Abstract
Background
In Germany the heptavalent pneumococcal conjugate vaccine (PCV7) has been recommended as a general infant vaccination since 2006. Data from similar programmes in the USA have reported a reduction of pneumococcal diseases in both vaccinated and unvaccinated populations, suggesting herd immunity effects. This study analyses the cost-effectiveness of a general vaccination with PCV7 in Germany based on these findings.
Methods
A Markov model adapts efficacy and herd immunity data to the German population. Further main model inputs are incidence, vaccination uptake, serotype distribution, case fatality rates, and vaccination and health-care costs.
Results
A general vaccination with PCV7 would avoid about 232,000 pneumococcal infections and 1,879 premature deaths per year in Germany. From the health-care payer's perspective, direct cost savings would outweigh vaccination expenditures by a ratio of 1:1.16. The sensitivity analysis shows that these estimates are quite conservative.
Conclusion
Based on the health-economic evaluation, the authors recommend the continuation of the general recommendation of PCV7 according to the 3 + 1 schedule within the German Statutory Health Insurance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is one of the most commonly encountered agents of invasive and non-invasive infections. Risk is particularly high in the elderly, the very young and those with a weakened immune system. Asymptomatic carriage is common and age-related: over 60% of children carried S. pneumoniae in their nasopharynx by their 3rd year of life; the rate of colonisation falls to 25–35% in school children and students and to 6% in adults who live in households without children [31]. Pneumococcal-related diseases start as local infections of the respiratory tract and can progress to bacteraemia, sepsis, or meningitis. Infections show a seasonal distribution: most invasive pneumococcal infections occur between October and March [38].
Ninety-one pneumococcal serotypes defined by the capsular polysaccharide have been identified [42]. A small group of virulent serotypes is responsible for the majority of invasive infections, and most cases are caused by only one single serotype. An earlier pneumococcal infection does not give immune protection against further pneumococcal infections with other serotypes.
In February 2001 the heptavalent pneumococcal conjugate vaccine (PCV7) received market approval in Europe [15]. PCV7 is a pneumococcal conjugate vaccine indicated for use in infants from 2 months up to 2 years and previously unvaccinated children with higher risks from 2 to 5 years. PCV7 protects against seven serotypes commonly associated with invasive diseases: 4, 9V, 14, 19F, 23F, 18C, and 6B. Effective since 1 July 2001, the German Standing Commission on Vaccination (STIKO) recommended the vaccination for children at increased risk for pneumococcal-related diseases. This recommendation was broadened further in July 2006 to all children under the age of 2 [72].
With the latest German Health-Care Reform Act (Gesetz zur Stärkung des Wettbewerbs in der Gesetzlichen Krankenversicherung) in 2007 [26], all vaccination programmes that were recommended by the STIKO in the past were included within the standard benefit package of the German Statutory Health Insurance [26] starting in July 2007. The highest decision-making body of the self-governing statutory health insurance scheme that defines mandatory treatments and the extent of reimbursement is the Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA). They usually base their decisions on recommendations from the STIKO. As all vaccinations recommended by the STIKO became standard benefits, also vaccination with PCV7 was included in the package. However, the G-BA made an amendment that they reserve the right to re-evaluate PCV7 at a later time [25].
A first economic evaluation of a universal vaccination programme in Germany was published in 2003 [14]. Subsequent data from the US [75, 76] have found reductions in the rate of invasive pneumococcal disease (IPD) exceeding vaccination uptake, suggesting that widespread use of PCV7 has led to herd immunity effects. Therefore, the previous German model from 2003 [14] was extended to reflect potential benefits from herd immunity in Germany.
This analysis evaluates costs and economic consequences of scenarios with all children receiving vaccination with PCV7 compared to no vaccination in Germany.
Methods
Model structure
A Markov model has been developed to analyse cost-effectiveness of no pneumococcal vaccination (branch A) compared to a general vaccination programme for all newborn children (branch B) in Germany. The primary health benefit outcomes are life years gained (LYG) and quality-adjusted life years (QALY). The model takes the perspectives of the German Statutory Health Insurance, which covers about 90% of the population, and a societal view. The timeframe of the model covers 99 cycles with a cycle length of 1 year.
