Abstract
Although the main features of salmonid life cycles are currently well known, marble trout (Salmo marmoratus) populations have been scarcely studied due to the present limited geographical distribution of the species. In this work we tested the hypothesis of density-dependent individual growth of marble trout with data gathered from multi-year on-going monitoring started in 1996 in three streams (Zakojska, Gorska and Gatsnik) in the Soca and Idrijca river basins (Slovenia). As observed for other salmonid species, marble trout exhibits high plasticity in body size and growth in response to environmental conditions. Age-specific mean lengths are significantly different among the three streams. Despite this variability, the statistical analyses outlined some clear patterns. Mean lengths of marble trout cohorts aged ≥1+ are correlated with total population density in the first year of life of the cohorts; the age-specific relationship between mean length and total density is well explained by negative power curves. Length of marble trout at age 1+ is significantly correlated with length at subsequent years up to age 4+. ANCOVAs performed on the stream-specific datasets showed a significant effect of total density of marble trout on annual age-specific individual growth rates. Von Bertalanffy’s body growth models were calibrated on length–age data of Zakojska and Gorska marble trout. Our analysis shows the existence of density-dependent effects on growth of marble trout that might be crucial in regulating population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying compensatory responses to changes in population density is of crucial importance to our understanding of the demography and dynamics of structured populations. Competition for limiting resources or predation are commonly claimed as the main factors underlying density-dependent patterns (i.e. Post et al., 1999). Density-dependent mortality and density-dependent emigration are phenomena widely described for stream-dwelling salmonids, while density-dependent growth is more difficult to detect in the wild (Grant & Kramer, 1990). However, density-dependent growth as a result of competition for food has been described for many fish populations (e.g. Beverton & Holt, 1957; Rochet, 1998; Jenkins et al., 1999; Post et al., 1999; Imre et al., 2005), even though, as noted by Lorenzen & Endberg (2002), its importance as a mechanism of population regulation remains little appreciated. Given the tight relationship between fecundity and body size (Jenkins et al., 1999; Klemetsen et al., 2003), density-dependence in individual growth in fish populations is a potentially powerful mechanism of population regulation. In fact, substantial reduction in fish abundance in lakes are often followed by high recruitment that either quickly restores population sizes to pre-manipulation levels or produces population cycles (Jenkins et al., 1999). Recent studies by Jenkins et al. (1999) on brown trout and by Imre et al. (2005) on Atlantic salmon have demonstrated the occurrence of density-dependent individual growth in salmonids in freshwater streams. A recent meta-analysis on density-dependent growth of juvenile stream-dwelling salmonids by Grant & Imre (2005) found that population density affects individual growth via exploitative competition, that is the depletion of food by competitors (Keddy, 1989), at intermediate population densities (≤1 fish m−2), while at higher densities, when space is limiting, density mainly affects survival via competition for suitable territory. Density-dependent growth in salmonid populations was also reported by Hartman & Scrivener (1990), Crisp (1993) and Newman (1993), suggesting that this might be a general bottom-up regulating mechanism for stream-dwelling salmonid populations (Imre et al., 2005).
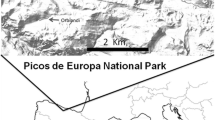
Here, we explore the issue of density-dependent growth in three translocated populations of the endangered marble trout Salmo marmoratus (Cuvier) in the Soca and Idrijca river basin. Among trout species, the marble trout Salmo marmoratus (Dorofoyeva et al., 1992; Kottelat, 1997) is of particular conservation interest, as only a few pure populations exist today in a restricted geographical distribution in the basin of the Po River in northern Italy (Sommani, 1961; Forneris et al., 1990; Giuffra et al., 1996), in the Adriatic basin of former Yugoslavia (Povz, 1995) and in Albania (Schoffmann, 1994). A conservation programme has been launched in Slovenia to duplicate the existing pure populations (Fig. 1) through translocation of pure marble trout in fishless streams (Crivelli et al., 2000). Since 1996, an annual monitoring programme has been carried out in order to assess the long-term persistence and the population dynamics of those new populations.
