities for samples of mine water that did not have visible particulates when collected and acidified following the method described by Hedin (2006). The acid balance error was calculated in a manner analogous to a charge balance error calculation, as follows:
The measured and calculated acidity values showed good correspondence. The influent and effluent sampling points had, on average, imbalances between − 2% and − 7%. It is not clear why the calculated acidities are slightly larger than the measured acidities.
Flow of the piped influents to the treatment systems was measured by the timed-volume method and is reported as liters per minute (L/min). Load (kg/day) was calculated using the product of flow and chemistry with appropriate unit adjustments.
Costs for the installation of the Anna S passive systems and two recent major maintenance events were obtained from PADEP Growing Greener grant documents. Costs for annual operation and maintenance of the Anna S systems were obtained from audited financial reports provided by BCWA. Treatment information and costs for the installation and operation of the lime treatment systems were obtained from the PADEP Bureau of Abandoned Mine Reclamation (PADEP 2019b) and from the PADEP Bureau of District Mining Operations (PADEP 2017). Costs were adjusted to 2018 US $ using the U.S. Bureau of Reclamation Construction Cost Trends composite cost index (US Bureau of Reclamation 2019). Future costs were estimated assuming a 20 year straight line depreciation and a 5% interest rate.
Results
Mine Drainage Characteristics
Table 2 shows the average flow, chemistry, and acidity loads for the discharges pre-daylighting (1975–1978), during daylighting (1979–1989), during the passive treatment system design (1995–1998), and since installation of the system in 2004. Individual flow and acidity measurements are shown in supplemental Figures S-1 and S-2. All three discharges are acidic with elevated concentrations of Fe, Al, and Mn. The HD and S1 discharges are the primary sources of flow and contamination. The S2 discharge always produced less flow with lower contaminant concentrations.
Pre-daylighting flows were higher than during and after daylighting. The combined pre-daylighting average flow was 3457 L/min while the combined daylighting flow averaged 2009 L/min, and the pre-treatment-system period averaged 2451 L/min. The differences in flow were not attributable to differences in precipitation, as a review of local weather records (Williamsport Municipal Airport, 53 km south) did not identify unusual precipitation before the daylighting period. The difference in flow was likely a consequence of the daylighting activities. Remining and reclamation of abandoned mines can lessen mine water flow due to more effective exclusion of surface water from the underground workings (Hawkins 1998).
However, daylighting significantly increased the release of contaminants. Concentrations of acidity and metals increased up to fivefold during the daylighting period (Fig. S2). Figure 3 shows combined loadings for the HD and S1 discharges between 1974 and 1999. During the daylighting period, several very high loading events were observed, while low loading events were less common. The increased contaminant loading coincided with the observed degradation of Pine Creek downstream of Babb Creek.
Since cessation of mining activities and reclamation of the site, concentrations of acidity and metals have decreased. Contaminant concentrations at HD have returned to levels observed before the daylighting operations; concentrations at S1 and S2 are 30% lower than pre-daylighting levels.
Major Maintenance
The parallel VFP design allows individual VFP units to be isolated for inspection or maintenance while maintaining treatment by the other units. The organic substrates in the VFPs have been periodically inspected to assess their reactivity. The alkaline organic substrates have a limited capacity for supporting beneficial chemical and microbial processes. When the capacity is exhausted, low pH metal-contaminated water will enter the underlying limestone aggregate and compromise its effectiveness. Because of the very large investment in the limestone aggregate (33,000 t of limestone with an installed cost of ≈ $1 Mio.), a primary goal of operation and maintenance activities is to protect the integrity of the limestone aggregate. This goal is accomplished by replacing the organic substrate before its failure results in degradation of the underlying aggregate.
