Abstract
Treatment evaluations were performed at four Pennsylvania hydrated lime treatment sites and one quicklime treatment site designed to remove ferrous iron from underground coal mine drainage. Two of the sites included a pretreatment decarbonation step to exsolve CO2(aq) and reduce hydrate consumption due to hydroxylation and calcite formation. Decarbonation reduced the daily hydrated lime dose by 22 % at one site and 28 % at the other. Field-measured CO2 mass transfer coefficients were determined for both decarbonation systems. CO2 mass transfer modeling predicted that Ca(OH)2 use would be reduced by an additional 19 and 28 % at these sites if the decarbonation systems were optimized. Hydroxylation of CO2 species and calcite formation consume between 40 and 90 % of the Ca(OH)2 dose. In terms of cost, more money is being spent on consumption due to hydroxylation and calcite formation than on removing the targeted parameter, ferrous iron. These processes can be minimized by improving decarbonation and oversizing ferrous reactor tanks to lower treatment pH.
Zusammenfassung
Die Auswertung der Behandlung mit Kalkhydrat zur Entfernung von zweiwertigem Eisen aus den Drainagewässern des Untertagekohlebergbau fand an vier Standorten in Pensylvania statt. Zwei Behandlungsanlagen beinhalteten eine Entkarbonisierungsstufe um CO2(aq) auszustrippen und damit den Hydratverbrauch durch Hydroxylierung und Calcitbildung zu senken. Durch die Entkarbonisierung konnte der Kalkhydratverbrauch in der einen Anlage um 22 % und in der anderen Anlage um 28 % gesenkt werden. Anhand vor Ort gemessener CO2-Werte wurden Massentransferkoeffizienten für beide Systeme zur Entkarbonisierung bestimmt. Die Modellierung des CO2-Massentransfers hat vorausgesagt, dass der Verbrauch an Ca(CO)2 um weitere 19 und 28 % durch die Optimierung der Entkarbonisierungsstufe gesenkt werden kann. Die Hydroxylierung der CO2-Spezies und die Calcitbildung zehren zwischen 40 und 90 % der Kalkhydratdosis auf. In Bezug auf die Kosten wird mehr Geld für das Kalkhydrat durch die Hydroxylierung und die Calcitbildung als für die Entfernung des Zielparameters (zweiwertiges Eisen) ausgegeben. Die Prozesse können durch eine Verbesserung der Entkarbonisierung und eine Überdimensionierung der Reaktionstanks zu einem niedrigeren pH-Wert minimiert werden.
Resumen
Las evaluaciones de tratamiento fueron realizadas en Pennsylvania en 4 sitios en los cuales se llevan a cabo tratamientos con lima hidratada y en un sitio donde se aplica tratamiento con cal viva diseñada para la remoción de hierro ferroso en el drenaje de una mina subterránea de carbón. Dos de los sitios incluyen un paso de pretratamiento de descarbonatación para separar CO2(ac) y reducir el consumo de hidrato debido a la hidroxilación y formación de calcita. La descarbonatación redujo la dosis diaria de lima hidratada en 22 % en uno de los sitios y 28 % en otro. Los coeficientes de transferencia de masa de CO2 medidos en campo fueron determinados para los dos sistemas de descarbonatación. El modelo de transferencia de masa de CO2 predijo que habría una reducción adicional de 19 y 28 % Ca(OH)2 en estos sitios si los sistemas de descarbonatación se optimizaran. La hidroxilación de especies de CO2 y la formación de calcita consume entre 40 y 90 % de la dosis de Ca(OH)2. En términos de costos, se gasta más dinero en el consumo debido a la hidroxilación y la formación de calcita que en la remoción del hierro ferroso. Estos procesos pueden ser minimizados mejorando el proceso de descarbonatación y sobredimensionando los tanques para el tratamiento de ferroso a menores valores de pH.
