Abstract
The reproductive cycle of female wild marble trout Salmo marmoratus was studied in a prealpine watercourse (Northeast Italy). Gonadosomatic index, oocyte growth and plasma levels of 17β-oestradiol and testosterone were bimonthly measured during one year to obtain a detailed dataset and to gain useful tools for the identification of the reproductive stage without impact for the species. Monitored features showed significant variations during the study period: gonadosomatic index and oocyte size increased slightly but significantly during the first part of the cycle (from February to June), while steroid levels remained quite constant; for all parameters, major increases were highlighted between August and the spawning season (which occurred from the middle of November to the middle of December). Ranges and trends observed for gonadosomatic index (from 0.87 ± 0.41% to 10.91 ± 3.37%) and oocyte diameter (from 0.692 ± 0.031 mm to 4.624 ± 0.208 mm) were generally in agreement with literature regarding salmonids, while plasma levels of 17β-oestradiol (from 0.214 ± 0.015 ng ml−1 to 78.090 ± 23.882 ng ml−1) and testosterone (from 0.327 ± 0.086 ng ml−1 to 71.800 ± 29.406 ng ml−1) showed wider ranges. A strong non-linear relationship was found between oocyte size and 17β-oestradiol plasma concentration (r 2 = 0.890) and especially between oocyte size and testosterone plasma concentration (r 2 = 0.947). This last relationship could likely be used to obtain information relative to the gonadal development, especially in the case of an endangered species like Salmo marmoratus, which needs non-invasive tools for management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
About one-third of the known European freshwater fish species are endangered or are facing the risk of extinction (Kottelat and Freyhof 2007; Vincenzi et al. 2012) and nearly all salmonid species have endemic populations that went extinct or became endangered (Williams et al. 1989; Behnke 1992). Among trout species, marble trout Salmo marmoratus is of particular conservation interest (Vincenzi et al. 2012; Lorenzoni et al. 2012), being endemic to the Adriatic River Basin of Northern Italy (Turin et al. 2006; Pujolar et al. 2011) and the Adriatic River system of the Western Balkans (Povž 1995; Povž et al. 1996; Crivelli et al. 2000; Fumagalli et al. 2002). Many authors have reported a progressive restriction of the original distribution area (Sommani 1961; Tortonese 1967; Crivelli et al. 2000; Turin et al. 2006: Meraner et al. 2007; Meraner et al. 2008) and the species is considered one of the most endangered freshwater fish of the Adriatic Basin (Povž et al. 1996; Crivelli et al. 2000), as listed in Annex II of the European Union Habitats Directive (92/43/EEC) and recently included as Critically Endangered in the IUCN Red List (Rondinini et al. 2013). In fact, hybridization with brown trout Salmo trutta (see Povž 1995; Berrebi et al. 2000; Delling et al. 2000; Meraner et al. 2008; Meraner et al. 2010), displacement by alien rainbow trout Oncorhynchus mykiss (see Vincenzi et al. 2011) and habitat alteration (Crivelli et al. 2000; Chiesa et al. 2016) are the most serious causes of concern for the survival of S. marmoratus. Due to these reasons, some rehabilitation projects have been led since the late 1980s, both in Italy (Specchi et al. 2004) and Slovenia (Crivelli et al. 2000; Vincenzi et al. 2012), and several studies have been carried out on the biology and ecology of S. marmoratus (see Povž et al. 1996; Delling et al. 2000; Meldgaard et al. 2007; Vincenzi et al. 2007a, b, 2008a, b, 2010a, b, 2011; Simčič et al. 2015) and on genetics (Berrebi et al. 2000; Snoj et al. 2000; Fumagalli et al. 2002; Jug et al. 2005; Pustovrh et al. 2014; Sušnik Bajec et al. 2015). Nevertheless, despite this great number of works, specific studies including characterizations of the wild marble trout reproductive cycle are still missing, even though this kind of research could give important information in the context of repopulation and management policies. In addition, the development of any useful management tool that could assist in conserving populations would be advantageous for the survival of the species, as suggested by Lorenzoni et al. (2012). Several published works include informations about sex steroid hormone levels for S. trutta (see Estay et al. 2003; Fregeneda-Grandes et al. 2013), O. mykiss (see Tyler et al. 1990; Tyler and Sumpter 1996; Wilkinson et al. 2010; Estay et al. 2012; Kagawa 2013) and other salmonids (Taranger et al. 1998; King and Pankhurst 2003; Jordanova et al. 2013; Taranger et al. 2015), almost all regarding farmed fish, but none addressed the marble trout.
In this context, it was deemed of interest to analyse the reproductive cycle of wild S. marmoratus females, within a community living in its typical habitat. Investigations have been led in a prealpine watercourse close to the boundary between Italy and Slovenia with the aim (i) to obtain a one-year dataset regarding variations of gonadosomatic index, oocyte diameters and plasma level of sex hormones (17β-oestradiol and testosterone) of wild S. marmoratus females; (ii) to check the correlations among the mentioned variables and between these variables and the chemical–physical features; (iii) to validate useful information for the identification of the reproductive stage without killing the specimens.
Materials and methods
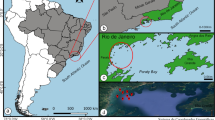
Study area. The study was carried out in the Uccea Brook, a right side tributary of the Soča River with a total length of 14 km (Fig. 1); the Uccea spring is placed at 1098 m above sea level in Northeast Italy, on the slopes of the Musi Mount, within the Special Area of Conservation called “Northern Julian Pre-alps” (SAC Site Code: IT3320012), which is included in the Special Protection Area identified as “Julian Alps” (SPA Site Code: IT3321002). The Uccea Brook flows in the west–east direction, partially within the SAC mentioned above; after 9 km from the spring, the watercourse crosses the state border between Italy and Slovenia and then flows into Soča River 5 km after the boundary. The sampling operations were always performed in a brook section of 1.7 km length, between the confluence with the Bianco Brook and the state border limit, outside the SPA and SAC zones.
