Abstract
Zeolite-bearing tuff (stilbite) was shown to be an effective and efficient adsorbent for treating acid mine drainage (AMD). We tested the adsorption of zinc ions from a synthetic acid aqueous solution and AMD from the Sasa mine. The concentration of zinc ions in the AMD decreased from 2.219 to 0.564 mg/dm3 (74% removal). The solution pH, prior to treatment with the zeolite-bearing tuff, was 3.90, and following treatment, it was 5.36. The physical and chemical properties of the tuff were characterized by X-ray diffraction, scanning electron microscopy, and energy dispersive spectroscopy. The concentrations of dissolved metal ions before and after treatment was determined by AES—ICP. The tuff’s maximum capacity was determined in equilibrium studies. Experimental data were fitted to adsorption models; the best fit was obtained with the Freundlich adsorption isotherm.
Zusammenfassung
Es wurde gezeigt, daß Zeolith (Stilbit) -führender Tuff ein effektives und wirksames Adsorbens zur Behandlung von saurem Bergbauwasser ist. Wir untersuchten die Adsorption von Zinkionen aus einer synthetischen Säurelösung und aus saurem Bergbauwasser der Sasa Mine. Die Konzentration der Zinkionen in dem sauren Bergbauwasser sank von 2.219 auf 0.564 mg/dm3 (74% Reduktion). Vor der Behandlung mit dem Zeolith-führendem Tuff hatte die Lösung pH 3.90, danach pH 5.36. Physikalische und chemische Eigenschaften des Tuffs wurden mittels Röntgendiffraktion, Rasterelektronenmikroskopie und Elektronenenergieverlustspektroskopie characterisiert. Die Konzentration der gelösten Metallionen vor und nach der Behandlung wurde mittels AES – ICP bestimmt. Die maximale Kapazität des Tuffes wurde durch Gleichgewichtsversuche bestimmt. Experimentelle Daten wurden mit Adsorptionsmodellen verglichen; die beste Passung entsprach einer Adsorptionsisotherme nach Freundlich.
Resumen
Se demostró que la toba que contiene zeolita (estilbita) es un adsorbente eficaz y eficiente para el tratamiento del drenaje ácido de la mina (AMD). Probamos la adsorción de iones cinc a partir de una solución ácida y de AMD de la mina Sasa. La concentración de cinc en la AMD disminuyó desde 2,219 a 0,564 mg/dm3 (74% de eliminación). El pH de la solución, antes del tratamiento, era 3,90 y después del tratamiento fue 5,36. Las propiedades físicas y químicas de la toba se caracterizaron por difracción de rayos X, microscopía electrónica de barrido y espectroscopía de energía dispersiva. Las concentraciones de iones metálicos disueltos antes y después del tratamiento se determinaron mediante AES-ICP. La capacidad máxima de la toba se determinó en estudios de equilibrio. Los datos experimentales se ajustaron a los modelos de adsorción; el mejor ajuste se obtuvo con la isoterma de adsorción de Freundlich.
抽象
含沸石凝灰岩(辉氟石)为处理酸性排放废水的有用和高效吸附剂。我们试验了含沸石凝灰岩去除合成酸性溶液和Sasa矿酸性废水(AMD)锌离子的吸附特性。AMD的锌离子浓度由2.219减小到0.564mg/dm3(去除率74%)。溶液pH值在含沸石凝灰岩处理前为3.9,处理后为5.36。通过XRD、扫描电子显微镜和能谱分析研究了凝灰岩的物理和化学特征。利用AES-ICP测试了溶溶在含沸石凝灰岩处理前后的金属离子浓度。通过平衡计算确定了含沸石凝灰岩最大吸附能力。试验数据最符合Freundlich吸附等温曲线。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining exposes mineral deposits containing pyrite, sphalerite, galena, or other sulphide minerals to weathering, often producing acid mine drainage (AMD). To meet environmental quality standards, zinc must be removed from polluted surface water and groundwater resources. Conventional methods typically involve such processes as coagulation, precipitation, ion-exchange, electrochemical methods, membrane processes, extraction, and adsorption. Some widely used adsorbents for metals include activated carbon (Kobya et al. 2005), clay minerals (Ammann 2003), biomaterials (Reddad et al. 2002; Sag and Aktay 2000), industrial solid wastes, and zeolites (Babel and Kurniawan 2003; Blanchard et al. 1984; Cabrera et al. 2005; Erdem et al. 2004; Motsi 2010; Pradeep et al. 2015; Taffarel and Rubio 2009; Zendelska et al. 2015).