The aim of the present study is to test the hypothesis of density-dependence in individual growth in the progeny of translocated marble trout in pristine Slovenian mountain streams. Although there are a number of studies dealing with the description of somatic growth of salmonid populations, most of them focus on more widespread species, such as brown trout Salmo trutta L. (e.g., Jenkins et al., 1999; Vollestad et al., 2002), rainbow trout Oncorhynchus mykiss (Walbaum) (e.g., Su et al., 1996; Gardeur et al., 2001), and Atlantic salmon Salmo salar L. (e.g., Gardeur et al., 2001; Imre et al., 2005). On the contrary, little is known on the individual growth dynamics of marble trout, which is unfortunate, as the quantitative analysis of somatic traits and investigation of density-dependence in individual growth is a crucial step to understand the population dynamics, to assess the long-term viability of the populations and the efficacy of rehabilitation projects for this species of great conservation interest. Specifically, we tested the following hypotheses: (1) population density significantly affects the age-specific annual growth rates; (2) mean body length at age x(1+ ≤ x ≤ 4+) of the individuals of a cohort is negatively correlated with total density of marble trout when the individuals of the same cohort were aged 1+.
Materials and methods
Study area and species description
The Soca and Idrijca river basins present a pristine environment with reduced agricultural and industrial activities, absence of erosion due to numerous deciduous forests—mainly Fagus sylvatica—, low population density and numerous areas officially protected, such as the Triglav National Park. Fly-fishing is one of the main attractions for tourists and one of the most profitable economic activities in the region, attracting many anglers from abroad. Fly-fishing is allowed only in the hybridisation zone in the lowland part of the rivers. Eight genetically pure populations (from 0% to 2% of foreign genes, Berrebi et al., 2000; Crivelli et al., 2000; Fumagalli et al. 2002), covering a length of 15 km (Fig. 1), were discovered in the headwaters of the Soca and Idrijca river basins, isolated from the hybridisation zone downstream by impassable waterfalls. Trout is the only fish species present within these streams and no predation by fish-eating birds and macroinvertebrates has been observed.
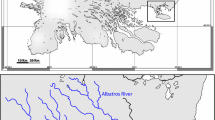
The remoteness of Zakojska, Gorksa and Gatsnik limits human disturbance linked to recreational activities (e.g., angling). Gorska and Zakojska are one-way streams, that is, marble trout can move only from upstream pools to downstream pools, while Gatsnik is a two-way stream; Gorska is more fragmented than Zakojska while Gatsnik does not present any discontinuity. The main features of the three streams are presented in Table 1 and in Fig. 2. Spawning takes place in November–December while marble trout eggs generally hatch in April. Marble trout reach sexual maturity at age 2+ and 3+ for males and females, respectively, and show a clear linear relationship between length of females and number of eggs produced (Crivelli, pers. comm.).
Stocking material
In June 1996, 500 marble trout reared in the fish farm were released as 1+ trout in fishless headwaters of Zakojska and Gorska streams. This stocking material was the progeny of marble trout females and marble trout males from the Zadlascica pure population (Fig. 1) caught in the wild in autumn 1994; females were stripped in the river and eggs reared up to fish aged 1+ within the fish farm (Tolminka, Tolmin, Slovenia).
In June 1998, 599 trout reared in the fish farm were released as 1+ trout in fishless headwaters of Gatsnik stream. The Gatsnik stocking material was the progeny of 26 marble trout females from the Trebuscica (Fig. 1) pure population and 12 marble trout males from the Lipocscek (Fig. 1) pure population caught in the wild in autumn 1996; females were stripped in the fish farm and eggs reared up to fish aged 1+ within the fish farm.
Sampling procedure
After the introduction, stocked trout successfully reproduced within the streams. Marble trout hatched in the streams, along with stocking materials, were sampled every June from 1997 to 2004 for Zakojska and Gorska and from 1999 to 2004 for Gatsnik on the whole length of each stream starting from downstream to the upstream extent. Using a gasoline-powered, portable backpack electrofishing unit, we electrofished every stream two times to produce a multiple-pass removal estimate of trout abundance using Microfish 3.0 (Van Deventer & Platts, 1989). All captured fish aged ≥1+ and with a minimum size of 115 mm (L T) were anaesthetized with benzocaine, marked with Carlin tags (Institute of Freshwater Research, Sweden), measured for total length (L T, to the nearest mm) and weight (W, g) and if sampled for the first time the adipose fin was removed. Marble trout then were returned live near the point of capture. Age was determined by tag examination. Age designation follows standard terminology; marble trout in the first year of life were denoted as 0+ and in the second year, subsequent to winter annulus formation, as 1+. At first marking, scales were taken to assess the age of the fish. The small size of marble trout aged 0+ prevents their sampling in June and they are not taken into account in this study. No sexing of trout caught could take place in June. A total of 1,305, 432 and 3,862 marble trout hatched within the streams were sampled in Zakojska, Gorska and Gatsnik, respectively.