All activities described in the present study were performed by permission of the Safeguard Fisheries Authority of the Region Friuli Venezia Giulia, as requested by local laws. Experimental procedures were carried out according to the Guidelines of the European Directive 2010/63/EU on the protection of animals used for scientific purposes and the principle of the 3Rs was applied. The Italian law (D. Lgs 26–14), which applies the European Directive, states that the used protocol does not require an authorization to the use of animals (art. 2, d).
At each sampling event, the values of the main chemical and physical data were recorded in the water column to support biological surveys: in particular, conductivity (µS cm−1), pH, temperature (°C) and dissolved oxygen (mg l−1 and percentage of saturation) were registered using field meters (HI 9033 conductivity meter; HI 9125 pH/ORP meter; HI 9147 dissolved oxygen meter; all instruments are manufactured by Hanna Instruments Inc., Woonsocket, Rhode Island, USA). Four values were measured approximately at mid-depth across a representative brook section, paying attention to cover different microhabitats. The mean values and relative standard deviations (sd) observed during the study period are reported in Table 1.
Fish sampling. Captures were performed by wading a watercourse section with a battery-powered backpack electrofisher (Model IG200–2: 15–25 A, 150–200 V; manufactured by Hans–Grassl GmbH, Schönau am Königssee, Germany).
During December 2014, a preliminary sample collection was carried out to check if the chosen population was sufficiently well structured to support the study without consequences for its conservation. As Salmo marmoratus is an annual spawning fish in nature field, preliminary sampling allowed to detect its spawning period within the Uccea Brook. Captures were carried out along a 180 m brook stretch, which was proportional to the riverbed width (Forneris et al. 2005), using the removal method (Seber and Le Cren 1967; Seber 1973) to obtain data about the marble trout community structure. Specimens of S. marmoratus were identified on the basis of the phenotypic characters. At the end of the preliminary sampling, all fish were released at the same site without consequences for their vitality.
As the Uccea marble trout community was sufficiently well structured to support the study, fish samples were bimonthly collected (February, April, June, August, October and December 2015) during the first week of the month. The last two samplings were performed in the middle of November and in the middle of December 2015 to gain information immediately before and after the spawning. Four mature female (20 cm length at least) were captured each time, moved into a tank and anesthetized using tricaine methane-sulfonate (MS-222) (Topic Popovic et al. 2012), to limit damages during the manipulation. Total length (L T ; x ± 0.1 cm) and weight (W; x ± 0.1 g) were measured for each specimen, and a blood sample was obtained from the caudal vein for hormone determinations. Then, specimens were killed by anaesthetic overdose and placed in frozen bags for the transport to the laboratory, where ovaries were dissected. Fulton condition factor (K) was calculated for each specimen (Ricker 1975) and the age was determined by scales lecture.
Gonadosomatic index and oocyte growth. Fresh gonad weights were measured immediately after dissection (x ± 0.0001 g) with an analytical balance, and the gonadosomatic index (I G %) was calculated.
Then, oocyte diameters were measured, using a technique based on the work of Thorsen and Kjesbu (2001) and used by several authors with some difference in details (Murua et al. 2003; Friedland et al. 2005; Klibansky and Juanes 2007, 2008; Alonso-Fernández et al. 2009; Gray et al. 2014; Diaha et al. 2016). For each specimen, an ovary subsample (approximately, 2 cm length) was removed from the centre of the left gonad and placed in 70% ethanol. Samples were then dissected to separate individual follicles, which were placed in glass Petri dishes filled with 33% glycerol solution and submitted to moderate stirring until they were adequately separated among each other. Subsequently, groups of four Petri dishes were captured as images using a flatbed scanner (Epson Perfection V500 Photo). Every image was 2400 dpi (6479 pixels × 22392 pixels) in 8-bit greyscale and saved in uncompressed tagged image file format (TIFF). Once scanned, each 44 MB image was analysed using the ImageJ Version 1.49 program (Schneider et al. 2012). The system was length calibrated (mm units) and diameter measurements were performed for 15 oocytes for each fish, using the greyscale images; two values were taken for each examined oocyte. Oocyte diameters were measured from February to November and fish caught in December (after deposition) were not considered.
Plasma level determination of sex steroids. To determine the sex steroid profiles, blood samples were obtained in 2 ml heparinized tubes, transferred in Eppendorf tubes, brought to the laboratory and kept at ambient temperature for less than two hours. Samples were immediately centrifuged at 3000 rpm for 5 minutes to obtain the plasma fractions, which were then frozen at −20 °C until hormone analysis. Competitive chemiluminescent immunoassays for the quantitative determination of 17β-oestradiol (E2; ng ml−1) and testosterone (T; ng ml−1) plasma levels were used to obtain steroid profiles (Access Immunoassay Systems, Beckman and Coulter Inc., Brea, CA, USA). Samples were assayed following the instructions provided by the manufacturer. The minimum detectable quantity was 0.02 ng ml−1 for E2 and 0.1 ng ml−1 for T; the cross-reactivity to other hormones was less than 2% in both analyses.