摘要
为评价井工煤矿排放废水中亚铁离子去除工艺的熟灰岩消耗量,本文研究了宾夕法尼亚4个熟石灰处理厂和1个生灰处理厂的熟石灰消耗特征。两个处理厂的处理系统包含了脱碳酸预处理和CO2(aq)出溶工艺,以减小羟化作用和碳酸钙生成引起的额外熟石灰消耗量。脱碳酸作用分别降低了两个处理厂每日熟石灰消耗量的22%和28%。同时,野外测量了两个处理系统的CO2质量转移系数。CO2质量转移预测模型表明,在优化脱碳酸系统以后,这些废水处理系统的熟石灰消耗量还可再减少19%和28%。CO2羟化作用和碳酸钙生成作用消耗了40%和90%的熟石灰。从处理成本角度分析,花费在CO2羟化作用和碳酸钙生成上的成本远比亚铁离子去除的直接成本高得多。该过程可通过提高脱碳酸效率和扩大亚铁离子反应池及降低pH值来实现。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrated lime [Ca(OH)2] is the most common alkaline reagent used to treat underground coal mine drainage (UCMD) in northern Appalachia according to the U.S. Office of Surface Mining’s 2000 annual report (Office of Surface Mining 2000). Typically, these pumped discharges range in flow from 5.6 to 38 million liters/day and ferrous iron [Fe(II)] concentrations range from 10 to over 500 mg/L. Hydrated lime is routinely selected because of the favorable cost per metric ton of alkalinity compared to other common alkaline reagents like caustic soda (NaOH), soda ash (Na2CO3), and magnesium hydroxide [Mg(OH)2]. However, focusing on chemical cost often creates an assumption that equates low chemical cost to a cost-effective and optimized treatment plant. Optimized treatment occurs when process chemistry achieves effluent parameter concentrations specified in a discharge permit while minimizing nuisance mineral precipitation as well as other hydroxyl consumptive processes. Most UCMD treatment systems in northern Appalachia are treating circumneutral pH mine water in which Fe(II) is the contaminant of concern. Applicable discharge criteria typically include an effluent pH of 6–9 and total iron <3.0 mg/L. The most common treatment approach involves pumping ferruginous mine water to the surface and dosing with hydrated lime to increase pH until insoluble Fe(OH)2 and Fe(OH)3 forms. A treatment strategy that is solely based on how pH relates to Fe(II) solubility may not optimize hydrated lime use if pH adjustment causes or intensifies avoidable hydroxyl-consuming reactions. These additional reactions can cause increased operation costs by increasing hydrated lime consumption and sludge production.
This paper quantifies and describes hydrated lime and calcium oxide (CaO) consumption at five treatment plants treating UCMD in Pennsylvania. First, a summary of each site is presented. Second, background information on hydrated lime and how it is consumed in mine drainage is presented. Third, measured and theoretical dosing requirements are compared to identify consumption reactions and compute chemical use efficiency amongst the sites. Lastly, treatment strategies that gave improvements in chemical use efficiency are presented to suggest ways to increase hydrated lime efficiency at other sites.
Site Descriptions
Site Descriptions and Treatment Configurations
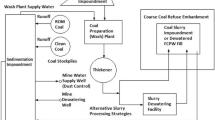
The five treatment plants contained varying treatment components, processes, and configurations. Table 1 shows treatment site location and operator information, and Tables 2 and 3 provide treatment system details. Noteworthy differences between treatments plants include onsite slaking, decarbonation, and high density sludge process. Every site, except Mine 31, purchased dry hydrated lime and every site but Lancashire used treated effluent water to prepare a hydrate slurry onsite. Mine 31 purchases calcium oxide and used an on-site slaker system to produce calcium hydroxide slurry. Lancashire pumped water that was low in total dissolved solids (TDS) from an adjacent mine pool for slurry make-up water.
Unlike the other sites, Lancashire and Renton included a decarbonation step before hydrate addition. Surface aerators are used to agitate and exsolve CO2(aq) from the untreated UCMD prior to the addition of an alkali reagent to reduce CO2-based acidity (Jageman et al. 1988). Decarbonation also affords control of effluent alkalinity concentrations and reduces CaCO3 formation. Lastly, Mine 31 and Lancashire use a sludge densification process developed by Bethlehem Steel (Bosman 1974; Haines and Kostenbader 1970; Kostenbader and Haines 1970), which involves pumping accumulated sludge to a conditioning tank where it is mixed with Ca(OH)2 slurry before being discharged to the reaction tank to react with the UCMD. This conditioning step adjusts the surface charge on FeOOH precipitates, resulting in improved precipitate particle growth and a less hydrated sludge. In addition, the recirculated solids act as seed particles, promoting precipitation and particle agglomeration (Yong et al. 2005). Sludge densities of up to 30 wt% can be achieved (Zick et al. 1999). Several investigators have reported the recirculation and conditioning process reduces Ca(OH)2 consumption, but did not identify the mechanisms or provide analysis (Kostenbader and Haines 1970; Zick et al. 1999).
Background
Physical and Chemical Characteristics of Hydrated Lime
Hydrated lime, Ca(OH)2, is manufactured as a 90+ % pure powder (<1–45 μm) in a two-step process. The first step involves calcining high-Ca content limestone at temperatures in kilns between 1,000 and 1,300 °C (above the theoretical calcination temperature of 851 °C) for rapid decomposition and evolution of CO2.