Data analysis
We carried out statistical analyses only on marble trout hatched in the streams after introduction. Stocking materials were not considered, as marble trout reared in the fish farm up to 1+ for the rehabilitation project may exhibit growth patterns quite different from those of marble trout naturally hatched within the streams and growing in the wild.
Length and weight data were log transformed to normalize the distributions. Assumption of normality of distribution and homogeneity of variances were tested through Shapiro–Wilk (Shapiro & Wilk, 1965) and Levene’s (Levene, 1960) tests, respectively. We use analyses of variance (ANOVA) to examine inter-population and intra-population variability in somatic traits across ages, and Tukey post-hoc tests (Tukey honest significant difference) for pair-wise comparison of means within group analyses yielding significant results.
Age-specific annual individual growth rates in length G L (year−1) was computed as follows: \( G_{\hbox{L}} (x) = \log \frac{{L_{\hbox{T}} (x + 1)}} {{L_{\hbox{T}} (x)}} \) where L T(x + 1) and L T(x) are the total length of marble trout at age x + 1 and age x, respectively.
Correlation between L T at age 1+ and L T at subsequent years was estimated by using Pearson’s r.
Total density of marble trout at the stream level was estimated by dividing the total number of marble trout (stocking material + marble trout hatched within the stream) sampled for the overall surface area of pools in each stream. Differences in mean density across years among streams were estimated by fitting a linear mixed-effect model (Pinheiro & Bates, 2000) with year of sampling modelled as random effect within streams. Differences in annual growth rate G L(x) were tested with analysis of covariance (ANCOVA) using, as explanatory variables, age, length, total density of trout when the marble trout is aged x and total density of trout when the marble trout was in its first year of life.
Statistics were computed on each stream separately. When testing the relationship between mean length at age x of the individuals of a cohort and total density of marble trout when the individuals of the same cohort were aged 1+, the analysis was carried out also by pooling together the stream-specific data sets to cover a wider range of trout densities. The relationship between mean length at age x of each cohort and total population density during the first year of life of the same cohort was tested by linear regression and by a negative power curve, estimated from linear regression after a logarithmic transformation of length and density data. Akaike Information Criterion (AIC, Motulsky & Christopoulos, 2004) was computed for each model; the model with the smallest AIC value was considered to offer a better fit if AICpower − AIClinear ≥ 2 (Motulsky & Christopoulos, 2004).
Mean stream-specific growth patterns were investigated by fitting Von Bertalanffy’s growth curves (Von Bertalanffy, 1938) in the form: \( L(x) = L_\infty \,\left[ {{\hbox{1}} - {\hbox{exp}}( - k(x - x_0 )} \right] \)
where x is the age (years), L ∞ is the maximum length (mm), k is the Brody’s growth coefficient relating age to length, and x 0 is the age at which length equals 0.
Von Bertalanffy’s growth parameters were estimated using non-linear least-squares estimation. Von Bertalanffy’s growth curve could not be estimated for Gatsnik, as marble trout have not reached the maximum age in Gatsnik, thus preventing the estimation of the asymptotic length L ∞. Differences in Von Bertalanffy’s growth curve parameters L ∞ and k between Gorska and Zakojska were tested by a t-test (Motulsky & Christopoulos, 2004) as follows: \( t = \frac{{b_{{\hbox{zakojska}}} - b_{{\hbox{gorska}}} }} {{\sqrt {{\hbox{Std}}{\hbox{.Err}}_{{\hbox{zakojska}}}^2 - {\hbox{Std}}{\hbox{.Err}}_{{\hbox{gorska}}}^2 } }} \) where b is the parameter estimate and the degrees of freedom for the t statistics are equal to the sum of the df from each model fitting.
The significance level α for all statistical tests is set at 0.05. All statistical analyses were performed by using the statistical software R (R development Core Team, 2005).
Results
Age and growth of marble trout
Table 2 reports the basic statistics relative to length, weight and age-specific growth rates in length G L(x) of marble trout hatched in Zakojska, Gorska and Gatsnik. The first cohorts of marble trout aged 1+ born in the streams were sampled in year 1999, 2000 and 2001 for Gorska, Zakojska and Gatsnik, respectively.
The mean age-specific lengths and weights varied significantly among the streams for all ages (ANOVA, p < 0.001 for all ages). In Gorska, age-specific mean length was higher than in Zakojska and in Gatsnik (Tukey test, p < 0.05 for all ages).