Statistical analysis. Mean values and relative standard deviations (sd) of condition factor (K), gonadosomatic index (I G), oocyte diameter, plasma level of 17β-oestradiol (E2) and plasma level of testosterone (T) were calculated for each sampling event. As the data were not normally distributed, analyses were performed using the non-parametric Kruskal–Wallis test. The Conover–Iman test (Conover and Iman 1979; Conover 1999) was used as post hoc test, to assess the presence of significant differences between values observed in two consequent sampling events. The correlations among biotic variables and between these variables and chemical and physical features were investigated with the Spearman rank correlation coefficient (ρ). In addition, the relations between sex steroids an oocyte size, expressed as oocyte diameter, were examined by non-linear regression. All statistical analyses were performed using StatSoft STATISTICA 7.1 (StatSoft 2005), except for the Conover–Iman test, which was performed using R software, version 3.3.1 (R Core Team 2013), with a P level of 0.05 used to interpret significance for all tests.
Results
During the bimonthly samplings, 28 mature females were captured, ranging from 21.0 cm to 40.5 cm length and from 89.4 g to 491.7 g weight (Table 2); most specimens were assigned to the 3+ age group (19 individuals), six specimens were 4+, two were 2+ and only one trout belonged to the 6+ group. Condition factor (K) mean values did not differ significantly during the study period, except in February, when the observed mean value was lower than that at the other months (Kruskal–Wallis test: H 6,28 = 14.00, P < 0.05; Conover–Iman test: P < 0.05 for all comparisons).
Gonadosomatic index and oocyte growth. The pattern observed for the gonadosomatic index (I G ) is reported in Figure 2a; significant variations were highlighted during the study period (Kruskal–Wallis test: H 6,28 = 23.71, P < 0.05). The I G values remained nearly at the same level from February to June, ranging from 0.87 ± 0.41% to 1.00 ± 0.27%; then values increased significantly in August (3.73 ± 0.90%) and October (7.72 ± 2.15%) (Conover–Iman test: P < 0.05 for both comparisons). I G increased further in November just prior to spawning, when the maximum mean value was recorded (10.91 ± 3.37%), but no significant difference was highlighted with the previous sample (Conover–Iman test: P = 0.39). The increasing pattern is quite linear from June to November. I G drastically decreased in December, immediately after spawning, as the minimum mean value was observed (0.44 ± 0.25%; Conover–Iman test: P < 0.001).
Bimonthly variations of gonadosomatic index I G (a) and oocyte diameters (b) observed for the female marble trouts sampled in the Uccea Brook [n = 4 for all cases; black dots correspond to mean values; boxes correspond to standard errors (se); whiskers correspond to standard deviations (sd); asterisks indicate significant differences with the previous bimonthly value; *: P < 0.05; **: P < 0.01; ***; P < 0.001]
Oocyte growth pattern is reported in Figure 2b and Figure 3. The minimum mean diameter was observed in February (0.692 ± 0.031 mm), after the end of the previous reproductive period and the beginning of the new cycle; then, the oocyte size increased always significantly from month to month (Kruskal–Wallis test: H 5,24 = 22.25, P < 0.05; Conover–Iman test: P < 0.01 for all comparisons). The maximum average size was observed in November (4.624 ± 0.208 mm), just prior to the spawning season (Figs. 2b, 3). A positive correlation between I G values and oocyte growth pattern was highlighted (Spearman rank correlation coefficient ρ = 0.874, P < 0.0001, n = 24). A positive correlation was found also between I G values and water temperature (Spearman rank correlation coefficient: ρ = 0.431, P < 0.05, n = 28) and between oocyte growth and water temperature (ρ = 0.463, P < 0.05, n = 24).
Plasma level of sex steroids. Plasma steroid patterns observed between February and December 2015 are shown in Fig. 4. Significant bimonthly variations were detected both for 17β-oestradiol (E2) and testosterone (T) (Kruskal–Wallis test: H 6,28 = 24.97, P < 0.01 for E2; H 6,28 = 24.28, P < 0.01 for T). During the February–June period that corresponds to the early vitellogenesis phase, low levels of E2 were detected, ranging between 0.214 ± 0.015 ng ml−1 and 0.442 ± 0.119 ng ml−1; no significant differences were recorded in this period (Conover–Iman test: P > 0.11 for all comparisons). E2 levels increased slightly but significantly in August, reached a peak in October (78.090 ± 23.882 ng ml−1), decreased clearly but not significantly in November and then significantly declined in December (spawning season) (Conover–Iman test: P < 0.001 for all comparisons except between October and November, where P = 0.11).
Bimonthly levels of 17β-oestradiol (E2, continuous black line), testosterone (T, continuous grey line) and variations of water temperature (broken black line) observed during the reproductive cycle of female marble trout in the Uccea Brook (February–December 2015; mean ± sd; n = 4 for each case). Asterisks (black for E2, grey for T) indicate significant differences with the previous bimonthly value; *: P < 0.05; **: P < 0.01; ***; P < 0.001)
The seasonal pattern of T levels lagged behind and followed that of E2 (Spearman rank correlation coefficient between E2 and T: ρ = 0.833, P < 0.0001, n = 28), with low values which did not differ significantly between February and June (Conover–Iman test: P > 0.05 for all comparisons), slightly increased in August and rapidly increased in October; the levels remained high at the beginning of the spawning period, when the maximum mean value was detected (71.800 ± 29.406 ng ml−1 observed in November) and then drastically decreased after the spawning (Conover–Iman test: P < 0.03 for all comparisons except between October and November, where P = 011).
