Abstract
Background
In 2009, the European Medicines Agency granted approval for two higher-valent pneumococcal conjugate vaccines. This study aims to evaluate the cost-effectiveness of universal infant (<2 years old) vaccination with a 13-valent pneumococcal conjugate vaccine (PCV13) in comparison with a 10-valent pneumococcal conjugate vaccine (PCV10) for the prevention of pneumococcal disease in Germany.
Methods
A population-based Markov model was developed to estimate the impact of PCV13 and PCV10 on invasive pneumococcal disease (IPD), non-invasive pneumonia (PNE), and acute otitis media (AOM) over a time horizon of 50 years. The model included the effects of the historical vaccination scheme in infants as well as indirect herd effects and replacement disease. We used German epidemiological data to calculate episodes of IPD, PNE, and AOM, as well as direct and indirect effects of the vaccination. Parameter uncertainty was tested in univariate and probabilistic sensitivity analyses.
Results
In the base-case analysis, the ICER of PCV13 versus PCV10 infant vaccination was EUR 9826 per quality-adjusted life-year (QALY) gained or EUR 5490 per life-year (LY) gained from the societal perspective and EUR 3368 per QALY gained or EUR 1882 per LY gained from the perspective of the German statutory health insurance. The results were particularly sensitive to the magnitude of indirect effects of both vaccines.
Conclusions
Universal infant vaccination with PCV13 is likely to be a cost-effective intervention compared with PCV10 within the German health care system, if additional net indirect effects of PCV13 vaccination are significant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Streptococcus pneumonia is a major cause of morbidity and mortality worldwide. Pneumococcal infections are the leading cause of death in children aged <5 years. Meningitis, bacteremia, and pneumonia with bacteremia and/or empyema are invasive diseases caused by pneumococci. Risk factors for invasive pneumococcal disease (IPD) include age, ethnicity, geographic location, concomitant chronic illnesses, and attendance in day care centers [1]. O’Brien et al. [2] estimated that about 14.5 million episodes of serious pneumococcal disease occurred in 2000 worldwide, resulting in about 826,000 deaths in children aged 1–59 months.
In 2001, a 7-valent pneumococcal vaccine (PCV7) was approved in Europe. It provides protection against seven of the 94 (to date) discovered serotypes. After the Standing Vaccination Committee (STIKO) at the German Robert Koch Institute had recommended PCV7 vaccination for children who were particularly vulnerable to pneumococcal disease [3], it expanded its recommendation to all children <2 years of age in mid-2006 [4]. As a result of the universal infant vaccination in Germany, the incidence of IPD decreased in children <16 years of age [5, 6]. In addition, indirect herd effects were observed in vaccine-type (VT) IPD [7, 8]. Replacement disease caused by non-vaccine serotypes had not been reported until mid-2008 [5], but during the following 2 years, the incidence of non-VT IPD had increased significantly in children <16 years old [6].
In 2009, the European Medicines Agency approved two other vaccines for the vaccination of infants against pneumococcal disease: a 10-valent conjugate vaccine (PCV10) and a 13-valent conjugate vaccine (PCV13). PCV10 includes the serotypes of PCV7 as well as three additional serotypes, whereas PCV13 covers the serotypes of PCV10 and three additional serotypes. While PCV7 and PCV13 are conjugated to a nontoxic diphtheria CRM197 carrier protein, PCV10 uses non-typeable Haemophilus influenzae protein D as its carrier.
In August of 2009, PCV10 was introduced to the German market. In the beginning of 2010, PCV7 was withdrawn from the market and completely replaced by PCV13. At the time of the launch of PCV10 and PCV13, about 54 % of IPD in children <16 years old and 36 % of IPD in people 16+ years old were caused by PCV10-serotypes, while 76 and 62 %, respectively, were caused by PCV13 serotypes [9].
After the introduction of the higher-valent conjugate vaccines in Germany, the number of reported PCV7-serotype IPD episodes has continued to decline in children <16 years of age. In addition, the additional six serotypes included in PCV13 have also started to decrease, while an ongoing increase in the number of IPD episodes caused by non-PCV13 serotypes has been observed [10].
Several studies [11–30] examined the cost-effectiveness of PCV13 in comparison with PCV10. The results of the analyses differed substantially due to varying assumptions on input parameters. Therefore, the objectives of this study were to evaluate the cost-effectiveness of universal infant vaccination using PCV13 only or PCV10 only in Germany, and to estimate the impact of higher-valent conjugate vaccines on the burden of pneumococcal disease compared with a reference scenario in which children <2 years old were still vaccinated with PCV7.
Methods
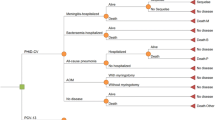
We developed a population-based Markov model in Microsoft EXCEL 2010 to estimate the effects of childhood vaccination (<2 years) over a time horizon of 50 years (2013–2062). The model took into account the historical vaccination scheme pre 2013 (burn-in see Fig. 1) to calculate the serotype-specific incidence of invasive pneumococcal disease at the start of the evaluation phase. The cycle length of the model was 1 year.
The model consists of three arms:
-
1.
Vaccination of infants with PCV7 from 2006 to 2009; vaccination of infants with PCV13 from 2010 to 2062
-
2.
Vaccination of infants with PCV7 from 2006 to 2009; vaccination of infants with PCV10 from 2010 to 2062
-
3.
Vaccination of infants with PCV7 from 2006 to 2062. This arm served as a reference scenario to estimate the effect of the higher-valent vaccines on the burden of pneumococcal diseases.
On average, transitions between states occur in the middle of each cycle [31]. Therefore, to compensate for the timing of transition, we performed half cycle corrections by averaging the outcomes of two cycles (\( {\text{outcome}_{\,half-cycle}}_{t} = \frac{{{\text{outcome}_{\,cycle}}_{t} + {\text{outcome}_{\,cycle}}_{t + 1} }}{2} \)) [32].
The evaluation was conducted from the perspective of the statutory health insurance (SHI) and the societal perspective. Quality-adjusted life-years (QALY) and life-years (LY) gained served as primary outcomes. Both costs and outcomes were discounted by 3 % following the recommendations of the Institute for Quality and Efficiency in Health Care (IQWiG) [33]. The price year was 2013.
Model structure
The population was divided into 101 age groups (0–100 years). The simulation started with the German population structure of 2005. Each cycle, the population aged 1 year and a new cohort of newborns was added. The entire population (including the new birth cohorts) was followed up until the end of the evaluation period (2062). Infants received vaccination during the first year of life. Over the course of time, immunized individuals might lose vaccine protection against pneumococcal disease (waning). The population-based Markov model was used to track vaccination states and simulate the aging process. We coupled the model with a decision tree to calculate the annual episodes of pneumococcal diseases and mortality for each age/vaccination state. Afterwards, the annual all cause death rates were corrected by the number of deaths related to pneumococcal disease. In case of IPD, the simulation distinguished between four groups of serotypes: serotypes included in PCV7 (PCV7), serotypes included in PCV10 but not in PCV7 (PCV10-7), serotypes included in PCV13 but not in PCV10 (PCV13-10) and serotypes not covered by PCV13 (N-PCV13). The model structure of the PCV13 arm is illustrated in Fig. 1.
Demography
To project the German population, we used a recently developed demographic model [34] that generated the all-cause death rates, number of newborns and life expectancy for each age group per calendar year. Results of the model were in line with projections of the German Federal Statistical Office [34]. Figure 2 shows the results of the projection for 2010, 2040, and 2060.
