Abstract
Daphnia, keystone herbivores in lakes, routinely produce immediately hatching eggs; additionally, they also produce resting eggs enveloped by an ephippial case, a thickened carapace that allows population survival under harsh environmental conditions. To examine differences in ephippial morphology between Daphnia species in different subgenera, we conducted microscopic observations and genetic analyses based on the mitochondrial 12S rRNA gene in ephippia from surface sediment in Lake Biwa, Japan. The lengths and heights of ephippia identified as Daphnia galeata Sars (Hyalodaphnia) were less than 0.82 and 0.50 mm, respectively, whereas those of Daphnia pulicaria Forbes (Daphnia) were greater than 0.87 and 0.53 mm, respectively, with the ephippial lengths of the two species differing significantly. The results indicate that D. galeata and D. pulicaria inhabiting Lake Biwa can be distinguished based on ephippium size, with a boundary ephippium length of approximately 0.86 mm. In concordance with this inference, historical data indicated that the length of ephippia recovered from sediment cores did not exceed 0.86 mm prior to the 1980s when D. galeata was the predominant species; however, it exceeded the threshold after 2000, coinciding with the coexistence of D. galeata and D. pulicaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daphnia, which are key herbivorous plankton in lakes and ponds (Carpenter and Kitchell 1993), routinely produce parthenogenetic eggs that develop directly in the brood chamber of the mother. However, they also produce resting (dormant) eggs enveloped by a thickened carapace, referred to as an ephippial case, which allows population survival during unfavorable periods. Indeed, such resting eggs are often well preserved in sediments for decades to centuries (Caceres 2012; Frisch et al. 2014), and can be successfully hatched even after several decades when stimulated with appropriate environmental cues (Decaestecker et al. 2004; Frisch et al. 2014; Hairston et al. 1999). Accordingly, resurrection studies based on analyses of resting eggs found in sediments are developing rapidly, with a view toward gaining a better understanding of biological responses to anthropogenic environmental change (Brede et al. 2009; Hairston et al. 1999), as well as determining the genetic structure of Daphnia populations related to long-term environmental change (Frisch et al. 2014; Möst et al. 2015; Geerts et al. 2015).
However, despite the utility of resting eggs for such resurrection studies, the hatching rates of these eggs are not always high in lakes (Faustová et al. 2004; Hairston et al. 1995; Rother et al. 2010). Additionally, for some lakes, species identification based on genetic analysis of collected resting eggs is often unsuccessful, probably owing to genome degradation (Faustová et al. 2004; Marková et al. 2006). Consequently, it may be difficult to reconstruct the dynamics of different Daphnia populations using resting eggs found in sediments (Faustová et al. 2004; Marková et al. 2006). Nevertheless, it is feasible that Daphnia ephippia might be distinguishable among species, owing to certain morphological differences, such as differences in the size of the ephippium of larger species in the subgenera Daphnia (Daphnia pulex group) and Ctenodaphnia (Daphnia magna group), and smaller species in the subgenus, Hyalodaphnia (Daphnia longispina group) (Vandekerkhove et al. 2004). Currently, however, few studies have investigated ephippium morphology in combination with molecular genetic analyses (Hamrová et al. 2010), which would enable identification at the species level (Juračka et al. 2010).
Although it has been reported previously that genetic analysis could not be applied to ephippia devoid of resting eggs (e.g., Brede et al. 2009), Ishida et al. (2012) developed a technique (UltraSHOT) that facilitated effective extraction of DNA from the ephippial carapace, thereby enabling genetic analysis, even in the absence of the resting eggs. In this regard, whereas polymerase chain reaction (PCR) sequencing is an ideal identification protocol, it tends to be relatively expensive; therefore, some studies have applied a PCR-restriction fragment length polymorphism (RFLP) approach to differentially restrict amplified DNA segments of Daphnia species (Billiones et al. 2004), which is more rapid and cheaper than traditional PCR sequencing protocols.
Lake Biwa in Japan is recognized as one of the most ancient lakes in the world (Hampton et al. 2018), which has experienced certain anthropogenic effects over the past century such as eutrophication and warming that have resulted in changes in the lake’s Daphnia community (Hsieh et al. 2011; Tsugeki et al. 2003). Between the 1960s and 1990s, Daphnia galeata Sars, belonging to subgenus Hyalodaphnia, was initially believed to be the only Daphnia species inhabiting the lake (Miura and Cai 1990; Tsugeki et al. 2003). However subsequent investigations have indicated that other Daphnia species of similar size appeared to have existed for limited periods of time during the 1970s (Liu et al. 2020). Moreover, a further large species, Daphnia pulicaria Forbes, belonging to the Daphnia subgenus, suddenly appeared in the lake in 1999 (Urabe et al. 2003). Although ephippia derived from D. galeata and D. pulicaria have been respectively found in sediments obtained from this lake (Urabe et al. 2003; Tsugeki et al. 2009), there have to date been no reports describing morphological differences between the ephippia of these two species, and consequently variations in the production of resting eggs during the period of their coexistence in the lake remain unclear.