All organic substrates in the VFPs were recently replaced using similar methodology. Flow into the VFP targeted for rehabilitation was diverted to other VFPs using the distribution infrastructure. Each targeted VFP was drained empty and the existing substrate was stripped off and set aside. The exposed underlying limestone aggregate was scarified by raking with an excavator. Approximately one foot of new alkaline organic substrate was placed on top of the limestone aggregate and the old substrate, which was not entirely exhausted, was placed on top of the new substrate. The new substrate was a 2:1 volume mixture of fresh spent mushroom compost and limestone fines. The substrate replacement process was developed in 2012 on one VFP and then used to rehabilitate the remaining three HD VFPs in 2013 and all four Anna VFPs in 2016. Effective treatment of the mine drainage was maintained during substrate replacement by assuring that all flows passed through functional VFPs. During the substrate replacement projects, general repairs were made to the systems including cleanout of channels, repairs to water level control structures, and rehabilitation of the S2 and HD collection systems.
Treatment Effectiveness
Table 3 shows the average chemistry of the system influents, VFP effluents, and final effluents of the two passive treatment systems. The effluents from the VFPs had a circumneutral pH and were strongly net alkaline. The VFPs in both systems decreased Al to less than 1 mg/L, had marginal impact on Mn, and had variable impact on Fe. The Anna VFPs did not markedly decrease Fe, while the HD VFPs decreased Fe concentrations by ≈ 50%. Additionally, the Fe removal by all of the VFPs was variable with respect to flow rate (Fig. 4). In many low flow conditions, the VFPs released Fe, while at all high flow conditions, the VFPs removed the Fe.
The VFPs had little effect on sulfate. If the changes in sulfate were attributable to the bacterial sulfate reduction, then the alkalinity generation attributable to sulfate reduction (100 mg/L CaCO3 generation per 96 mg/L SO4 removal) only accounted for 3% of the net alkalinity generation by the Anna VFPs and 6% of the alkalinity net generation of the HD VFPs. Calcite dissolution was the dominant source of alkalinity in both systems.
The VFPs discharge to aerobic wetlands and ponds that were intended to remove residual Fe and Mn by oxidative processes. The aerobic units were effective. Fe was decreased to 1.1 mg/L at the final effluent of the Anna system and to 0.5 mg/L at the final effluent of the HD system. The passive removal of Mn requires alkaline aerobic conditions and low concentrations of ferrous iron (Hedin et al. 1994). This was accomplished in both systems, but was more effective in the HD system, which has a larger aerobic wetland.
The oxidative removal of Fe and Mn in the wetlands consumes alkalinity, yet the effluents of both systems are still strongly net alkaline. The average net generation of alkalinity, calculated from the difference of acidity between the influent and effluent, was 229 mg/L CaCO3 for the Anna system and 444 mg/L CaCO3 for the HD system. The average generation of net alkalinity by the systems, calculated from the net alkalinity generation and the influent flow rates, was 266 kg/day CaCO3 for the Anna system and 642 kg/day CaCO3 for the HD system. On average, the combined systems generated 332 t/year alkalinity as CaCO3. Over the 5598 days of operations (Jan 1, 2004–Apr 30, 2019), the systems generated a combined 5,033 t of alkalinity as CaCO3.
The treatment was reliable. Figure 5 shows effluent concentrations of alkalinity and net acidity over the 15 year monitoring period for both systems. The lowest concentration of alkalinity was 60 mg/L CaCO3 and the highest concentration of acidity was − 50 mg/L CaCO3. Effluent pH was always greater than 6.5.
The replacement of organic substrate resulted in short-term changes in water chemistry. Figure 4 notes two samples with elevated Fe that were collected from two VFPs effluents within a month of their organic substrate replacement. These temporary changes in chemistry were not detected when the systems were sampled several months later.