Epidemiology of pneumococcal disease in Germany
In Germany, two independent reporting systems perform nationwide surveillance of IPD for children <16 years old [6]. The German pediatric surveillance unit (ESPED) collects IPD data from all children’s hospitals and all pediatric wards in general hospitals [35]. The second system is a laboratory-based passive (web-based) sentinel surveillance operated by the Robert Koch Institute [6, 8]. IPD incidences are estimated by capture recapture calculations [5, 6, 36]. Due to stable IPD numbers, the hospital surveillance was stopped in 2003 and was resumed in January 2007 after the recommendation for universal infant PCV7 vaccination [5]. Therefore, pre-vaccination pneumococcal meningitis and non-meningitis IPD incidences from 1997 to 2003 served as model inputs.
In the absence of nationwide notification requirements for IPD cases and an active hospital-based surveillance, there is a lack of national data on IPD incidence in adults. The only German source that reported incidences in the population >15 years of age, was a laboratory-based surveillance study conducted in the state North-Rhine Westphalia between 2001 and 2003 [37]. Due to poor blood culturing practices in Germany, we adjusted non-meningitis IPD incidence rates for under-ascertainment [37, 38]. The pre-vaccination serotype distribution for the period from 2005 to 2006 was obtained from the National Reference Center for Streptococci (NRZ) [9].
A detailed microbiological diagnosis is usually not performed in non-invasive pneumonia (PNE) and acute otitis media (AOM). Therefore, we used data of all-cause PNE and AOM. Age-specific incidences of hospitalized PNE were estimated based on German pre-vaccination hospital admission data [39]. Due to significant annual fluctuations in the reported cases, we took the average incidence rates of the period 2003–2005. Outpatient PNE data were derived from a representative pharmaceutical prescription panel [40]. Schnoor et al. estimated an incidence of PNE ranging from 370 to 1230 cases per 100,000 adults [41], which was in line with the hospital admission data (approximately 1,200 cases per 100,000 adults).
The majority of AOM episodes occur in childhood with a peak in children <5 years old. Schnabel et al. [42] observed 3097 German children born between November 1997 and January 1999. Of these, 18.7, 30.0, and 21.2 % suffered from AOM in their first, second until fifth, and sixth year of life, respectively. Grueber et al. [43] prospectively observed 1314 children born in 1990 in the MAS-90 study. For children from 7 to <12 years old, the annual risk of developing an AOM episode was 8 %. Finally, incidence of AOM in adolescents (12–13 years of age: 4.3 %, and 14–17 years of age: 3.5 %) was taken from the KiGGS-Study [44]. In 15 per 100,000 hospitalized children <2 years old, and 5 per 100,000 hospitalized children >2 years old surgical treatment was needed (e.g., tympanostomy tube placement) [45, 46] (Tables 1, 2).
A significant proportion of survivors of pneumococcal meningitis and non-meningitis IPD suffer from serious sequelae, such as neurological disorders or hearing loss. According to ESPED, sequelae were present in 20.6 % of children with pneumococcal meningitis and in 3.8 % with non-meningitis IPD. Hearing loss occurred in 9.1 % of meningitis survivors [36]. As the ESPED data comprised only acute incidents with the observation ending at hospital discharge, late sequelae were not documented.
In Germany, the pediatric case fatality ratios (CFR) of pneumococcal meningitis and non-meningitis IPD were 7.5 and 2.0 % [36], respectively. IPD-specific CFRs for adults were taken from an analysis of 22,000 IPD patients in England (March 2002–March 2009) [47]. Age-specific CFRs for hospitalized PNE were estimated based on national hospital statistics [39]. For outpatient PNE, we assumed CFRs of 0 % for patients <60 years old and 0.5 % for patients ≥60 years old (Table 3).
Vaccine uptake
Recent analyses hinted high acceptance of the primary immunization in Germany. However, deficits existed in the application of the booster dose in the second year of life [48]. Surveillance of vaccination coverage based on data from 17 regional associations of SHI physicians suggested that the current uptake for the first three doses was about 90 % [49] and for the fourth dose about 75 % [50]. In the model, we applied an uptake of 90 % for all four doses.
Direct effects of vaccination
IPD efficacy data (Table 4) were derived from the trial of the Northern California Kaiser Permanente (NCKP) Health Maintenance Organization [51] and the FinIP trial [52]. In the intention to treat analysis of the NCKP trial, the effectiveness of PCV7 against vaccine serotypes was 93.9 %. We assumed that the efficacy of PCV13 was equal to PCV7 for all vaccine serotypes. In the FinIP trial, PCV10 showed an effectiveness of 100 % against VT IPD in children receiving a 3 + 1 schedule and 92 % in children receiving a 2 + 1 schedule.
In the absence of pathogen-specific data, we used estimates of the effectiveness against all causes of non-invasive pneumonia. PCV7 and PCV10 showed comparable effects against pneumonia [53, 54], while clinical data on the impact of PCV13 on non-invasive pneumococcal pneumonia in children does not exist to date. Due to a lack of clinical evidence, we assumed that the effectiveness of the higher-valent vaccine increased proportionally to the serotype coverage in IPD [17]. PCV7 effectiveness data from the NCKP trial [55] (11.1 % against hospitalized PNE and 6.0 % against outpatient PNE) provided the basis for the calculations.
In a systematic literature review of the impact of pneumococcal conjugate vaccine on AOM, Taylor et al. [56] identified three randomized controlled trials (RCT) for PCV7. While two trials [51, 57–59] reported comparable effects of 5.8–8.9 %, one study [60] in high-risk American natives failed to demonstrate effectiveness of PCV7 against AOM. However, the failure to detect a statistically significant impact might be due to a lack of statistical power [56, 60]. Again, we took data from the NCKP trial [58] and increased the effectiveness of PCV13 proportionally to the serotype coverage in IPD. Two studies [61, 62] suggested an additional benefit of PCV10 in preventing AOM due to its effectiveness against Haemophilus influenza AOM. Therefore, we applied an effectiveness of 19 % against all cause AOM [62], instead of extrapolating the PCV7-effectiveness.
For children >5 years old, we adjusted the effectiveness of each vaccine against non-invasive pneumococcal disease according to its serotype coverage for IPD.
Melegaro et al. [63] calculated an average duration of vaccine protection of 8 years. In the absence of empirical data on the effects of waning immunity for pneumococcal conjugated vaccines, we applied the estimate of Melegaro in our model. We assumed a constant waning rate. Hence, every year 11.75 % (1 − exp[−1/8]) of the immunized people lost their protection completely.
Indirect effects
The introduction of PCV7 vaccination in infants had led to indirect herd effects and had changed the epidemiology of pneumococcal disease [64–70]. In Germany, indirect herd effects after the implementation of universal childhood vaccination with PCV7 were also detected [7, 8]. The emergence of replacement disease was not observed after 2 years of universal pneumococcal conjugate infant vaccination [5]. However, after 4 years a significant increase in the incidence of IPD caused by non-vaccine serotypes was reported [6].
Jiang et al. [71] used cumulative gamma distributions fitted to United States (US) data to calculate indirect herd effects and serotype replacement as a function of the cumulative vaccination coverage in children. We modeled indirect effects as a function of the cumulative vaccine coverage adjusted for waning (CVCW). The CVCW at time t was an outcome of the Markov model.
We used two parametric sigmoid functions of the form \( \frac{\alpha }{{1 + \beta \cdot \left( {{\text{CVCW}}_{t} } \right)^{ - \gamma } }} \) to calculate indirect effects. The decline of VT IPD incidence and replacement disease in unvaccinated persons and vaccinated individuals who lost protection against S. pneumonia at time t (t = 0: start of the vaccination program) was modeled as follows:
Indirect herd effects
Replacement disease
The sum of both functions gave the net indirect effects at time t:
Replacement disease also occurred in vaccinated infants with direct protection against VT IPD and reduced the overall effectiveness of the vaccine.
Figure 3 illustrates the indirect effects in a simple scenario without vaccine replacement (from PCV7 to higher-valent PCVs) for the fitted parameters α HE, β HE, γ HE, α RD, β RD and γ RD (Table 7), the vaccination coverage shown in Fig. 4 and a waning rate of 11.75 % per year. The effects are presented relative to the VT IPD incidence in 2005 and can easily be calculated relative to the IPD incidence by multiplying with the proportion of VT IPD in 2005 (Table 2).