The second step involves hydrating the quicklime (CaO) to form Ca(OH)2. The conversion of CaO–Ca(OH)2 is referred to as “hydration” when water is added in stoichiometric amount to produce a dry or very low (1–2 wt%) moisture finished product. The term “slaking” is commonly used to describe hydration using an excess of water to produce a pumpable lime slurry. In most CMD treatment situations, a commercially available treatment reagent, dry hydrate, is used; however, treatment plant operators can opt to slake quicklime on site using a slaker or purchase manufactured lime slurry (e.g. milk of lime).
The use of hydrated lime is more common on mine sites, as slaking requires a high degree of process control to produce highly reactive Ca(OH)2 slurry, and commercially available hydrated lime is produced to have highly reactive particles. Also, slakers contribute to site capital and maintenance cost, and manpower requirements. Quicklime must hydrate before dissolution occurs (Boynton 1980); efficient slaking depends on several factors, including the slaking temperature and duration, CaO–H2O ratio, and the water quality used for slaking. Uncontrolled and direct application of CaO–CMD results in incomplete hydration and highly inefficient use of CaO.
Some CMD treatment plants simply dry feed hydrate directly into the CMD; however, most plants produce Ca(OH)2 slurry before mixing with the CMD. Advantages of Ca(OH)2 slurry production include the ability to pump the hydrate to mixing tanks and produce saturated and readily-reactive alkaline water for efficient neutralization. Other advantages of Ca(OH)2 slurry include deagglomeration, increased surface area of the hydrate particles, and better dose rate control. Slurrying hydrated lime is a practical way to produce an alkaline treatment stream since the solubility of Ca(OH)2 in water is low at 1.6 g/L at 20 °C (Wiersma and Nekami 1986). The dissolved aqueous neutralization capacity of slurry is 2,100 mg/L as CaCO3 based on solubility; however, the remaining neutralization capacity is derived from suspended Ca(OH)2 particles contained in the slurry. Dissolution rates of Ca(OH)2 particulates in relatively dilute water ranges from 5 to 80 s, depending on particle size and aqueous chemistry (Johannsen and Rademacher 1999; Van Dijk and Wilms 1991). Dissolution inefficiencies may arise if slurry particulates become encapsulated by metal hydroxide precipitates when the slurry and CMD are mixed.
Evolution and Chemical Characteristics of UCMD
CMD from flooded underground coal mines is notably different than other Pennsylvania CMD. CMD originating from surface mines or minimally-flooded underground mines is typically low pH with varying concentrations of Fe(III), Fe(II), Mn(II), and Al(III). Pyrite oxidation liberates iron and acidity, which dissolves Al(III) from clay-rich strata that underlie the coal. In Pennsylvania, elevated Mn(II) is common in surface mine drainage but is less common UCMD since manganous minerals are mostly associated with overburden.
Since the passage of the Surface Mining Control and Reclamation Act of 1977, underground coal mines are designed to completely flood after closure. Flooding causes a geochemical evolution of mine drainage that is distinctly different from surface mines or partially-flooded underground mines. Infiltration of alkaline groundwater results in varying degrees of in situ treatment, minimizes mine atmosphere development, and minimizes pyrite oxidation. These processes produce UCMD with a circumneutral pH and elevated Fe(II).
Circumneutral pH UCMD can be either net acidic or net alkaline. Net acidic UCMD occurs if the equivalence of proton-donating species is greater than proton-consuming species when the water equilibrates with atmospheric conditions. When equilibrated to ambient atmospheric conditions,the pH decreases for net acidic waters while pH is likely to increase for net alkaline water as a result of CO2(aq) exsolution (Geroni et al. 2012). Since Fe(II) is the main acid-producing pollutant in UCMD, net acidic conditions will occur if Fe(II) concentrations (mg/L) are 1.9 times larger than HCO3 − concentrations (mg/L as CaCO3).
Hydrated lime is often selected to treat both net alkaline and net acidic waters, but for different reasons. Hydrated lime is added to net acidic UCMD to buffer against pH drop when Fe(II) oxidizes and precipitates. Hydrated lime is added to net alkaline UCMD to increase the rate of Fe(II) precipitation to minimize the size of the treatment footprint. In either case, UCMD is typically dosed with Ca(OH)2 until a pH of 8.3–8.5 is achieved. In this pH range, Fe(II) is removed by two different mechanisms occurring simultaneously. The rapid increase in pH causes most of the Fe(II) to be removed as Fe(OH)2, as indicated by the formation of green precipitate. However, Fe(II) concentrations in equilibrium with Fe(OH)2 can still exceed 20 mg/L because of the increased solubility caused by \( {\text{FeHCO}}_{{3({\text{aq)}}}}^{ + } \) and FeCO3(aq) complexing. The remaining Fe(II) is reduced to compliance concentrations by rapid oxidation due to the increased pH. Using rate constants reported by Dempsey et al. (2001), kinetic modeling predicts the dissolved Fe(II) concentration is reduced by half in <10 s at pH 8.5. Iron effluent standards are routinely achieved by dosing to pH 8.5 because of the combination of Fe(OH)2 formation and rapid Fe(II) oxidation removal mechanisms.