Costs
Table 4 shows the costs to construct, operate, and maintain the Anna and HD systems. In 2003, the construction cost was $2,215,699 and the cost for design, engineering, permitting, and project management was $301,000. For this analysis, operation and maintenance (O&M) cost was divided into routine activities and major maintenance events. Routine O&M is conducted by BCWA and includes monthly inspections, semi-annual sampling, and simple maintenance activities. BCWA maintains nine passive treatment systems located at six sites. In 2018, the total cost to maintain all nine sites was $64,267, of which $10,711 was allocated to the Anna S passive treatment systems. Major maintenance includes tasks that require the hiring of engineering support, contractors, mobilization of heavy equipment, and major materials purchases. Two major maintenance events occurred, replacing the organic substrates in the HD VFPs in 2012 ($210,008) and in the Anna VFPs in 2016 ($201,706). As noted previously, these budgets included general and site-specific system improvements.
Costs to install and operate the Anna S passive systems were realized at different times throughout the 15 year operational period. All costs were adjusted to 2018 U.S. $ using the U.S. Bureau of Reclamation Construction Cost Trends composite cost index (U.S. Bureau of Reclamation 2019). Between 2003 and 2018, construction costs increased by 66% or 3.45% per year (compounded basis). Table 4 shows all costs converted to 2018 values. The cost to design, permit, and install the Anna S Mine passive treatment complex systems in 2018 is estimated at $4,200,000.
Discussion
The HD and Anna passive treatment systems effectively treated acidic coal mine drainage contaminated with Al, Fe, and Mn for 15 years. Every effluent water sample collected from the system (114 samples) had a pH above 6.5 and at least 60 mg/L alkalinity. Based on influent flows and differences in influent and effluent chemistry, the two systems removed ≈ 3100 t of acidity (as CaCO3), 310 t of Al, 290 t of Fe, and 60 t of Mn from the influent waters.
The success of the treatment systems contrasts with current policies regarding the use of passive treatment for coal mine waters in the United States. In West Virginia, passive treatment is considered only appropriate for mine water with less than 100 mg/L acidity (Mack et al. 2010). The U.S. Office of Surface Mining Reclamation and Enforcement (OSMRE) and PADEP have developed criteria for evaluating proposed passive treatment projects (PADEP 2019a). The guidance, shown in Table 5, assigns “risk of failure” to proposed projects based on influent chemistry (summed concentrations of Fe plus Al) and hydrologic loading (flow per treatment cell). The Anna system was designed to treat up to 409 L/min per VFP containing 26 mg/L Fe + Al while the HD system was designed to treat up to 303 L/min per VFP containing 79 mg/L Fe + Al. Both systems would have been classified as having a high risk of failure.
As noted previously, the influent chemistry of both systems improved between their design and the construction, resulting in lower influent metal concentrations and loads. An evaluation was made of the actual chemical and hydraulic conditions received by each system. The Anna system was classified as having a medium risk of failure 30 times and a low risk 7 times. The HD system was classified as having a high risk of failure 45 times and a medium risk 10 times. Based on these evaluations and the high cost of the projects, neither would be fundable under current PADEP and OSMRE project evaluation criteria. As a result, the recommended remedial action would be more expensive and energy intensive chemical treatment. The success of the Anna and Hunters Drift passive treatment systems suggests reconsideration of these criteria.
The 15 year effectiveness of the Anna and HD passive treatment systems can be attributed to several factors. The primary reason for success is the conservative system sizing and design. The Anna and HD systems were designed for 90th and 75th percentile acidity loads, respectively. This feature ensures that the VFPs are oversized under most operating conditions. The VFPs were designed with raised berms and upslope diversion channels that protect them from stormwater damage during extreme precipitation events. Both systems contain functional bypasses that prevent spikes in flow that could damage the VFPs. The systems receive influent flows through adjustable distribution structures that deliver water to multiple treatment units in parallel. This feature allows individual VFPs to be taken off-line for inspection and O&M activities, while maintaining treatment through the other VFPs. By scheduling O&M activities for low flow periods when there is excessive treatment capacity, no degradation of the final effluent quality occurs.