Diel et al. analyzed retrospective data from the German IMS-Health-VIP® and found indirect herd effects in PNE [72] and AOM [73] of the infant vaccination with PCVs. We modeled the impact of indirect effects on non-invasive pneumococcal disease incidences as follows:
-
The direct vaccine effect was reduced proportionally with the decrease in direct effects against IPD due to replacement disease.
-
Net indirect effects in unprotected individuals were calculated by multiplying the net indirect effects on IPD in unvaccinated individuals by the quotient of vaccine effectiveness against non-invasive pneumococcal disease and vaccine effectiveness against invasive pneumococcal disease (Table 7).
The most recent German IPD data in adults [74] showed that the number of serotype 3 cases remained stable after the introduction of the universal infant vaccination with PCV13, while the number of all other PCV13-serotype IPD decreased. Therefore, we reduced the indirect effects in PCV13-PCV10 serotypes by the proportion of serotype 3 in 2009 (the year before the introduction of universal infant vaccination with PCV13). This meant that PCV13 still had an indirect effect on serotype 3, since, in contrast to non-vaccine serotypes, serotype 3 was stopped from spreading further.
Health economic parameters
To evaluate costs from the perspective of the SHI (Table 5), we applied the current German recommendations [75] for the valuation of resource usage. It was assumed that all IPD cases were treated in hospitals. Hospitalization costs were derived from official German Diagnosis Related Groups codes (G-DRGs) [76]. For each disease, the weighted average of different DRGs was calculated according to Cleas et al. [77]. We applied the official German Uniform Evaluation Scheme (EBM) [78] to calculate outpatient physician costs. In 2013, a physicians received a flat payment of EUR 37.60, EUR 29.00, EUR 26.20, EUR 29.70 and EUR 35.00 for standard health care services in outpatient care for children <4 years old, 5- to 18-year-old, 19- to 54-year-old, 55- to 75-year-old and elderly ≥76 years old, respectively [78].
Systemic antibiotics made up for the main part of the prescriptions in AOM, which on average cost EUR 8.09 per episode [40]. According to expert opinions (a Delphi panel survey of German pediatricians experienced as clinical investigators) about 22 % of the AOM patients needed an operation in the ambulatory setting (e.g., tympanostomy tube placement) which cost EUR 385. Costs of outpatient PNE treatment included the capitation fee and average compensation for prescriptions of EUR 23.91 per episode [40]. Costs of long-term health services for children with sequelae were derived from a German cost-benefit analysis of pediatric cochlear implantation [79] and a German cost-effectiveness analysis of PCV7 [77].
Indirect costs of productivity loss were calculated according to the friction cost approach (FCA). Thus, costs of long-term production loss were limited to a friction period that was assumed to be 77 days [80]. Data from the German Federal Health monitoring [81] were used to calculate days off work in patients with IPD and PNE. Age-specific work loss ranged between 7.4 and 18.7 days. Employment rates were derived from the Federal Statistical Office of Germany [82]. We assumed that hospitalized children <12 years of age caused seven days of parental work absence and children in need of outpatient treatment 3 days [77]. Daily cost of work disability was calculated from the average compensation of employees in 2013 [83].
Prices of PCV10 and PCV13 were EUR 52.57 and EUR 55.82 per dose (pack of 10 price excluding discounts to the SHI), respectively [84]. The average physician fee was EUR 6.95 based on a sample of German vaccination agreements between the Association of SHI Physicians and the SHI. Costs of adverse reactions were not included in the model due to the low incidence of the vaccine side effects [57].
The baseline QALY weights were obtained from studies of the EuroQol Group [85]. Utility losses related to pneumococcal disease were derived from Melegaro et al. [86] and Rozenbaum et al. [17] (Table 6).
Sensitivity analysis
Univariate and a probabilistic sensitivity analyses (PSA) were performed to explore parameter uncertainty and to ensure stability of the results. Parameters used in the sensitivity analyses were estimated from the referenced literature or assumed. The PSA was run for 10,000 iterations.
Calibration of indirect effects parameters
Calibration (nonlinear optimization) of the parameters α HE, β HE, γ HE, α RD, β RD and γ RD (Table 7) was performed with the Excel Solver tool by minimizing the squared differences between modeled and observed pneumococcal meningitis episodes in children <16 years of age. The Excel Solver routine iteratively solved the whole model (Markov model, decision tree disease model, demographic model) and changed the parameters of the functions systematically using gradient methods until the residual sum of squares reached a minimum.
It is likely that ascertainment bias due to a change in blood culturing practices has significantly impacted the reported incidence rates of non-meningitis IPD [6]. Hence, we only used pneumococcal meningitis data for fitting purposes. The fit between model outcomes and observed pneumococcal meningitis incidences are shown in Figure S1 of the supplementary material.
Results
Base-case analysis of PCV13 and PCV10 versus the PCV7 reference scenario
In the period from 2013 to 2062, 44,161 IPD episodes, 556,497 PNE episodes (including 149,700 hospitalized cases), and 5,019,108 AOM episodes could be prevented in Germany, if infants were vaccinated with PCV10 instead of vaccination with PCV7. Vaccinating children <2 years of age with PCV13 could further reduce the burden of pneumococcal disease by 21,057 IPD episodes, 188,138 PNE episodes (including 81,390 hospitalized cases), but AOM episodes would increase by 3,990,948 episodes. Figure S3 in the supplementary material shows the development of IPD episodes in the period from 2013 to 2062 for each vaccination strategy in comparison with the reference scenario (maintenance of PCV7). The prevented cases of pneumococcal disease corresponded to a total (discounted) gain of 134,372 life-years or 111,565 QALYs for PCV10, and 209,291 life-years or 153,423 QALYs for PCV13 (Table 8).
Incremental analysis of PCV13 versus PCV10
Compared with PCV10, the universal infant vaccination with PCV13 would result in a gain of 74,919 life-years or 41,859 QALYs in the base-case analysis. In addition, EUR 43.36 million of direct disease costs could be saved from the perspective of the SHI. From the societal perspective, the disease costs would increase by EUR 270.35 million due to the indirect costs of AOM episodes. Additional costs of vaccination would sum up to EUR 184.3 million. From the societal perspective, the ICER of PCV13 versus PCV10 was EUR 9826 per QALY gained or EUR 5490 per LY gained. Excluding co-payments and indirect disease costs (SHI perspective), ICERs decreased to EUR 3368 per QALY gained or EUR 1882 per LY gained (Table 9).
Sensitivity analyses
The results were robust to variations of most model input parameters (Table S1 of the supplementary material). Indirect vaccine effects had a major impact on the cost-effectiveness of the vaccines. Without indirect effects, PCV10 vaccination of infants would dominate the PCV13 strategy or would be highly cost-effective. Substantial additional net-indirect effects of PCV13 resulted in the vaccine being cost-effective or dominant. PCV10 would also be cost-effective, if its effectiveness against AOM was 33.6 % and PCV13 did not provide additional benefits in the prevention of PNE at the same time.
The probability that PCV13 was cost-effective at a willingness-to-pay threshold of EUR 20,000 per QALY gained was 79 % from the SHI perspective and 64 % from the societal perspective. At a threshold of EUR 30,000 the probability would increase to 85 and 74 %, respectively. At ICER thresholds above EUR 100,000, there was still a small probability that PCV13 was not cost-effective (Fig. 5) due to the combined uncertainty of indirect effects, the effectiveness of PCVs against all cause PNE and the magnitude of additional benefits of PCV10 in preventing all case AOM.