Hydrated Lime Consumption in UCMD
The Ca(OH)2 requirement to achieve a desired target treatment pH is a function of the total hydroxyl-consuming reactions that occur when the pH is adjusted. Identifying the reactions responsible for hydroxyl consumption is important for predicting hydrated lime requirement and development of treatment strategies to reduce avoidable consumption. Common hydroxyl-consuming reactions encountered when treating UCMD include Fe(II) removal, hydroxylation of aqueous species, and CaCO3 precipitation.
Fe(II) Removal
Equations (3) and (4) show the two Fe(II) removal mechanisms that occur simultaneously in the pH range of 8.3–8.5 during treatment with hydrated lime. Equation (3) describes the formation of ferrous hydroxide and Eq. (4) provides the overall reaction for oxidation of Fe(II) and subsequent precipitation of ferric hydroxide. In either case, the molar ratio of Fe(II)–OH− is equivalent; both reactions consume the same amount of hydrated lime. Figure 1 shows the pH-dependent solubility for both iron hydroxides and that iron concentrations will be below 1.0 mg/L at a treatment pH of 8.5 if ferric hydroxide formation controls iron solubility.
Hydroxylation of Aqueous Species
Hydroxylation is defined herein as the reaction of hydroxyl ion (OH−) with aqueous species to form water and other aqueous species. For example, as Ca(OH)2 dissociates in solution, hydroxylation of anions, cations, and aqueous complexes occur as represented in Eqs. (5)–(8).
Hydroxylation of anion:
Hydroxylation of cation:
Hydroxylation of aqueous complexes:
Generally, the hydroxylation of anions and aqueous complexes yields water, whereas the hydroxylation of cations yields hydroxyl complexes. Hydroxylation of CO2-based species is a significant source of hydroxyl consumption when pH is adjusted to 8.5 in UCMD treatment systems. Hydrated lime consumption from hydroxylation of aqueous species can be computed by using aqueous speciation modeling to predict changes in species concentrations as pH is increased by hydrated lime addition.
CaCO3 Formation
Two mechanisms cause calcite (CaCO3) formation in Ca(OH)2 treatment systems. Both mechanisms increase the hydrated lime requirement by consuming OH− to precipitate CaCO3. In the first mechanism, termed “dissolve-precipitate”, hydrated lime dissolves into mine drainage and increases [Ca2+] and [OH−] until the solution becomes supersaturated and induces CaCO3 precipitation. Elevated aqueous CO2 species concentrations in UCMD can cause CaCO3 precipitation to occur at a treatment pH as low as 7. Equation (9) shows that CaCO3 precipitation will buffer against pH increase.
The other mechanism, termed recarbonation, occurs when hydrated lime slurry particulates adsorb CO2(aq) to create a CaCO3 shell around hydrate particulates before they can completely dissolve (Wiersma and Nekami 1986).
Sampling Methods
Aqueous and Solids Sampling
The sampling techniques used at the study sites included collection of aqueous and solid materials. Field alkalinity was determined on filtered (0.45 μm) samples using a Hach digital titration kit. Field pH and temperature were measured using a calibrated Hanna HI98107 meter.
Raw influent, final effluent, and samples from each major process unit were obtained at all of the treatment sites for laboratory analysis. Less complex treatment systems at the Russellton and Banning sites required only three sampling stations, an influent sample, a sample at the mixing tank outfall, and the final effluent after clarification. The Renton site required one additional sampling station, as this facility employed a decarbonation process before lime treatment. The Mine 31 facility uses a sludge recirculation process; therefore, additional sample locations were needed to evaluate the impact of recirculating sludge in the treatment process. The most complex of the study sites was the Lancashire 15 facility, which uses a dense sludge recirculation process, a pre-aeration process, and a separate source water for lime slurry makeup; it required six sampling stations in order to characterize the treatment process.
Unfiltered and filtered water samples were collected and preserved on ice with metals samples acidified and shipped by overnight courier for analysis by the Pennsylvania Department of Environmental Protection, Bureau of Laboratories. Total inorganic carbon (TIC) samples were collected by completely submerging and filling 40 mL glass bottles containing a septa. The samples were placed on ice and analyzed within 24 h. Laboratory aqueous chemistry was determined using inductively coupled plasma mass spectrometry, and ion chromatography. Analysis included pH, specific conductance, TDS, suspended solids, alkalinity, hot acidity, TIC, and total and dissolved determinations of all major cations and anions. Cations analyzed included aluminum, calcium, iron, potassium, magnesium, manganese, sodium, silica, and zinc. Anions included sulfate, chloride, and bicarbonate. These parameters permitted QA/QC checks of each sample by means of cation anion balance. Field and laboratory data are presented in Table 4.
