Abstract
The seagrass Zostera marina is characterized by either an annual phenotype, which flourishes during spring and dies off in other seasons, or a perennial phenotype, which lives for several years. To determine the influences of such differences in seagrass life cycles on fish assemblage structures, fish sampling and environmental measurements were conducted in annual and perennial seagrass beds, as well as on bare sand/mud flats, in Lake Hamana, central Japan. The perennial and annual seagrass beds harbored similar levels of fish species richness and abundances when the latter beds were flourishing, whereas the numbers of fish species and individuals showed a relative decrease in the annual beds and sand/mud flats during seasonal die-off. Such fish occurrence patterns were primarily determined by permanent seagrass habitat residents, seasonal residents and transients, which showed different occurrence patterns in each seagrass bed. Permanent residents preferred perennial seagrass beds, with the constant availability of seagrass vegetation structure likely being an essential habitat requirement. Seasonal residents showed different occurrence patterns, being abundant in the annual seagrass beds during periods of flourishing growth, sometimes exceeding levels in the perennial beds. Such fishes may use complex seagrass structures for only restricted periods. In contrast, transients did not show any elevated habitat use for a particular seagrass habitat structure, probably because of the lesser importance of the seagrass habitat as a feeding ground.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass beds are one of the most productive habitats in coastal ecosystems (Williams and Heck 2001), having abundant populations of small invertebrates, as well as detritus, which serve as important food resources for fishes (Kikuchi 1974; Edgar et al. 1994; Edgar and Shaw 1995a; Yamada et al. 2010). In addition, the complex structure of seagrass provides shelter for small fishes from predation and/or strong water movement, as well as increases microhabitat availability (Heck et al. 2003; Heck and Orth 2006; Horinouchi 2009). Seagrass beds therefore support large numbers of fish species and individuals and provide nursery habitats for juveniles of many species, whereas unvegetated areas are usually characterized by fewer species and individuals (Orth and Heck 1980; Heck et al. 1989; Edgar and Shaw 1995b; Jenkins and Wheatley 1998; Heck et al. 2003; Cote et al. 2013).
The seagrass Zostera marina is a widely distributed species, found in the northern hemisphere from warm temperate waters to near arctic conditions (Green and Short 2003). Although it is generally perennial, annual forms have been recorded from time to time (Phillips et al. 1983; Imao and Fushimi 1985; van Lent and Verschuure 1994; Morita et al. 2007; Jarvis et al. 2012). Although the mechanisms of annual seagrass occurrence (e.g., plastic changes from a perennial form or genetic adaptation to the environment) are likely to vary among locations (Gagnon et al. 1980; Heij and Nienhuis 1992; Reusch 2002; Muñiz-Salazar et al. 2005), many studies have indicated that environmental factors such as significant temperature increases and/or a decrease in the salinity of ambient water are related to annual seagrass occurrences (Imao and Fushimi 1985; van Lent et al. 1995; van Katwijk et al. 1998; Jarvis et al. 2012). Ocean temperatures have been increasing over the past several decades as a result of global climate change (Belkin 2009), which also affects eelgrass through increased frequency and intensity of storm events. Thus, climate change and accompanying environmental transitions likely increasingly favor annual life cycles over perennial, especially in the southern range of Z. marina distribution (Jarvis et al. 2012).
In central Japan, one of the southernmost regions of Z. marina distribution, annual seagrass beds flourish in spring and wither in summer after seed production, with almost all of the shoots subsequently disappearing until autumn (Imao and Fushimi 1985; Morita et al. 2007, 2010). Seedlings appear from October to March (Morita et al. 2010). Accordingly, such beds almost entirely lack an aboveground habitat structure over the autumn/winter period when resident fishes still use seagrass beds (Horinouchi 2009), whereas perennial seagrass beds maintain such structure all year round. Although the drastic change in habitat structure of annual seagrass beds may have a significant impact on resident fishes, no information has been obtained to date as to how such changes influence fish assemblages.
Although a great variety of fish species inhabits seagrass beds, the degree of association with seagrass varies among the species. For example, in temperate Japanese waters, small filefish Rudarius ercodes and sculpin Pseudoblennius cottoides are permanent seagrass residents, being strongly dependent upon seagrass beds (Horinouchi 2009; Horinouchi et al. 2013), whereas rockfish Sebastes species and Japanese seabass Lateolabrax japonicus are seasonal residents, using seagrass beds only during juvenile and sub-adult stages in spring and early summer (Kikuchi 1966, 1974). Such seagrass habitat use groups may respond differently to seagrass life cycle types.
To assess the responses of fishes to different seagrass life cycle types, we investigated fish assemblage structures and environmental factors, including seagrass vegetation structure and water quality, in annual and perennial seagrass beds in Lake Hamana, central Japan. The following questions were addressed: (1) how do seasonal patterns of the fish assemblage structures differ in annual and perennial seagrass beds and (2) which factors are responsible for variations in fish assemblage structures?
Materials and methods
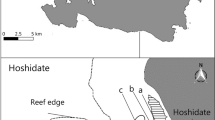
Study area. Lake Hamana (34° 75’ N, 137° 57’ E), located in Shizuoka Prefecture, central Japan (Fig. 1), is a brackish water lake opening directly to the Pacific Ocean via a narrow channel. The northern part (water depth 2 to 5 m) is characterized mainly by annual seagrass and the southern part (water depth less than 3 m) by perennial seagrass. In the northern part, annual seagrass flowers and matures from April to May, with mature seeds from the reproductive shoots falling to the bottom in June (Imao and Fushimi 1985). During the withering period from July to September, shoots do not occur on the site. Seedlings, appearing primarily in November and December, grow rapidly in February and March. Imao and Fushimi (1985) suggested that the low salinity and high temperature environment may be factors responsible for the occurrence of annual seagrass in the northern part of the lake. For the present study, seven sampling sites (A–G) were established: Sites A and B in perennial seagrass beds, Sites C and D in annual seagrass beds and Sites E–G (controls) on bare sand/mud flats (Fig. 1).