Discussion
This study is the first German economic evaluation of universal infant pneumococcal vaccination programs that included time-dependent indirect herd effects and replacement disease for the entire German population. Base-case results of our analysis indicated that PCV13 was cost-effective compared with PCV10 from the perspective of the SHI and the societal perspective.
Impact of pneumococcal conjugate vaccination of infants on pneumococcal disease in Germany
Our model predicted a rapid decline of pneumococcal diseases covered by PCVs (Figure S1–S3 of the supplementary material) and an increase of non-VT disease in all age groups. Indirect herd effects and replacement disease have been reported in several studies for PCV7 [6, 88–92] and PCV13 [93–97] infant vaccination programs. German incidence data of invasive pneumococcal disease in adults does only exist for the pre-PCV period. Therefore, we simulated the impact of PCV7 infant vaccination on all age groups based on pneumococcal meningitis data in <16 years old and extrapolated the effects for the higher-valent vaccines. Comparing the modeled serotype distribution in IPD in adults with the observed distribution (Figure S2 of the supplementary material) reveals that the model gave good prediction for the age group 16–59 years old but underestimated either indirect herd effects or replacement disease in the age group 60+ years old.
Table S2 of the supplementary material compares the predicted short-term impact of PCV infant vaccination predicted by our model with reported IPD incidences from other Western European countries. The results of our model were in the range of the observed data, which varied considerably between countries. The exception were vaccination programs with PCV10 which did not have an impact on the IPD incidence in adults in Finland but reduced the total number of IPD cases substantially in our model, if we assumed indirect effects of PCV10.
The long-term effects of PCV infant vaccination estimated by our model are shown in Table S3 of the supplementary material. Depending on the administered PCV vaccine, infant vaccination programs could prevent about 1649–2953 IPD episodes, 7397–12,216 hospitalized PNE episodes, 8963–19,036 PNE episodes treated in outpatient care and 13,759-114,141 AOM episodes on average per year compared with the number of pneumococcal disease cases (adjusted for demographic trends) in the pre-PCV period.
Pneumococcal disease data in Germany
This study is limited by a lack of German epidemiologic data. In Germany, nationwide notification requirements for pneumococcal disease do not exist to date. One passive surveillance unit [8] collects IPD cases in adults, but only a fraction of German laboratories send samples to the system and the number of participating laboratories changes every year [8]. The only study which reports IPD incidences in adults is a laboratory-based surveillance study conducted in the state North-Rhine Westphalia between 2001 and 2003 [37]. However, several correction factors (for laboratories not participating, hospitals not sending samples to laboratories and a lack of blood culturing in pneumonia cases) were applied to generate reasonable incidence estimates [37].
Furthermore, it is very likely that an ascertainment bias due to a change in blood culturing practices has a substantial impact on the reported incidence rates of non-meningitis IPD in <16 years old [6]. For instance, the estimated incidence in 2–15 years old increased by a factor of two after the introduction of universal infant vaccination with PCV7 [6]. Therefore, we adjusted the non-meningitis IPD incidence using an underreporting factor of 2.7 derived from the study in North-Rhine Westphalia [37] and only included pneumococcal meningitis cases in the calibration process of indirect herd effect parameters.
The burden of non-invasive pneumococcal disease is unknown in Germany. The CAPNETZ study [98] estimates that around 30 % of all pneumonia cases are caused by S. pneumonia but data on the serotype distribution do not currently exist.
Direct effects of higher-valent PCVs
PCV10 efficacy against VT-IPD was derived from the FinIP trial [52]. For PCV13, we assumed an efficacy of 93.9 % against all VT-IPD. This figure is based on efficacy estimates for PCV7 in the NCKP trial [51]. Andrews et al. [99] calculate a lower effectiveness of PCV13 against serotype 3 but the CAPITA study does not provide any evidence for a lower effectiveness against serotype 3 in comparison with other vaccine serotypes [100]. In addition, adjusting for the impact of age at vaccination in the CAPITA data, van Werkhoven et al. [101] estimate an effectiveness of PCV13 against VT bacteremic pneumococcal pneumonia of >90 % in 50 years old which corresponds with the efficacy applied in our model.
In the absence of pathogen-specific epidemiologic data in Germany, we used effectiveness estimates against all causes of non-invasive pneumonia and AOM. We assumed that the effectiveness of the higher-valent vaccine increased proportionally to the serotype coverage in IPD [17]. Since the CAPITA study does not provide evidence for a reduced effectiveness of PCV13 against non-PCV7 serotypes in preventing non-invasive pneumonia [100], this assumption seems to be justified if the serotype distribution in non-invasive pneumococcal disease is comparable to the distribution in IPD. The letter may not be the case, hence, we tested different scenarios in the sensitivity analyses. If both higher-valent PCVs do not provide additional benefits in preventing non-invasive pneumonia, the cost-effectiveness of PCV13 will be questionable.
Indirect effects of higher-valent PCVs
The evolution of pneumococcal serotypes in Western Europe showed a decrease of the total IPD incidence in all age groups due to the implementation of childhood vaccination programs with pneumococcal conjugate vaccines [97]. While positive net indirect effects have been well documented for PCV7 [6, 88–92] and PCV13 in the prevention of IPD [93–97] and pneumococcal pneumonia [95], this has not been the case for PCV10. The introduction of infant vaccination programs with PCV10 has not reduced the overall incidence of IPD in non-vaccinated age groups in Finland [97]. In the Netherlands, a reduction of PCV10-PCV7 serotypes has been observed in unvaccinated persons in the third year after the implementation of PCV10 vaccination [102], but the effects on the overall IPD incidence are unclear [103]. Hence, by assuming positive net indirect effects for PCV10, we may have overestimated its effects on the entire population substantially.
In contrast to previous German analyses [18, 71, 104], we estimated indirect effects based on German instead of US data which led to more conservative estimates. A dynamic transmission model of universal infant vaccination with PCV7 fitted to UK data [105] calculated a 9 % reduction of IPD cases in the long term, which is in line with the results of our model. However, a similar model calibrated to US data calculated a 34 % decline of IPD cases [63].
Model type
Dynamic transmission models are the preferred method to evaluate vaccination programs that induce indirect effects. Markov cohort models are not able to simulate transmission dynamics of infectious disease and, therefore, do not endogenously capture indirect effects of vaccination. However, the lack of data on pneumococcal carriage and disease incidences in Germany, as well as poor knowledge of the mechanism of serotype competition, cause substantial additional uncertainty in dynamic models that may counter its advantages.
We included additional parameters to model protection against pneumococcal disease in unvaccinated individuals. Herd effects and replacement disease were estimated using German pneumococcal meningitis data of children <16 years old and applying the results to other age groups. This approach is problematic since it neglects social contact patterns and other factors that may affect the transmission process. However, epidemiologic outcomes of our model are comparable to a dynamic model that evaluated PCV7 vaccination of infants in the UK [105]. Another UK-model [106], which included PCV13, predicted the elimination of PCV13-serotype IPD around 8 years after the implementation of a vaccination program with the higher-valent PCV. In our model, infant vaccination with PCV13 did not result in a complete elimination of VT pneumococcal diseases in the long term. A small fraction of PCV10 serotypes would persist and PCV13 serotypes not covered by PCV10 would still cause a substantial number of pneumococcal diseases due to the adjustment of indirect effects for serotype 3.
Comparison with previously published studies comparing the cost-effectiveness of PCV10 and PCV13
Several recently published economic evaluations of universal pneumococcal infant vaccination (direct or indirect) compared PCV13 with PCV10 [11–23]. While the majority of studies [11, 12, 16–18, 21, 23, 25, 27] found PCV13 cost saving or at least cost-effective compared with PCV10, some analyses reported the exact opposite [15, 19, 20, 22, 30]. The contradictory results can be explained through different assumptions on the vaccine effects, which varied significantly among the different studies [107]. We did not incorporate cross protection in our model but included positive net indirect effects of PCV10 vaccination, additional benefits of PCV10 in the prevention of all cause AOM and reduced indirect herd effects of PCV13 against PCV13-PCV10 serotypes. Thus, our model may underestimate the value of infant vaccination programs with PCV13 compared with PCV10 in Germany.