Length of marble trout at age 2+, 3+ and 4+ was positively correlated with length of the same individual at age 1+ in each stream, except for Gorska where length at age 4+ was not significantly correlated with length at age 1+, probably due to the low number of samples (Table 3). The correlations were significant also when pooling together the stream-specific datasets (Table 3).
Significant differences in mean G L(x) among streams were found only from age 2+ to age 3+, but subsequent comparison of means revealed significant difference only between Gorska and Gatsnik (Tukey test, p < 0.05). Growth of marble trout is greatly reduced after the first years of life (Table 2). Growth from age 2+ to 3+ was only 54, 44 and 46% of growth from age 1+ to 2+ in Zakojska, Gorska and Gatsnik, respectively.
Density-dependent effects
Data relative to marble trout population density is reported in Table 4. Density of marble trout varied annually within each stream. Density of marble trout in ind. m−2 is significantly different among streams, with Gorska presenting the lowest mean density. The ANCOVAs performed on stream-specific datasets (Table 5) revealed that G L(x) is affected by total density of marble trout in the first year of life for Gorska and Gatsnik trout populations and by total population density when marble trout is aged x for the Gorska and Zakojska marble trout populations. Analyses performed using body weight instead of length showed similar results.
There was no significant effect of total population density during the first year of life of the cohort on mean length at age x of marble trout of the same cohort when we tested each of the three streams separately, neither when modelling the pattern with negative power curve nor with linear regression. However, when pooling together the data of the three streams, mean length of each cohort from age 1+ to age 4+ is negatively correlated with total density of marble trout (ind. m−2) when the individuals of the same cohort where aged 1+ (Fig. 3). In each case, the negative power curve provided a significantly better fit of the density-dependent pattern than the linear model. It was not possible to compute the correlation between average length of 5+ cohorts and density during the first year of life of the same cohorts due to the low number of individuals in the sample. When density is measured in biomass (g m−2), fitting negative power curves explain a smaller, yet significant, portion of the variability of mean length of marble trout cohorts for all ages.
Von Bertalanffy’s growth model
The Von Bertalanffy’s growth curve was estimated on 1,305 and 432 length–age data for Zakojska and Gorska, respectively (Fig. 4). Von Bertalanffy’s growth curve parameters are reported in Table 6. The t-tests showed significant differences in L ∞ but not in k between Zakojska and Gorska.
Von Bertalanffy’s growth curve (solid line) and 95% confidence intervals (dashed lines) as estimated from marble trout sampled in Zakojska and in Gorska. Parameter estimates are reported in Table 6
Discussion and conclusions
It is well known that salmonid species usually present remarkable variations in somatic and demographic traits resulting from either genetic differences or from plasticity in growth rates in response to variable environmental conditions (Klemetsen et al., 2003). Our analysis shows that variations in somatic traits, like mean age-specific length and weight, also were notable across the three translocated marble trout populations in Slovenian mountain streams. Despite this variability, some common patterns can be detected in all the three populations.
The annual variation in total density of marble trout within and across streams might be partially explained by the topographic and physical features of the streams (Fig. 2). It is well known that the availability of shelters and suitable microhabitat for juveniles are limiting factors for population abundance (Lobón-Cerviá & Rincón, 2004). Further studies will be necessary to determine whether stream discharge combined with stream morphology is a crucial determinant of population size, as argued by Lobón-Cerviá & Rincón (2004), also for the three streams under study. Despite this caveat, the results of our analysis suggest that density-dependence in individual growth of marble trout may indeed occur in the studied system (Table 5). The relationship between average length of marble trout cohorts from age 1+ to 4+ and total population density during the first year of life of the cohorts was non-significant at the stream level, probably because of the small range of variation of population density within each stream. On the other hand, as argued by Jenkins et al. (1999), the detection of density-dependence patterns is obviously easier when data span over a wide range of densities. In fact, when pooling the datasets of the three streams together, our study provides statistical evidence of the effect of total density during the first year of life on mean length of marble trout cohorts from age 1+ to age 4+. Our results are in agreement with those of Jenkins et al. (1999) for S. trutta and Imre et al. (2005) for S. salar, who hypothesized that body size of underyearlings follows a negative power curve in relation to fish density. Other studies suggest a curvilinear relationship between average body size or growth rate and population density (e.g. Hartman & Scrivener, 1990; Crisp, 1993; Newman, 1993), thus implying that this might be a general pattern for stream-dwelling salmonids.
