Abstract
We obtained the following five results in 10 rivers while elucidating the potential threat of introduced rainbow trout to native salmonids. First, native salmonid density decreased with increasing trout density. Second, four environmental variables, namely altitude, distance from the sea, river width, and river depth, did not significantly affect trout density. Third, the distribution of trout expanded, and trout were the dominant species in an unconfined river. Fourth, the trout invasion has changed salmonid faunas over the last 20 years. Lastly, smolt trout were collected from a river. Therefore, the potential threat to native salmonids due to trout introduction is continuously increasing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rainbow trout, Oncorhynchus mykiss, is the most widely introduced salmonid fish species worldwide (Welcomme 1992); however, the introduction of rainbow trout negatively affects native species and ecosystems (e.g., Dunham et al. 2004; Fausch 2007). The introduction of rainbow trout has also additionally occurred widely in Japan (Kitano 2004), and thus a number of studies have reported on natural reproduction and establishment of rainbow trout (e.g., Hokkaido, Aoyama et al. 1999; Nara, Kato and Yanagawa 2000). In Japan, rainbow trout is included in the top 100 of the worst invasive alien species in Japan (Murakami and Washitani 2002) and has been given a rank of “A2” in the Hokkaido Blue List (Hokkaido 2010). On Hokkaido Island, where the impact of rainbow trout on native species is a concern, the private stocking of rainbow trout is believed to be a major cause of their recent expansion. However, few studies have described the current status of the privately stocked rivers and the potential threat posed by privately stocked rainbow trout to native salmonids.
Several studies have examined the regulated factors for the establishment of trout and the interspecific relationship between the trout and native salmonids (Taniguchi et al. 2000; Fausch et al. 2001; Taniguchi et al. 2002; Inoue et al. 2009). Additionally, previous studies have suggested that the density of rainbow trout is affected by several environmental factors, such as flow disturbance, flow variability, altitude, and river gradient (Fausch et al. 2001; Inoue et al. 2009; Kitanishi et al. 2010). However, ecological information for the established rainbow trout population is still scarce to establish an adequate measure of rainbow trout management.
In this study, we examined the following four points to elucidate the potential threat posed by the introduced rainbow trout to native salmonids in 10 rivers in which rainbow trout had been privately stocked: 1) the effect of the density of rainbow trout on the density of native salmonids, 2) the effect of four environmental variables on the density of rainbow trout, 3) the longitudinal distribution of salmonid fishes, and 4) a comparison of the status of salmonid fauna between previous and present years. Our objective was to sound the alarm on the private stocking of rainbow trout by describing the current status of the privately stocked rivers.
Materials and methods
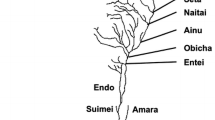
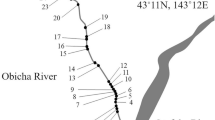
Study area. The present study was conducted in 10 rivers, where information of the inhabitation status of rainbow trout was obtained in advance, on 12-28 May 2015 (Fig. 1a). In the studied rivers, rainbow trout stocking had never been conducted by public institutions. To estimate the density of fish and to measure environmental variables, study stations were established in each river (reach length of the station: 40 m; number of the stations in each river: two to five; distance between the stations: ≥300 m; [Electronic Supplementary Material (ESM) Table S1]. In the river 2, 4, 5, 6, 9, and 10, impassable physical barrier (i.e., natural waterfall or erosion control dam) exists below the study stations, thus salmonid fishes cannot access the study stations from the mainstream and sea. The erosion control dams do not have reservoirs. In the Todo River, we established five study stations to investigate the longitudinal distribution of salmonid fishes (Fig. 1b). An impassable artificial dam exists below the study stations located at the Todo River (Fig. 1b); however, no physical barriers for fish migration or extreme water temperature gradient between the study stations existed.
a Study rivers at the southern part of Hokkaido, Japan. The Todo River is labeled as River 9. The names of the other rivers are given in Table 1. b Study stations along the Todo River. Dotted lines show contours and bold gray line shows boundary lines of the watershed. Densities of rainbow trout and native salmonids, and associated environmental variables are described in Table 1 and ESM Table S1
Sampling of fishes. The fishes were sampled using an electrofisher (200–300 V DC, model 12B Smith-Root, Inc., Vancouver, Washington, USA) with a dip net (30 cm in width, 3-mm mesh). The densities of the rainbow trout and native salmonids (i.e., white-spotted charr Salvelinus leucomaenis, Dolly Varden charr Salvelinus malma, and masu salmon Oncorhynchus masou) at each station were estimated using the two-pass removal method (model M(b), program CAPTURE, available at http://www.mbr-pwrc.usgs.gov/software/index.html). In certain rivers, 0+ juveniles of the white-spotted charr and masu salmon were collected; however, they were excluded from density estimation because quantitative collection is difficult for the 0+ juveniles during spring due to their small body size.