Zeolites are crystalline micro-porous minerals that are broadly distributed in nature and are often used as absorbents. Natural zeolite is an attractive alternative for wastewater treatment because of its low cost (Cui et al. 2006) and high surface area, which in turn is due to its porous and rigid structure (Alvarez-Ayuso et al. 2003). Zeolites also act as molecular sieves and this property can easily be modified to increase its performance (Sprynskyy et al. 2006). Zeolites have a favourable cation exchange capacity (Yuan et al. 1999), high selectivity for cations (Malliou et al. 1994), good structural stability in acidic conditions, and can be easily regenerated. They can also have a buffering effect, in that they assist in neutralising acidic solutions via cation exchange (Leinonen and Letho 2001).
The objective of this research was to examine the potential treatment of AMD using a zeolite-bearing tuff from the Vetunica deposit in Macedonia. The zeolite-bearing tuff was characterised using classical chemical analysis, X-ray diffraction (XRD), and scanning electron microscopy with energy dispersive spectroscopy (SEM/EDS). The research was conducted using a synthetic acid aqueous solution and AMD from the Sasa lead and zinc mine (Makedonska Kamenica) in Macedonia. The Sasa mine is one of the largest production facilities on the Balkan Peninsula for lead and zinc ore extraction, flotation separation, and recovery of Pb and Zn concentrate. One of the Sasa mine’s negative effects on the environment is its AMD discharge.
Materials and Methods
Analytical Methods
The mineralogical structure of the zeolite-bearing tuff sample was studied using an X-ray diffractometer (Shimadzu 6100). The X-ray diffractometer was equipped with a Cu anode with a radiation wavelength of CuKα = 1.54178 Å. The operating voltage was U = 40.0 kV and current intensity I = 30.0 mA. Samples were examined within 10.0–80.0 with 2.0 s on each step in a controlled rotational mode at 60.0 rpm. The data were compared to the International Centre for Diffraction Data database, to identify the material in the solid samples.
The surface morphology of the sample was studied using a VEGA3 LMU SEM, which was fitted with an Inca 250 EDS system. An Agilent inductively coupled plasma atomic emission spectroscope (ICP-AES) was used to analyse the concentration of metal ions in solution before and after treatment. Based on material balance, the adsorption capacity was calculated by using the following expression:
where qe is the mass of adsorbed metal ions per unit mass of adsorbent (mg/g), Co and Ce are the initial and final metal ion concentrations (mg/dm3), respectively, V is the volume of the aqueous phase (dm3), and m is the mass of adsorbent used (g). The degree of adsorption, in percentage, is calculated as:
Experimental Procedure and Conditions
The rate of adsorption is a complex function of several factors including: the initial solution pH and concentration, mass of adsorbent, adsorbent particle size, temperature, flow rate in columns, and in the case of batch experiments, agitation speed. Overall reaction rate may be influenced by the separate or combined effect of these factors. In this study, some of these factors were investigated with regards to their effect on the efficiency of zeolite-bearing tuff in removing zinc from solution.
Contact time is one of the most important parameters that influence the adsorption process. For this reason, all experiments were performed within certain time intervals.