The evidence gathered by Jenkins et al. (1999) and by Imre et al. (2005) in favour of density-dependent growth of underyearling of salmonid and the results of our study seem to suggest that the total population density during the first year of life may affect the growth pattern of marble trout during the entire life span. In fact, we also found a positive correlation between length at age 1+ and length at subsequent years (Table 3); this means that larger marble trout at age 1+ tend to remain larger also at older ages. This might have important implications for the population dynamics of the marble trout: if larger trout are expected to spawn more and/or produce larger eggs (Koops et al., 2004), density-dependency in individual growth rate from hatching up to age 1+ may affect the overall reproductive output of the trout during its entire reproductive life span, as already argued by Lobón-Cerviá et al. (1997) for S. trutta in the Esva River (Spain). A number of studies reported a general correlation between rapid growth and early sexual maturation (Hutchings & Jones, 1998; Utrilla & Lobón-Cerviá, 1999); accordingly, trout growing relatively slowly could delay sexual maturation in order to reach the minimum size required for gonad development. This phenomenon was already observed for brown trout in the Esva River (Spain) (e.g. Lobón-Cerviá et al., 1997), though the nature of phenotypic covariation between body size and age of sexual maturity in salmonids is still not completely understood (Martyniuk et al., 2003). In lakes, reductions in density are commonly followed by high recruitment, a mechanism possibly leading to fluctuation in fish abundance (Hamrin & Persson, 1986; Townsend & Perrow, 1989; Persson et al., 1993; Tonn et al., 1994). In streams, these density-related fluctuations could be more difficult to detect; in fact, it is well known that exogenous environmental factors (such as floods or droughts) can cause the population to collapse to very low densities before endogenous intrinsic density-dependent regulation mechanisms take place (Lobón-Cerviá & Rincón, 2004). However, given the observational nature of our data and the sampling interval of one year, we cannot exclude that other factors affecting growth, such as food abundance (Imre et al., 2004) or water temperature (Egglishaw & Shackley, 1985), might explain the observed pattern of body growth. As for the potential mechanism of density-dependent growth, the analysis carried out in our three experimental streams suggests that density-dependent growth is probably caused by exploitation rather than interference competition, as evidenced also by Grant & Imre (2005) for juvenile stream-dwelling salmonids at densities ≤1 fish m−2. Post et al. (1999) observed that in organisms with plastic growth, exploitative competition typically results in reduction in the growth rate of all individuals when food abundance is limiting. As noted by Imre et al. (2005), in the case of exploitative competition for a renewing resource, such as stream drift, the per capita foraging rate will decrease as 1/n − n being the number of competitors (Kramer et al., 1997)—thus following a negative power curve.
From a population regulation point of view, high individual growth at low densities may enable the marble trout populations to rebound quickly after catastrophic events, such as landslides, droughts and floods. On the other hand, as noted by Jenkins et al. (1999), density-dependent growth may impose an upper limit to population abundance, through at least two mechanisms. First, density-dependent growth, especially during the first year of life, could translate into density-dependent survival over the first winter as argued by Post et al. (1999); second, as fecundity in marble trout is positively related to female length, density-dependence in growth should limit the egg production of a population.
The presence of density-dependence in individual growth in marble trout is an important issue for conservation managers, particularly for translocations of marble trout in fishless streams to enhance the viability of the species. As stressed by Grant & Imre (2005), a successful recovery programme needs to maximize recruitment without wasting the stocking resource. In this sense, our results suggest that particular attention must be paid to the number of fish introduced when stocking marble trout; in fact, given the tight relationship between body length and number of eggs produced, the density-dependence in individual growth and the other compensatory responses observed in freshwater salmonids (Rose et al. 2001) are likely to regulate the population abundance and allow it to reach its upper limit in a few generations. In addition, to avoid the negative effects of density-dependent growth, scatter stocking is preferable to point stocking. As noted by Berg & Jørgensen (1991) stocking fish at higher densities than the carrying capacity of the stream could be more harmful than beneficial, as the consequent overall reduced growth rate may result in a collapse of the trout population in the stream.