Measurement of environmental variables. To investigate the effects of environmental variables on the densities of rainbow trout, we measured the following four environmental variables as indices of environmental conditions for each station: altitude, distance from the sea, river width, and river depth (Table S1). The environmental variables were selected to evaluate the effect of study site location in a river system and its environmental suitability on the densities of rainbow trout. The altitude was measured for each station using a portable GPS receiver (GPSMAP62SJ, Garmin, Olathe, KS, USA). The distance from the sea was measured for each station using the GPS receiver and 1:25,000 scale topographic maps (http://www.gsi.go.jp/). River width was measured at 5-m intervals at each study station. River depth was measured at 5-m intervals with three evenly spaced transects (1/4, 1/2, and 3/4 of the river width).
Data analyses. To test the effect of density of rainbow trout on the density of native salmonids, we used a linear mixed model (LMM). In the LMM, the density of native salmonids was treated as a response variable, the density of rainbow trout was treated as an explanatory variable, and the river was treated as a random intercept. We additionally used an LMM to test the effect of the environmental variables on the density of rainbow trout. The density of rainbow trout was treated as a response variable, the environmental variables (i.e., altitude, distance from the sea, river width, or river depth) were treated as explanatory variables, and the river was treated as a random intercept in the LMM. The significance of the explanatory variables in the LMMs was evaluated using the F test. All statistical analyses were performed using RStudio Version 0.98.1103.
Results
A total of 545 rainbow trout and 191 native salmonid individuals (i.e., white-spotted charr, Dolly Varden charr, and masu salmon) were collected in the study stations (Table 1). Most of the sampled rainbow trout were mature fish or immature parr; however, two fishes, which were collected at the Rebunge River, were identified as smolts (ESM Fig. S1). The smolts were classified at the smolt stage of pre-seaward migration, during which body color is silver and the color of the tip of the dorsal fin is black (ESM Fig. S1). Rainbow trout were collected at all but one of the study sites (i.e., 23/24), whereas native salmonids were not identified at approximately 29 % of the study sites (i.e., 7/24) (Table 1).
The density of native salmonids significantly decreased with increasing density of rainbow trout (F = 6.033, P = 0.026; Fig. 2). All of the four environmental variables showed no significant relationship with the density of rainbow trout (altitude: F = 1.317, P = 0.283; distance, F = 0.214, P = 0.655; width: F = 1.970, P = 0.177; depth: F = 0.751, P = 0.397).
Relationship between the density of rainbow trout and native salmonids at 24 study stations in 10 rivers. Each symbol represents a river: black square River 1; black circle River 2; black triangle River 3; black inverted triangle River 4, black diamond River 5; gray square River 6; gray circle River 7; gray triangle River 8; gray inverted triangle River 9; gray diamond River 10
The proportions of rainbow trout in the fish assemblage were higher than 75 % at all five study stations along the Todo River (Table 1). In the river, native salmonids were not the dominant species, even at the upstream study stations (Table 1).
Considering the results of the present study (Table 1) with the references to previous results of salmonid fauna (ESM Supplement A), rainbow trout invaded the Komatazawa and Todo rivers in the last 20 years. The native salmonid faunas at the Todo and Raruishi rivers have experienced a considerable change from the dominance by native salmonids to dominance by rainbow trout (Table 1, ESM Supplement A). In particular, this change in the Todo River has occurred in the last 20 years.
Discussion
In the present study, the density of native salmonids decreased with increasing density of rainbow trout. Previous studies have similarly shown that the abundance of native salmonids decrease with increasing density of rainbow trout (Morita et al. 2004; Baxter et al. 2007; Inoue et al. 2009). Rainbow trout are competitively superior to white-spotted charr (Hasegawa et al. 2004) and induce interspecific competition between sympatric native salmonids, white-spotted charr, and masu salmon (Hasegawa and Maekawa 2006). The introduction of rainbow trout induces an appreciable reduction of terrestrial prey in the diet and growth of native Dolly Varden charr (Baxter et al. 2007). Therefore, the density of native salmonids might be decreased through interspecific competition between the native salmonids and rainbow trout.