In branch A, incidence rates as well as the German population structure in 2005 were used to compute annual cases of four pneumococcal-related diseases in Germany: meningitis, sepsis, pneumonia, and acute otitis media (AOM). The model combines incidence rates with resource usage and unit costs to estimate annual health-care costs attributive to pneumococcal infections in Germany.
Branch B examines a steady state in which all children are recommended to receive vaccination using the recommended four-dose schedule with older children already being immunised. The model structure does not illustrate the initiation and implementation of a vaccination programme over time. Instead it portrays a steady state, in which all children are vaccinated (or not), and the theoretical vaccination programme has been in place for years (or not). Experience from the US shows that a steady state can be achieved within 3 or 4 years after introduction of a general vaccination programme [74]. Vaccination of German elderly is not considered in this model since the vaccination rate with the approved 23-valent polysaccharide vaccine has been constant (5–7%) during the last years [57].
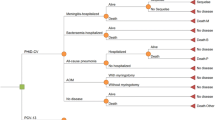
The analysis has been conducted using DATA version TreeAge Pro 2006. The model structure is shown in (Fig. 1).
Incidence rates and epidemiology outcomes
The incidence rate of paediatric IPD used in the model is the average annual rate as reported in the Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland (ESPED) surveillance system. Data for the period 1997–2003 were available for modelling purposes [38, 39]. According to that source, cases of sepsis are divided into septicaemia (60%) and bacteraemic pneumonia (40%). Data on the incidence of adult IPD are less comprehensive: surveillance data from North-Rhine Westphalian hospitals reported by the German National Reference Centre for Streptococci (NRZ) has been used as the best available data source [56]. In addition, there is evidence that in these cases there is an underreporting [56] of underlying causalities (with a factor of 2.7) since there is no routine use of blood cultures for pathogen analysis in Germany [61]. The incidence of community-acquired pneumonia (CAP) is based on diagnosis data from prescription indices recorded by IMS Health [32]. Specific German AOM incidence rates are not available. Instead, Finnish incidence data were incorporated within the model [20]. Incidence data built into the model are summarised in Table 1.
Outcomes
The model considered case fatality resulting from IPD and pneumonia, and sequelae resulting from IPD. ESPED data report a case fatality rate of 7.5% in paediatric patients with pneumococcal meningitis in Germany [39]. Furthermore, 20.6% of patients experience sequelae [39], of whom 40.5% experience hearing disabilities and hearing losses. In the model late sequelae are assumed to be 29.4% since ESPED focusses only on acute sequelae.
Among paediatric patients with pneumococcal sepsis, case fatality rates are reported to be 2% and multiple sequelae occur in 3.8% of all cases, of whom 7.7% reported disability or hearing loss [39]. The case fatality rate of IPD in adults is estimated to be 20% in those aged under 65 and 30% in those aged 65 years and over [2, 5, 22].
A conservative assumption was made in the estimation of case fatality rates in pneumonia: within the model in outpatient care, no deaths occur in adults under 55 years old, and the case fatality rate in adults older than 55 years is assumed to be 0.5%. German hospital statistics report an average case fatality rate for hospitalised CAP of 10.1% (2005) [69]. No additional case fatality has been included for AOM as invasive infections originating from AOM are already captured as IPD [64].
Life years lost due to premature death were estimated with German life tables from 2001/2003 [18] and the population structure of 2005 taking data of the Federal Statistical Office in Germany [68]. Patients who suffered pneumococcal-related sequelae are assumed to have a reduced life expectancy of 65 years.
Effectiveness and herd immunity of PCV7
Effectiveness in Germany is estimated taking into account vaccine efficacy, German serotype distribution of IPD and pneumonia, and herd immunity. Basis of individual effectiveness in paediatric IPD is the German serotype coverage of PCV7, including cross-reactive serotypes 6A/6B derived from ESPED data covering 1997–1998 (Table 1) [39]. In the US study, vaccine efficacy was reported with 97.4% against susceptible IPD in fully vaccinated individuals [8]. IPD was reduced by 94% in the target population, due to an uptake of 83% [8]. Surveillance data from the US reported the vaccination uptake in usual care to be 68% in 2003 [3] and showed a reduction in IPD in the target population by 92% [74]. Hence, within the model it is conservatively assumed that an uptake rate of 70% would be achievable in Germany. A recent analysis of vaccination uptake in Germany testifies high acceptance of the basic immunisation in the 1st life year, but shows deficits in uptake of the booster vaccination in the 2nd life year [53]. This may also be applicable for PCV7 due to its similar vaccination scheme.