For marble trout, the individual annual growth rate in length G L(x) decreases as age increases, therefore it is appropriate to fit a Von Bertalanffy growth curve to model the growth of marble trout. Von Bertalanffy’s growth curves were calibrated on age-length data only for marble trout from Zakojska and Gorska (Table 6). Beverton & Holt (1957) argued that density-dependent growth mediated by food competition is expected to affect the asymptotic length L ∞. Our findings indeed suggest a crucial role of population density in determining asymptotic length L ∞ (Table 6). Comparison of Von Bertalanffy growth parameters among different species of salmonids living in different environments should be interpreted cautiously, not only because of species-specific differences but also because individual growth is strongly influenced by environmental factors such as temperature (Elliot, 1975a, b; Edwards et al., 1979), invertebrate food (McFadden & Cooper, 1962) and water current (Mason, 1976). Von Bertalanffy growth parameters have been estimated for S. trutta individuals sampled in Old Castile (Spain) (L ∞ = 659 mm; k = 0.18 year−1) (Lobón-Cerviá et al., 1986), Schleswig-Holstein (Germany) (L ∞ = 570; k = 0.28) (Froese & Pauly, 2005) and Afon Cwm (Wales) (L ∞ = 216; k = 0.31) (Crisp & Beaumont, 1995). The amount of variation observed in growth patterns of salmonids seem to be a phenotypic adaptation to local ecological conditions, as shown by the experiment by Mann et al. (1983).
The results presented here are not conclusive yet, however, we are confident that our analysis of individual marble trout growth in Slovenian streams evidences the existence of density-dependent effects on growth that might be crucial to regulate its population dynamics.
References
AFNOR (Association Francaise de Normalisation), 1992. Essais des eaux. Determination de ĺindice biologique global normalisé (IBGN). AFNOR, NF T90-350, Paris.
Berg, S. & J. Jørgensen, 1991. Stocking experiment with 0+ and 1+ trout parr, Salmo trutta L., of wild and hatchery origin: 1. Post-stocking mortality and smolt yield. Journal of Fish Biology 39: 151–169.
Berrebi, P., M. Povz, D. Jesensek & A. J. Crivelli, 2000. The genetic diversity of native, stocked and hybrid populations of Marble trout in the Soca River, Slovenia. Heredity 85: 277–287.
Beverton, R. J. H. & S. J. Holt, 1957. On the Dynamics of Exploited Fish Populations. Chapman and Hall, London.
Crisp, D. T., 1993. Population densities of juvenile trout (Salmo trutta) in 5 upland streams and their effects upon growth, survival and dispersal. Journal of Applied Ecology 30: 759–771.
Crisp, D. T. & W. R. C. Beaumont, 1995. The trout (Salmo trutta) population of the Afon Cwm, a small tributary of the Afon Dyfi, mid-Wales. Journal of Fish Biology 46: 703–716.
Crivelli, A. J., G. Poizat, P. Berrebi, D. Jesensek & J. F. Rubin, 2000. Conservation biology applied to fish: The example of a project for rehabilitating the Marble trout in Slovenia. Cybium 24: 211–230.
Dorofeyeva, Ye. A., T. Vukovich & D. Seratlich, 1992. Osteological features of the endemic Balkan Marbled trout, Salmo marmoratus Cuv. (Salmonidae). Journal of Ichthyology 31: 113–121.
Edwards, R. W., J. W. Densem & P. A. Russell, 1979. An assessment of the importance of temperature as a factor controlling the growth rate of brown trout in streams. Journal of Animal Ecology 58: 501–507.
Egglishaw, H. J. & P. E. Shackley, 1985. Factors governing the production of juvenile Atlantic salmon in Scottish streams. Journal of Fish Biology 27: 27–33.
Elliott , J. M., 1975a. The growth rate of brown trout (Salmo trutta L.) fed on maximum rations. Journal of Animal Ecology 44: 805–821.
Elliott, J. M., 1975b. The growth rate of brown trout (Salmo trutta L.) fed on reduced rations. Journal of Animal Ecology 44: 823–842.
Forneris, G., G. B. Del Mastro & S. Bellardi, 1990. Attuale distribuzione di Salmo marmoratus Cuvier, 1817 in Provincia di Torino. Rivista di Idrobiologia 29: 213–221.
Froese, R. & D. Pauly, 2005. www.fishbase.org, version (07/2005).
Fumagalli, L., A. Snoj, D. Jesensek, F. Balloux, T. Jug, O. Duron, F. Brossier, A. J. Crivelli & P. Berrebi, 2002. Extreme differentiation among the remnant populations of marble trout (Salmo marmoratus) in Slovenia. Molecular Ecology 11: 2711–2716.
Gardeur, J. N., M. Paspatis, A. Gélineau & T. Boujard, 2001. Biostatistical implications of individual variability in growth in rainbow trout and Atlantic salmon. Aquaculture 195: 51–59.