Adsorption of zinc ions on zeolite-bearing tuff was performed with different initial concentration (5, 25, 50, 200, and 400 mg/dm3) of synthetic single ion solutions of zinc ions, and AMD from the Sasa mine with an initial zinc concentration of 2.219 mg/dm3. Synthetic single component solutions of this metal were prepared by dissolving a weighed mass of the analytical grade salt ZnSO4·7H2O in 1 dm3 distilled water. The multi-component solution of the metals were prepared by dissolving a weighed mass of the analytical grade salts, CuSO4·5H2O, ZnSO4·7H2O, MnSO4·H2O, and Pb(NO3)2 in 1 dm3 distilled water.
The initial pH of the prepared solutions was adjusted by adding 2% sulphuric acid and controlled by a 210 Microprocessor pH meter. The initial pH of the tested solutions was 2.5, 3.5, and 4.5. The experiments were performed in a batch mode in a series of beakers equipped with magnetic stirrers using 2, 5, and 10 g of the tuff in 0.4 dm3 of zinc ions solution. A magnetic stirrer was used for agitation at 400 rpm, up to 360 min, at temperatures of 20 °C and 60 °C. The final pH was also measured. After a predetermined time, the suspension was filtered and the filtrate’s concentration of metal ions in solution was analysed by ICP-AES.
Equilibrium Studies
Equilibrium studies generally involve the determination of the adsorption capacity of a given material. This determination is important in accessing the potential of the material as an economic and commercially viable adsorber. The adsorption of zeolite-bearing tuff was predicted by fitting the experimental data to conventional adsorption mathematical models, namely the Freundlich and Langmuir models.
Langmuir Model
The Langmuir isotherm model (Langmuir 1918), based on monolayer coverage of adsorbent surfaces by the adsorbate at specific homogeneous sites within the adsorbent, is represented as:
where \({q_e}\), mg/g, is the amount of solute adsorbed per unit mass of adsorbent at equilibrium; \({C_e}\), mg/dm3, is the residual adsorbate concentration in solution at equilibrium; \({q_m}\), mg/g, is the amount of solute adsorbed per unit mass of adsorbent corresponding to complete coverage of available sites, \({K_l}\), dm3/mg, is the Langmuir adsorption coefficient, this constant is related to the affinity between the adsorbent and solute, which is evaluated by linearization of Eq. 4:
The essential characteristics of the Langmuir isotherm can be described by a dimensionless constant called the equilibrium parameter, RL, which is usually defined by:
where \({K_l}\) is the Langmuir constant that indicates the nature of adsorption and \({C_0}\) is the highest initial metal concentration (mg/dm3). The value of RL indicates if the adsorption isotherm is irreversible (RL = 0), favourable (0 < RL < 1), linear (RL = 1), or unfavourable (RL > 1).
Freundlich Model
The Freundlich isotherm model (Freundlich 1906), based on monolayer adsorption on heterogeneous surfaces with a non-uniform distribution of adsorption heat, is represented as:
where \({q_e}\) is the amount of solute adsorbed per unit mass of adsorbent at equilibrium (mg/g), \({C_e}\) is the residual adsorbate concentration in solution at equilibrium (mg/dm3), and \({k_f}\) and \(n\) are empirical Freundlich constants. \({k_f}\) (mg/g) is an indicator of adsorption capacity, while \(n\) (g/dm3) is related to the adsorption intensity or binding strength. Their values were determined from the linear form of the Freundlich equation:
where 1/n is the heterogeneity factor. Values of 1/n < < 1 indicate heterogeneous adsorbents, while values closer to or equal to 1 indicate a material with relatively homogeneous binding sites (Papageorgiou et al. 2006). The zeolite should be a heterogeneous adsorbent due to its porous nature. Alvarez-Ayuso et al. (2003), Avila (2005) and Gunay et al. (2007) successfully used the Freundlich adsorption isotherm to model their results from equilibrium experiments.
Results and Discussion
Characteristics of the Adsorbent
The zeolite-bearing tuff was from the Vetunica deposit, which is localised in the northern marginal parts of the well-known Kratovo-Zletovo volcanic area in the Republic of Macedonia. The particle sizes ranged from 0.8 to 2.5 mm. The general characteristics of the zeolite-bearing tuff, such as chemical composition and physical characteristics, are presented in Table 1.