Sampling design. Fishes were collected using a seine net (16 m long, 2.5 m height, 13 mm mesh size) between 0700 and 1300 hours in September and December 2010 and April, May and June 2011 at Sites A–F. At Site G (sand/mud flat) adjacent to the annual seagrass beds (Sites C and D), sampling was conducted only during the period when annual seagrass flourished (from April through June 2011). Four consecutive sampling sweeps were conducted at each site on each sampling occasion, in the following manner. Within each site, four different starting points were established, the net being extended in a semi-circle and the end ropes then pulled by three or four persons to sweep approximately 160 m2 on each occasion (n = 4). All fish samples were preserved in 10 % formalin, identified to species level (Okiyama 1988; Nakabo 2013) and counted. Standard lengths were measured to the nearest 0.1 mm.
Following fish sampling at each site, water temperature (°C), salinity, dissolved oxygen (mg/l) and turbidity (NTU) were measured using a Quanta multi water quality monitoring system (HYDROLAB, Loveland, USA) at four different points within each site (n = 4). Seagrass shoot density and leaf height were measured using the following procedure: at each seagrass site, four 0.50 × 0.50 m2 quadrats were established randomly. The entire exposed length of all seagrass shoots within each quadrat was then collected by divers, and the number of shoots retrieved from each quadrat was later counted in the laboratory to calculate mean seagrass shoot density. Additionally, five shoots were selected randomly from each quadrat and leaf length measured (being the length from the bottom of the stem to the tip of the longest leaf of each shoot). In instances of less than five seagrass shoots collected from a quadrat, the leaf lengths of all shoots were measured. To determine the complexity of seagrass vegetation, a modified index of seagrass vegetation structure (Veg index) was also calculated as shoot density × mean leaf length in each quadrat (modified from Tolan et al. 1997). Although the original vegetation index was defined as “shoot density × leaves per shoot × leaf length” (Tolan et al. 1997), we considered that the Veg index used here was appropriate, expressing three-dimensional seagrass vegetation structure compared to shoot density or leaf length alone.
Fish groups utilizing seagrass habitats. Fishes collected were divided into the following four groups with respect to their patterns of seagrass habitat use (Kikuchi 1966, 1974; Horinouchi 2007a): (1) permanent residents, which occur in seagrass beds during both juvenile and adult stages, (2) seasonal residents, which utilize seagrass beds during either their juvenile or adult stage in a specific season, (3) transients, which occur in seagrass beds in the course of foraging over a variety of habitats and (4) casual species, which appear only occasionally in seagrass beds. Actual assignment of species to each habitat use group was based on classifications used in previous studies (Kikuchi 1966, 1974; Koike and Nishiwaki 1977; Shimizu 1979; Kimura et al. 1983; Shiobara and Suzuki 1985; Horinouchi 2009) or information on habitat use patterns (Dotu 1954; Minami 1981; Tabeta 1996; Kawai et al. 2007; Otsuka et al. 2009).
Data analysis.
Univariate analyses. –Variations in mean shoot density, leaf length and Veg index of seagrass between the two life cycle types (annual vs. perennial) in each sampling month were tested using generalized linear mixed models (GLMMs). The models were fitted with Poisson error distributions for shoot density (count data) and normal error distributions for leaf length and log (x + 1)-transformed Veg index (Bolker et al. 2009). The explanatory variables were life cycle type as a fixed factor and site nested in the life cycle type as a random factor. The likelihood ratio test (LRT) was used to indicate any significant differences between the two life cycle types for each month.
Differences in mean species richness and individual number per haul of overall fishes and each seagrass habitat use group (permanent residents, seasonal residents and transients) between habitat types (annual seagrass beds, perennial seagrass beds and sand/mud flats) were tested with GLMMs under the assumption of Poisson error distributions (species richness and individual numbers being count data) (Bolker et al. 2009). Casual species on the seagrass beds were excluded from the analyses because of their lower densities. The explanatory variables were habitat type, month and the interaction term between habitat type and month. Site, nested within habitat type, was also included as a random factor. Focusing on the fish assemblages of the sand/mud flats, differences in mean species richness and individual number per haul of overall fishes and each seagrass habitat use group between the southern (Sites E and F) and northern (Site G) parts were tested to examine the effects of locality on the fish occurrence patterns. The explanatory variables were locality, month and the interaction term between locality and month. Site, nested within locality, was also included as a random factor. Due to non-sampling on the sand/mud flats of the northern part (Site G) in September and December, comparisons were conducted only in and after April. The LRT was used to determine the significance of explanatory variables in the model. When the interaction term was significant, multiple comparisons between habitat types were conducted each month using the LRT with the False Discovery Rate adjustment (Benjamini and Hochberg 1995). All analyses were conducted using R version 3. 1. 2 (R Development Core Team 2014) with the package lme4 (Bates et al. 2013).
Multivariate analyses. –Differences in the fish assemblage structures between habitat types were tested using permutational multivariate analysis of variance (PERMANOVA; Anderson 2001). This test was conducted separately for each seagrass habitat use group (permanent residents, seasonal residents and transients) using a matrix of zero-adjusted Bray–Curtis dissimilarity based on the density of each species. Casual species in seagrass beds were not included in this analysis. The zero-adjusted Bray–Curtis dissimilarity measure was calculated by adding a dummy species to the original density matrix, which enables calculations of entirely blank or nearly empty samples by forcing a pair of blank samples to a dissimilarity of 0 % (Clarke et al. 2006). Prior to the analysis, the density was fourth-root transformed to normalize distributions, stabilize variances and lower the contributions of the dominant species (Clarke and Green 1988; Clarke 1993). The explanatory variables were habitat type, month and the interaction term. Site, nested within habitat type, was also included as a random factor. When the interaction term was significant, multiple comparisons between habitat types were conducted each month. The analysis was also conducted for the sand/mud flats to examine the difference in the fish assemblage structures between localities (southern and northern parts). The explanatory variables were locality, month and the interaction term. Site, nested within habitat type, was included as a random factor. As in GLMM, the comparisons between the localities were conducted in and after April. Nonmetric multidimensional scaling (MDS) ordination, based on the dissimilarity matrix of the same measure, was used to visually represent the fish assemblage structure of each seagrass habitat use group at each site for each month. The ordinations were performed using data averaged over the four replicates per site for each month to simplify the presentation.