In this study, we retrieved Daphnia ephippia from surface sediments in Lake Biwa and examined the interspecific differences (in terms of ephippial size) as a potential tool to distinguish between species based on ephippium morphology. To identify Daphnia ephippia, we sequenced mitochondrial ribosomal DNA and applied a newly developed inexpensive and rapid PCR–RFLP method. Owing to its predominance in freshwater environments, the D. pulex species complex, including D. pulicaria, has been extensively studied for several decades (Benzie 2005). However, as it is difficult to definitively resolve the taxonomy of this complex, owing to a lack of diagnostic morphological characteristics and the tendency of species to undergo hybridization, the mitochondrial NADH dehydrogenase subunit 5 (ND5) has been used to elucidate relationships among groups (Colbourne et al. 1998; Crease et al. 2012; Cristescu et al. 2012). Thus, in the present study, we analyzed DNA sequence variation of the mitochondrial ND5 gene to clarify the phylogenetic lineages of D. pulicaria present in Lake Biwa. Furthermore, to evaluate the inference accuracy of ephippial morphological differences between species from a long-term perspective, we investigated historical variation in the size of ephippia preserved in sediment cores covering the past century, over which time there have been changes in the Daphnia community of this lake.
Materials and methods
Sampling and microscope observation
Lake Biwa has a maximum depth of 104 m and a surface area of 674 km2. It consists of a small shallow southern basin (surface area = 58 km2; mean depth = 3.5 m) and a large deep northern basin (surface area = 616 km2; mean depth = 45.5 m). Ephippium samples were collected from the surface sediment in August 2017 using an Ekman Bottom Grab at a pelagic site in the northern basin (35° 15′ 017″ N, 136° 04′ 008″ E; water depth = 71 m) onboard the CER research boat Hasu. Surface sediment samples were sealed in bags and stored at − 80 °C until used for analysis.
Daphnia ephippia were sieved from the 500-g wet surface sediment through a 100-μm mesh and measured under a dissecting microscope at × 20 to × 40 magnification. Three-hundred and thirty ephippia were collected from the surface sediment samples. All ephippia were photographed using a digital camera and the size (length and height) of each ephippium was measured (Fig. 1). Some samples are not presented in figures owing to incomplete formation of the ephippium. After microscopic observation, individual ephippia were placed in 0.2-mL tubes and stored at − 80 °C until further genetic analysis. Genetic analysis was performed on a random sample of 237 of the 330 observed ephippia, among which, we did not check for the presence/absence of resting eggs.
Core sampling and chronology
We subsequently examined whether the size distribution of Daphnia ephippia in the sediment core fluctuated concomitantly with fluctuations in the Daphnia community in the past to evaluate the validity of using ephippial size differences to differentiate between species. For this investigation, we collected three sediment cores (LB1, LB4, and LB7) at a pelagic site (35° 15′ 017″ N, 136° 04′ 013″ E; water depth = 71 m) in the northern basin of Lake Biwa using a gravity corer (inner diameter = 10.9 cm) in August 2017. These cores were carefully sliced at 1-cm intervals from the surface to the bottom and each sliced sample stored at − 80 °C prior to further analyses.
Sediment chronology analysis was performed for the LB7 core. Chronology was determined based on the constant rate of supply (CRS) method of 210Pb dating (Appleby and Oldfield 1978) and verified using the 137Cs peak traced in the period 1962–1963 (Appleby 2001). Details of the chronological method have been reported elsewhere (Hyodo et al. 2017). Briefly, dried samples were sealed in holders for a month to allow 222Rn and its short-lived decay product (214Pb) to equilibrate. The activity of supported 210Pb was estimated by measuring the activity of 214Pb, whereas that of 210Pbexcess was determined according to the difference between the total and the supported 210Pb (210Pbexcess = 210Pbtotal–214Pb). 137Cs, 210Pb, and 214Pb activities were determined by gamma counting using a germanium detector (GXM25P; EG & G ORTEC, Tokyo, Japan) equipped with a multi-channel analyzer (MCA7700; SEIKO EG & G, Tokyo, Japan) at the Center for Marine Environmental Studies, Ehime University. The age of a given sample mass depth was calculated using the 210Pbexcess inventory, which was obtained by numerical integration of the radioactivity of 210Pbexcess versus the mass depth profile (Appleby 2001). The chronology of the other cores (LB1 and LB4) was estimated indirectly by comparison with the profiles of proxies such as chlorophyll pigments and magnetic susceptibilities in the LB7 core. To compare the proxies, the marked peak or trough layers were used as reference layers. Magnetic susceptibility and chlorophyll pigments were measured using an SM-30 m (ZH instruments, Brno, Czech Republic) and a UV–Vis mini 1240 spectrophotometer (Shimadzu, Kyoto, Japan), respectively. The concentrations of chlorophyll-a and phaeopigments were calculated according to the method of Lorenzen (1967). Ephippia were extracted from the LB1 and LB4 sediment cores as described above, after sieving the sediment through a 100-μm-mesh sieve and observing under a dissecting microscope at × 20 to × 40 magnification. After retrieval, at least 10 ephippia in each sliced sample were photographed using a digital camera. Thereafter, ephippial size was measured, excluding those from the samples in which fewer than 10 ephippia were detected (Tsugeki et al. in preparation). Prior to the 1980s, D. galeata was the predominant species of Daphnia in Lake Biwa, (Miura and Cai 1990; Tsugeki et al. 2009), whereas D. pulicaria appeared after 1999 (Urabe et al. 2003). Accordingly, we separated and compiled the ephippial data from the sediment cores (LB1 and LB4) into two periods, i.e., prior to the early 1980s and after 2000, corresponding to differences in the Daphnia community structure.