Conclusions
In comparison with PCV10, universal infant vaccination with PCV13 is likely to be a cost-effective intervention within the German healthcare system, if additional net indirect effects of vaccination with the higher-valent vaccine are significant.
References
World Health Organization: Pneumococcal conjugate vaccine for childhood immunization: WHO position paper. Wkly Epidemiol. Rec. 82(12), 93–104 (2007)
O’Brien, K.L., Wolfson, L.J., Watt, J.P., Henkle, E., Deloria-Knoll, M., McCall, N., Lee, E., Mulholland, K., Levine, O.S., Cherian, T.: Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374(9693), 893–902 (2009). doi:10.1016/S0140-6736(09)61204-6
Robert Koch-Institut: Zu den Neuerungen in den Impfempfehlungen der STIKO. Epidemiol. Bull. 29, 219–233 (2001)
Robert Koch-Institut: Neuerungen in den aktuellen Empfehlungen der Ständigen Impfkommission (STIKO) am RKI vom Juli 2006. Epidemiol. Bull. 32, 271–280 (2006)
Rückinger, S., van der Linden, M., Reinert, R.R., von Kries, R., Burckhardt, F., Siedler, A.: Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine 27(31), 4136–4141 (2009). doi:10.1016/j.vaccine.2009.04.057
van der Linden, M., Weiß, S., Falkenhorst, G., Siedler, A., Imöhl, M., von Kries, R.: Four years of universal pneumococcal conjugate infant vaccination in Germany: impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine 30(40), 5880–5885 (2012). doi:10.1016/j.vaccine.2012.06.068
Imöhl, M., Reinert, R.R., van der Linden, M.: Temporal variations among invasive pneumococcal disease serotypes in children and adults in Germany (1992–2008). Int. J. Microbiol. 2010, 874189 (2010). doi:10.1155/2010/874189
Robert Koch-Insitut: Pneumoweb
Nationales Referenzzentrum für Streptokokken: Distribution of serotypes in invasive pneumococcal disease. Data on file (2013)
Bialkowski, A., Gärtner, J., Giani, G., Haas, W., Henneke, P., Jakob, A., Kölker, S., Kries, R. von, Liese, J., Poets, C.-F., Schaible, T., Wabitsch, M.: ESPED-Jahresbericht (2013)
Chuck, A.W., Jacobs, P., Tyrrell, G., Kellner, J.D.: Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine 28(33), 5485–5490 (2010). doi:10.1016/j.vaccine.2010.05.058
Kim, S.-Y., Lee, G., Goldie, S.J.: Economic evaluation of pneumococcal conjugate vaccination in the Gambia. BMC Infect. Dis. 10, 260 (2010). doi:10.1186/1471-2334-10-260
Urueña, A., Pippo, T., Betelu, M.S., Virgilio, F., Giglio, N., Gentile, A., Jimenez, S.G., Jáuregui, B., Clark, A.D., Diosque, M., Vizzotti, C.: Cost-effectiveness analysis of the 10- and 13-valent pneumococcal conjugate vaccines in Argentina. Vaccine 29(31), 4963–4972 (2011). doi:10.1016/j.vaccine.2011.04.111
Newall, A.T., Creighton, P., Philp, D.J., Wood, J.G., MacIntyre, C.R.: The potential cost-effectiveness of infant pneumococcal vaccines in Australia. Vaccine 29(45), 8077–8085 (2011). doi:10.1016/j.vaccine.2011.08.050
Robberstad, B., Frostad, C.R., Akselsen, P.E., Kværner, K.J., Berstad, A.K.: Economic evaluation of second-generation pneumococcal conjugate vaccines in Norway. Vaccine 29(47), 8564–8574 (2011). doi:10.1016/j.vaccine.2011.09.025
Tyo, K.R., Rosen, M.M., Zeng, W., Yap, M., Pwee, K.H., Ang, L.W., Shepard, D.S.: Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine 29(38), 6686–6694 (2011). doi:10.1016/j.vaccine.2011.06.091
Rozenbaum, M.H., Sanders, E.A.M., van Hoek, A.J., van der Jansen, A.G.S.C., Ende, A., van den Dobbelsteen, G., Rodenburg, G.D., Hak, E., Postma, M.J.: Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ 340, c2509 (2010)
Strutton, D.R., Farkouh, R.A., Earnshaw, S.R., Hwang, S., Theidel, U., Kontodimas, S., Klok, R., Papanicolaou, S.: Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J. Infect. 64(1), 54–67 (2012). doi:10.1016/j.jinf.2011.10.015
Knerer, G., Ismaila, A., Pearce, D.: Health and economic impact of PHiD-CV in Canada and the UK: a Markov modelling exercise. J. Med. Econ. 15(1), 61–76 (2012). doi:10.3111/13696998.2011.622323
Bakır, M., Türel, Ö., Topachevskyi, O.: Cost-effectiveness of new pneumococcal conjugate vaccines in Turkey: a decision analytical model. BMC Health Serv. Res. 12(1), 386 (2012). doi:10.1186/1472-6963-12-386
Earnshaw, S.R., McDade, C.L., Zanotti, G., Farkouh, R.A., Strutton, D.: Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect. Dis. 12(1), 101 (2012). doi:10.1186/1471-2334-12-101
Gomez, J.A., Tirado, J.C., Rojas, A.A.N., Alba, M.M.C., Topachevskyi, O.: Cost-effectiveness and cost utility analysis of three pneumococcal conjugate vaccines in children of Peru. BMC Public Health 13, 1025 (2013). doi:10.1186/1471-2458-13-1025
Klok, R.M., Lindkvist, R.-M., Ekelund, M., Farkouh, R.A., Strutton, D.R.: Cost-effectiveness of a 10-versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin. Therap. 35(2), 119–134 (2013). doi:10.1016/j.clinthera.2012.12.006
Vučina, V.V., Filipović, S.K., Kožnjak, N., Stamenić, V., Clark, A.D., Mounaud, B., Blau, J., Hoestlandt, C., Kaić, B.: Cost-effectiveness of pneumococcal conjugate vaccination in Croatia. Vaccine 33(Suppl 1), A209–A218 (2015). doi:10.1016/j.vaccine.2014.12.043
Mezones-Holguin, E., Canelo-Aybar, C., Clark, A.D., Janusz, C.B., Jaúregui, B., Escobedo-Palza, S., Hernandez, A.V., Vega-Porras, D., González, M., Fiestas, F., Toledo, W., Michel, F., Suárez, V.J.: Cost-effectiveness analysis of 10- and 13-valent pneumococcal conjugate vaccines in Peru. Vaccine 33(Suppl 1), A154–A166 (2015). doi:10.1016/j.vaccine.2014.12.039
Kieninger, M.P., Caballero, E.G., Sosa, A.A., Amarilla, C.T., Jáuregui, B., Janusz, C.B., Clark, A.D., Castellanos, R.M.: Cost-effectiveness analysis of pneumococcal conjugate vaccine introduction in Paraguay. Vaccine 33(Suppl 1), A143–A153 (2015). doi:10.1016/j.vaccine.2014.12.078
Ordóñez, J.E., Orozco, J.J.: Cost-effectiveness analysis of the available pneumococcal conjugated vaccines for children under 5 years in Colombia. Cost Eff. Resour. Alloc. 13, 6 (2015). doi:10.1186/s12962-015-0032-1
Vemer, P., Postma, M.J.: A few years later. Update of the cost-effectiveness of infant pneumococcal vaccination in Dutch children. Hum. Vaccines Immunother. 10(7), 1841–1849 (2014). doi:10.4161/hv.29008
Ayieko, P., Griffiths, U.K., Ndiritu, M., Moisi, J., Mugoya, I.K., Kamau, T., English, M., Scott, J.A.G.: Assessment of health benefits and cost-effectiveness of 10-valent and 13-valent pneumococcal conjugate vaccination in Kenyan children. PLoS One 8(6), e67324 (2013). doi:10.1371/journal.pone.0067324