A multivariate distance-based linear regression model (DISTLM) was used to examine the contribution of environmental variables to temporal and spatial variations in the assemblage structures of three seagrass habitat use groups (permanent residents, seasonal residents and transients) (Anderson et al. 2008). A zero-adjusted Bray–Curtis dissimilarity measure was constructed based on the averaged density of each fish species over the four replicates per site for each month. Prior to the analysis, the density was fourth-root transformed as in PERMANOVA. The environmental variables were averaged data of Veg index, minimum Veg index over the study period (min-Veg), water temperature, salinity, dissolved oxygen and turbidity at each site for each month. Min-Veg was used to express the constant availability of seagrass vegetation structure at each site over the study period. A permutation test was conducted to assess the significance of individual environmental variables for variation in the fish assemblage structure, and the best procedure, based on Akaike’s information criterion (AIC) (Anderson et al. 2008), used to find a reduced model that retained only the best combination of predictor variables. A distance-based redundancy analysis (dbRDA) was conducted to visualize the reduced model (Anderson et al. 2008). Species vectors, which showed the highest correlation to the dbRDA axes (multiple correlation coefficient >0.3), were also overlaid to identify which species contributed to the observed variation in the assemblages. Co-linearity of environmental variables was tested using the variance inflation factor (VIF) test (Sokal and Rohlf, 1995). Although VIF > 10 indicates harmful co-linearity as a rule of thumb, the values were <3.45 for all environmental variables.
Multivariate analyses were performed using PRIMER 6 (Clarke and Gorley 2006) with the PERMANOVA add-on (Anderson et al. 2008) and the co-linearity checked using R version 3. 1. 2 (R Development Core Team 2014).
Results
Life cycles of two types of seagrasses. The perennial seagrass beds (Sites A and B) retained aboveground leaf structures throughout the study period, whereas the annual seagrass beds (Sites C and D) did not (Fig. 2). In September 2010, an aboveground structure of seagrass leaves was not present in either annual seagrass bed. In December, the annual seagrass bed at Site D still lacked an aboveground structure, although a few short seedlings were present in the bed at Site C. In April and May 2011, annual seagrass flourished in both beds, with leaves being longer than those of perennial seagrass and shoot densities similar. Because long flowering shoots constitute the bulk of annual seagrass compared with mainly vegetative shoots in perennial seagrass, a clear contrast in leaf length between the two seagrass types was observed. In June, however, most annual seagrass died off, with only a few live shoots remaining. Shoot densities in the perennial beds were greater than those in the annual beds in September and June, although not differing between the two seagrass types in other months (Fig. 2). Leaf lengths were longer in the perennial beds in September and December, but longer in the annual beds from April to June (Fig. 2). Veg index was greater in the perennial beds in September, December and June, and there were no differences found between the two bed types in other months (Fig. 2). Min-Veg at each seagrass site was as follows: 1023.4 for Site A and 86.5 for Site B in the perennial seagrass beds, and 0 for Sites C and D in the annual seagrass beds. No seagrass vegetation was observed on the three bare sand/mud flats (Sites E–G), resulting in zero values for Veg index and Min-Veg over the study period.
Mean shoot density, leaf length and Veg index (±SD) of perennial and annual seagrass at each site (A–D) in each sampling month. Asterisk denotes significant differences (P < 0.05) between annual and perennial seagrass beds for each month. A constant value of 1 was added to indicate zero in the logarithmic scale for Veg index. See text for details of Veg index
Water quality. Seasonal water temperature patterns were similar at all sites, reaching 25 °C in September but decreasing to 13 °C in December and April (Fig. 3). Salinity was slightly lower in the annual seagrass beds (Sites C and D) than in the perennial beds (Sites A and B), except in September (Fig. 3). Although large differences in dissolved oxygen were not found among habitats in September and December, they became greater in the annual beds (Sites C and D) than in the perennial beds (Sites A and B) in April and May (Fig. 3). Water turbidity was clearly greater in September than in other months and was relatively greater in the annual beds than in the perennial beds from April through June (Fig. 3).
Fish assemblage structures. During the study period, a total of 3833 individuals belonging to 63 species (39 families) were collected at the sampling sites in Lake Hamana (Table 1). The most abundant species in the perennial seagrass beds were goldlined seabream Rhabdosargus sarba (13.5 % of total individual numbers in the perennial beds), Japanese black rockfish Sebastes ventricosus (11.1 %) and tidepool gunnel Pholis nebulosa (10.2 %), while in the annual seagrass beds, R. sarba (35.1 %), Japanese seabass Lateolabrax japonicus (22.3 %) and Japanese anchovy Engraulis japonica (18.3 %) were most dominant. On the sand/mud flats, E. japonica (49.7 %) and sand goby Favonigobius gymnauchen (16.0 %) were the dominant species. Seventeen species, such as small filefish Rudarius ercodes, sea sculpin Pseudoblennius cottoides and chameleon goby Tridentiger trigonocephalus, were found only in the perennial seagrass beds, and seven species, including black rockfish Sebastes schlegelii and small goby Gymnogobius opperiens, only in the annual seagrass beds. Three species, including spiny red gurnard Chelidonichthys spinosus, were exclusive to the sand/mud flats. In total, 36 species were recorded in multiple habitats. Twelve species, such as E. japonica, L. japonicas and R. sarba, were found in all three habitats. Ten species were found in both perennial and annual seagrass beds; three of them were rock fishes Sebastes inermis, S. ventricosus and Sebastes cheni. Eleven species, including sweetfish Plecoglossus altivelis altivelis and rabbitfish Siganus fuscescens, were found in perennial seagrass beds and on sand/mud flats. Three species, such as silver-stripe round herring Spratelloides gracilis, were found in annual seagrass beds and on sand/mud flats.