A correlation was found also between steroid profiles and water temperature (Spearman rank correlation coefficient: ρ = 0.463, P < 0.02, n = 28 for E2; ρ = 0.421, P < 0.05, n = 28 for T). Sex steroid profiles were also correlated with I G (Spearman rank correlation coefficient: ρ = 0.671, P < 0.0001, n = 28 for E2; ρ = 0.757, P < 0.0001, n = 28 for T) and were strongly linked with oocyte growth pattern (Spearman rank correlation coefficient: ρ = 0.846, P < 0.0001, n = 24 for E2; ρ = 0.932, P < 0.0001, n = 24 for T): the relationship between oocyte diameters and E2 plasma levels and the relationship between oocyte diameters and T plasma levels both resulted in a highly significant non-linear regression, with r 2 values equal to 0.890 and 0.947, respectively (Fig. 5; Table 3).
Relationship between mean oocyte diameter and plasma levels of 17β-oestradiol (E2) (a) and testosterone (T) (b), where x = E2 or T and y = oocyte diameter. The curve for E2 was fitted by: y = 0.5772ln(x) + 1.7711 (r 2 = 0.890, P < 0.0001, n = 24); the curve for T was fitted by: y = 0.6871ln(x) + 1.5368 (r 2 = 0.947, P < 0.0001, n = 24)
Discussion
The present study reports bimonthly patterns for variations of gonadosomatic index, oocyte diameters and plasma levels of sex steroids (17β-oestradiol and testosterone) for wild female Salmo marmoratus, living in its typical habitat.
The patterns observed in the Uccea Brook are generally in line with those reported in other studies concerning the biology and reproduction of salmonids. From the beginning of the analysis to the spawning phase, the I G mean values observed in the present work underwent an 11- or 12-fold increase (Table 2; Fig. 2a). This range is comparable with those provided by Jelli et al. (1991), which reported I G mean values for S. marmoratus captured in different river basins of the Po Plain area, ranging between 0.609 ± 0.204% and 15.948 ± 1.854% during the March–December period (Adige River basin) and between 0.556 ± 0.033% and 9.674 ± 2.170% between March and October (Piave River). The I G mean values observed in the Uccea Brook showed a quite constant level from February to June, then rapidly increased in August (at the beginning of the secondary vitellogenesis), reached its peak in November just prior the deposition and finally rapidly decreased after spawning. This pattern is generally in agreement with those observed by other authors with reference to other salmonids such as brown trout Salmo trutta (Linnaeus 1758) (Billard 1987; Estay et al. 2003; Arslan and Aras 2007; Nusrat and Ulfat 2013), Caspian brown trout Salmo trutta caspius (see Jamalzadeh et al. 2013), rainbow trout Oncorhynchus mykiss (see Tyler et al. 1990), amago salmon Oncorhynchus rhodrus (see Kagawa 2013), Ohrid trout Salmo letnica (see Jordanova et al. 2013) and Atlantic salmon Salmo salar (see King and Pankhurst 2003; Taranger et al. 2015). However, although the general pattern is the same, the decrease observed in the early post spawning period (December) is faster than that in some other studies (Jordanova et al. 2013; Nusrat and Ulfat 2013), which reported more gradually declining values, and the maximum values recorded just prior to the spawning are generally lower than those indicated for other salmonids (Tyler et al. 1990; Estay et al. 2003; Jordanova et al. 2013; Kagawa 2013; Taranger et al. 2015), where maximum I G values were equal to 20–22%. However, these differences could depend on climate variations, watercourse characteristics and could occur from zone to zone and/or from year to year (Jelli et al. 1991). An example of this variability could also be found in the work of Arslan and Aras (2007) on the reproductive cycle of two brown trout populations in Turkey, as the authors reported values of I G which differed in two subsequent years for both populations.
Concomitant with the variations in I G , the mean oocyte diameter underwent a 7-fold increase from the beginning of the study until the spawning period (Table 2; Figs. 2b, 3). Diameters recorded during the mature phase are in agreement with those reported for S. marmoratus in the Piave River basin (4.430 ± 0.242 mm), but lower than those obtained in the Adige River area (5.580 ± 0.380 mm and 5.508 ± 0.565 mm) during a work carried out in Northern Italy by Jelli et al. (1991). The observed mature oocyte diameters are also in line with values reported for wild brown trout in Central Italy (4.810 ± 0.028 mm) by Bicchi et al. (2010). The oocyte growth pattern reported in the present study is also comparable with those provided by Estay et al. (2003) for farmed brown trout in Chile and with data reported in literature for O. mykiss (see Tyler and Sumpter 1996; Kwon et al. 2001; Gülsoy 2007) and for S. salar (see King and Pankhurst 2003). Bimonthly values are generally slightly lower than those observed in other studies regarding salmonids (Tyler et al. 1990; Kagawa 2013; Taranger et al. 2015), but this could be due to several factors, such as female fish size (Wootton 1998; King and Pankhurst 2003; Heinimaa and Heinimaa 2004) which influences the timing of the first sexual maturation stage and the duration of the vitellogenesis phases (Bromage and Cumaranatunga 1988; Estay et al. 2003).