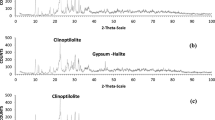
The sample was analyzed to determine the content and type of exchangeable cations. The dominant ion, in the exchangeable position, was K+ (66.5 meq/100 g), followed by Ca2+ (21.5 meq/100 g), Mg2+ (8.5 meq/100 g), and Na+ (3.5 meq/100 g). The total cation exchange capacity was 0.94–1.07 meq/g. The XRD results (Fig. S-1) show that the minerals present in the sample were: stilbite, albite, anorthite, kaolinite, and quartz.
The surface morphology of the sample was studied using the SEM (Fig. 1). The micrographs clearly show a number of micro-pores and well defined crystals of stilbite in the zeolite structure.
Effect of Initial Metal Concentration in Solution
The effect of the initial metal concentration was investigated by contacting 5 g of the zeolite-bearing tuff, at pH 3.5, with different concentrations of the single-component solutions (5, 25, 50, 200, and 400 mg/dm3). An increase in concentration generally results in an increase in the amount of zinc adsorbed. This may be due to more collisions between the reactants, leading to an observed increase in reaction rate and capacity (Connors 1990). Increasing the initial metal concentration in solution until the system reaches a saturation point will increase the amount adsorbed (qe). After reaching saturation, increasing the zinc concentration will not result in any significant change in the amount adsorbed. Results of this investigation are presented (Table 2; Fig. 2).
These results indicate that the amount of zinc adsorbed by the zeolite-bearing tuff at equilibrium is dependent on the initial dissolved metal concentration. This was expected because it is a consequence of an increase in the concentration-driving force. The concentration-driving force is important because it is responsible for overcoming the mass transfer resistance associated with the adsorption of metals from solution by the adsorbent (Barrer 1978). An increase in initial metal concentration in solution not only results in an increase in the amount adsorbed (qe) but also decreases the efficiency of adsorbents used for the removal of zinc (Table 2). Golomeova and Zendelska (2016), Motsi (2010), and Sprynskyy et al. (2006) also found a similar trend, that is, less efficient on metal adsorption from solution by zeolite.
Effect of the Mass of the Adsorbent
The effect of the mass of the adsorbent was investigated by contacting 2, 5, and 10 g of zeolite-bearing tuff, at pH 3.5, with the 25 mg/dm3 Zn solution. An increase in the adsorbent mass should increase the adsorption of zinc ions, because more adsorption sites are available per unit mass of adsorbent. The results confirmed that an increase in the adsorbent mass slightly increased the adsorption of zinc ions (Fig. 3); the adsorption of zinc ions was 92% with 2 g of adsorbent and 98% using 10 g of adsorbent.
Effect of the Initial pH of the Solution
The effect of the initial pH of the solution was investigated by contacting 5 g of zeolite-bearing tuff at three pH values (2.5, 3.5, and 4.5), with the 25 mg/dm3 Zn solution. As expected, as the pH was decreased, zinc removal efficiency also decreased. This is because H+ ions compete with zinc cations for the same exchange sites (Alvarez-Ayuso et al. 2003), and the electrostatic repulsion between the zinc cations in solution and the protonated zeolite surface increases as more H+ ions are adsorbed (Cabrera et al. 2005). With an increasing pH, the adsorbent surface becomes more negatively charged, thus facilitating greater metal uptake (Turan and Mesci 2011).
Figure 4 shows how the adsorption capacity of zeolite-bearing tuff was affected by the solution’s pH. Similar results have been obtained by others (Alvarez-Ayuso et al. 2003; Moreno et al. 2001; Motsi 2010).