Giuffra, E., R. Guyomard & G. Forneris, 1996. Phylogenetic relationships and introgression patterns between incipient parapatric species of Italian brown trout (Salmo trutta L. complex. Molecular Ecology 5: 207–220.
Grant, J. W. A. & I. Imre, 2005. Patterns of density-dependent growth in juvenile stream-dwelling salmonids. Journal of Fish Biology 67: 100–110.
Grant, J. W. A. & D. L. Kramer, 1990. Territory size as a predictor of the upper limit to population density of juvenile salmonids in streams. Canadian Journal of Fisheries and Aquatic Sciences 47: 1724–1737.
Hamrin, S. F. & L. Persson, 1986. Asymmetrical competition between age classes as a factor causing population oscillations in an obligate planktivorous fish species. Oikos 47: 223–232.
Hartman, G. F. & J. C. Scrivener, 1990. Impacts of forestry practices on a coastal stream ecosystem, Carnation Creek, British Columbia. Canadian Bulletin of Fisheries and Aquatic Sciences 223: 1–148.
Hutchings, J. A. & M. E. B. Jones, 1998. Life history variation and growth rate thresholds for maturity in Atlantic Salmon, Salmo Salar. Canadian Journal of Fisheries and Aquatic Sciences 55: 22–47.
Imre, I., J. W. A. Grant & E. R. Keeley, 2004. The effect of food abundance on territory size and population density of juvenile steelhead trout (Oncorhynchus mykiss). Oecologia 138: 371–378.
Imre, I., J. W. A. Grant & R. A. Cunjak, 2005. Density-dependent growth of young-of-the-year Atlanti salmon Salmo salar in Catamaran Brook, New Brunswick. Journal of Animal Ecology 74: 508–516.
Jenkins, T. M. Jr., S. Diehl, K. W. Kratz & S. D. Cooper, 1999. Effects of population density on individual growth of brown trout in streams. Ecology 80: 941–956.
Keddy P. A., 1989. Competition. Chapman and Hall.
Klemetsen, A., P.-A. Amundsen, J. B. Dempson, B. Jonsson, N. Jonsson, M. F. O’Connell & E. Mortensen, 2003. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Artic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish 12: 1–59.
Koops, M. A, J. A. Hutchings, & T. M. McIntyre, 2004. Testing hypotheses about fecundity, body size and maternal condition in fishes. Fish and Fisheries 5: 120–130.
Kottelat, M., 1997. European freshwater fishes. Biologia, Bratislava 52: 1–271.
Kramer, D. L., R. W. Rangeley & L. J. Chapman, 1997. Habitat selection: Patterns of spatial distribution from behavioural decisions. In Godin J.-G. J. (ed.), Behavioural Ecology of Teleost Fishes. Oxford University Press, Oxford: 37–80.
Levene, H., 1960. Robust tests for equality of variance. In Olkin I. (ed.), Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press, Stanford, CA: 278–292.
Lobón-Cerviá, J. & P. A. Rincón, 2004. Environmental determinants of recruitment and their influence on the population dynamics of stream-living brown trout Salmo trutta. Oikos 105: 641–646.
Lobón-Cerviá, J., C. Montanes & A. de Sostoa, 1986. Reproductive ecology and growth of a population of brown trout (Salmo trutta L.) in a aquifer-fed stream of Old Castile (Spain). Hydrobiologia 135: 81–94.
Lobón-Cerviá, J., C. Utrilla, P. Rincón & F. Amezcua, 1997. Environmentally induced spatio-temporal variations in the fecundity of brown trout Salmo trutta L.: trade-offs between egg size and number. Freshwater Biology 38: 277–288.
Lorenzen, K. & K. Endberg, 2002. Density-dependent growth as a key mechanism in the regulation of fish populations: Evidence from among-population comparison. Proceedings of the Royal Society of London B 269: 49–54.
Mann, R. H. K., C. A. Mills & D. T. Crisp, 1983. Geographical variation in the life history tactics of some species of freshwater fish. In Miller J. T. (ed.), Fish Reproduction. Academic Press, London: 171–186.
Martyniuk, C. J., G. M. L. Perry, H. K. Mogahadam, M. M. Ferguson & R. G. Danzmann, 2003. The genetic architecture of correlations among growth related traits and male age at maturation in rainbow trout. Journal of Fish Biology 63: 746–764.
Mason, J. C., 1976. Response of underyearling coho salmon to supplemental feeding in a natural stream. Journal of Wildlife Management 40: 775–788.