Genetic analysis
DNA extraction and PCR amplification
DNA extraction and PCR amplification from ephippial carapaces were performed according to the methods described by Ishida et al. (2012), which have been specifically developed for the ephippium of Daphnia. Briefly, 235 ephippial carapaces were individually transferred into 200-μL PCR tubes, to which 50 μL of alkaline lysis buffer (100 mM NaOH and 50 mM of disodium EDTA, pH 12) was added. The samples were then subjected to five cycles of thermal shock, consisting of 5 min at − 80 °C and 20 s at 70 °C. Thereafter, the samples were vortexed and sonicated for at least 1 min using an HD 2070-U ultrasonic homogenizer (BANDELIN Electronic, Berlin, Germany). The samples were then incubated at 95 °C for 30 min and immediately thereafter stored on ice for > 3 min, after which a further 50 μL of neutralizing buffer (Tris–HCl, pH 5) was added to each tube. The samples were again briefly vortexed prior to storing at − 80 °C. In addition, 10 ephippial carapaces, the lengths and heights of which were greater than 1.0 mm and 0.61 mm, respectively, were mixed as a single sample (sample ID: LB-RE1), and then DNA was extracted as described above (Table S1). DNA was also extracted from two single ephippial carapaces, sample IDs: LB-RE2 and LB-RE3, whose lengths and heights were 0.765 and 0.471 mm, and 0.941 and 0.618 mm, respectively (Tables S1 and S2).
PCR amplification of 12S rRNA gene fragments was carried out using a Type-it Microsatellite PCR Kit (Qiagen) for the initial PCR, as described by Ishida et al. (2012). For those samples for which no target amplicons were obtained using the initial PCR, additional PCRs were performed using the initial PCR products with Ex Taq Hot Start (Takara) (Ishida et al. 2012). Amplifications were performed using the primers, 5′-ATGCACTTTCCAGTACATCTAC-3′ and 5′-AAATCGTGCCAGCCGTCGC-3′, designed by Colbourne and Hebert (1996). Negative PCR controls (containing no template DNA) were run for each PCR and no contamination was detected.
PCR restriction fragment length polymorphism (RFLP) analysis and sequences
We developed a rapid and reliable RFLP marker to distinguish between D. galeata and D. pulicaria using PCR products from the 12S rRNA gene region. This PCR–RFLP analysis was performed on the randomly selected 235 ephippia, among which, we did not verify the presence or absence of resting eggs. PCR products were digested with the restriction enzymes HindIII HF (New England Biolab., Beverly, MA, USA) and incubated for 2 h at the enzyme-specific optimal temperature (37 °C), after which the digestion products were electrophoresed on agarose gels for 30 min, stained with GelGreen (Biotium, Hayward, CA, USA), visualized using a UV transilluminator, and photographed. As a marker, Daphnia species were differentiated based on digestion patterns using HindIII HF, which has two and one restriction site(s) for the target sequences of D. galeata and D. pulicaria, respectively, and is expected to give three (135, 145, and 323 bp) and two (289 and 326 bp) fragments, respectively.
For D. pulicaria, a fragment (877 bp) including part of the gene coding was amplified by PCR using a Type-it Microsatellite PCR Kit (Qiagen) (Ishida et al. 2012) with the DpuND5a forward primer (5′-ATAAAACTCCAATCAACCTTG-3′) and the DpuND5b reverse primer (5′-GGGGTGTATCTATTAATTCG-3′) (Colbourne et al. 1998). The PCR thermal cycling conditions were as follows: one cycle of initial denaturation at 94 °C for 1 min, followed by 35 cycles at 94 °C for 1 min, annealing at 50 °C for 1 min, and extension for 1 min at 72 °C (Ishida et al. 2012).
To sequence the 12S rRNA gene, one PCR product was used for each D. galeata (LB-RE2) and D. pulicaria (LB-RE1) sample (Table S1). To sequence the ND5 gene, two PCR products were used for D. pulicaria (LB-RE1 and LB-RE3) (Table S2). Each PCR product was purified using ExoSAP-IT Express (Thermo Fisher Scientific), and sequencing was performed commercially. The 12S rRNA gene sequences of D. pulicaria and D. galeata from Lake Biwa have been deposited in the DDBJ/GenBank database under the accession IDs LC534941 (LB-RE1) and LC534942 (LB-RE2), respectively (Table S1). Similarly, the ND5 gene sequences of D. pulicaria from Lake Biwa have been deposited in the DDBJ/GenBank database under accession IDs LC534943 (LB-RE1) and LC534944 (LB-RE3), respectively (Table S2).
Phylogenetic and statistical analysis
To analyze the phylogenetic relationship between the two Daphnia species from Lake Biwa studied here and related taxa, previously reported DNA sequences of 12S rRNA genes were obtained from the GenBank database (Table S1). To determine the potential origin of D. pulicaria in Lake Biwa, we also conducted phylogenetic analyses on partial sequences of the ND5 gene using reported sequences of accessions from different geographical regions, including those of related species (Table S2). Multiple sequence alignment was performed using CLUSTALW (Thompson et al. 1994) with standard parameters in MEGA 6.06 (Tamura et al. 2013). Phylogenetic trees were constructed using neighbor-joining methods implemented in MEGA 6.06 (Tamura et al. 2013). Evolutionary distances were computed using the maximum composite likelihood method (Tamura et al. 2004) and are presented in terms of the number of base substitutions per site. Codon positions included were 1st + 2nd + 3rd + noncoding. All positions containing gaps and missing data were eliminated (complete deletion option). Bootstrap percentages were computed using 1000 pseudo-replications.