The 63 species captured in this study were assigned to four seagrass habitat use groups: 14 species, including R. ercodes, P. cottoides and small waspfish Hypodytes rubripinnis, were permanent residents, 20 species, such as R. sarba and L. japonicas, were seasonal residents, 12 species, such as P. altivelis altivelis and F. gymnauchen, were transients and 17 species were casual visitors (Table 1).
Mean overall species richness and individual number of fishes per haul in the perennial seagrass beds were constantly high during the study period, while those in the annual seagrass beds showed large seasonal fluctuations (Fig. 4). Species richness and individual density in the latter were relatively low in September, but increased with seagrass leaf length (Figs. 2, 4). Significant interactions between habitat and month were apparent for species richness and density (Table 2); both of the latter in the perennial seagrass beds were greater than those in the annual seagrass beds throughout almost all of September and December, whereas no differences were found from April to June (Fig. 4). Compared with the sand/mud flats, fish species richness and density were greater in the perennial seagrass beds in almost all months, whereas those in the annual beds tended to be higher only in May and June (Fig. 4).
Mean numbers (±SD) of species and individuals of all fishes per haul (160 m2, n = 4) in each habitat type at each site (A–G) in each sampling month. A constant value of 1 was added to indicate zero in the logarithmic scale for numbers of individuals. Different letters denote a pair of habitat types with a significant difference (P < 0.05) in each month detected by multiple comparisons
Significant interactions between habitat and month were also apparent for the mean numbers of species and individuals per haul for each of the three seagrass habitat use groups (Table 2). Species richness and individual density of permanent residents were consistently greater in the perennial seagrass beds than in other habitats in almost all months (Fig. 5). In the annual seagrass habitats and on sand/mud flats, however, most permanent seagrass residents occurred infrequently or not at all (Fig. 5). Species richness and density of seasonal residents were high in the perennial seagrass beds in most months, except December when such fishes seldom occurred (Fig. 5). Seasonal residents were seldom found in annual seagrass beds lacking aboveground seagrass structure in September and December. In and after April, when annual seagrass started flourishing, however, fish species richness and density increased, sometimes exceeding levels in the perennial seagrass beds, although differences between the seagrass types were not statistically significant. The species richness and density of seasonal residents were generally lower on the sand/mud flats throughout the study period. In the case of transients, species richness and density were often similar among habitat types, including the bare sand/mud flats, although differences were sometimes detected (Fig. 5).
Mean numbers (±SD) of species and individuals of each seagrass habitat use group per haul (160 m2, n = 4) in each habitat type at each site (A–G) in each sampling month. A constant value of 1 was added to indicate zero in the logarithmic scale for the numbers of individuals. Different letters denote a pair of habitat types with a significant difference (P < 0.05) in each month detected by multiple comparisons. Asterisk denotes significant difference (P < 0.05) between southern (Sites E and F) and northern (Site G) parts of sand/mud flats in each month
For the comparison of sand/mud flats, species richness and density of overall fishes and permanent residents did not differ between the southern (Sites E and F) and northern (Site G) parts from April to June (Table 2; Figs. 4 and 5). On the other hand, the species richness of seasonal residents was significantly greater in the northern part, while the species richness and density of transients were greater in the southern part (Table 2; Fig. 5).
PERMANOVA detected a significant interaction between habitat type and month for the three seagrass habitat use groups, indicating that the effects of habitat type on the fish assemblage structures varied among sampling months (Table 3). Multiple comparisons revealed significant or marginal differences in the assemblage structure of permanent residents between the perennial beds and other habitats in almost all months, although no differences were found between the annual beds and sand/mud flats (Table 3). For seasonal residents, the assemblage structure differed among all habitats in September, whereas no differences were detected in December (Table 3). In and after April, when annual seagrass started to flourish, a high degree of similarity was observed between the perennial and annual beds in the seasonal resident assemblage, although those assemblages tended to differ from those on sand/mud flats (Table 3). The assemblage structure of transients, unlike those of the other two habitat use groups, did not differ among habitats in many cases. MDS plots provided a graphical representation supporting the results of PERMANOVA, the map of the permanent resident assemblage showing a clear separation between the perennial seagrass beds and other habitats (Fig. 6a). For seasonal residents, separations were observed among all the habitats in September, and between the two seagrass beds and sand/mud flats in and after April (Fig. 6b). A clear grouping among habitats was limited to only a few cases for the transient assemblage (Fig. 6c).
Results of nonmetric multidimensional scaling (MDS) ordination showing the dissimilarity of assemblage structures of (a) permanent residents, (b) seasonal residents and (c) transients in each habitat type at each site (A–G) in each sampling month. Zero-adjusted Bray–Curtis dissimilarities were calculated based on fourth-root transformed abundance. S September 2010, D December 2010, A April 2011, M May 2011, J June 2011
For the comparison of sand/mud flats, PERMANOVA found no significant differences in the fish assemblages of three seagrass habitat use groups between the southern (Sites E and F) and northern (Site G) parts (Table 3). MDS plots also showed no clear separations of the three seagrass groups between the two localities (Fig. 6).
Relationships between fish assemblage structures of three seagrass habitat use groups and environmental variables. The assemblage structure of permanent residents was significantly correlated with min-Veg, and the latter plus turbidity were selected as the most significant combination of best predictor variables, explaining 21.2 % of the total variability of the assemblage structure (min-Veg = 16.1 %, turbidity = 5.1 %) (Fig. 7a; Table 4). Species which contributed to the variation observed in the permanent resident assemblages (multiple correlation >0.30) were Japanese seahorse Hippocampus mohnikei, Hypodytes rubripinnis, S. schlegeli, grass pufferfish Takifugu niphobles and panther pufferfish Takifugu pardalis (Fig. 7d). Sebastes schlegeli and T. pardalis were positively correlated with Min-Veg, while H. rubripinnis was shown to have a positive correlation with turbidity (Fig. 7a, d).