McFadden, J. T. & E. L. Cooper, 1962. An ecological comparison of six populations of brown trout (Salmo trutta). Transactions of the American Fisheries Society 91: 53–62.
Motulsky, H. & A. Christopoulos, 2004. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press, Oxford.
Newman, R. M., 1993. A conceptual model for examining density dependence in the growth of stream trout. Ecology of Freshwater Fish 2: 121–131.
Persson, L., G. Andersson, L. Johansson, S. Diehl & S. F. Hamrin, 1993. Density dependent interactions in lake ecosystems: Whole lake perturbations. Oikos 66: 193–208.
Pinheiro, J. & B. Douglas, 2000. Mixed-effect model in S and S-plus. Springer-Verlag, New York.
Post, J. R., E. A. Parkinson & N. T. Johnston, 1999. Density-dependent processes in structured fish populations: Interaction strength in whole-lake experiments. Ecological Monographs 69: 155–175.
Povz, M., 1995. Status of freshwater fishes in the Adriatic catchment of Slovenia. Biological Conservation 72: 171–177.
R Development Core Team, 2005. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna, Austria ISBN 3-900051-07-0 http://www.R-project.org.
Rochet, M. J., 1998. Short-term effects of fishing on life history traits of fishes. ICES Journal of Marine Science 55: 371–391.
Rose, K. A., J. H. Cowan Jr., K. O. Winemiller, R. A. Myers & R. Hilborn, 2001. Compensatory density dependence in fish populations: Importance, controversy understanding and prognosis. Fish and Fisheries 2: 293–327.
Schoffmann, J., 1994. Zur gegenwätigen Situation des marmorierten Forelle (Salmo marmoratus Cuvier, 1817) in Albanien, ihrem südlichsten Verbreitungsraum. Osterreichs Fischerei 47: 132–136.
Shapiro, S. S. & M. B. Wilk, 1965. An analysis of variance test for normality. Biometrika 52: 591–611.
Sommani, E., 1961. Il Salmo marmoratus Cuv.: sua origine e distribuzione nell’Italia settentrionale. Bolletino di Pesca, Piscicoltura e Idrobiologia 15: 40–47.
Su, G. S, L. E. Liljedahl & G. A. E. Gall, 1996. Genetic and environmental variation of body weight in rainbow trout (Oncorhynchus mykiss). Aquaculture 144: 71–80.
Tonn, W. M., I. J. Holopainen & C. A. Paszkowski, 1994. Density-dependent effects and the regulation of crucian carp populations in single-species ponds. Ecology 75: 824–834.
Townsend, C. R. & M. R. Perrow, 1989. Eutrophication may produce population cycles in roach, Rutilus rutilus (L.) by two contrasting mechanisms. Journal of Fish Biology 34: 161–164.
Utrilla, C. G. & J. Lobón-Cerviá, 1999. Life-history patterns in a southern population of Atlantic salmon. Journal of Fish Biology 55: 68–73.
Van Deventer, J. S. & W. S. Platts, 1989. Microcomputer Software System for Generating Population Statistics from Electrofishing Data-Users Guide for Microfish 3.0. U.S. Forest Service General Technical Report INT-254.
Vollestad, L. A., E. M. Olsen & T. Forseth, 2002. Growth-rate variation in brown trout in small neighbouring streams: Evidence for density dependence? Journal of Fish Biology 61: 1513–1527.
Von Bertalanffy, L., 1938. A quantitative theory of organic growth (Inquiries on growth laws II). Human Biology 10: 181–213.
Acknowledgements
We thank Fondation Tour du Valat and the Angling Association of Tolmin for funding this study. All the people who have participated each year to the fieldwork are warmly thanked. We thank Patrick Clevestam and Magnus Gehlin for helping us to get the Carlin marks, and S. Sumer for undertaking IBGN and benthos biomass investigations. We also thank Marino Gatto for helpful review of the manuscript. The author also thanks three anonymous referees for their valuable suggestions to improve a first draft of the MS. The authors thank the Italian Minister of Research for having partially supported the present work under an internationalization project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. Trexler
Rights and permissions
About this article
Cite this article
Vincenzi, S., Crivellì, A.J., Jesensek, D. et al. Density-dependent individual growth of marble trout (Salmo marmoratus) in the Soca and Idrijca river basins, Slovenia. Hydrobiologia 583, 57–68 (2007). https://doi.org/10.1007/s10750-006-0470-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0470-z