The patterns of plasma levels observed herein for gonadal steroid E2 and T are strictly related to the gonadal/oocyte development and were similar and broadly consistent with those reported for salmonids elsewhere. In particular, the trends observed in the present study are in agreement with those described by Kagawa (2013) and with those reported by other authors for other trout species and generally for salmonids (Taranger et al. 1998; Estay et al. 2003; King and Pankhurst 2003; Estay et al. 2012; Hoogenboom et al. 2012; Fregeneda-Grandes et al. 2013; Taranger et al. 2015), though the values provided in the present work for the Uccea Brook population show a wider range (0.214 ± 0.015–78.090 ± 23.882 ng ml−1 for E2; 0.327 ± 0.086–71.800 ± 29.406 ng ml−1 for T) and lower values in the initial samplings (Table 2; Fig. 4). In fact, E2 and T levels remained very low from February to June (≤0.442 ± 0.119 ng ml−1 for E2 and ≤0.820 ± 0.238 ng ml−1 for T), slightly increased in August (Table 2; Fig. 4) and then increased greatly. These differences in plasma concentration of the steroids could be due to several factors such as body size, age, nutritional condition, dominance status and environmental condition, or could be related to the different methodologies used, the geographical zones studied or the different species analysed (Fregeneda-Grandes et al. 2013). In addition, differences could be due to the fact that literature datasets are often referred to farmed/cultured salmonids, or to experimental treatments carried out under simulated conditions on fish collected from hatcheries, while the present study considered only wild fish. The specimen origin from a natural environment could also explain the intra-sample variability, expressed as standard deviations (sd), observed in the present work for I G , oocytes growth and especially for steroid levels (in particular, during the mid- and late vitellogenesis in the August–November period), which is generally higher than in other datasets. Despite these differences, general patterns are consistent with literature data. Moreover, non-linear relationships have been highlighted between oocyte size (expressed as diameter) and E2 concentration, and between oocyte size and T concentration (Table 3; Fig. 5), with a very strong correlation in both cases (r 2 = 0.890 and r 2 = 0.947 respectively). A strong non-linear relationship between E2 and oocyte size (r 2 = 0.834) was highlighted in a study on S. salar by King and Pankhurst (2003), which reported that an exponential relationship could reflect the increase in E2 levels, and that I G and oocyte size also increased following an exponential pattern. However, the relationship between T and oocyte diameter presented herein is stronger and could likely better reflect the progress of the oocyte maturation in terms of temporal scale. In fact, T has been detected in the plasma of females in almost all teleost fish species during the reproductive season (Fregeneda-Grandes et al. 2013) and its role as a precursor for the synthesis of E2 by aromatase activity in the ovary is well known (Fostier et al. 1983; Kagawa et al. 1983; Norberg et al. 1989; King and Pankhurst 2003; Kagawa 2013). Follicles produce E2 in response to gonadotropin stimulation, but as this production increases during oocyte growth, it decreases rapidly in association with the ability of the oocyte to mature (Kagawa et al. 1983; Young et al. 1983; Kanamori et al. 1988; Kagawa 2013). Moreover, T production increases during oocyte growth and peaks during the postvitellogenetic phase. These high plasma T levels observed in salmonid mature females are related to the decrease of the aromatase activity which converts T to E2. Using in vitro experiments, Kagawa (2013) described trends for Oncorhynchus rhodurus, indicating that E2 levels increase during vitellogenesis, reach a peak and then rapidly decrease in mature and ovulated fish, while the pattern of T levels follows that of E2 but remains high in mature and ovulated specimens. Estay et al. (2003) reported that in farmed brown trout, T levels remain high during most of oogenesis and then fall down during ovulation, while the E2 level falls down in the spawning period, when the oocyte size is greater. T levels reported herein for S. marmoratus remained high at the beginning of the spawning period, when the maximum mean value was detected, and then drastically decreased after the deposition. These differences could conceivably be due to the factors mentioned above. In particular, differences in plasma steroid could be due to the source of fish, i.e. farmed/cultured or wild (Fregeneda-Grandes et al. 2013). Norberg et al. (1989) found that differences in plasma levels between wild and farmed female brown trout might be due to differences in stress susceptibility, environmental conditions, nutritional status or genetic divergences. In addition, some datasets were obtained from experimental treatments carried out under simulated conditions (Taranger et al. 1998; Estay et al. 2003; Wilkinson et al. 2010; Estay et al. 2012; Fregeneda-Grandes et al. 2013; Taranger et al. 2015) on fish collected from hatcheries or from in vitro experiments (Kagawa et al. 1983; Kagawa 2013), instead of analysing wild specimens and/or studying another species.
However, the observed patterns imply that higher levels of E2 do not correspond to the maximum oocyte size (Table 3; Fig. 5a) at the end of the reproductive cycle in mature fish, even if the correlation between E2 and the oocyte diameter is strong, while this problem did not occur with T plasma concentrations, which can always correspond to a homologous oocyte size. Therefore, it could be likely possible to obtain information about the oocyte maturation stage from this relationship. This fact is of pivotal importance, especially in the case of an endangered species like S. marmoratus, which needs the development of several non-invasive management tools.
In conclusion, the patterns observed for I G , oocyte growth, E2 and T for S. marmoratus in the Uccea prealpine brook are consistent with literature data and could represent a general reproduction pattern for S. marmoratus. In addition, the significant relationship between oocyte growth and plasma steroid concentrations (especially for testosterone) is clearly validated by the data of the present study and the quantification of plasmatic testosterone levels will be a valuable tool for the identification of reproductive females, without requiring their sacrifice, in future programs for the management of marble trout.