Student’s t test analyses were performed following an equal variance test to compare ephippial lengths of the Daphnia species, using R version 3.6 (Comprehensive R Archive Network [CRAN] at https://CRAN.R-project.org/) with minimal significance designated at p < 0.05.
Results
Genetic analysis
Over 600 bp of 12S rRNA gene fragments were detected from ephippia of each of the two target Daphnia species. Using BLAST sequence comparison, the identities of the analyzed sequences were confirmed as D. galeata (100%) and D. pulicaria (greater than 99%) for single (LB-RE2) and mixed (10) (LB-RE1) ephippial samples, respectively. The lengths of the aligned sequences for D. galeata and D. pulicaria in Lake Biwa were 603 and 605 bp, respectively. Phylogenetic analysis based on the 12S rRNA gene sequence indicated occurrence of D. galeata and D. pulicaria ephippia in the lake sediments (Fig. 2). Further analyses using PCR–RFLP for the sequenced samples yielded specific bands for D. galeata (LB-RE2) and D. pulicaria (LB-RE1,3), confirming that bands corresponding to these two organisms could be differentiated clearly in agarose gel using HindIII restriction (Fig. 3). Based on the location of HindIII digestion sites in the D. galeata 12S rRNA gene sequence, we expected to obtain three (135, 145, and 323 bp) fragments. However, because of the very small difference in size of 135 and 145 bp fragments of the D. galeata 12S rRNA gene, these bands could not be resolved by electrophoresis. Consequently, only two bands (approximately 140 and 323 bp) were detected (yellow arrowheads in lane 5 of Fig. 3). In case of D. pulicaria, specific bands were also detected at 289 and 326 bp (yellow arrowheads in lane 4: LB-RE1 and lane 8: LB-RE3 of Fig. 3) using two different samples, which confirmed the reproducibility of species discrimination. Thus, RFLP patterns were well defined and enabled the identification of 30 ephippia as either D. galeata (n = 12) or D. pulicaria (n = 18). We also performed phylogenetic analysis for ND5 mtDNA at 877-bp length using mixed (10) (LB-RE1) and single (LB-RE3) ephippial samples, and were accordingly able to identify both ephippial samples as D. pulicaria, and revealed that the Lake Biwa population of D. pulicaria is genetically more closely related to populations distributed across the western regions of North America than to those distributed in Europe (Fig. 4). Consequently, by using a combination of sequencing and RFLP methods, we were able to obtain a dataset of 32 ephippia for D. galeata (n = 13) and D. pulicaria (n = 19), with individually measured ephippial sizes and a percentage identification of 13.5% (32 identified among 237 ephippia).
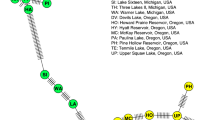
Neighbor-joining phylogenetic tree of two Daphnia species (D. pulicaria and D. galeata) based on partial sequences of the 12S rRNA gene. We used nucleotide substitutions, treating transitions and transversions equally, and conducted multiple sequence alignment using CLUSTALW (Thompson et al. 1994). Support values greater than 70% are shown above branches. Bold letters indicate sequences obtained from the present study. Superscript letters indicate sequences obtained from the following studies: a: Lehman et al. 1995, b: Crease 1999, c: Gieβler and Englbrecht 2009 and d: Tokishita et al. 2017. Detailed information on the sequences used, such as accession number, is shown in Table S1. The scale bars indicate the mean number of nucleotide substitutions per site
Restriction fragment length polymorphism (RFLP) validation. Agarose gels showing the results of digestion with HindIII. Lanes 1 and 6 show standard DNA size markers. Lanes 2, 3, and 7 show undigested bands of the PCR products obtained for the 12S rRNA gene. Lanes 4, 5, and 8 show the specific band patterns for each species following digestion with HindIII. The yellow arrowheads indicate the bands of the digested PCR products. Specific bands for D. galeata and D. pulicaria were detected at 140 and 323 bp, and 289 and 326 bp, respectively. The 140-bp band of D. galeata was expected to contain the 135 and 145 bp DNA fragments (based on the location of HindIII digestion sites in the 12S rRNA gene sequence) as the resolution of the gel was not sufficient to allow us to distinguish between these two bands
Neighbor-joining phylogenetic tree of two Daphnia species based on partial sequences of the ND5 gene. We used nucleotide substitutions, treating transitions and transversions equally, and conducted multiple sequence alignment using CLUSTALW (Thompson et al. 1994). Support values greater than 80% are shown above branches. Bold letters indicate sequences obtained in the present study. Detailed information on the sequences used, such as accession number, are shown in Table S2. The scale bars indicate the mean number of nucleotide substitutions per site
Morphological variation in ephippial size and statistical analysis
On the basis of our analysis of surface sediments, we detected 330 Daphnia ephippia, which had lengths and heights ranging from 0.47 to 1.30 mm and 0.36 to 0.82 mm, respectively (Fig. 5). Sequencing and PCR–RFLP analyses of Daphnia ephippia revealed that the length and height of ephippia identified as D. galeata (n = 13) were smaller than 0.82 and 0.50 mm, respectively, whereas those of D. pulicaria (n = 19) were larger than 0.87 and 0.53 mm, respectively (Fig. 5). Moreover, we detected a significant difference between the length of the ephippia of these two species (unpaired sample Student’s t test, t30 = 9.868, p < 0.001).