Effect of Temperature
The effect of the temperature of the solution was investigated by contacting 5 g of the zeolite-bearing tuff with the 25 mg/dm3 Zn solution at a pH of 3.5 and temperatures of 20 and 60 °C. Adsorption was quicker at the higher temperature, though the adsorption of zinc ions reached the same level at equilibrium. The duration of the adsorption process can be reduced to 240 min with the same degree of adsorption (Fig. 5).
Effect of Competing Cations
The effect of competing cations was also investigated using a mixture of four metal ions: Cu2+, Mn2+, Zn2+, and Pb2+. The experiments were completed by contacting 5 g of the zeolite-bearing tuff, at pH 3.5 and 20 °C with multi-component solutions of 25 mg/dm3 Cu, 25 mg/dm3 Zn, 25 mg/dm3 Mn, and 25 mg/dm3 Pb.
Figure 6 compares the adsorption of zinc ion from the single- and multi-component solutions. The results show that the amount of Zn2+ adsorbed from the multi-component solution was more than 50% less than from the single component solution. This was expected because of the increased amount of competing cations.
The difference in the zeolite-bearing tuff’s adsorption capacity for the metal ions can be affected by factors such as the hydration radii, hydration enthalpies, and cation solubility. The hydration radii of the cations are: rHZn2+ = 4.30 Å, rHCu2+ = 4.19 Å, rHPb2+ = 4.01 Å, and rHMn2+ = 4.38 Å (Nightingale 1959). The smallest cations should ideally be adsorbed faster and in larger quantities than the larger cations, since the smaller cations can pass through the micro-pores and channels of the zeolite structure with ease (Calvo et al. 2009). Furthermore, adsorption should be described using hydration enthalpy, which is the energy that permits the detachment of water molecules from cations and thus reflects the ease with which the cation interacts with the adsorbent. Therefore, the more a cation is hydrated, the stronger its hydration enthalpy, and the less it can interact with the adsorbent (Motsi 2010). Because of its high Si:Al ratio, zeolite has a low structural charge density. Therefore, divalent cations with low hydration energies are sorbed preferably compared to cations with high hydration energies (Colella 1991). The hydration energies of the cations are: − 2010, − 1955, − 1760, and − 1481 kJmol− 1 for Cu2+, Zn2+, Mn2+, and Pb2+ respectively (Mobasherpour et al. 2012; Nightingale 1959). According to the hydration radii, the order of adsorption should be Pb2+ > Cu2+ > Zn2+ > Mn2+, and according to the hydration enthalpies, the order should be Pb2+ > Mn2+ > Zn2+ > Cu2+.
Of the studied cations, the lead cations have the smallest hydration energy and hydration radius and the zeolite-bearing tuff will prefer Pb over Cu, Zn, and Mn in multi-component solutions. Therefore, it is to be expected that Pb concentrations will limit the uptake of Zn, Cu, and Mn.
Equilibrium Experiments
Equilibrium experiments were carried out to determine the maximum Zn adsorption capacity of the zeolite-bearing tuff by contacting 5 g of adsorbent at pH 3.5 and temperature of 20 ± 1 °C with different concentrations of single component solutions 5, 25, 50, 200, and 400 mg/dm3 of Zn2+. The experimental data from these experiments were fitted to the Langmuir and Freundlich adsorption isotherms (Table 3).
According to the Freundlich model, the values for the heterogeneity factor, 1/n, indicate that the zeolite-bearing tuff is a heterogeneous adsorbent, because the value of heterogeneity factor is < < 1. Based on the correlation coefficients (R2), the adsorption isotherms can be better described by the Freundlich model. The RL values (Table 3) confirmed that the behaviour of Zn2+ adsorption onto used adsorbent was favorable (0 < RL < 1).
Figures 7 and 8 compares the adsorption isotherms based on the Langmuir and Freundlich models with the experimental data. The Freundlich adsorption isotherm is a better fit to the experimental results (Fig. 8). Figure 8 also shows that as the initial concentration of zinc cations increases, the amount of metal adsorbed per gram of adsorbent (qe) increases too. This is mainly due to the fact that at high metal concentrations, there is a higher solute concentration gradient, which provides the necessary driving force for metal ions to displace exchangeable cations on the surface and from the zeolite’s internal micro-pores (Cagin 2006; Motsi 2010). However, this increasing trend is valid up to the point at which the maximum capacity of the adsorbent samples for the respective metal cation is achieved, that is, its saturation point.