Another factor related to this success is the ongoing operation and maintenance provided by BCWA. The systems are inspected and sampled regularly, and minor maintenance is conducted as a routine operation. BCWA is able to support its O&M activities through funding received from a dedicated tipping fee at a local landfill. Major maintenance, such as replacement of the organic substrates, cleanout of ditches and channels, and repairs to collection systems and hydrologic controls, is conducted as necessary. The latter activities require the BCWA to obtain funding to support these activities. The organization has successfully met these objectives.
Finally, the chemistry of the influent mine water has improved since the systems were designed. Influent concentrations of acidity and metals to the HD and Anna systems have averaged 30% and 50% less, respectively, then those assumed in the design. These changes resulted in lower contaminant loading to the systems. Gradual improvement in mine water chemistry is a common characteristic of mine drainage discharges from abandoned coal mines in Appalachia (Burrows et al. 2015; Demchak et al. 2004; Mack et al. 2010). Passive treatment systems are intended to provide decades of treatment and natural amelioration of contaminant concentrations in the water being treated can be a component of their long-term success.
Reliability is an important aspect of mine water treatment, especially when the treatment system is essential to maintaining ecosystem function in the receiving stream. Short-term failures of chemical treatment systems can create large and rapid changes in effluent chemistry that have long-lasting impacts on the receiving stream ecology (Kruse et al. 2012). Except under catastrophic events, the failure of a passive treatment system is a gradual process that, with proper monitoring, can be recognized and corrected before the receiving stream has been seriously degraded. In the case of the Anna S passive systems, the declining VFP performance was recognized and corrected through planned major maintenance actions.
The generation of excess alkalinity at the effluents is an important benefit of the Anna S treatment systems. Many streams degraded by legacy mining receive flows of acidic water that cannot be cost-effectively collected and treated. The in-stream treatment of these flows is achieved through inputs of excess alkalinity from treatment systems. Approximately 40% of the alkalinity generated by the Anna S systems is realized as alkalinity in the system effluents, which provides valuable neutralization and buffering capacity for Babb Creek.
The reliable treatment and net alkalinity generation provided by the Anna S systems has contributed significantly to the restoration of improved water quality in Babb Creek and downstream in Pine Creek. A native brook trout fishery has reestablished in Babb Creek below Wilson Creek and both Babb Creek and Pine Creek have been removed from EPA’s 303(d) list of degraded streams (PADEP 2012).
Comparative Cost Evaluations
The high cost of the conservative design and ongoing maintenance raises the question: are the Anna S passive treatment systems cost-effective? The conventional method for active treatment of acidic mine water is lime. Current costs and performance data were obtained for three lime treatment plants constructed and operated by the PADEP. Like the Anna S systems, these three treatment plants discharge circumneutral pH water with net alkalinity and low metal concentrations. Table 6 shows costs realized at the time of expenditure and adjusted to 2018 dollars. These costs were used to calculate the annual cost of treatment, assuming that 2018 construction and engineering costs are depreciated over 20 years at 5% interest rate. The total annualized cost of treatment is compared to the annual alkalinity generation (t/years as CaCO3) to calculate the $/t as CaCO3. The Anna and HD systems, combined, generate 327 t/years of alkalinity at a unit cost of $1168/t.
The Hollywood hydrated lime plant treats multiple low pH mine discharges with aeration, lime neutralization, and clarification. The discharges are collected by an extensive gravity flow system and the sludge is pumped into a nearby abandoned underground mine. The plant was installed in 2013 at a cost of ≈ $16,800,000 and had an operating cost in 2017 of $670,248 (PADEP 2019b). The plant generates 911 t/years of alkalinity (as CaCO3) at a 20 year cost of $2,491/t as CaCO3.
The Smail–Orcutt system treats two flows of high-Fe acidic water with lime slurry in reaction tanks followed by earthen settling ponds. The plant was installed in 2015 at a cost of $682,000 and had an operational cost in 2016 of $77,000 (PADEP 2017). The sludge is periodically removed and buried on-site. The plant generates 52 t/years of alkalinity at a unit cost of $2707/t as CaCO3.