Relationship between the length and height of Daphnia ephippia collected from the sediments of Lake Biwa (open circle). Some ephippia were identified as D. pulicaria (n = 19, filled squares) and D. galeata (n = 13, filled triangles) based on mitochondrial 12S rRNA gene and PCR–RFLP (restriction fragment length polymorphism) analyses
Radioactivity of 210Pb, 214Pb, and 137Cs CRS-based chronology and historical variation in ephippial size
210Pb radioactivity in Lake Biwa sediments declines non-exponentially with depth (Supplementary Fig. S1), thereby implying that sediment accumulation rates are not constant, and that the CRS model was the most appropriate for determining sediment age (Appleby and Oldfield 1978). The 210Pb chronologies revealed that the calendar year corresponding to a core depth of 28.5 cm (layer of 28–29 cm depth) was 1924 (Supplementary Fig. S2). Although it was possible to estimate ages below a depth of 28.5 cm based on the CRS model, the calendar year estimates had considerably large errors (> 100 years), indicating that the chronology below the depth was not well defined. Above the 28.5-cm depth, such calendar age errors were estimated to be < 2 years after the 1980s, < 10 years after the 1950s, and < 25 years in the 1930s. We expected the peak of 137Cs radioactivity (Supplementary Fig. S1) to be detected at a depth of between 20.5 cm (layer of 20–21 cm depth) and 18.5 cm (layer of 18–19 cm depth), which is assigned to the fallout maxima around 1963 (Appleby 2001). The depth–age model estimated by 210Pb dating was consistent with the depth/age of the 137Cs fallout maxima (Supplementary Fig. S2).
Profiles for magnetic susceptibilities and pigments were compared between the cores to estimate the chronology of the LB1 and LB4 cores according to that of the LB7 core. On the basis of a comparison of chlorophyll pigments, we identified two peaks and an inflection point as reference layers (Supplementary Fig. S3a). The first peak of the 13.5 cm layer (each layer was expressed as mid-depth; e.g., 13.5 cm for the 13–14 cm depth layer) in LB7 was comparable to that of the 12.5 and 14.5 cm layers in LB1 and LB4, respectively. Simultaneously, the second peak of the 5.5 cm layer in LB7 was comparable to that of the 4.5 and 5.5 cm layers in LB1 and LB4, respectively. In all cores, an inflection layer indicating the start of an increasing trend was commonly detected at the 20.5 cm depth layer. Similarly, we detected two reference layers for the comparison of magnetic susceptibilities (Supplementary Fig. S3b). The first reference layer was at the 17.5 cm depth layer in LB7 and was comparable to that of the 16.5 cm depth layer in LB1 and LB4. The second layer was at a depth of 9.5 cm in LB1 and LB7 and was comparable to that at 8.5 cm in LB4. Therefore, the depth differences between the LB7 chronological core and those of LB1 and LB4 were at most 1 cm, indicating that the estimated age difference between cores were less than 3 years after 2000, and throughout most of the core were even less than 5 years prior to the 1980s.
We separated and compiled the ephippial data from the sediment cores (LB1 and LB4) into two periods, i.e., prior to the 1980s (13.5–27.5 cm depth layers), during which D. galeata predominated, and after 2000 (0.5–7.5 cm depth layers), during which D. galeata and D. pulicaria coexisted (Liu et al. 2020). Figure 6a shows the difference in ephippial length between D. galeata and D. pulicaria identified from DNA analysis as shown in Fig. 5. Figure 6b, c shows the compiled data of the lengths ephippia recovered from the sediment cores after around 2000 and before the 1980s (n = 126 and 213, respectively; LB1: 55 and 110, LB4: 71 and 103, respectively). We found that prior to the 1980s, ephippial length showed a peak at approximately 0.6 mm and did not exceed 0.85 mm (Fig. 6c), and its distribution was almost concordant with that of identified D. galeata (Fig. 6a). After 2000, ephippial length showed bimodal peaks at approximately 0.6 and 1.0 mm, with a wide range from 0.5 to 1.3 mm (Fig. 6b), and its distribution was consistent with those of identified D. galeata and D. pulicaria in Fig. 6a.