Solid samples of the dry treatment reagent, the produced hydrate slurries, and the resulting fresh treatment sludge were collected at each facility. Sludge samples were obtained and dried by a moisture balance instrument at no more than 125 °C. This drying temperature and short duration of drying (<1 h) ensures preservation of calcium hydroxide, carbonate, and oxide species. Immediately after drying, the sample was placed in air-evacuated containers and analyzed within 24 h of collection by the Carmeuse Lime and Stone Technology Center laboratory facilities in Pittsburgh, Pennsylvania. Additional sludge samples were collected by the same methodology and transported to the Pennsylvania Geological Survey’s Lab in Harrisburg for X-ray diffraction analysis using a PANalytical Empyrean x-ray diffractometer. Interpretation was carried out using a PANalytical HighScare Plus solftware using the PDF-4 database published by the International Centre for Diffraction Data. The Carmeuse lab facility also performed elemental analysis by X-ray fluorescence (XRF), inductively coupled plasma (ICP) and thermogravimetric analysis (TGA) of the dry reagents, sludges, and liquors.
Hydrate Consumption Analysis
Dosing Measurements and Quantifying Ca(OH)2-Consuming Reactions
Dosing rates were measured by quantifying the mass increase in total [Ca] between the influent and effluent of the reactor tanks. Computed dosing rates were adjusted to reflect the purity of Ca(OH)2 measured at site. Measured dosing rates agreed with plant records for all sites. At Lancashire, the dose rate was measured from plant usage records.
The amount of Ca(OH)2 consumed by Fe(II) removal, hydroxylation reactions, and CaCO3 formation was quantified at each site. Hydrate consumption due to Fe(II) precipitation was computed by measuring the reduction in dissolved Fe(II) that occurred in each of the reactor tanks. Both of the Fe(II) removal mechanism consume identical equivalents of hydroxyl [Eqs. (3, 4)]. Hydrate consumption due to dissolved Fe(II) precipitation was computed in mg/L:
Aqueous speciation modeling was used to compute hydrate consumption due to hydroxylation reactions. Geochemist Workbench (Bethke 2008; Bethke and Yeakel 2012) software was used to speciate both the untreated CMD entering the reactor tank and the treated water leaving the reactor tank. Changes in concentrations of aqueous species undergoing hydroxylation between the two sampling points were noted and the equivalent amount of Ca(OH)2 addition required to provide the OH− for hydroxylation was calculated. For example, speciation modeling shows hydrate addition in the reactor tank causes a decrease of H2CO3 and an increase in HCO3 − and CO3 2− concentrations as the water equilibrates to the greater pH. Concentrations of complexes, like CaCO3(aq), also increase. The Ca(OH)2 dose required for the increase in species like HCO3 − and CaCO3(aq) can be computed from the following relationship:
The total Ca(OH)2 consumption due to hydroxylation was computed by tracking changes in modeled concentrations for the 19 species prone to hydroxylation reactions.
Hydrate consumption due to calcite formation was determined by identifying differences in total and dissolved TIC concentrations between the reactor tank influent and effluent. TIC was determined by both collecting total and dissolved samples and by computing from field-measuring pH, temperature, and total and dissolved alkalinity. The differences between total and dissolved TIC in the reactor effluent were interpreted as calcite formation that occurred in the reactor tank.
Validation of Consumption Analysis Using Alkalinity Accounting
The hydrated lime dose was measured by conducting a mass balance analysis of [Ca] between the influent and effluent of the reactor tank. Identifying the geochemical pathway of the hydrated lime consumption was indirectly determined by tracking the changes in hydroxyl-consuming species, speciation modeling, and water samples between the influent and effluent of the reactor tank. System understanding was validated by performing a mass balance accounting of the alkalinity inputs and consumption within the reactor tank. The alkalinity inputs consist of the influent alkalinity of the untreated CMD and that from hydrate dosing. Alkalinity consumption is caused by the hydroxyl-consuming reactions noted above. Subtracting the alkalinity consumption from the alkalinity inputs was used to predict the effluent alkalinity concentration from the reactor tank. Agreement between computed and measured effluent alkalinities validates an understanding of the consumptive processes.