The pH was measured before and after treatment for each equilibrium study. Figure 9 presents the variation in the equilibrium pH values with respect to the initial zinc concentration. The pH values at equilibrium were greater than the initial pH values. As initial zinc concentration increases, the pH increases to 6, then eases and settles at ≈ 5.8, regardless of concentration. The adsorption of H+ ions from solution will cause a pH increase. As the initial zinc concentration increases, the concentration-driving force begins to favor the adsorption of zinc ions over H+. According to the results (Fig. 9), it can be confirmed that the zeolite-bearing tuff has a buffering effect.
Acid Mine Drainage Treatment
According to the results obtained with the synthetic acid solutions, it can be concluded that zinc ions were adsorbed efficiently onto zeolite-bearing tuff at the studied conditions. Based on that, the investigation continued with AMD from the Sasa mine. The experiment was performed by contacting 5 g of zeolite-bearing tuff in 0.4 dm3 of AMD. The magnetic stirrer was agitated at 400 rpm for up to 360 min, at 20 °C. The initial zinc concentration and pH of the AMD are presented in Table 4.
The initial concentrations and pH values confirmed that the drainage from the Sasa mine is acidic, with a high concentration of zinc ions. Table 4 also shows the remaining zinc concentration and the pH of the AMD after treatment. Figures 10 and 11 presents the results of the reaction of the AMD with the zeolite-bearing tuff and its effect on рН with respect to contact time.
It can be seen that the zinc ions in the AMD were efficiently adsorbed by the zeolite-bearing tuff; more than 74% of the zinc ions were removed. However, the zinc adsorption was lower than in the synthetic acid solution experiment, since other metal ions were present to compete with the zinc ions. The presence of lead ions limited the uptake of others ions because of its small hydrated energy and hydrated radius. Again, the pH zeolite-bearing tuff had a buffering effect, even with AMD.
Conclusion
The adsorption of zinc ions onto a zeolite-bearing tuff was shown to occur efficiently. It was most effective at lower concentrations of zinc ions, a higher adsorbent mass, and higher pH and was faster at a higher temperature. The maximum capacity of the tuff for removal of zinc ions from solution was determined and, in general, the Freundlich adsorption isotherm was a better fit with the experimental results. While investigating the effect of the competing cations, it was concluded that the presence of cations with smaller hydration energies and hydration radii, such as lead cations, limits the uptake of zinc cations. The investigation then tested the adsorption of zinc ions onto the zeolite-bearing tuff from the Sasa mine AMD and more than 74% of the zinc ions were removed.