Distance-based redundancy analysis (dbRDA) plots showing the assemblage structure of each seagrass habitat use group in each habitat type at each site (A–G) in each sampling month, its relationship to (a–c) environmental variables selected from the best model and (d–f) the abundance of key species correlated with the two axes (multiple correlation >0.30). The circle is a unit circle (radius = 1) of relative size and arbitrary position of origin with respect to the underlying plot (Anderson et al. 2008). Points of the assemblage structure and vectors of environmental variables were omitted in plots (d) to (f) for clarity. S September 2010, D December 2010, A April 2011, M May 2011, J June 2011, Veg seagrass vegetation structure, Min-Veg minimum seagrass vegetation structure over the study period, Temp water temperature, Sal salinity, DO dissolved oxygen, Turb turbidity
For seasonal residents, Veg index, salinity, dissolved oxygen and turbidity were significantly correlated with assemblage structure (Table 4). The combination of best predictor variables, including Veg index, water temperature, dissolved oxygen and turbidity, explained 38.8 % of the total variability in the assemblage structure (Fig. 7b; Table 4). Veg index showed the highest percentage of variance explained (20.2 %), followed by temperature (8.3 %), turbidity (6.3 %) and dissolved oxygen (4.0 %). Floating goby Gymnogobius heptacanthus, L. japonicus, P. nebulosa, R. sarba, and S. fuscescens contributed mainly to the variation observed in the assemblage structure (multiple correlation >0.30) (Fig. 7e), G. heptacanthus and L. japonicus were positively correlated with Veg index, and P. nebulosa and S. fuscescens with dissolved oxygen and turbidity, respectively (Fig. 7e).
Environmental variables significantly correlated with the transient assemblage structure were water temperature, dissolved oxygen and turbidity, although salinity, dissolved oxygen and turbidity were selected as the most significant combination of best predictors, explaining 38.3 % of the total variability in the assemblage structure (turbidity = 24.1 %, dissolved oxygen = 9.6 %, salinity = 4.6 %) (Fig. 7c; Table 4). Small gobies, including Gymnogobius breunigii and G. urotaenia, Sumatran silverside Hypoatherina valenciennei, spotnape ponyfish Nuchequula nuchalis and sharpnose tigerfish Rhynchopelates oxyrhynchus contributed mainly to the variation observed in the transient assemblage structure (multiple correlation >0.30) (Fig. 7f). Both G. breunigii and G. urotaenia were positively correlated with dissolved oxygen and negatively with salinity, while N. nuchalis and H. valenciennei showed a positive correlation with turbidity (Fig. 7f).
Discussion
The present study demonstrated different fish assemblage structures between annual and perennial seagrass habitats. Perennial seagrass beds apparently harbored greater numbers of fish species and individuals compared with annual seagrass beds during the die-off period of the latter. However, overall species richness and abundance in flourishing annual beds increased to perennial bed levels, with Fonseca et al. (1990) reporting similar levels of fish abundance between natural and transplanted eelgrass beds, the latter persisting for few months like annual beds. Such differences in the fish assemblage structure were largely determined by the occurrence patterns of three seagrass habitat use groups (permanent residents, seasonal residents and transients) in each seagrass habitat.
An important point of consideration is whether or not these results were due to differences in seagrass life cycles or the geographical separation of the two types of seagrass beds (i.e., annual seagrass beds located in the northern part, perennial seagrass beds in the southern part). However, comparisons of sand/mud flats between localities (southern and northern parts) detected no significant effects of locality on the fish assemblages of all three seagrass habitat use groups. Based on these results, seagrass life cycles rather than geographical separation may be the main cause for the differences in the fish assemblage between the two types of seagrass habitats.
Permanent residents constituted a large proportion of the fish assemblage in the perennial seagrass beds, being constantly abundant in such habitats. In the annual seagrass beds (and on sand/mud flats), however, they were scarce throughout the study period, and species richness and individual densities did not increase even when annual seagrass flourished. This resulted in differences in the fish assemblage structure between the perennial seagrass beds and other habitats throughout the study period. Permanent residents are dependent on and have strong affinity with seagrass habitats throughout their life cycles (Kikuchi 1974). Many past studies have already described the features provided by complex seagrass structure, such as sheltering effects against predators/wave action and greater microhabitat/food availability (e.g., Kikuchi 1974; Fonseca and Cahalanb 1992; Edgar and Shaw 1995a; Heck et al. 2003; Horinouchi 2009). Annual seagrass beds, however, lack such features over autumn and winter because of the lack of aboveground seagrass structure. Accordingly, even though larvae of permanent residents can settle in annual seagrass habitats during the flourish period, subsequent juveniles have difficulty in growing and surviving there over autumn and winter, resulting in their low frequency of occurrence in annual beds in the next flourish period. In addition, some permanent resident species (e.g., pipefish and seahorse) are direct developers and do not have a pelagic larval stage in which fish can disperse widely. Larvae of such fishes would be less likely to recruit into annual seagrass habitats because (a) they cannot disperse from other habitats, and (b) parental adults are absent from the former due to the lack of seagrass structure over autumn and winter. Therefore, temporally constant availability of the aboveground structure of seagrass may be essential for permanent residents. Indeed, the DISTLM indicated that the permanent resident assemblages were mainly determined by minimum seagrass vegetation structure over the study period (min-Veg), supporting the essential nature of a stable seagrass habitat for this group. If annual seagrass beds were located closer to perennial ones, permanent residents could migrate to the former from the latter, depending on the availability of the aboveground seagrass structure. Such a landscape effect of seagrass beds may also be important in determining the distribution patterns of permanent residents.