Discussion
In this study, we examined the ephippia of Daphnia that have accumulated in the sediments of Lake Biwa over recent and historical periods. We successfully collected 330 ephippia from the surface sediments, among which 32 were identified as D. galeata (n = 13) or D. pulicaria (n = 19). PCR–RFLP analysis of Daphnia ephippia revealed different restriction patterns for D. galeata and D. pulicaria, which is consistent with the findings of previous studies that have distinguished Daphnia species based on ephippial carapace and resting eggs using RFLP analysis (Alric et al. 2016; Brede et al. 2009; Ohtsuki et al. 2015; Petrusek et al. 2007). In the present study, we also found that ephippia of both D. galeata and D. pulicaria were abundant in surface sediments, which is consistent with the findings of previous studies that have reported on the presence of ephippia of D. galeata in sediments over the past century (Tsugeki et al. 2009) and the relatively sudden appearance of ephippia of D. pulicaria after 1999 (Urabe et al. 2003). Our simultaneous detection of ephippia of these two species imply that they have been co-existing in recent years and respectively producing resting eggs. Under favorable conditions, Daphnia typically reproduce parthenogenetically, but produce resting eggs to survive unfavorable conditions. Although species of Daphnia are among the major types of zooplankton present in Lake Biwa, they are virtually undetectable during some seasons (Liu et al. 2020; Yoshida et al. 2001). Therefore, it is highly probable that resting eggs enable each Daphnia species to maintain their populations under severe conditions.
In the present study, the length of Daphnia ephippia collected from recent surface sediments differed significantly between the two assessed species, with the ephippia of D. pulicaria in the D. pulex group (subgenus Daphnia) being larger than those of D. galeata in the D. longispina group (subgenus Hyalodaphnia). Moreover, we estimated the boundary length differentiating the two species to be approximately 0.86 mm. These findings tend to be consistent with the sedimentary records. We found that historical variations in the length of ephippia recovered from sediment cores were less than 0.85 mm between the 1980s and the early twentieth century during which time D. galeata was the predominant species in Lake Biwa (Miura and Cai 1990; Tsugeki et al. 2009). Conversely, in the years subsequent to 2000, during which these species have coexisted (Liu et al. 2020; Urabe et al. 2003), the maximum length of ephippia exceeded 0.85, reaching up to 1.3 mm. Similarly, a previous study has also shown that the ephippial length of Daphnia, mainly from D. galeata, in Lake Constance (Germany) has never exceeded 0.86 mm (Table 1; Jankowski and Straile 2003). Furthermore, the lengths of the ephippia of D. longispina (subgenus Hyalodaphnia) in Lake Fogo, Portugal (Skov et al. 2010), and lake Mamasin, Turkey (Kaya and Erdoğan 2014), have been found to be considerably smaller than 0.86 mm. Moreover, the minimum length of the ephippia of D. pulex (subgenus Daphnia) collected from a permanent pool in Belgium is approximately 0.91 mm (Pinceel et al. 2016), which is notably larger than 0.86 mm. Such evidence indicates that as a morphological characteristic, ephippial length has potential utility with respect to distinguishing Daphnia species belonging to different subgenera, although further analyses are required to evaluate the potential application of ephippia in this regard.
It has previously been established that there is a large genetic divergence between North America and Europe populations of D. pulicaria (Černý and Hebert 1999; Colbourne et al. 1998; Weider et al. 1999), which is reflected in larger differences in sequences of the ND5 mitochondrial gene across large geographical scales (Colbourne et al. 1998). In the present study, the ND5 gene of D. pulicaria is phylogenetically more closely related to that of populations distributed across the western regions of North America than to those in Europe, corroborating a previous report regarding the sudden appearance of D. pulicaria in Lake Biwa (Urabe et al. 2003). Accordingly, it is reasonable to deduce that the phylogeny of D. pulicaria has remained unchanged since its appearance in this lake.
Conclusion
In the present study, ephippia of two Daphnia species, D. galeata and D. pulicaria, which inhabit Lake Biwa, were abundant in recent surface sediments. Considering the fact that Daphnia are almost undetectable in the water column during certain seasons (Liu et al. 2020; Yoshida et al. 2001), the existence of ephippia in the sediments indicates that resting eggs have facilitated population survival and reestablishment of the two Daphnia species over the years. Furthermore, the length of Daphnia ephippia could be used to discriminate between D. galeata and D. pulicaria in Lake Biwa. In the lake, populations of the two Daphnia species have fluctuated substantially over the past several decades (Liu et al. 2020; Tsugeki et al. in preparation). As ephippial production is closely related to Daphnia population growth (McCauley et al. 1999), further investigations on the production of different sizes of resting eggs could shed light on the factors influencing Daphnia population dynamics. Although the data obtained in the present study are admittedly limited, the information presented here could stimulate additional resurrection and reconstruction studies using ephippia, and could enhance our understanding of the biological and evolutionary responses of Daphnia to environmental change.