The present study examined the relationship between four environmental factors and the density of rainbow trout to assess the effect of study site location within a river system and the environmental suitability of the study site. In particular, rainbow trout occupy locations that have deeper water and are closer to cover than are masu salmon (Inoue et al. 2009). Thus, the river depth and width may affect the rainbow trout density. However, our results illustrated that the density of rainbow trout was not significantly related to the four environmental variables, implying that the invasion and presence of rainbow trout are not affected by the four environmental variables. However, caution is needed when interpreting our results, because the effect of other environmental factors on the density of rainbow trout was not controlled by the study. For example, the unconsidered factors, such as food availability and the number of visual barriers, may affect the density of rainbow trout by altering inter- or intra-specific competition. Thus, further studies should independently evaluate the effect of the four environmental factors on rainbow trout density.
A number of previous studies have shown that longitudinal variation in the distribution of native and exotic salmonids and, in many cases, the abundance of native salmonids increases from downstream to upstream (e.g., Dunham et al. 2002; Morita et al. 2004; Baxter et al. 2007). However, in the present study, rainbow trout were widely distributed in a single river and native salmonids were not the dominant species, even at the upstream study stations. In upstream river reaches where native salmonids have become the dominant species, physical barriers or a water temperature gradient are the critical factors limiting the upstream invasion of exotic salmonids (e.g., Dunham et al. 2002). However, in the present study, both of these factors would not have affected the distribution of rainbow trout, since physical barriers and an extreme temperature gradient are not present in the Todo River. Thus, the present study suggests that the effect of an expansion of rainbow trout distribution in a river occurs when physical barriers or a temperature gradient is absent.
In Hokkaido, the distribution of rainbow trout has increased widely from 1970 to 2012 (1970, five river systems; 1996, 72 systems; 2012, more than 120 systems) (Takami and Aoyama 1999; Shimoda 2012). In addition, at our study sites, rainbow trout have been recently introduced in at least two rivers in the last 20 years. In particular, the invasion by upstream migration by rainbow trout in the Todo River is thought to be impossible due to the unpassable dam that was constructed at the lower reach. Thus, the extension of the distribution of rainbow trout would only be continued by private stocking. Moreover, the current study showed that the invasion of rainbow trout has largely changed the salmonid fauna in the last 20 years. Nagasawa et al. (2009) showed that the proportion of native salmonids in the Shoro River system has decreased in conjunction with the invasion of rainbow trout over the last 30 years. Thus, the invasion of rainbow trout would rapidly change salmonid faunas in the river.
Not only resident rainbow trout, but also outmigrating smolt fish were collected at the studied river. A part of the rainbow trout (i.e., steelhead) population exhibits partial migration in which anadromous fish undergo marine migration and complete their life cycle in freshwater (Kendall et al. 2015). The alternative migratory tactics of the partial migration have been reported in an introduced population (Pascual et al. 2001). However, in Japan, most previous studies focused on resident populations of rainbow trout in streams, and no studies have reported on the presence of rainbow trout smolt. Even if the smolting rate is low, smolts often emerge above the migration barrier (e.g., Morita et al. 2000). Similarly, a rainbow trout population keeps the potential of smolt emergence even after 100 years of isolation above an unpassable waterfall (Phillis et al. 2016). Our result suggests that introduced rainbow trout populations in Japanese rivers have the potential to produce anadromous fish, and this potential has been retained in populations confined above physical barriers. Thus, further studies are required to examine the existence of anadromous fish in Japanese rivers to precisely quantify the potential threat posed by introduced rainbow trout.
In conclusion, the private stocking of rainbow trout has been continued in the rivers of Japan to this day, and salmonid faunas have consequently undergone rapid changes in these sites. Currently in Japan, there is no legal control applied to rainbow trout stocking, and thus the establishment of a legal control or a license system is urgently required for the management of rainbow trout.