References
Alonso-Fernández A, Carole Vallejo A, Saborido-Rey F, Murua H, Trippel EA (2009) Fecundity estimation of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) of Georges Bank: Application of the autodiametric method. Fish Res 99:47–54
Arslan M, Aras NM (2007) Structure and reproductive characteristics of two brown trout (Salmo trutta) populations in the Çoruh River Basin, Northeastern Anatolia, Turkey. Turk J Zool 31:185–192
Behnke RJ (1992) Native trout of western North America, American Fisheries Society Monograph 6. American Fisheries Society, Bethesda
Berrebi P, Povž M, Jesensek D, Cattaneo-Berrebi G, Crivelli AJ (2000) The genetic diversity of native, stocked and hybrid populations of marble trout in the Soča river, Slovenia. Heredity 85:277–287
Bicchi A, Angeli V, Pedicillo G, La Porta G, Carosi A, Viali P, Lorenzoni M (2010) Biologia riproduttiva di Salmo trutta trutta L., 1758 nel torrente Monterivoso. Studi Trent Sci Nat 87:145–147
Billard R (1987) The reproductive cycle of male and female brown trout (Salmo trutta fario): a quantitative study. Reprod Nutr Dévelop 27(1A):29–44
Bromage NR, Cumaranatunga R (1988) Egg production in the rainbow trout. In: Muir JF, Robert JR (eds) Recent advances in aquaculture Vol 3. Timber Press, Portland, pp 63–138
Chiesa S, Filonzi L, Ferrari C, Vaghi M, Bilò F, Piccinini A, Zuccon G, Wilson RC, Ulheim J, Nonnis Marzano F (2016) Combinations of distinct molecular markers allow to genetically characterize marble trout (Salmo marmoratus) breeders and stocks suitable for reintroduction plans. Fish Res 176:55–64
Conover WJ (1999) Practical Nonparametric Statistics 3rd edition. Wiley, Hoboken
Conover WJ, Iman RL (1979) On multiple-comparisons procedures. Technical Report LA-7677-MS. Los Alamos Scientific Laboratory, Los Alamos
Crivelli AJ, Poizat G, Berrebi P, Jesensek D, Rubin JF (2000) Conservation biology applied to fish: the example of a project for rehabilitating the marble trout in Slovenia. Cybium 24:211–230
Delling B, Crivelli AJ, Rubin JF, Berrebi P (2000) Morphological variation in hybrids between Salmo marmoratus and alien Salmo species in the Volarja stream, Soča River basin, Slovenia. J Fish Biol 57:1199–1212
Diaha NC, Zudaire I, Chassot E, Barrigah BD, Irié YD, Gbeazere DA, Kouadio D, Pecoraro C, Romeo MU, Murua H, Amandè MJ, Dewals P, Bodin N (2016) Annual monitoring of reproductive traits of female yellowfin tuna (Thunnus albacares) in the eastern Atlantic Ocean. Collect Vol Sci Pap ICCAT 72:534–548
Estay F, Dìaz A, Pedrazza R, Colihueque N (2003) Oogenesis and plasma levels of sex steroids in cultured females of brown trout (Salmo trutta Linnaeus, 1758) in Chile. J Exp Zool 298:60–66
Estay F, Colihueque N, Araneda C (2012) Comparison of oogenesis and sex steroid profiles between twice and once annually spawning of rainbow trout females (Oncorhynchus mykiss). Sci World J. doi 10.1100/2012/986590
Forneris G, Merati F, Pascale M, Perosino GC (2005) Materiali e metodi per i campionamenti e monitoraggi dell’ittiofauna (determinazione della qualità delle comunità ittiche). Digital Print, Torino
Fostier A, Jalabert B, Billard R, Breton R, Zohar Y (1983) The gonadal steroids. In: Hoar AWS, Randall DJ, Donaldson EM (eds) Fish physiology, Vol IX. Academic Press, New York, pp 277–372
Fregeneda-Grandes JM, Hernàndez-Navarro S, Fernandez-Coppel IA, Correa-Guimaraes A, Ruìz-Potosme N, Navas-Garcia LM, Aller-Gancedo JM, Martìn-Gil FJ, Martin-Gil J (2013) Seasonal and sex-related variations in serum steroids hormone levels in wild and farmed brown trout Salmo trutta L. in the north-west Spain. J Water Health 11:720–728
Friedland KD, Ama-Abasi D, Manning M, Clarke L, Kligys G, Chambers RC (2005) Automated egg counting and sizing from scanned images: rapid sample processing and large data volumes for fecundity estimates. J S Res 54:307–316
Fumagalli L, Snoj A, Jesensek D, Balloux F, Jug T, Duron O, Brossier F, Crivelli AJ, Berrebi P (2002) Extreme genetic differentiation among the remnant populations of marble trout Salmo marmoratus in Slovenia. Mol Ecol 11:2711–2716
Gray CA, Barnes LM, van der Meulen DE, Kendall BW, Ochwada-Doyle FA, Robbins WD (2014) Depth interactions and reproductive ecology of sympatric Sillaginidae: Sillago robusta and S. flindersi. Aquat Biol 21:127–142
Gülsoy N (2007) Development of the yolk nucleus of previtellogenetic oocytes in Rainbow trout, Oncorhynchus mykiss, studied by light microscopy. J Appl Biol Sci 1:33–35
Heinimaa S, Heinimaa P (2004) Effect of the female size on egg quality and fecundity of the wild Atlantic salmon in the sub-artic River Teno. Boreal Environ Res 9:55–62
Hoogenboom MO, Metcalfe NB, Groothuis TGG, de Vries B, Costantini D (2012) Relationship between oxidative stress and circulating testosterone and cortisol in pre-spawning female brown trout. Comp Biochem Physiol A Mol Integr Physiol 163:379–387
Jamalzadeh HR, Akhundian M, Kabir M, Khara H, Mirrasuli E, Hajirezaee S, Golpour A (2013) Changes of gonadosomatic index and plasma levels of cortisol in the male and female captive Caspian brown trout, Salmo trutta Caspius (Kessler, 1877) during the reproductive cycle. J Appl Anim Res 4:133–136