References
Alvarez-Ayuso E, Garcia-Sanchez A, Querol X (2003) Purification of metal electroplating waste waters using zeolites. Water Res 37:4855–4862
Ammann L (2003) Cation exchange and adsorption on clays and clay minerals. PhD Diss, Christian—Albrechts—Univ, Kiel
Avila MAS (2005) Experiment and modelling of the competititive sorption and transport of chlorinated ethenes in porous media. Cuvillier Verlag, Gottingen
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97(1):219–243
Barrer VRM (1978) Zeolites and clay minerals as sorbents and molecular sieves. Academic Press Inc, London
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18(12):1501–1507
Cabrera C, Gabaldon C, Marzal P (2005) Sorption characteristics of heavy metal ions by a natural zeolite. J Chem Technol Biotechnol 80:477–481
Çagin V (2006) Use of clinoptilolite for copper and nickel removal from aqueous solutions. McS diss, Middle East Technical Univ, Turkey
Calvo B, Canoira L, Morante F, Martínez-Bedia JM, Vinagre C, García-González JE, Elsen J, Alcantara R (2009) Continuous elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from acidic waters by ionic exchange on natural zeolites. J Hazard Mater 166(2–3):619–627
Colella C (1991) Ion exchange equilibria in zeolite minerals. Miner Deposita 31:554–562
Connors KA (1990) Chemical Kinetics: The study of reaction rates in solution. VCH Publishers, Hoboken
Cui H, Li LY, Grace JR (2006) Exploration of remediation of acid rock drainage with clinoptilolite as sorbent in a slurry bubble column for both heavy metal capture and regeneration. Water Res 40:3359–3366
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interf Sci 280(2):309–314
Freundlich H (1906) Über die adsorption in lösunge. Zeitschrift für Physikalische Chemie 62(5):121–125
Golomeova M, Zendelska A (2016) Application of some natural porous raw materials for removal of lead and zinc from aqueous solutions. In: Dariani RS (ed) Microporous and mesoporous materials. InTech, Croatia, pp 21–49. https://doi.org/10.5772/62347
Gunay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371
Kobya M, Demirbas E, Senturk E, Ince M (2005) Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour Technol 96(13):1518–1521
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leinonen H, Letho J (2001) Purification of metal finishing waste waters with zeolites and activated carbons. Waste Manag Res 19:45–57
Malliou E, Loizidou M, Spyrellis N (1994) Uptake of lead and cadmium by clinoptilolite. Sci Total Environ 149:139–144
Mobasherpour I, Salahi E, Pazouki M (2012) Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: adsorption isotherm study. Arab J Chem 5(4):439–446
Moreno N, Querol X, Ayora C (2001) Utilization of zeolites synthesised from coal fly ash for the purification of acid mine waters. Environ Sci Technol 35:3526–3534
Motsi T (2010) Remediation of acid mine drainage using natural zeolite. PhD Diss, Univ of Birmingham, UK
Nightingale ER (1959) Phenomenological theory of ion solvation. Effective radii of hydrated ions. J Phys Chem 63(9):1381–1387
Papageorgiou KS, Katsaros KF, Kouvelos PE, Nolan WJ, LeDeit H, Kanellopoulos KN (2006) Heavy metal sorption by calcium alginate beads from Laminaria digitata. J Hazard Mater B137:1765–1772
Pradeep PT, Yeole PM, Shrivastava VS (2015) The study of Sr(II) metal ion exchange by stilbite a natural zeolite and its adsorbed derivative. Br J Appl Sci Technol 7(5):436–448
Reddad Z, Gerente C, Andres Y, Cloirec PL (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36(9):2067–2073
Sag Y, Aktay Y (2000) Mass transfer and equilibrium studies for the sorption of chromium ions onto chitin. Process Biochem 36:157–173
Sprynskyy M, Boguslaw B, Terzyk AP, Namiesnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+ and Cd2+) adsorption on clinoptilolite. J Colloid Interf Sci 304:21–28
Taffarel SR, Rubio J (2009) On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner Eng 22:336–343
Turan GN, Mesci B (2011) Adsorption of copper(II) and zinc(II) ions by various agricultural by-products. Experimental studies and modelling. Environ Protect Eng 31(4):143–161
Yuan G, Seyama H, Soma M, Theng BKG, Tanaka A (1999) Adsorption of some heavy metals by natural zeolites: XPS and batch studies. J Environ Sci Health C 34:625–648
Zendelska A, Golomeova M, Blažev K, Boev B, Krstev B, Golomeov B, Krstev A (2015) Kinetic studies of manganese removal from aqueous solution by adsorption on natural zeolite. Maced J Chem Chem Eng 34(1):1857–5625
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zendelska, A., Golomeova, M., Golomeov, B. et al. Removal of Zinc Ions from Acid Aqueous Solutions and Acid Mine Drainage Using Zeolite-Bearing Tuff. Mine Water Environ 38, 187–196 (2019). https://doi.org/10.1007/s10230-018-0560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-018-0560-y