Additional Evaluation Metrics
In the USA, industrial discharges are regulated by the National Pollutant Discharge Elimination System (NPDES). Efficient treatment is achieved when chemical consumption solely targets NPDES effluent criteria and avoids nuisance consumption reactions and additional sludge production. A metric, termed NPDES Treatment Efficiency, was computed to compare Ca(OH)2 use among the treatment plants and against other treatment chemicals. The metric was computed by comparing Ca(OH)2 consumption due to Fe(II) removal [Eq. (13)] with total measured consumption:
The performance of the decarbonation step used at Lancashire and Renton was evaluated by calculating the rate of CO2(aq) mass transfer from the liquid to gas phase (Stumm and Morgan 1995):
K L and a were combined to compute a bulk mass transfer coefficient (\( {\text{K}}_{{{\text{LCO}}_{2} ,{\text{a}}}} \)) for the surface aerator/tank system. Values for \( {\text{K}}_{{{\text{LCO}}_{2} ,{\text{a}}}} \) were calculated by measuring field pH and temperature and collecting TIC and aqueous chemistry samples at the influent and effluent of the decarbonation tank. Geochemist Workbench (Bethke and Yeakel 2012) was used to speciate the samples to quantify the decrease in CO2(aq) concentrations across the tank. Values for CO2(aq)atm were modeled by equilibrating the raw water with a partial pressure of CO2 of 10−3.4 atm to simulate ambient atmosphere conditions. A differentiated form of Eq. (17) was used to model the decarbonation systems as continuous stirred reactors.
Results and Discussion
All untreated water was undersaturated with respect to calcite (SIcalcite <0) (Table 3) and evolved to supersaturated conditions after Ca(OH)2 dosing. Field application of HNO3 to fresh precipitate affirmed carbonate formation at all sites. Furthermore, calcite was the only mineral identified by XRD analysis of fresh precipitate at all sites.
The computed Ca(OH)2 consumption due to the different reactions is shown in Table 4 and the corresponding daily chemical cost is shown in Table 5. Table 4 shows that treatment of the Renton drainage required the highest Ca(OH)2 dose (1,066 mg/L) but the drainage also contained the highest initial concentrations of Fe(II) and TIC. Interestingly, Lancashire had the lowest treatment dose (131 mg/L) but contained third highest concentration of Fe(II) and the lowest concentration of TIC, which illustrates the control that TIC has on Ca(OH)2 consumption. TIC concentrations control calcite formation and also directly influence hydroxylation of carbonate species, so it is not surprising that 420 mg/L of Renton’s Ca(OH)2 dose was consumed by hydroxylation and calcite reactions, compared to 84 mg/L at Lancashire. Over 90 % of the Ca(OH)2 dose at Banning was consumed by hydroxylation and calcite formation reactions. In terms of cost, every site but Renton spent more money on calcite formation and hydroxylation reactions than on the target parameter, Fe(II). These results show the importance of developing a treatment strategy that minimizes calcite formation and hydroxylation when treating UCMD.
Table 6 provides the results of the alkalinity accounting method that was used to validate the consumption analysis. Table 6 shows that the calculated effluent alkalinity [Eq. (16)] was over-predicted for all sites but Mine 31. The good agreement between calculated and measured alkalinity provides confidence in identifying and modeling the Ca(OH)2-consuming reactions for Lancashire, Russellton, and Banning. Mine 31 had poor agreement with a predicted effluent alkalinity of −18 mg/L, as Ca(OH)2. There are two plausible causes that can explain the discrepancy, with the first being errors in field or laboratory measurements. Another plausible explanation is to assume the predicted alkalinity of −18 mg/L is correct. This would indicate the existence of an additional alkaline source that was not considered in the alkalinity accounting validation. The additional source could be the calcite contained in the recirculated sludge that is part of the high-density sludge process employed at Mine 31. XRD analysis of the sludge confirmed the presence of calcite and mass balance calculations for Mine 31 predict that 1,432 mg/L of calcite was being recirculated, within the sludge, and remixed with the untreated water in the reactor tank. If calcite dissolution is contributing to treatment, then sludge recirculation reduced Ca(OH)2 dosing by 1.08 metric t/day at the Mine 31 site and constitutes 20 % of the alkalinity requirement. Table 5 does not suggest that dissolution of recirculated calcite occurred at the Lancashire site since the alkalinity discrepancy is only 5 mg/L. It is hypothesized that sludge recirculation to lessen consumption would be more beneficial for low pH CMD.
Table 6 shows the measured Ca(OH)2 dose for Renton and Mine 31 was less than the computed Ca(OH)2 consumption. From this standpoint, these treatment systems were efficient, in that they used influent alkalinity to lessen their dosing requirement for treatment.