Shiragami, M., Mizukami, A., Leeuwenkamp, O., Mrkvan, T., Delgleize, E., Kurono, Y., Iwata, S.: Cost-effectiveness evaluation of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine and 13-valent pneumococcal vaccine in Japanese children. Infect. Dis. Ther. (2014). doi:10.1007/s40121-014-0053-7
Siebert, U., Alagoz, O., Bayoumi, A.M., Jahn, B., Owens, D.K., Cohen, D.J., Kuntz, K.M.: State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Value Health 15(6), 812–820 (2012). doi:10.1016/j.jval.2012.06.014
Gray, A.: Applied methods of cost-effectiveness analysis in health care. Handbooks in health economic evaluation series. Oxford University Press, Oxford (2011)
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): General methods for evaluating the relation between cost and benefit—version 1.0 general methods for evaluating the relation between cost and benefit—version 1.0 (2009)
Bowles, D., Zuchandke, A.: Entwicklung eines Modells zur Bevölkerungsprojektion—Modellrechnungen zur Bevölkerungsentwicklung bis 2060 (2012)
Göbel, U., Heinrich, B., Krauth, K.A., Steingrüber, H.-J., von Kries, R.: Evaluation der Prozess- und Ergebnisqualität der Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland (ESPED) (Process and outcome quality of the German Paediatric Surveillance Unit (ESPED)). Klin. Padiatr. 222(2), 92–97 (2010). doi:10.1055/s-0030-1247587
von Kries, R., Toschke, A.M., Siedler, A.: Population-based Nationwide Study on Invasive Pneumococcal Infections among Children in Germany (1997–2003) (unpublished)
Reinert, R.R., Haupts, S., van der Linden, M., Heeg, C., Cil, M.Y., Al-Lahham, A., Fedson, D.S.: Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin. Microbiol. Infect. 11(12), 985–991 (2005). doi:10.1111/j.1469-0691.2005.01282.x
Rüggeberg, J.U., Ketteler, K., MacKenzie, C.R., von Kries, R., Reinert, R.R., Schroten, H.: Blood culture sampling rates at a German pediatric university hospital and incidence of invasive pneumococcal disease. Infection 32(2), 78–81 (2004). doi:10.1007/s15010-004-3104-2
Federal Statistical Office of Germany (Statistisches Bundesamt): Diagnosedaten der Krankenhäuser ab 2000 (2013)
IMS Health Deutschland: Verschreibungsindex für Pharmazeutika (VIP) (unpublished) (2009)
Schnoor, M., Hedicke, J., Dalhoff, K., Raspe, H., Schäfer, T.: Approaches to estimate the population-based incidence of community acquired pneumonia. J. Infect. 55(3), 233–239 (2007). doi:10.1016/j.jinf.2007.04.355
Schnabel, E., Sausenthaler, S., Brockow, I., Liese, J., Herbarth, O., Michael, B., Schaaf, B., Krämer, U., von Berg, A., Wichmann, H.-E., Heinrich, J.: Burden of otitis media and pneumonia in children up to 6 years of age: results of the LISA birth cohort. Eur. J. Pediatr. 168(10), 1251–1257 (2009). doi:10.1007/s00431-008-0921-9
Grüber, C., Keil, T., Kulig, M., Roll, S., Wahn, U., Wahn, V.: History of respiratory infections in the first 12 years among children from a birth cohort. Pediatr. Allergy Immunol. 19(6), 505–512 (2008). doi:10.1111/j.1399-3038.2007.00688.x
Kamtsiuris, P., Atzpodien, K., Ellert, U., Schlack, R., Schlaud, M.: Prävalenz von somatischen Erkrankungen bei Kindern und Jugendlichen in Deutschland. Ergebnisse des Kinder- und Jugendgesundheitssurveys (KiGGS) (Prevalence of somatic diseases in German children and adolescents. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50(5–6), 686–700 (2007). doi:10.1007/s00103-007-0230-x
Kværner, K.J., Kristiansen, H.A., Russell, M.B.: Otitis media history, surgery and allergy in 60-year perspective: a population-based study. Int. J. Pediatr. Otorhinolaryngol. 74(12), 1356–1360 (2010). doi:10.1016/j.ijporl.2010.09.002
Pang, L.H., Barakate, M.S., Havas, T.E.: Mastoiditis in a paediatric population: a review of 11 years experience in management. Int. J. Pediatr. Otorhinolaryngol. 73(11), 1520–1524 (2009). doi:10.1016/j.ijporl.2009.07.003
van Hoek, A.J., Andrews, N., Waight, P.A., Stowe, J., Gates, P., George, R., Miller, E.: The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J. Infect. 65(1), 17–24 (2012). doi:10.1016/j.jinf.2012.02.017
Poethko-Müller, C., Kuhnert, R., Schlaud, M.: Durchimpfung und Determinanten des Impfstatus in Deutschland. Ergebnisse des Kinder- und Jugendgesundheitssurveys (KiGGS) (Vaccination coverage and predictors for vaccination level. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50(5–6), 851–862 (2007). doi:10.1007/s00103-007-0248-0
Bader, H.-M.: Impfschutz in Schleswig-Holstein 2011 (2013)
Rieck, T.: Impfquoten aus KV-Daten - Sinnvolle Ergänzung zu den Schuleingangsuntersuchungen (2013)
Black, S., Shinefield, H., Fireman, B., Lewis, E., Ray, P., Hansen, J.R., Elvin, L., Ensor, K.M., Hackell, J., Siber, G., Malinoski, F., Madore, D., Chang, I., Kohberger, R., Watson, W., Austrian, R., Edwards, K.: Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19(3), 187–195 (2000)
Palmu, A.A., Jokinen, J., Borys, D., Nieminen, H., Ruokokoski, E., Siira, L., Puumalainen, T., Lommel, P., Hezareh, M., Moreira, M., Schuerman, L., Kilpi, T.M.: Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease. A cluster randomised trial. Lancet 381(9862), 214–222 (2013). doi:10.1016/S0140-6736(12)61854-6
Hansen, J., Black, S., Shinefield, H., Cherian, T., Benson, J., Fireman, B., Lewis, E., Ray, P., Lee, J.: Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr. Infect. Dis. J. 25(9), 779–781 (2006). doi:10.1097/01.inf.0000232706.35674.2f
Tregnaghi, M.W., Sáez-Llorens, X., López, P., Abate, H., Smith, E., Pósleman, A., Calvo, A., Wong, D., Cortes-Barbosa, C., Ceballos, A., Tregnaghi, M., Sierra, A., Rodriguez, M., Troitiño, M., Carabajal, C., Falaschi, A., Leandro, A., Castrejón, M.M., Lepetic, A., Lommel, P., Hausdorff, W.P., Borys, D., Ruiz Guiñazú, J., Ortega-Barría, E., Yarzábal, J.P., Schuerman, L.: Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 11(6), e1001657 (2014). doi:10.1371/journal.pmed.1001657
Black, S.B., Shinefield, H.R., Ling, S., Hansen, J., Fireman, B., Spring, D., Noyes, J., Lewis, E., Ray, P., Lee, J., Hackell, J.: Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21(9), 810–815 (2002). doi:10.1097/01.inf.0000027926.99356.4c
Taylor, S., Marchisio, P., Vergison, A., Harriague, J., Hausdorff, W.P., Haggard, M.: Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin. Infect. Dis. 54(12), 1765–1773 (2012). doi:10.1093/cid/cis292
Black, S., Shinefield, H.: Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from Northern California. Eur. J. Pediatr. 161(Suppl 2), S127–S131 (2002). doi:10.1007/s00431-002-1064-z