Up to the age of 16, PCV7 on average reduces pneumococcal pneumonias by 17.7% as well as 6% of all clinically diagnosed pneumonias [9]. The latter value is incorporated into the model linked to the incidences based on clinical diagnoses. Effectiveness against AOM has been taken from a large randomised study conducted in Finland that found a reduction of 57% in vaccine serotype-related otitis media episodes [20] and 6% in overall AOM. Again, taking a conservative approach, the lowest reported rate of 6% was used.
PCV7 has also an impact on the severity of pneumococcal diseases, which results in less utilisation of health-care services. In the NCKP study the number of paracentesis decreases (23.2%, intention-to-treat) [23]. This effect is adjusted with the decrease of incidence. In the US PCV7 has been available for vaccination of children under 2 years old since 2000. In surveillance data covering a population of over 16 million, a decline in IPD in adults between 20 and 41% (period 1998–2003) depending on age groups [74] has been found. In the target population for PCV7 vaccination, IPD fell by 53% in infants under 2 months (i.e. before the first vaccination) and by 51% in children between the ages of 5 and 17. In addition, a reduction of 27% in pneumococcal pneumonia across all age groups has been reported [74]. Data from a comprehensive Kaiser Permanente Study confirm these results [4, 7].
Recently, different authors demonstrated evidence of herd immunity in peer-reviewed publications [41, 55, 76] as well as conference proceedings [4, 74]. This model uses herd immunity data from the most recent publications (Table 1) [55]. By transferring that data to a German model, one has to consider that the serotype distribution differs from that in the US. The effective range of herd immunity in adults therefore is estimated considering different distributions of serotype coverage in the US (1998/1999) [76] and Germany (July 2001–June 2003) [56]. As a result of that, the effective range of herd immunity in paediatric IPD and CAP is assumed to be 60%. A declining effect is not modelled, since herd immunity can be expected to close this gap in individual immunity.
Health-care cost and quality-adjusted life years
One health economic outcome is QALY, which is literature based (Table 2) [54]. The model covers costs of purchasing and administration of the vaccine, costs for treating infections, costs of long-term sequelae of meningitis, and indirect costs.
All patients with IPD and children under 2 years of age with pneumococcal pneumonia require hospital treatment. The hospitalisation of all other cases is estimated based on national hospital statistic [69]. The rate of hospitalisation is 14.1% for children <5 years, 6.9% in those aged 5–54 years, 14% in those aged 55–64 years, and 38.4% in those over 65, which is consistent with data from the US [37]. Eight percent of all patients with otitis media are treated with a paracentesis (reflecting German treatment reality based on expert opinion).
As Germany has a DRG system, inpatient lump-sum payments in cases of IPD and CAP are estimated as weighted average values from the German-DRG 2007 scheme (2005) [34]. The G-DRG 2007 distinguishes between cases with artificial respiration, which therefore have to be considered in the average costs. According to the literature about 13% of adults with IPD [33] and 11% with CAP [36] need artificial respiration during inpatient care.
The costs of outpatient treatment of AOM is based on average outpatient remuneration figures from the literature [78] plus drug prescription costs [32]. The costs of outpatient care of pneumonia are assumed to be similar to outpatient care of otitis media. The treatment costs of paracentesis were estimated by an expert panel. Complications of AOM are assumed to include 8% of patients treated with paracentesis at a cost of €490 (materials, anaesthetic, and nursing care). Inpatient treatment of otitis media is rare in Germany (0.5%), and costs of €1,340 per admission have been used [34].
The lifetime discounted cost of bilateral deafness is estimated to be €90,000 [66, 67], assuming that 50% of cases of pneumococcal meningitis require cochlear implants and that on average one repeated implantation is needed over a patient’s lifespan (assumption). The life-long costs of other sequelae were estimated to be €50,000. All prices are based on the year 2005 or are price-level adjusted.