The Brandy Camp system was originally installed as a hydrated lime plant to treat a high-Fe acidic discharge. The plant has been retrofitted several times over the last 20 years at a total cost of ≈ $2,400,000. The plant was recently redesigned for treatment with lime slurry and hydrogen peroxide (H2O2) and had an operating cost in 2017 of $263,499 (PADEP 2019b). The plant generates 264 t/years of alkalinity at a unit cost of $2,278/t as CaCO3.
The Anna S passive systems are generating alkalinity and removing metals over a 20 year period at a cost that is 49–57% less than lime systems. Extending the analysis to a longer time frame increases the savings because the annual costs of passive treatment are so much lower than lime treatment. This differential is an underestimate because the long-term major maintenance costs of the Anna S systems are known and accounted for in its costs. The lime systems are all relatively new construction with unknown major maintenance needs. It is likely that expensive repairs or equipment replacement will be required in the next 10–15 years, which will increase their unit treatment costs.
A common reason for excluding passive treatment from consideration is the difficulty of finding a suitable site that is accessible by gravity. Pumping of mine water to a passive system is common practice in the United Kingdom (Coal Authority 2012), but it is rarely considered in the U.S. By disregarding pumping, many feasible passive treatment projects are not installed. If the Anna S passive systems included pump stations to raise the mine water 30 m, the capital costs would increase by ≈ $100,000 and the annual costs would increase by ≈$26,500 (calculated with AMDTreat (U.S. OSMRE 2019) assuming 1910 L/min flow, 30 m lift, 75% pump efficiency, 85% motor efficiency, $0.10/kwh, and 18%/years pump maintenance). The addition of these costs would increase the unit cost to $1,274/t CaCO3. Even with pumping costs included, the passive option would still be 49% less expensive than lime treatment.
Conclusion
The Anna S Mine passive treatment complex has provided 15 years of reliable treatment of low pH coal mine drainage containing elevated Al, Fe, and Mn. The effectiveness of the treatment systems has contributed to the reestablishment of cold-water fisheries in Babb Creek and Pine Creek. The success of the systems is attributable to conservative design, diligent maintenance by the Babb Creek Watershed Association, and natural attenuation of the mine drainage chemistry. The unit cost of alkalinity generation in the passive systems is 50% the cost of comparable conventional lime treatment operations in Pennsylvania. The analysis presented in this paper speaks to the need for reconsideration of the current regulatory understanding of passive treatment’s reliability and cost-effectiveness in the United States.
References
American Public Health Association (1999) Standard Methods for the examination of water and wastewater. In: Clesceri LS, Greenberg AE, Eaton AD (eds), (20th Edit), American Public Health Assoc, American Water Works Assoc, and the Water Environment Federation, Washington DC, USA
Boyer Kantz and Associates (1976) Operation Scarlift, Babb Creek Mine Drainage Abatement Project. Abandoned Mine Reclamation Clearinghouse. https://amrclearinghouse.org/Sub/SCARLIFTReports/ Accessed 22 June 2019
Burrows JE, Peters SC, Cravotta CA (2015) Temporal geochemical variations in above- and below-drainage coal mine discharge. Appl Geochem 62:84–95
Coal Authority (2012) Mine water treatment schemes, code of practice. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/356529/pos-tca-mine-water-treatment-schemes_codeofpractice_2012.pdf. Accessed 18 June 2019
Demchak J, Skousen J, McDonald LM (2004) Longevity of acid discharges from underground mines located above the regional water table. J Environ Qual 33:356–668
Hawkins JW (1998) Remining. In: Brady K, Smith M, Schueck J (eds) Coal mine drainage prediction and pollution prevention in Pennsylvania. PA Department of Environmental Protection, Harrisburg