Table 7 shows the calculated NPDES treatment efficiency [Eq. (16)] for each facility. This metric compares the theoretical dose required to treat the NPDES target parameter to the actual dose, which includes consumption due to nuisance mineral precipitation and treatment inefficiencies. Table 7 shows that 60 % of the dose at Renton neutralizes Fe(II) acidity compared to only 7 % at Banning. The high NPDES treatment efficiency at Renton is due to the high Fe(II) concentration and the benefit of decarbonation, which lowers nuisance consumption. At Banning, Ca(OH)2 consumption due to hydroxylation and calcite formation was 11 times greater than consumption due to Fe(II) removal. NPDES treatment efficiencies were similar for Banning and Russellton even though Russellton dosed dry hydrate into the CMD and had no mechanical mixing step for reacting the hydrate with the drainage. The low NPDES treatment efficiencies in Table 7 for Lancashire, Russellton, and Banning indicate that alternative treatment processes could improve efficiency and lower costs.
Benefit of the Decarbonation Step
Lancashire and Renton incorporated a decarbonation step to exsolve CO2(aq) and reduce chemical consumption. Decarbonation at Lancashire consisted of a 556.3 kL tank with a 22.38 kW (30 hp) WesTech Landy 7 surface aerator configured as a continuous stirred reactor. Renton had a 260.8 kL tank with an 18.65 kW (25 hp) surface area that was also configured as a continuous stirred reactor. Influent and effluent data for the decarbonation tank (Table 3) were collected and showed CO2(aq) was reduced by 52 % at Lancashire and 68 % at Renton. The daily cost to operate the aerators was 40 US$. Without the decarbonation step, daily chemical costs would increase due to additional hydroxyl consumption from hydroxylation of carbonate species (Eqs. 5, 6). Geochemical modeling predicted the Ca(OH)2 dose would have increased from 131 to 183 mg/L at Lancashire and from 1,066 to 1,359 mg/L at Renton.
Bulk Gas Transfer Coefficients
Equation 17 was used to compute bulk gas transfer coefficients (\( {\text{K}}_{{{\text{LCO}}_{2} ,{\text{a}}}} \)) for the decarbonation system at Lancashire (5.2 × 10−4 s−1) and Renton (8.23 × 10−4 s−1). Since both decarbonation systems were configured as continuous stirred reactors, decarbonation was evaluated by simulating effluent CO2(aq) as a function of tank volume (Fig. 2). Both sites contained substantial CO2(aq) concentrations post decarbonation (Table 8). Figure 2 shows that increasing the decarbonation tank volume from 260 to 412 kL at Renton would decrease the effluent CO2(aq) from 143 to 103 mg/L. This would decrease the Ca(OH)2 dose at Renton by 33 mg/L, thus reducing the daily hydrate cost by 50 US$. The increased capital cost for the additional tank volume would result in a payback in <4 years from the Ca(OH)2 savings. At Lancashire, increasing the tank volume from 556 to 812 kL would decrease the Ca(OH)2 dose by 10 mg/L. Considering the 15,519 L/s flow rate, the dose reduction would decrease the chemical cost by $40/day and the payback for the increased tank volume would be 7.3 years.
Strategies to Reduce Ca(OH)2 Consumption
A major focus of this study was to identify treatment processes that would lower Ca(OH)2 consumption while preserving NPDES compliance. The results show hydroxylation and calcite formation consume significant amounts of Ca(OH)2. These processes can be minimized by improving decarbonation and reducing treatment pH. Other strategies to reduce Ca(OH)2 consumption include proper selection of calcium reagents and proper slurrying, slaking, storing, and mixing practices.
Decarbonation
Only two of the five treatment plants used decarbonation, even though every site contained elevated TIC concentrations. Recognition of the effect that TIC has on Ca(OH)2 consumption through hydroxylation reactions and calcite formation is necessary to understand the benefit of decarbonation. The decarbonation analysis for Lancashire and Renton show the importance of properly selecting the optimum tank volume when configured as a continuous stirred reactor. Decarbonation simulations using the calculated \( {\text{K}}_{{{\text{LCO}}_{2},{\text{a}}}} \) showed that both sites would have achieved a greater financial benefit from increased decarbonation.
Treatment pH
Treatment pH controls the amount of hydroxylation and calcite formation. Conventional treatment to a pH of 8.5 to precipitate Fe(OH)2 produces a supersaturated calcite condition. Calcite precipitation can occur if the treatment pH exceeds the pH shown in Table 9.