Seasonal residents showed different occurrence patterns, compared with permanent residents. They generally responded to the presence of seagrass habitat structure. As an exception, they seldom occurred in both seagrass habitats in December even though above-ground structure was available in the perennial seagrass beds. This may have been due to a lack of recruitment of such species during the colder season. Seasonal residents utilize seagrass beds during some stages in their life histories, often as a nursery (Kikuchi 1974), because of the above-mentioned merits provided by seagrass. For example, in Japan, larvae of rockfishes (species of Sebastes) settle on seagrass habitats and grow there during March to May, subsequently migrating to deeper habitats in summer depending on water temperature (Plaza et al. 2003; Kamimura et al. 2011). Therefore, their seagrass-associated period matches the flourishing season of annual seagrass, probably resulting in their high abundance in those beds. In contrast, juveniles of rabbitfish (Siganus fuscescens) were not observed in the annual seagrass beds, whereas they appeared in high density in the perennial ones. The different occurrence patterns of rabbitfish between the two seagrass habitats may be due to the lack of aboveground structure in the annual seagrass beds during the recruitment period of the former from July to October (Fujita et al. 2002; Akiyama et al. 2009). Unlike permanent residents, seasonal residents need seagrass habitats only for a restricted period, and the highly complex seagrass structure during such a period may be exclusively important for them. Terazono et al. (2012) also reported fish responses to ephemeral habitats; high-level recruitment of juvenile fishes occurred in both temperate and tropical Sargassum beds during the biomass peak of both algae from late spring to early summer in southern Japan, despite the latter bed disappearing over the autumn and winter seasons.
In contrast to permanent and seasonal residents, transients did not show any consistent patterns in response to the structural complexity and/or temporally constant availability of seagrass habitats. Transients generally occur in seagrass beds in the course of foraging over a variety of habitats (Kikuchi 1974). Gut content analysis found that many of them had fed on zooplankton such as calanoida and/or decapoda larvae (unpublished data, M. Sato), the distributions of which are strongly influenced by water quality and circulation patterns (Siokou-Frangou et al. 1998; Marques et al. 2006, 2007). For transients, therefore, seagrass beds may simply represent an opportunistic feeding habitat, there being little response to seagrass vegetation.
Although the seagrass fish assemblage patterns possibly resulted from a combination of different fish responses to seagrass-life cycle traits, other environmental factors may also be important. The DISTLM found, for example, that turbidity was related to variations in the assemblage structures of all seagrass habitat use groups, as many previous studies have suggested (e.g., Cyrus and Blaber 1987, 1992; Akin et al. 2005). Piscivorous fishes avoid turbid water due to lower visibility and foraging efficiency, while small or juvenile fishes appear to use such an environment as a refuge (Maes et al. 1998), suggesting that spatial and temporal variations in turbidity affect seagrass fish assemblage structures. Food availability may also contribute to variations in fish assemblage structures (e.g., Horinouchi 2007b; Horinouchi et al. 2013). Further studies are needed to clarify the relative contribution of seagrass-life cycle traits and other possible environmental factors to variations in seagrass fish assemblage structures.
Conclusions
The present study demonstrated different responses of each seagrass habitat use group (permanent residents, seasonal residents and transients) to different seagrass life cycles. Global climate change and accompanying environmental transitions may lead to loss of seagrass beds (Short and Neckles 1999; Orth et al. 2006) and/or alter seagrass from perennial to annual forms (Jarvis et al. 2012). While the loss of seagrass habitats impacts negatively on seagrass fishes (Pihl et al. 2006; Nakamura 2010), a change in seagrass life cycle would likely reduce the species diversity of associated fishes, because permanent residents do not frequently appear in annual seagrass beds and seasonal residents cannot use such habitats during periods of die-off. Because some seasonal residents are commercially important species and used perennial seagrass beds when annual beds were disappearing (e.g., Stephanolepis cirrhifer), such a change in seagrass life cycle may also have a negative impact on fisheries in the region. Furthermore, if perennial seagrass habitats vanish completely in an area, permanent residents are absolutely threatened, because temporally constant availability of seagrass structure is essential for them and alternative habitats for these species (e.g., perennial macroalgal beds) are not present (Washiyama et al. 2002). In contrast, more than seventy percent of fish species collected in the annual seagrass beds were also found to comprise part of the fish assemblage in the perennial beds, with only Sebastes schlegelii being exclusive to the former beds among the seagrass-dependent species (i.e., permanent and seasonal residents). This study, therefore, indicates the greater importance of conservation of perennial seagrass than of annual seagrass and is useful in understanding and predicting the effects of habitat change on fish assemblages.