Hedin RS, Nairn RW, Kleinmann RLP (1994) Passive treatment of polluted coal mine drainage. US Bureau of Mines Information Circular 9389, United States Department of Interior, Washington DC, p 35
Hedin RS (2006) The use of measured and calculated acidity values to improve the quality of mine drainage datasets. Mine Water Environ 25:146–152
Hedin RS (2008) Iron removal by a passive system treating alkaline coal mine drainage. Mine Water Environ 27:200–209
Hedin RS, Weaver T, Wolfe N, Weaver K (2010) Passive treatment of acid mine drainage: the Anna S Mine passive treatment complex. Mine Water Environ 29:165–175
Kruse NA, Bowman JR, Mackey AL, McCament B, Johnson KS (2012) The lasting impacts of offline periods in lime doses streams: A case study in Racoon Creek, Ohio. Mine Water Environ 31:266–272
Mack B, McDonald LM, Skousen J (2010) Acidity decay of above-drainage underground mines in West Virginia. J Environ Qual 39:1–8
PADEP (Pennsylvania Dept of Environmental Protection) (2012) Pennsylvania nonpoint source management program FFFY2011 annual report Commonwealth of Pennsylvania. Dept of Environmental Protection, Harrisburg
PADEP (2017) Mining and reclamation advisory board April 2017 meeting, Chris Yeakle Presentation. https://www.dep.pa.gov/PublicParticipation/AdvisoryCommittees/Mining/MiningReclamation/Pages/2017.aspx. Accessed 3 May 2019
PADEP (2019a) Acid mine drainage set-aside program implementation guidelines. https://www.dep.pa.gov/Business/Land/Mining/AbandonedMineReclamation/Pages/AMD-Set-Aside-Program.aspx. Accessed 22 June 2019
PADEP (2019b) Acid mine drainage (AMD) set-aside program, summary of BAMR’s active mine drainage treatment plants, https://www.dep.pa.gov/Business/Land/Mining/AbandonedMineReclamation/Pages/AMD-Set-Aside-Program.aspx. Accessed 22 June 2019
Reed LA (1980) Effects of strip mining the abandoned deep Anna S Mine on the hydrology of Babb Creek, Tioga County, Pennsylvania. USGS WRI-80–53, Washington DC, p 41
Stream Restoration Inc. (2019) Datashed. https://www.datashed.org/. Accessed 22 June 2019
U.S. Bureau of Reclamation (2019) Construction cost trends. https://www.usbr.gov/tsc/techreferences/mands/cct.html. Accessed 22 June 2019
U.S. OSMRE (Office of Surface Mining, Reclamation, and Enforcement (2019) AMD treat. https://amd.osmre.gov/default.htm. Accessed 22 June 2019
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, Remediation. Springer, New York
Acknowledgements
The Babb Creek Watershed Association provided water sampling data, system information, and has been a continuous steward of the Anna S passive treatment systems. Michael Smith, Neil Wolfe, Naomi Anderson, and an anonymous reviewer provided valuable feedback about draft versions of this papers.
Funding
This research was supported by Pennsylvania Department of Environmental Protection (Grant WR140163).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hedin, R.S. Long-term Performance and Costs for the Anna S Mine Passive Treatment Systems. Mine Water Environ 39, 345–355 (2020). https://doi.org/10.1007/s10230-020-00676-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-020-00676-9




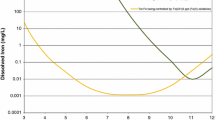




 ) and effluent (
) and effluent ( ) concentrations of Fe plotted against influent flow rate for the a HD and b Anna VFPs. “OS” is organic substrate
) concentrations of Fe plotted against influent flow rate for the a HD and b Anna VFPs. “OS” is organic substrate
 ) and net acidity (
) and net acidity ( ) for the a HD and b Anna systems. The vertical lines indicate the substrate replacement events. A measurement of − 505 mg/L acidity for the Anna system immediately after the substrate replacement is not shown
) for the a HD and b Anna systems. The vertical lines indicate the substrate replacement events. A measurement of − 505 mg/L acidity for the Anna system immediately after the substrate replacement is not shown