Indirect costs
Indirect costs quantify the social loss due to pneumococcal-related absence from work, decreased productivity or premature death. In the evaluation of PCV7 indirect costs result from parents’ absence from work taking care of their sick child and from persons, who would have normally worked up to retirement, who suffer a reduction in earning capacity or a premature death from a pneumococcal disease. In the context of herd immunity, the model includes pneumoccocal-related absence from work and loss of future working years in the sub-population of working age (between 20 and 60 years old). In Markov models it is usually necessary to discount future values to reflect that costs and benefits occur at different times. The special construction of the presented Markov model as a steady-state model allows for ignoring discounting – with the exception of loss of future life incomes linked to pneumococcal-related sequelae and deaths.
Estimation of indirect costs follows the human capital approach on the basis of the Hannoveraner Konsens [65]. The German per capital income in 2005 was €32,894 per year (€90.12 per day) [71]. In accordance with the micro census of the Federal Statistical Office, 74.7% of the females with children were gainfully employed in the year 2005 [70]. It is assumed that women do not work in the first 6 months. It was assumed that a pneumococcal-related inpatient treatment of a child younger than 12 years of age caused 7 days of parental work absence, whereas an outpatient treatment caused only 3 days. Having a child of up to 12 years in outpatient care, parents miss 3 days at work and 1 week in inpatient care (assumption). Adults between 20 and 61 years of age lose 3 weeks at work when they suffer from a pneumococcal disease. The loss of future years in gainful work due to premature death is calculated by subtracting the death age from 61, since the average male (female) started his retirement in 2005 at the age of 60.7 (61) [17]. Therefore, the discounted (5%) life income of a 20-year-old person in gainful work is €422,131.
PCV7 vaccination
The recommended immunisation schedule for young children consists of three doses given at intervals of at least 4 weeks and a booster dose between 12 and 15 months of age [51]. In 2005 the pharmacy retail price per dose was €62.42 [58] and the remuneration for vaccine administration was €6.86 (on average) [29]. An uptake rate of 70% was assumed. The model does not consider expenditures for immunisation of older age groups that might take place when the vaccine is introduced in the model. Altogether the vaccine is well tolerated, so that neither less serious side effects such as pain and skin irritations nor severe side effects are included.
Sensitivity analysis
A deterministic sensitivity analysis is carried out to explore robustness of results to variation in the input data. In future decades it is expected for Germany that birth cohorts will decrease, but the number of elderly will increase. To test how this affects the results, the analysis has been repeated using official estimates for the population structure in 2030 and 2050 [19].
While paediatric pneumococcal-related meningitis in Germany arises in similar frequency as in other European countries, the incidence of pneumococcal-related sepsis turned out to be substantially lower. Rüggeberg et al. [61] show that bacteriamic pneumonias were clearly underreported in a German hospital sample looking at pneumococcal sepsis. A mandatory analysis of pathogens via blood cultures in inpatient pneumonias would increase the incidence of pneumococcal sepsis. Thus, a study from the US reported that a 2.2-fold higher incidence of pneumococcal sepsis was based on improved diagnosis by a 2.3-fold higher number of blood cultures [11]. Therefore, in sensitivity analyses the incidence of adult IPD is increased by the factor 4 instead of 2.7 in the base case.
A recent study taking data from the German Community Acquired Pneumonia Network (CAPNETZ) [63] estimates 680,000 annual cases of CAP in German adults. Although an underestimation can be expected, the incidence is varied in the sensitivity analysis due to data adjusting the rate of the hospitalisation. In most European countries value-added tax is either not applicable or reduced for reimbursable medications or prescriptions. Exceptions are Germany, Norway, Austria, and the Slovakian Republic [12]. To avoid a bias Wisloff et al. [77] and Salo et al. [62] took the net vaccine price. To guarantee the comparability in the sensitivity analysis a similar approach is chosen. The vaccine price is set at €53.81 for each dose (2005 without value-added tax) [59]. In the base case analysis the vaccine price is calculated for 2005 including 16% value-added tax. In the sensitivity analysis the vaccine price is also computed including 19% value-added tax as it was raised in 2007 (€64.63).