Jelli F, Alessio G, Duchi A (1991) Biologia della trota marmorata, Salmo (trutta) marmoratus Cuv. In: Provincia Autonoma di Trento, Istituto Agrario di S. Michele all’Adige (eds) Atti del 4° Convegno Nazionale AIIAD – Distribuzione della fauna ittica italiana. Riva del Garda, 12– 13 dicembre 1991. Privincia Autonoma di Trento, Trento, pp 46–75
Jordanova M, João Rocha M, Rebok K, Rocha E (2013) Variations in the volumes of parenchyma and stroma of the liver and in the cytology of hepatocytes are associated with gonadal stages in female Ohrid trout (Salmo letnica). Ichthyol Res 60:26–35
Jug T, Berrebi P, Snoj A (2005) Distribution of non native trout in Slovenia and their introgression with native trout populations as observed through microsatellite DNA analysis. Biol Conserv 123:381–388
Kagawa H (2013) Oogenesis in Teleost Fish. Aqua-BioSci Monogr 6:99–127
Kagawa H, Young G, Nagahama Y (1983) Relationship between seasonal plasma estradiol-17β and testosterone levels and in vitro production by ovarian follicles of amago salmon (Oncorhynchus rhodurus). Biol Reprod 29:391–309
Kanamori A, Adachi S, Nagahama Y (1988) Developmental changes in steroidogenic responses of ovarian follicles of amago salmon (Oncorhynchus rhodurus) to chum salmon gonadotropin during oogenesis. Gen Comp Endocrinol 72:13–24
King HR, Pankhurst NW (2003) Ovarian growth and plasma sex steroids and vitellogenin profiles during vitellogenesis in Tasmanian female Atlantic salmon (Salmo salar). Aquaculture 219:797–813
Klibansky N, Juanes F (2007) Species-specific effects of four preservative treatments on oocytes and ovarian material of Atlantic cod (Gadus morhua), haddock (Melanogrammus aeglefinus), and American plaice (Hippoglossoides platessoides). Fish Bull 105:538–547
Klibansky N, Juanes F (2008) Procedures for efficiently producing high-quality fecundity data on a small budget. Fish Res 89:84–89
Kottelat M, Freyhof J (2007) Handbook of European Freshwater Fishes. Kottelat, Cornol & Freyhof, Berlin
Kwon JY, Prat F, Randall C, Tyler CR (2001) Molecular characterization of putative yolk processing enzymes and their expression during oogenesis and embryogenesis in rainbow trout (Oncorhynchus mykiss). Biol Reprod 65:1701–1709
Lorenzoni M, Giannetto D, Maio G, Pizzul E, Pompei L, Turin P, Vincenzi S, Crivelli A (2012) Empirical standard mass equation for Salmo Marmoratus. J Fish Biol 81:2086–2091
Meldgaard T, Crivelli AJ, Jesensek D, Poizat G, Rubin JF, Berrebi P (2007) Hybridization mechanism between the endangered marble trout (Salmo marmoratus) and the brown trout (Salmo trutta) as revealed by in-stream experiments. Biol Conserv 136:602–611
Meraner A, Baric S, Pelster B, Dalla Via J (2007) Trout (Salmo trutta) mitochondrial DNA polymorphism in the centre of the marble trout distribution area. Hydrobiologia 579:337–349
Meraner A, Baric S, Dalla Via J (2008) The selection of the wild: a combined molecular approach for the identification of pure indigenous fish from hybridized populations. Comp Biochem Physiol D 3:36–42
Meraner A, Baric S, Pelster B, Dalla Via J (2010) Microsatellite DNA data point to extensive but incomplete admixture in a marble and brown trout hybridization zone. Conserv Genet 11:985–999
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northwest Atl Fish Sci 33:33–54
Norberg B, Björnsson BT, Brown CL, Wichardt UP, Deftos LJ, Haux C (1989) Changes in plasma vitellogenin, sex steroids, calcitonin and thyroid hormones related to sexual maturation in female brown trout (Salmo trutta). Gen Comp Endocrinol 75:316–326
Nusrat R, Ulfat J (2013) Study on the fecundity of Salmo trutta fario (Brown trout) in Kashmir. J Biol Life Sci 4(1):181–193
Povž M (1995) Status of freshwater fishes in the Adriatic catchment of Slovenia. Biol Conserv 72:171–177
Povž M, Jesensek D, Berrebi P, Crivelli AJ (1996) The Marble Trout, Salmo trutta marmoratus, Cuvier 1817, in the Soca River Basin, Slovenia. Tour du Valat Publication, Arles
Pujolar JM, Vincenzi S, Zane L, Jesensek D, De Leo GA, Crivelli AJ (2011) The effect of recurrent floods on genetic composition of marble trout populations. PLoS One 6:e23822
Pustovrh G, Snoj A, Sušnik Bajec S (2014) Molecular phylogeny of Salmo of the western Balkans, based upon multiple nuclear loci. Genet Sel Evol 46:1–12
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 19 October 2016
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board of Can 191:203–234
Rondinini C, Battistoni A, Peronace V, Teofili C (2013) Li sta rossa IUCN dei vertebrati Italiani, Comitato Italiano IUCN’e. Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Seber GAF (1973) The estimation of animal abundance and related parameters. Charles Griffin Company, London
Seber GAF, Le Cren ED (1967) Estimating population parameters from catches large relative to the population. J Anim Ecol 36:631–643
Simčič T, Jesensek D, Brancelj A (2015) Metabolic characteristics of early life history stages of native marble trout (Salmo marmoratus) and introduced brown trout (Salmo trutta) and their hybrids in the Soča River. Ecol Freshw Fish. doi 10.1111/eff.12264
Snoj A, Jug T, Milkic E, Susnik S, Pohar J, Dovc P, (2000) Mitochondrial and microsatellite DNA analysis of marble trout in Slovenia. J Freshw Biol (Quaderni ETP) 29:5–11