References
Alric B, Möst M, Domaizon I et al (2016) Local human pressures influence gene flow in a hybridizing Daphnia species complex. J Evol Biol 29:720–735. https://doi.org/10.1111/jeb.12820
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Last WM, Smol JP (eds) Basin analysis, coring, and chronological techniques. Tracking environmental change using lake sediments, vol 1. Kluwer Academic Publishers, Dordrecht, pp 171–203. https://doi.org/10.1007/0-306-47669-x_9
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 5:1–8. https://doi.org/10.1016/S0341-8162(78)80002-2
Benzie JAH (2005) Cladocera: the genus Daphnia (including Daphniopsis). Backhuys, Leiden
Billiones R, Brehm M, Klee J, Schwenk K (2004) Genetic identification of Hyalodaphnia species and interspecific hybrids. Hydrobiologia 526:43–53
Brede N, Sandrock C, Straile D, Spaak P, Jankowski T, Streit B, Schwenk K (2009) The impact of human-made ecological changes on the genetic architecture of Daphnia species. PNAS 106:4758–4763
Caceres CE (2012) Eggs interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79:1699–1710
Carpenter SR, Kitchell JF (1993) The trophic cascade in lakes. Cambridge University Press, Cambridge
Černý M, Hebert PDN (1999) Intercontinental allozyme differentiation among four holarctic Daphnia species. Limnol Oceanogr 44:1381–1387
Colbourne JK, Hebert PD (1996) The systematics of North American Daphnia (Crustacea: Anomopoda): a molecular phylogenetic approach. Philos Trans R Soc B 351:349–360
Colbourne JK, Crease TJ, Weider L, Hebert PD, Duferesne F, Hobaek A (1998) Phylogenetics and evolution of a circumarctic species complex (Cladocera: Daphnia pulex). Biol J Linn Soc Lond 65:347–365
Crease TJ (1999) The complete sequence of the mitochondrial genome of Daphnia pulex (Cladocera: Crustacea). Gene 233:89–99
Crease TJ, Omilian AR, Costanzo KS, Taylor DJ (2012) Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 7:e46620
Cristescu ME, Constantin A, Bock DG, Caceres CE, Crease TJ (2012) Speciation with gene flow and the genetics of habitat transitions. Mol Ecol 21:1411–1422
Decaestecker E, Lefever C, DeMeester L, Ebert D (2004) Haunted by the past: evidence for dormant stage banks of microparasites and epibionts of Daphnia. Limnol Oceanogr 49:1355–1364
Faustová M, Petrusek A, Černý M (2004) Status of Daphnia resting egg banks in Bohemian forest lakes affected by acidification. Hydrobiologia 526:23–31
Frisch D, Morton PK, Chowdhury PR, Culver BW, Colbourne JK, Weider LJ, Jeyasingh PD (2014) A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol Lett 17:360–368. https://doi.org/10.1111/ele.12237
Geerts AN, Vanoverbeke J, Vanschoenwinkel B, Doorslaer WV, Feuchtmayr H, Atkinson D, Moss B, Davidson TA, Sayer CD, De Meester L (2015) Rapid evolution of thermal tolerance in the water flea Daphnia. Nat Clim Change 5:665–668. https://doi.org/10.1038/nclimate2628
Gieβler S, Englbrecht CC (2009) Dynamic reticulate evolution in a Daphnia multispecies complex. J Exp Zool 311:530–548
Hairston NG, Van Brunt RA, Kearns CM, Engstrom DR (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711. https://doi.org/10.2307/1940704
Hairston NG Jr, Lampert W, Cáceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, Fox JA, Post DM (1999) Rapid evolution revealed by dormant eggs. Nature 401:446
Hampton SE, McGowan S, Ozersky T et al (2018) Recent ecological change in ancient lakes. Limnol Oceanogr 63:2277–2304. https://doi.org/10.1002/lno.10938
Hamrová E, Goliáš V, Petrusek A (2010) Identifying century-old long-spined Daphnia: species replacement in a mountain lake characterised by paleogenetic methods. Hydrobiologia 643:97–106. https://doi.org/10.1007/s10750-010-0127-9
Hsieh CH, Sakai Y, Ban S et al (2011) Eutrophication and warming effects on long-term variation of zooplankton in Lake Biwa. Biogeosci Discuss 8:593–629. https://doi.org/10.5194/bgd-8-593-2011
Hyodo F, Kuwae M, Sasaki N, Hayashi R, Makino W, Kusaka S, Tsugeki KN, Ishida S, Ohtsuki H, Omoto K, Urabe J (2017) Variations in lignin-derived phenols in sediments of Japanese lakes over the last century and their relation to watershed vegetation. Org Geochem 103:125–135. https://doi.org/10.1016/j.orggeochem.2016.11.001
Ishida S, Ohtsuki H, Awano T, Tsugeki NK, Makino W, Suyama Y, Urabe J (2012) DNA extraction and amplification methods for ephippial cases of Daphnia resting eggs in lake sediments: a novel approach for reconstructing zooplankton population structure from the past. Limnology 13:261–267
Jankowski T, Straile D (2003) A comparison of egg-bank and long-term plankton dynamics of two Daphnia Species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnol Oceanogr 48:1948–1955
Juračka PJ, Kořínek V, Petrusek A (2010) A new Central European species of the Daphnia curvirostris complex, Daphnia hrbaceki sp. nov. (Cladocera, Anomopoda, Daphniidae). Zootaxa 2718:1–22
Kaya M, Erdoğan S (2014) Morphological examination of the resting egg structure of 3 cladoceran species [Ceriodaphnia quadrangula (OF Müller, 1785), Daphnia longispina (OF Müller, 1776), and D. magna Straus, 1820]. Turk Zool Derg 38:131–135
Lehman N, Pfrender ME, Morin PA, Crease TJ, Lynch M (1995) A hierarchical molecular phylogeny within the genus Daphnia. Mol Phylo Evol 4:395–407