References
Akin S, Buhan E, Winemiller KO, Yilmaz H (2005) Fish assemblage structure of Koycegiz Lagoon-Estuary, Turkey: Spatial and temporal distribution patterns in relation to environmental variation. Estuar Coast Shelf Sci 64:671–684
Akiyama S, Naganuma M, Katayma S (2009) Annual life cycle of rabbitfish Siganus fuscescens in Tateyama Bay, Chiba prefecture. Fish Eng 46:107-115
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth
Bates D, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using S4 classes, Ver 1.0-5
Belkin IM (2009) Rapid warming of large marine ecosystems. Prog Oceanogr 81:207–213
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57:289–300
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens M, Henry H, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 3:127–135
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Ausratl J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Clarke KR, Green RH (1988) Statistical design and analysis for a ‘biological effects’ study. Mar Ecol Prog Ser 46:213–226
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray−Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
Cote D, Gregory RS, Morris CJ, Newton BH, Schneider DC (2013) Elevated habitat quality reduces variance in fish community composition. J Exp Mar Biol Ecol 440:22-28
Cyrus DP, Blaber SJM (1987) The influence of turbidity on juvenile marine fishes in estuaries. Part 1. Field studies at Lake St. Lucia on the southeastern coast of Africa. J Exp Mar Biol Ecol 109:53–70
Cyrus DP, Blaber SJM (1992) Turbidity and salinity in a tropical northern Australian estuary and their influence on fish distribution. Estuar Coast Shelf Sci 35:545–563
Dotu Y. (1954) On the life history of a goby, Chaenogobius castanea O’SHAUGHNESSY. Jpn J Ichthyol 3:133–138
Edgar GJ, Shaw C (1995a) The production and trophic ecology of shallow-water fish assemblages in southern Australia II. Diets of fishes and trophic relationships between fishes and benthos at Western Port, Victoria. J Exp Mar Biol Ecol 194:83–106
Edgar GJ, Shaw C (1995b) The production and trophic ecology of shallow-water fish assemblages in southern Australia I. Species richness, size-structure and production of fishes in Western Port, Victoria. J Exp Mar Biol Ecol 194:53–81
Edgar GJ, Shaw C, Watsona GF, Hammond LS (1994) Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western Port, Victoria. J Exp Mar Biol Ecol 176:201–226
Fonseca MS, Cahalanb JA (1992) A preliminary evaluation of wave attenuation by four species of seagrass. Estuar Coast Shelf Sci 35:565–576
Fonseca MS, Kenworthy WJ, Colby DR, Rittmaster KA, Thayer GW (1990) Comparisons of fauna among transplanted eelgrass Zostera marina meadows: criteria for mitigation. Mar Ecol Prog Ser 65:251–264
Fujita S, Kinoshita I, Takahashi I, Azuma K (2002) Species composition and seasonal occurrence of fish larvae and juveniles in the Shimanto Estuary, Japan. Fish Sci 68:364–370
Gagnon P, Vadas R, Burdick D, May B (1980) Genetic identity of annual and perennial forms of Zostera marina L. Aquat Bot 8:157–162
Green E, Short F (2003) World atlas of seagrasses. University of California Press, Berkley
Heck KL, Orth RJ (2006) Predation in seagrass beds. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: Biology, Ecology and Conservation. Springer, New York, pp 537–550
Heck KL, Able KW, Fahay MP, Roman CT (1989) Fishes and decapod crustaceans of Cape Cod seagrass meadows - species composition, seasonal abundance patterns and comparison with unvegetated substrates. Estuaries 12:59–65
Heck KL, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136
Heij H, Nienhuis P (1992) Intraspecific variation in isoenzyme patterns of phenotypically separated populations of Zostera marina L. in the south-western Netherlands. J Exp Mar Biol Ecol 161:1–14
Horinouchi M. (2007a) Review of the effects of within-patch scale structural complexity on seagrass fishes. J Exp Mar Biol Ecol 350:111–129
Horinouchi M. (2007b) Distribution patterns of benthic juvenile gobies in and around seagrass habitats: effectiveness of seagrass shelter against predators. Estuar Coast Shelf Sci 72:657–644
Horinouchi M (2009) Horizontal gradient in fish assemblage structures in and around a seagrass habitat: some implications for seagrass habitat conservation. Ichthyol Res 56:109–125
Horinouchi M, Mizuno N, Jo Y, Fujita M, Suzuki Y, Aranishi F, Sano M (2013) Habitat preference rather than predation risk determines the distribution patterns of filefish Rudarius ercodes in and around seagrass habitats. Mar Ecol Prog Ser 488:256–266
Imao K, Fushimi H (1985) Ecology of the seagrass (Zostera marina L.), especially environmental factors determining the occurrence of annual seagrass in Lake Hamana-ko. Jpn J phycology 33:320–327
Jarvis J, Moore K, Kenworthy WJ (2012) Characterization and ecological implication of seagrass life history strategies near the species’ southern limit in the western North Atlantic. Mar Ecol Prog Ser 444:43–56
Jenkins GP, Wheatley M J (1998) The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: comparison of shallow seagrass, reef-algal and unvegetated sand. J Exp Mar Biol Ecol 221:147–172
Kamimura Y, Kasai A, Shoji J (2011) Production and prey source of juvenile black rockfish Sebastes cheni in a seagrass and macroalgal bed in the Seto Inland Sea, Japan: estimation of the economic value of a nursery. Aquat Ecol 45:367–376
Kawai T, Akino H, Tanaka Y, Muto T (2007) Habitat and behavior of juvenile and young black rockfish, Sebastes schlegeli, in Tomari, in the southwestern area of the Sea of Japan, Hokkaido, Japan. Saibai Giken 35:59–62
Kikuchi T (1966) An ecological study on animal communities of the Zostera marina belt in Tomioka Bay, Amakusa, Kyushu. Publ Amakusa Mar Biol Lab Kyushu Univ 1:1–106
Kikuchi T (1974) Japanese contributions on consumer ecology in seagrass (Zostera marina L.) beds, with special reference to trophic relationships and resources in inshore. Aquaculture 4:145–160
Kimura S, Nakamura Y, Aritaki M, Kimura F, Mori K, Suzuki K (1983) Ecological studies on fishes of the Zostera bed at the mouth of Ago bay, Mie Prefecture–I: Fish fauna and its seasonal change. Bull Fac Fish Mie Univ 10:71–93