Sommani E (1961) Il Salmo marmoratus Cuv.: sua origine e distribuzione nell’Italia settentrionale. Bollettino di Pesca, Piscicoltura e Idrobiologia 15:40–47
Specchi M, Battistella S, Amirante GA, Sigalotti GM, Tibaldi E, Pizzul E (2004) Il recupero della trota marmorata nel Friuli Venezia Giulia. Ente Tutela Pesca del Friuli Venezia Giulia, Udine
StatSoft Italia s.r.l. (2005) STATISTICA (sistema software di analisi dei dati), versione 7.1. http://www.statsoft.it. Accessed 27 March 2016
Sušnik Bajec S, Pustovrh G, Jesenšek D, Snoj A (2015) Population genetic SNP analysis of marble and brown trout in a hybridization zone of the Adriatic watershed in Slovenia. Biol Conserv 184:239–250
Taranger GL, Haux C, Stefansson SO, Björnsson BT, Walther BTh, Hansen T (1998) Abrupt changes in photoperiod affect age at maturity, timing of ovulation and plasma testosterone and oestradiol-17b profiles in Atlantic salmon, Salmo salar. Aquaculture 162:85–98
Taranger GL, Munacaster S, Norberg B, Thorsen A, Andersson E (2015) Environmental impacts on the gonadotropic system in female Atlantic salmon (Salmo salar) during vitellogenesis: Photothermal effects on pituitary gonadotropins, ovarian gonadotropin receptor expression, plasma sex steroids and oocyte growth. Gen Comp Endocrinol 221:86–93
Thorsen A, Kjesbu OS (2001) A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. J Sea Res 46:295−308
Topic Popovic N, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Persin Berakovic A, Sauerborn Klobucar R (2012) Tricaine methane-sulfonate (MS-222) application in fish anesthesia. J Appl Ichthyol 28:553–564
Tortonese E (1967) La trota marmorata o padana. Rivista italiana di Piscicoltura e Ittiopatologia 2:7–8
Turin P, Zanetti M, Bilò MF (2006) Distribuzione e stato delle popolazioni di trota marmorata nelle acque del bacino dell’alto Adriatico. Biologia Ambientale 20:39–44
Tyler CR, Sumpter JP (1996) Oocyte growth and development in teleosts. Rev Fish Biol Fish 6:287–318
Tyler CR, Sumpter JP, Witthames PR (1990) The dynamics of oocyte growth during vitellogenesis in the rainbow trout (Oncorhynchus mykiss). Biol Reprod 43:202–209
Vincenzi S, Crivelli AJ, Jesensek D, Rubin JF, De Leo GA (2007a) Early survival of marble trout Salmo marmoratus: evidence for density dependence? Ecol Freshw Fish 16:116–123
Vincenzi S, Crivelli AJ, Jesensek D, Rubin JF, De Leo GA (2007b) Density-dependent individual growth of marble trout (Salmo marmoratus) in the Soca and Idrijca river basins, Slovenia. Hydrobiologia 583:57–68
Vincenzi S, Crivelli AJ, Jesensek D, Rubin JF, Poizat G, De Leo GA (2008a) Potential factors controlling the population viability of newly introduced endangered marble trout populations. Biol Conserv 141:198–210
Vincenzi S, Crivelli AJ, Jesensek D, De Leo GA (2008b) The role of density-dependent individual growth in the persistence of freshwater salmonid populations. Oecologia 156:523–534
Vincenzi S, Crivelli AJ, Jesensek D, De Leo GA (2010a) The management of small, isolated salmonid populations: do we have to fix it if it ain’t broken? Anim Conserv 13:21–23
Vincenzi S, Crivelli AJ, Jesensek D, De Leo GA (2010b) Individual growth and its implications for the recruitment dynamics of stream-dwelling marble trout (Salmo marmoratus). Ecol Freshw Fish 19:477–486
Vincenzi S, Crivelli AJ, Jesensek D, Rossi G, De Leo GA (2011) Innocent until proven guilty? Stable coexistence of alien rainbow trout and native marble trout in a Slovenian stream. Naturwissenschaften 98:57–66
Vincenzi S, Crivelli AJ, Jesensek D, De Leo G (2012) Traslocation of stream-dwelling salmonids in headwaters: insight from a 15-year reintroduction experience. Rev Fish Biol Fish 22:437–455
Wilkinson RJ, Longland R, Woolcott H, Porter MJR (2010) Effect of elevated winter–spring water temperature on sexual maturation in photoperiod manipulated stocks of rainbow trout (Oncorhynchus mykiss). Aquaculture 309:236–244
Williams JE, Johnson JE, Hendrickson DA, Contreras-Balderas S, Williams JD, Navarro-Mendoza M, McAllister DE, Deacon JE (1989) Fishes of North America endangered, threatened, or of special concern. Fisheries 14:2–20
Wootton RJ (1998) Ecology of teleost fishes. Kluwer Academic Publishers, Dordrecht
Young G, Kagawa K, Nagahama Y (1983) Evidence for a decrease in aromatase activity in the ovarian granulosa cells of amago salmon (Oncorhynchus rhodurus) associated with final oocyte maturation. Biol Reprod 29:310–315
Acknowledgements
This work was funded and sustained by the Safeguard Fisheries Authority of the Region Friuli Venezia Giulia (Ente Tutela Pesca in Italian). Many thanks are due to Prof. Giorgio Valli for his support.
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Marchi and M. Bertoli contributed equally to this work.
About this article
Cite this article
Marchi, I., Bertoli, M., Mosco, A. et al. Analysis of the reproductive cycle of female wild marble trout Salmo marmoratus in a prealpine brook of the Soča River basin (Northeast Italy). Ichthyol Res 64, 221–231 (2017). https://doi.org/10.1007/s10228-016-0563-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0563-2