Liu X, Dur G, Ban S, Sakai Y, Ohmae S, Morita T (2020) Planktivorous fish predation masks anthropogenic disturbances on decadal trends in zooplankton biomass and body size structure in Lake Biwa, Japan. Limnol Oceanogr 65:667–682
Lorenzen CJ (1967) Determination of chlorophyll and pheopigments spectrophotometric equation. Limnol Oceanogr 12:343–346
Marková S, Černý M, Rees D, Stuchlík E (2006) Are they still viable? Physical conditions and abundance of Daphnia pulicaria resting eggs in sediment cores from lakes in the Tatra Mountains. Biologia (Bratisl) 61:S135–S146. https://doi.org/10.2478/s11756-006-0126-5
McCauley E, Nisbet RM, Murdoch WW, de Roos AM, Gurney WSC (1999) Large-amplitude cycles of Daphnia and its algal prey in enriched environments. Nature 402:653–656
Miura T, Cai QH (1990) Annual and seasonal occurrences of the zooplankters observed in the North Basin of Lake Biwa from 1965 to 1979. Lake Biwa Research Institute, Otsu
Möst M, Oexle S, Marková S, Aidukaite D, Baumgartner L, Stich HB, Wessels M, Martin-Creuzburg D, Spaak P (2015) Population genetic dynamics of an invasion reconstructed from the sediment egg bank. Mol Ecol 24:4074–4093
Ohtsuki H, Awano T, Tsugeki NK, Ishida S, Oda H, Makino W, Urabe J (2015) Historical changes in the ecosystem condition of a small mountain lake over the past 60 years as revealed by plankton remains and Daphnia ephippial carapaces stored in lake sediments. PLoS ONE 10:e0119767
Petrusek A, Černý M, Mergeay J, Schwenk K (2007) Daphnia in the Tatra Mountain lakes: multiple colonisation and hidden species diversity revealed by molecular markers. Fundam Appl Limnol 169:279–291. https://doi.org/10.1127/1863-9135/2007/0169-0279
Pinceel T, Brendonck L, Vanschoenwinkel B (2016) Propagule size and shape may promote local wind dispersal in freshwater zooplankton—a wind tunnel experiment. Limnol Oceanogr 61:122–131
Rother A, Pitsch M, Hülsmann S (2010) The importance of hatching from resting eggs for population dynamics and genetic composition of Daphnia in a deep reservoir. Freshw Biol 55:2319–2331. https://doi.org/10.1111/j.1365-2427.2010.02441.x
Skov T, Buchaca T, Amsinck SL, Landkildehus F, Odgaard BV, Azevedo J, Gonçalves V, Raposeiro PM, Andersen TJ, Jeppesen E (2010) Using invertebrate remains and pigments in the sediment to infer changes in trophic structure after fish introduction in Lake Fogo: a crater lake in the Azores. Hydrobiologia 654:13–25
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thompson J, Higgins D, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tokishita S, Shibuya H, Kobayashi T, Sakamoto M, Ha J-Y, Yokobori S, Yamagata H, Hanazato T (2017) Diversification of mitochondrial genome of Daphnia galeata (Cladocera, Crustacea): comparison with phylogenetic consideration of the complete sequences of clones isolated from five lakes in Japan. Gene 611:38–46
Tsugeki NK, Oda H, Urabe J (2003) Fluctuation of zooplankton community in Lake Biwa during the 20th century: a paleolimnological analysis. Limnology 4:101–107
Tsugeki NK, Ishida S, Urabe J (2009) Sedimentary records of reduction in resting egg production of Daphnia galeata in Lake Biwa during the 20th century: a possible effect of winter warming. J Paleolimnol 42:155–165
Urabe J, Ishida S, Nishimoto M, Weider LJ (2003) Daphnia pulicaria, a zooplankton species that suddenly appeared in 1999 in the offshore zone of Lake Biwa. Limnology 4:0035–0041
Vandekerkhove J, Declerck S, Vanhove M, Brendonck L, Jeppesen E, Porcune JMC, DeMeester L (2004) Use of ephippial morphology to assess richness of anomopods: potentials and pitfalls. J Limnol 63:75–84
Weider LJ, Hobaek A, Colbourne JK, Crease TJ, Dufresne F, Hebert PD (1999) Holarctic phylogeography of an asexual species complex I. Mitochondrial DNA variation in arctic Daphnia. Evolution 53:777–792
Yoshida T, Kagami M, Bahadur Gurung T, Urabe J (2001) Seasonal succession of zooplankton in the north basin of Lake Biwa. Aquat Ecol 35:19–29
Acknowledgements
We are grateful to H. Kudoh, S. Nakano, H. Iwata, M. Ochiai, S. Goda, T. Akatsuka, M. Shoji, M. Sakamoto, S. Kawabuchi, I. Hashimoto, and G. Mitamura for their assistance with laboratory analysis and field sampling. This work was supported by a Grant-in-Aid for Scientific Research C (No. 17K00528 to NT) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and also supported by the Kurita Water and Environment Foundation (Grant No.17B050 to MH). The Joint Usage/Research Grant of Center for Ecological Research (2017–2019 jurc-cer), Kyoto University and a project on Joint Usage/Research Center–Leading Academia in Marine and Environment Pollution Research (LaMer) of the Center for Marine Environmental Studies, Ehime University, also supported this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Mingbo Yin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsugeki, N.K., Honjo, M.N. & Kuwae, M. Interspecific variation in ephippial size between Daphnia galeata and D. pulicaria in Lake Biwa, Japan. Limnology 22, 197–207 (2021). https://doi.org/10.1007/s10201-020-00646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-020-00646-8