References
Aoyama T, Takami T, Fujiwara M, Kawamula H (1999) Natural reproduction of rainbow trout, Oncorhynchus mykiss, in the Shiribetsu River in Hokkaido, Japan. Sci Rep Hokkaido Fish Hatchery 53:29–38
Baxter CV, Fausch KD, Murakami M, Chapman PL (2007) Invading rainbow trout usurp a terrestrial prey subsidy from native charr and reduce their growth and abundance. Oecologia 153:461–470
Dunham JB, Adams SB, Schroeter RE, Novinger DC (2002) Alien invasions in aquatic ecosystems: toward an understanding of brook trout invasions and potential impacts on inland cutthroat trout in western North America. Rev Fish Biol Fish 12:373–391
Dunham JB, Pilliod DS, Young MK (2004) Assessing the consequences of nonnative trout in headwater ecosystems in western North America. Fisheries 29:18–26
Fausch KD (2007) Introduction, establishment and effects of non-native salmonids: considering the risk of rainbow trout invasion in the United Kingdom. J Fish Biol 71:1–32
Fausch KD, Taniguchi Y, Nakano S, Grossman GD, Townsend CR (2001) Flood disturbance regimes influence rainbow trout invasion success among five holarctic regions. Ecol Appl 11:1438–1455
Hasegawa K, Maekawa K (2006) The effects of introduced salmonids on two native stream-dwelling salmonids through interspecific competition. J Fish Biol 68: 1123–1132
Hasegawa K, Yamamoto T, Murakami M, Maekawa K (2004) Comparison of competitive ability between native and introduced salmonids: evidence from pairwise contests. Ichthyol Res 51:191-194
Hokkaido (2010) Hokkaido Blue list. http://bluelist.hokkaido-ies.go.jp/. Accessed 29 September 2015
Inoue M, Miyata H, Tange Y, Taniguchi Y (2009) Rainbow trout (Oncorhynchus mykiss) invasion in Hokkaido streams, northern Japan, in relation to flow variability and biotic interactions. Can J Fish Aquat Sci 66:1423–1434
Kato K, Yanagawa T (2000) A reproductive population of rainbow trout introduced into the Sanjo river, western Japan. Suisanzoshoku 48:603–608
Kendall NW, McMillan JR, Sloat MR, Buehrens TW, Quinn TP, Pess GR, Kuzishchin KV, McClure MM, Zabel RW (2015) Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): a review of the processes and patterns. Can J Fish Aquat Sci 72:319–342
Kitanishi S, Yamamoto T, Nakagawa M (2010) Abiotic factors associated with the occurrence of introduced rainbow trout in the Atsuta River. Ichthyol Res 57:305–309
Kitano S (2004) Ecological impacts of rainbow, brown and brook trout in Japanese inland waters. Glob Environ Res 8:41–50
Morita K, Yamamoto S, Hoshino N (2000) Extreme life history change of white-spotted char (Salvelinus leucomaenis) after damming. Can J Fish Aquat Sci 57:1300–1306
Morita K, Tsuboi J, Matsuda H (2004) The impact of exotic trout on native charr in a Japanese stream. J Appl Ecol 41:962–972
Murakami O, Washitani I (2002) Handbook of alien species in Japan. Chijin-shokan, Tokyo
Nagasawa T, Morita K, Tsuboi, J (2009) Longitudinal distribution and changes in the fish fauna of a mid-scale river, Shoro River system, eastern Hokkaido, with notes on signal crayfish. Jpn J Ichthyol 56:31–45
Pascual M, Bentzen P, Riva Rossi C, Mackey G, Kinnison MT, Walker R (2001) First documented case of anadromy in a population of introduced rainbow trout in Patagonia, Argentina. Trans Am Fish Soc 130:53–67
Phillis CC, Moore JW, Buoro M, Hayes SA, Garza JC, Pearse DE (2016) Shifting thresholds: rapid evolution of migratory life histories in Steelhead/rainbow trout, Oncorhynchus mykiss. J Hered 107:51–60
Shimoda K (2012) Alien fish problems in Hokkaido (introduced salmonidae fishes). Nippon Suisan Gakkaishi 78:754–757
Takami T, Aoyama T (1999) Distributions of rainbow and brown trouts in Hokkaido, northern Japan. Wildlife Conserv Jpn 4:41–48
Taniguchi Y, Miyake Y, Saito T, Urabe H, Nakano S (2000) Redd superimposition by introduced rainbow trout, Oncorhynchus mykiss, on native charrs in a Japanese stream. Ichthyol Res 47:149–156
Taniguchi Y, Fausch KD, Nakano S (2002) Size-structured interactions between native and introduced species: can intraguild predation facilitate invasion by stream salmonids? Biol Invasions 4:223–233
Welcomme RL (1992) A history of international introductions of inland aquatic species. ICES Mar Sci Symp 194:3–14
Acknowledgements
We thank K. Hasegawa for help with fieldwork, K. Ohkuma for obtaining the sampling permit issued by the Governor of Hokkaido, and K. Miyashita for his support. This work was supported by JSPS KAKENHI Grant Number 15J01925 and 25450293.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sahashi, G., Morita, K. Potential threat of introduced rainbow trout Oncorhynchus mykiss to native salmonids in the western part of Hokkaido, Japan. Ichthyol Res 63, 540–544 (2016). https://doi.org/10.1007/s10228-016-0521-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0521-z