Fireman, B., Black, S.B., Shinefield, H.R., Lee, J., Lewis, E., Ray, P.: Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr. Infect. Dis. J. 22(1), 10–16 (2003). doi:10.1097/01.inf.0000045221.96634.7c
Eskola, J., Kilpi, T., Palmu, A., Jokinen, J., Haapakoski, J., Herva, E., Takala, A., Käyhty, H., Karma, P., Kohberger, R., Siber, G., Mäkelä, P.H.: Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344(6), 403–409 (2001). doi:10.1056/NEJM200102083440602
O’Brien, K.L., David, A.B., Chandran, A., Moulton, L.H., Reid, R., Weatherholtz, R., Santosham, M.: Randomized, controlled trial efficacy of pneumococcal conjugate vaccine against otitis media among Navajo and White Mountain Apache infants. Pediatr. Infect. Dis. J. 27(1), 71–73 (2008). doi:10.1097/INF.0b013e318159228f
Prymula, R., Peeters, P., Chrobok, V., Kriz, P., Novakova, E., Kaliskova, E., Kohl, I., Lommel, P., Poolman, J., Prieels, J.-P., Schuerman, L.: Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367(9512), 740–748 (2006). doi:10.1016/S0140-6736(06)68304-9
Tregnaghi, M.W., Sáez-Llorens, X., López, P., Abate, H., Smith, E., Pósleman, A., Calvo, A., Wong, D., Cortes-Barbosa, C., Ceballos, A., Tregnaghi, M., Sierra, A., Rodriguez, M., Troitiño, M., Carabajal, C., Falaschi, A., Leandro, A., Castrejón, M.M., Lepetic, A., Lommel, P., Hausdorff, W.P., Borys, D., Ruiz Guiñazú, J., Ortega-Barría, E., Yarzábal, J.P., Schuerman, L.: Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 11(6), e1001657 (2014). doi:10.1371/journal.pmed.1001657
Melegaro, A., Choi, Y.H., George, R., Edmunds, W.J., Miller, E., Gay, N.J.: Dynamic models of pneumococcal carriage and the impact of the Heptavalent Pneumococcal Conjugate Vaccine on invasive pneumococcal disease. BMC Infect. Dis. 10, 90 (2010). doi:10.1186/1471-2334-10-90
Whitney, C.G., Farley, M.M., Hadler, J., Harrison, L.H., Bennett, N.M., Lynfield, R., Reingold, A., Cieslak, P.R., Pilishvili, T., Jackson, D., Facklam, R.R., Jorgensen, J.H., Schuchat, A.: Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348(18), 1737–1746 (2003). doi:10.1056/NEJMoa022823
Lexau, C.A., Lynfield, R., Danila, R., Pilishvili, T., Facklam, R., Farley, M.M., Harrison, L.H., Schaffner, W., Reingold, A., Bennett, N.M., Hadler, J., Cieslak, P.R., Whitney, C.G.: Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294(16), 2043–2051 (2005). doi:10.1001/jama.294.16.2043
Grijalva, C.G., Poehling, K.A., Nuorti, J.P., Zhu, Y., Martin, S.W., Edwards, K.M., Griffin, M.R.: National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 118(3), 865–873 (2006). doi:10.1542/peds.2006-0492
Grijalva, C.G., Nuorti, J.P., Arbogast, P.G., Martin, S.W., Edwards, K.M., Griffin, M.R.: Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369(9568), 1179–1186 (2007). doi:10.1016/S0140-6736(07)60564-9
Ray, G.T., Pelton, S.I., Klugman, K.P., Strutton, D.R., Moore, M.R.: Cost-effectiveness of pneumococcal conjugate vaccine: an update after 7 years of use in the United States. Vaccine 27(47), 6483–6494 (2009). doi:10.1016/j.vaccine.2009.08.045
Ray, G.T., Whitney, C.G., Fireman, B.H., Ciuryla, V., Black, S.B.: Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr. Infect. Dis. J. 25(6), 494–501 (2006). doi:10.1097/01.inf.0000222403.42974.8b
Roche, P.W., Krause, V., Cook, H., Barralet, J., Coleman, D., Sweeny, A., Fielding, J., Giele, C., Gilmour, R., Holland, R., Kampen, R., Brown, M., Gilbert, L., Hogg, G., Murphy, D.: Invasive pneumococcal disease in Australia, 2006. Commun. Dis. Intell. Q Rep. 32(1), 18–30 (2008)
Jiang, Y., Gauthier, A., Annemans, L., van der Linden, M., Nicolas-Spony, L., Bresse, X.: Cost-effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev. Pharmacoecon. Outcomes Res. 12(5), 645–660 (2012). doi:10.1586/erp.12.54
Diel, M., Laurenz, M., Krause, K., Sprenger, R., Busse, A.: Impact of pneumococcal conjugate vaccines on pneumonia among children in Germany. 31st annual meeting of the European Society of Paediatric Infectious Diseases (ESPID), Milan, Italy, May 28-June 1, Poster A-534-0044-00928 (2013)
Diel, M., Laurenz, M., Krause, K., Sprenger, R., Busse, A.: Impact of pneumococcal conjugate vaccines on acute otitis media among children in Germany. 31st annual meeting of the European Society of Paediatric Infectious Diseases (ESPID), Milan, Italy, May 28–June 1, Poster A-534-0044-00926 (2013)
van der Linden, M., Falkenhorst, G., Perniciaro, S., Imöhl, M.: Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS One 10(7), e0131494 (2015). doi:10.1371/journal.pone.0131494
Braun, S., Prenzler, A., Mittendorf, T., von der Schulenburg, J.M.: Bewertung von Ressourcenverbräuchen im deutschen Gesundheitswesen aus Sicht der Gesetzlichen Krankenversicherung [Appraisal of valuation of resource use in the German healthcare system from the perspective of the statutory health insurance]. Gesundheitswesen 71, 19–23 (2009)
Institut für das Entgeltsystem im Krankenhaus GmbH: G-DRG-System 2013, Reportbrowser 20011/2013 (2013)
Claes, C., Reinert, R.R., von der Schulenburg, J., Graf, J.-M.: Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur. J. Health Econ. 10(1), 25–38 (2009). doi:10.1007/s10198-008-0098-1
National Association of Statutory Health Insurance Physicians: Uniform Value Scale 2013 (2013)
Schulze-Gattermann, H., Illg, A., Schoenermark, M., Lenarz, T., Lesinski-Schiedat, A.: Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol. Neurotol. 23(5), 674–681 (2002)
Federal Employment Agency of Germany (Bundesagentur für Arbeit): Analyse der gemeldeten Arbeitsstellen (2013)
Gesundheitsberichterstattung des Bundes: Arbeitsunfähigkeit bei AOK-Pflichtmitgliedern ohne Rentner (Arbeitsunfähigkeitsfälle, Arbeitsunfähigkeitsfälle je 100.000 Pflichtmitglieder, Arbeitsunfähigkeitstage, Arbeitsunfähigkeitstage je 100.000 Pflichtmitglieder,Tage je Fall). Gliederungsmerkmale: Jahre, Deutschland, Geschlecht, ICD-10 (2008)
Federal Statistical Office of Germany (Statistisches Bundesamt): Bevölkerung, Erwerbstätige, Erwerbslose, Erwerbspersonen, Nichterwerbspersonen: Deutschland, Jahre, Altersgruppen
Federal Statistical Office of Germany (Statistisches Bundesamt): VGR des Bundes - Bruttonationaleinkommen, Volkseinkommen (2013)
Lauertaxe: Arzneimittelpreise (2013)
The EuroQol Group’s International Task Force: Measuring Self-Reported Population Health: An International Perspective based on EQ-5D (2004)