Koike K., Nishiwaki S. (1977) Seasonal Change of Fish Fauna in the Zostera Zone in Shimoda Bay and Nabeta Cove, the Izu Peninsula. Jpn J Ichthyol 24:182–192
Maes J, Taillieu A, Van Damme PA, Cottenie K, Ollevier F (1998) Seasonal patterns in the fish and crustacean community of a turbid temperate estuary (Zeeschelde Estuary, Belgium). Estuar Coast Shelf Sci 47:143–151
Marques S, Azeiteiro U, Marques JC, Neto JN, Pardal M (2006) Zooplankton and ichthyoplankton communities in a temperate estuary: spatial and temporal patterns. J Plankton Res 28:297–312
Marques S, Pardal M, Pereira M, Gonçalves F, Marques J, Azeiteiro U (2007) Zooplankton distribution and dynamics in a temperate shallow estuary. Hydrobiologia 587:213–223
Minami T (1981) The early life history of a flounder Limanda yokohamae. Nippon Suisan Gakkaishi 47:1411–1419
Morita T, Okumura H, Abe M, Kurashima A, Maegawam M (2007) Density and distribution of seeds in bottom sediments in Zostera marina beds in Ago Bay, central Japan. Aquat Bot 87:38–42
Morita T, Kakinuma M, Mizuno G, Okumura I, Kokubu H, Kurashima A, Maegawam M (2010) Morphological characteristics of annual Zostera marina shoots at various germination temperatures Aquat Bot 92:49–54
Muñiz-Salazar R, Talbot SL, Sage GK, Ward DH, Cabello-Pasini A (2005) Population genetic structure of annual and perennial populations of Zostera marina L. along the Pacific coast of Baja California and the Gulf of California. Mol Ecol 14:711–22
Nakabo T (ed) (2013) Fishes of Japan with pictorial keys to the species, third edition. Tokai University Press, Hadano
Nakamura Y (2010) Patterns in fish response to seagrass bed loss at the southern Ryukyu Islands, Japan. Mar Biol 157:2397–2406
Okiyama M (ed) (1988) An atlas of the early stage fishes in Japan. Tokai University Press, Tokyo
Orth RJ, Heck KL (1980) Structural components of eelgrass (Zostera marina) meadows in the lower Chesapeake Bay–Fishes. Estuaries 3:278–288
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM and others (2006) A global crisis for seagrass ecosystems. Bioscience 56:987−996
Otsuka Y, Suzuki H, Akagawa I (2009) Occurrence, gonad morphology and maturity of Japanese seahorse Hippocampus mohnikei in Matsushima Bay, Japan. J Sch Mar Sci Tech Tokai Univ 7:11–22
Pihl L, Baden S, Kautsky N, Ronnback P, Soderqvist T, Troell M, Wennhage H (2006) Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuar Coast Shelf Sci 67:123–132
Phillips RCR, Grant WS, McRoy CP (1983) Reproductive strategies of seagrass (Zostera marina L.). Aquat Bot 16:1–20
Plaza G, Katayama S, Omori M (2003) Otolith Timing of parturition, planktonic duration, and settlement patterns of the black rockfish, Sebastes inermis. Environ Biol Fishes 68:229–239
R Development Core Team. (2014) R: a language and environment for statistical computing, ver 3.1.2. R Foundation for Statistical Computing, Vienna, Austria
Reusch TBH (2002) Microsatellites reveal high population connectivity in seagrass (Zostera marina) in two contrasting coastal areas. Limnol Oceanogr 47:78–85
Shimizu T (1979) Fishes of Zostera zone in Odawa Bay II: fluctuations of the number of species and the number of individuals and locality and continuity of fish communities. Bull Kanagawa Pref Fish Exp Stn 79:1–13
Shiobara Y, Suzuki K (1985) An ecological study on the fish community in Zostera bed at the Uchiura Coast, Suruga Bay, Japan. J Sch Mar Sci Tech Tokai Univ 21:129–143
Short FT, Neckles HA (1999) The effects of global climate change on seagrasses. Aquat Bot 63:169–196
Siokou-Frangou I, Papathanassiou E, Lepretre A, Frontier S (1998) Zooplankton assemblages and influence of environmental parameters on them in a Mediterranean coastal area. J Plankton Res 20:847–870
Sokal RR, Rohlf FJ (1995) Biometry, 3rd ed. W.H. Freeman, New York
Tabeta O (1996) Distributional and ecologocal aspects of pufferfish Takifugu radiatus. Nippon Suisan Gakkaishi 62:946-947
Terazono Y, Nakamura Y, Imoto Z, Hiraoka M (2012) Fish response to expanding tropical Sargassum beds on the temperate coasts of Japan. Mar Ecol Prog Ser 464:209–220
Tolan JM, Holt SA, Onuf CP (1997) Distribution and community structure of ichthyoplankton in Laguna Madre seagrass meadows: potential impact of seagrass species change. Estuaries 20:450–464
van Katwijk MM, Schmitz GHW, Hanssen LSAM, den Hartog C (1998) Suitability of Zostera marina populations for transplantation to the Wadden Sea as determined by a mesocosm shading experiment. Aquat Bot 60:283–305
van Lent F., Verschuure J.M. (1994) Intraspecific variability of Zostera marina L. (seagrass) in the estuaries and lagoons of the southwestern Netherlands. I. Population dynamics. Aquat Bot 48:31–58
van Lent F, Verschuure JM, van Veghel MLJ (1995) Comparative study on populations of Zostera marina L.(seagrass): in situ nitrogen enrichment and light manipulation. J Exp Mar Biol Ecol 185:55–76
Washiyama H, Yoshizawa Y, Nagatani T, Ishiwata T (2002) Distribution of eelgrass Zostera marina in Lake Hamana. Bull Shizuoka Pref Fish Exp Stn 37:37–40
Williams S, Heck K (2001) Seagrass community ecology. In: Bertness M, Gaines S, Hay M (eds) Marine community ecology. Sinauer Press, Sunderland, pp 317–337
Yamada K, Hori M, Tanaka Y, Hasegawa N, Nakaoka M (2010) Contribution of different functional groups to the diet of major predatory fishes at a seagrass meadow in northeastern Japan. Estuar Coast Shelf Sci 86:71–82
Acknowledgments
We are grateful to Yuzuru Suzuki, Naoki Mizuno, Sho Hosoya, Yuka Jo, Yukinori Nakane, Kusuto Nanjo, Yuichi Tanaka, Kazuya Takigasaki and all the staff of the Fisheries Laboratory, The University of Tokyo, for their generous assistance with field and laboratory work. Constructive comments on the manuscript from David Cote, Graham Hardy, Ken Okamoto, Shigeru Aoki and the anonymous reviewers are much appreciated. Our thanks are also due to the fishermen’s unions in Lake Hamana for their permission to collect fish samples.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sato, M., Horinouchi, M., Fujita, M. et al. Responses of fish assemblage structures to annual and perennial life cycles of seagrass Zostera marina in Lake Hamana, central Japan. Ichthyol Res 63, 445–459 (2016). https://doi.org/10.1007/s10228-016-0514-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0514-y