The low NPDES treatment efficiency calculated for Lancashire, Banning, and Russellton indicates that the sites may benefit by lowering treatment pH or using other strategies to increase treatment efficiency. One strategy to decrease hydrate consumption would be to design a treatment system that would use Fe(II) oxidation as the primary Fe(II) removal mechanism, as opposed to Fe(OH)2 precipitation. Reaction tank size would be based on the time required to oxidize Fe(II) at a pH that will prevent or minimize calcite precipitation. For example, Lancashire exhibited a treatment pH of 8.5 with a 30 min retention time in the reaction tank. Homogenous Fe(II) oxidation rate constants presented by Stumm and Morgan (1995) predict that Fe(II) removal would be achieved if the reaction tank was increased to 60 min of retention time at a treatment pH of 7.7. Employing this strategy of adding hydrate to increase the pH to accelerate Fe(II) oxidation while staying below calcite saturation could reduce Ca(OH)2 consumption by 38 mg/L (150 US$/day) at Lancashire (Table 5).
Conclusions
The fate of hydrated lime in mine drainage treatment was established at five study sites. The results showed that calcite formation and hydroxylation of CO2 species were responsible for 40–90 % of the hydrated lime dosage. The decarbonation step used at Lancashire and Renton reduced the Ca(OH)2 dosage by 28 and 22 %, respectively, but appreciable CO2(aq) remained after decarbonation. Modeling using the field-measured CO2(aq) mass transfer coefficients showed that Lancashire and Renton would have benefited from larger decarbonation tanks to further reduce CO2(aq). The results show decarbonation optimization and treatment pH control is an important factor when designing UCMD treatment plants
References
Bethke CM (2008) Geochemical and biogeochemical reaction modeling, 2nd edn. Cambridge University Press, Cambridge
Bethke CM, Yeakel S (2012) The geochemist’s workbench user’s guide, version 9.0. Aqueous Solutions LLC, Champaign
Bosman DJ (1974) The improved densification of sludge from neutralized acid mine drainage. J S Afr Inst Min Metall 74:340–348
Boynton RS (1980) Chemistry and technology of lime and limestone, 2nd edn. Wiley, New York
Dempsey BA, Roscoe HC, Ames R, Hedin R, Jeon B (2001) Ferrous oxidation chemistry in passive abiotic systems for the treatment of mine drainage. Geochem Explo Environ Anal 1(1):81–88
Geroni JN, Cravotta CA, Sapsford DJ (2012) Evolution of the chemistry of Fe bearing waters during CO2 degassing. Appl Geochem 27:2335–2347
Haines GF Jr, Kostenbader PD (1970) High density sludge process for treating acid mine drainage. In: Proceedings of the 3rd symposium in coal mine drainage research, Mellon Institute, Pittsburgh, PA, USA, pp 12–26
Jageman TC, Yokley RA, Heunisch HE (1988) The use of pre-aeration to reduce the cost of neutralizing acid mine drainage. In: Proceedings of the mine drainage and surface mine reclamation, USBM, Pittsburgh, PA, USA, pp 131–135. http://www.asmr.us/Publications/Conference%20Proceedings/1988%20papers/Jageman%20131-135.pdf
Johannsen IK, Rademacher S (1999) Modelling the kinetics of calcium hydroxide dissolution in water. Acta Hydrochim Hydrobiol 27:72–78
Kostenbader PD, Haines GF (1970) High density sludge treats acid mine water. Coal Age 75(9):90
Office of Surface Mining (2000) Pennsylvania annual report (unpublished report)
Stumm W, Morgan J (1995) Aquatic chemistry, 3rd edn. Wiley, New York
Van Dijk JC, Wilms DA (1991) Water treatment without waste material—fundamentals and state of the art of pellet softening. J Water Supply Res Technol 40:263–280
Wiersma DJ, Nekami B (1986) Water softening with pellet reactors. In: Proceedings of the 6th International Lime Congress, London, UK, pp 1–12
Yong GW, Selomulya C, Tapsell G, Amal R (2005) Densification of iron (III) sludge in neutralization. Int J Miner Process 76:149–162
Zick RL, Leon MH, Finn DC (1999) Dense-sludge process for reduced AMD sludge disposal. Min Eng 51:46–50
Acknowledgments
The authors express their sincere thanks and appreciation to the owners and operators at each of the facilities included in the study: the Renton plant operators, Don and Mike Charlton of AMD Industries, Inc., the operators at the Banning and Russellton plants, Larry Neff of Pristine Resources at the Bethlehem Mine 31 plant, and Dennis Lloyd of Lloyd Environmental Services at the Lancashire 15 plant. All of these individuals provided full access to their facilities and greatly assisted the authors by graciously sharing their insights and vast experience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Means, B., Beam, P.G.R. & Mercer, J. Analysis of Hydrated Lime Consumption in Circumneutral Underground Coal Mine Drainage Treatment. Mine Water Environ 34, 10–19 (2015). https://doi.org/10.1007/s10230-014-0308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-014-0308-2