Melegaro, A., Edmunds, W.J.: Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 22(31–32), 4203–4214 (2004). doi:10.1016/j.vaccine.2004.05.003
§ 61 Zuzahlungen: Sozialgesetzbuch (SGB) Fünftes Buch (V) - Gesetzliche Krankenversicherung - (SGB V). Artikel 1 G. v. 20.12.1988 BGBl. I S. 2477, 2482; zuletzt geändert durch Artikel 1 G. v. 22.12.2013 BGBl. I S. 4382 (2013)
Pilishvili, T., Lexau, C., Farley, M.M., Hadler, J., Harrison, L.H., Bennett, N.M., Reingold, A., Thomas, A., Schaffner, W., Craig, A.S., Smith, P.J., Beall, B.W., Whitney, C.G., Moore, M.R.: Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201(1), 32–41 (2010). doi:10.1086/648593
Vestrheim, D.F., Høiby, E.A., Bergsaker, M.R., Rønning, K., Aaberge, I.S., Caugant, D.A.: Indirect effect of conjugate pneumococcal vaccination in a 2 + 1 dose schedule. Vaccine 28(10), 2214–2221 (2010). doi:10.1016/j.vaccine.2009.12.054
Rodenburg, G.D., de Greeff, S.C., Jansen, A.G.C.S., de Melker, H.E., Schouls, L.M., Hak, E., Spanjaard, L., Sanders, E.A.M., van der Ende, A.: Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg. Infect. Dis. 16(5), 816–823 (2010). doi:10.3201/eid1605.091223
Weinberger, D.M., Malley, R., Lipsitch, M.: Serotype replacement in disease after pneumococcal vaccination. Lancet 378(9807), 1962–1973 (2011). doi:10.1016/S0140-6736(10)62225-8
Loo, J.D., Conklin, L., Fleming-Dutra, K.E., Knoll, M.D., Park, D.E., Kirk, J., Goldblatt, D., O’Brien, K.L., Whitney, C.G.: Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr. Infect. Dis. J. 33(Suppl 2), S161–S171 (2014). doi:10.1097/INF.0000000000000084
Harboe, Z.B., Dalby, T., Weinberger, D.M., Benfield, T., Mølbak, K., Slotved, H.C., Suppli, C.H., Konradsen, H.B., Valentiner-Branth, P.: Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin. Infect. Dis. 59(8), 1066–1073 (2014). doi:10.1093/cid/ciu524
Steens, A., Vestrheim, D.F., de Blasio, B.F.: Pneumococcal vaccination in older adults in the era of childhood vaccination: public health insights from a Norwegian statistical prediction study. Epidemics 11, 24–31 (2015). doi:10.1016/j.epidem.2015.01.001
Rodrigo, C., Bewick, T., Sheppard, C., Greenwood, S., Mckeever, T.M., Trotter, C.L., Slack, M., George, R., Lim, W.S.: Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur. Respir. J. 45(6), 1632–1641 (2015). doi:10.1183/09031936.00183614
Moore, M.R., Link-Gelles, R., Schaffner, W., Lynfield, R., Lexau, C., Bennett, N.M., Petit, S., Zansky, S.M., Harrison, L.H., Reingold, A., Miller, L., Scherzinger, K., Thomas, A., Farley, M.M., Zell, E.R., Taylor, T.H., Pondo, T., Rodgers, L., McGee, L., Beall, B., Jorgensen, J.H., Whitney, C.G.: Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA. Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 15(3), 301–309 (2015). doi:10.1016/S1473-3099(14)71081-3
Htar, M.T.T., Christopoulou, D., Schmitt, H.-J.: Pneumococcal serotype evolution in Western Europe. BMC Infect. Dis. 15, 419 (2015). doi:10.1186/s12879-015-1147-x
Pletz, M.W., von Baum, H., van der Linden, M., Rohde, G., Schütte, H., Suttorp, N., Welte, T.: The burden of pneumococcal pneumonia-experience of the German competence network CAPNETZ. Pneumologie 66(8), 470–475 (2012). doi:10.1055/s-0032-1310103
Andrews, N.J., Waight, P.A., Burbidge, P., Pearce, E., Roalfe, L., Zancolli, M., Slack, M., Ladhani, S.N., Miller, E., Goldblatt, D.: Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect. Dis 14(9), 839–846 (2014). doi:10.1016/S1473-3099(14)70822-9
Bonten, M.J., Huijts, S.M., Bolkenbaas, M., Webber, C., Patterson, S., Gault, S., van Werkhoven, C.H., van Deursen, A.M., Sanders, E.A., Verheij, T.J., Patton, M., McDonough, A., Moradoghli-Haftvani, A., Smith, H., Mellelieu, T., Pride, M.W., Crowther, G., Schmoele-Thoma, B., Scott, D.A., Jansen, K.U., Lobatto, R., Oosterman, B., Visser, N., Caspers, E., Smorenburg, A., Emini, E.A., Gruber, W.C., Grobbee, D.E.: Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 372(12), 1114–1125 (2015). doi:10.1056/NEJMoa1408544
van Werkhoven, C.H., Huijts, S.M., Bolkenbaas, M., Webber, C., Schmoele-Thoma, B., Patterson, S.D., Gruber, W., Grobbee, D.E., Bonten, M.: 109913-valent pneumococcal conjugate vaccine efficacy is declining with old age: results from an exploratory analysis of the CAPiTA trial. Open Forum Infect. Dis. 1(Suppl 1), S324–S325 (2014). doi:10.1093/ofid/ofu052.807
Knol, M.J., Wagenvoort, G.H.J., Sanders, E.A.M., Elberse, K., Vlaminckx, B.J., de Melker, H.E., van der Ende, A.: Invasive pneumococcal disease 3 years after Introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg. Infect. Dis. 21(11), 2040–2044 (2015). doi:10.3201/eid2111.140780
Mangen, M.-J.J., Rozenbaum, M.H., Huijts, S.M., van Werkhoven, C.H., Postma, D.F., van Deursen, A.M.M., van der Ende, A., Grobbee, D.E., Sanders, E.A.M., Sato, R., Verheij, T.J.M., Vissink, C.E., Bonten, M.J.M., de Wit, G.A.: Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur. Respir. J. 46(5), 1407–1416 (2015). doi:10.1183/13993003.00325-2015
Claes, C., von der Schulenburg, J.-M.G.: Gesundheitsökonomische Modellierung eines Szenarios zum Serotypen-Catch-up bei der Impfung gegen Pneumokokken mit PCV13 (Prevenar 13®) in Deutschland. Pharmacoecon. Ger. Res. Artic. 8(2), 85–95 (2010). doi:10.1007/BF03320767
Choi, Y.H., Jit, M., Gay, N., Andrews, N., Waight, P.A., Melegaro, A., George, R., Miller, E., Borrow, R.: 7-Valent pneumococcal conjugate vaccination in England and Wales: Is it still beneficial despite high levels of serotype replacement? PLoS One 6(10), e26190 (2011). doi:10.1371/journal.pone.0026190
Choi, Y.H., Jit, M., Flasche, S., Gay, N., Miller, E., Beall, B.: Mathematical modelling long-term effects of replacing Prevnar7 with Prevnar13 on invasive pneumococcal diseases in England and Wales. PLoS One 7(7), e39927 (2012). doi:10.1371/journal.pone.0039927
Farkouh, R.A., Klok, R.M., Postma, M.J., Roberts, C.S., Strutton, D.R.: Cost-effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev. Vaccines 11(10), 1235–1247 (2012). doi:10.1586/erv.12.99
Authors’ contributions
AK constructed and implemented the model in Excel, performed the analysis of the results and drafted the manuscript. JMGvdS reviewed the manuscript and revised it critically for important intellectual content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuhlmann, A., von der Schulenburg, JM.G. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ 18, 273–292 (2017). https://doi.org/10.1007/s10198-016-0770-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-016-0770-9