On the European level there is an ongoing discussion about the vaccination scheme. The 2 + 1 scheme has been approved by the European Agency for the Evaluation of Medicinal Products. In the absence of reliable clinical data supportive of the 2 + 1 scheme, only a sensitivity analysis with the 2 + 1 scheme is modelled assuming the same uptake, effectiveness, and herd immunity as under the 3 + 1 scheme. Nevertheless, both direct and indirect effects of a 2 + 1 vaccination programme can deviate substantially from what has been found for the four-dose schedule.
The attainable uptake rate of vaccination is a result of the national situation and respective efforts made to promote prevention measures. In the base case an uptake of 70% is assumed. Under favourable conditions it would be possible to reach higher uptake rates in Germany, so that uptake rates of 80% as well as of 90% with an efficacy of 92 and 94%, respectively, are examined in the sensitivity analysis.
The evidence of herd immunity is based on two different samples. On the one hand this effect was observed in the NCKP study and on the other hand in the data of the ABCS [74, 76]. The first is used in the base case and the second is analyzed in the sensitivity analysis. In the model the effectiveness is modelled with incidences of pneumococcal diseases in accordance with the clinical studies. Apart from the proven influence on the number of infections PCV7 also influences the course of related diseases. Grijalva et al. [27] elicited the hospitalisation of CAP in the US by comparison of hospitalisation with CAP as the first diagnosis from a national hospital sample (Nationwide Inpatient Sample) of the years 1997–1998 versus the years 2001–2004. In another study Grijalva et al. [28] also analysed diagnosis data about otitis media (all causes) from the same source of hospitals with diagnosis data of outpatient physicians (National Ambulatory Medical Care Survey). Hospitalisation of pneumococcal CAP decreased at a substantially stronger rate than expected, around 65% in the age group 0 to 1 years old and 73, 46, 30, 11, and 20% in the age groups 2–4, 5–17, 18–39, 40–64, and 65+ years. The diagnosis of pneumoccocal CAP is methodically demanding and uncertain. So the authors confirm these results with an analysis of data with CAP of all causes (age groups 0–1, 2–4, 5–17, 18–39, 40–64, and 65+ years: −39, −17, −18, −26, −19, and −15% respectively). Therefore, in the sensitivity analysis the effectiveness of PCV7 in hospitalisation of CAP (all causes) is modelled with the smaller German serotype coverage (60% of the US figures). Additionally, it is assumed that PCV7 did not change the number of cases of CAP in outpatient treatment due to the results of Grijalva et al. [28], who found a decrease in the demand of outpatient services in paediatric otitis media, but not in CAP.
Since the start of the German G-DRG system, the cost of an average admission tends to fall with sample expansion. A comparison of the DRG payments of pneumococcal-related diseases reveals strong fluctuations, since the relative calculation of respective DRGs changes from year to year. To quantify these effects, the DRG lump-sum compensation of IPD and CAP is calculated within the sensitivity analysis without artificially respiration. Besides a break-even DRG lump sum determined for CAP is calculated in the sensitivity analysis.
Results
First, the number of cases in Germany was estimated in the model via combining incidences and the population structure in 2005 (Table 3). It should be noted that CAP and otitis media cases estimated are all cause related and not only resulting from pneumococcal infections. The proportion of the adult population in IPD overall is 95% and in CAP 65%, respectively. The sequelae after IPD, in particular after pneumococcal meningitis, come to about 160 children annually. The total number of 12,850 pneumococcal deaths comes mainly from adult patients.
With an estimated uptake of 70%, the number of pneumococcal infections can be reduced by about 232,000 per year (Table 3). For an interpretation of the 4% preventable episodes, one has to consider that CAP and otitis media cases are all cause related. An estimated 35 paediatric premature deaths and 1,834 adult premature deaths per year could be prevented. Additionally, 83 cases of paediatric sequelae after IPD could be avoided.
Estimated expenditures for the vaccination programme within the German Statutory Health Insurance would be up to €133 million per year using the recommended 3 + 1 schedule with an estimated uptake of 70%. From the health-care payers perspective, cost savings would outweigh expenditure by a ratio of 1:1.16.
Altogether €0.77 of each € spent for the vaccination programme directly would be refinanced by savings in direct health-care costs for avoided cases in children and €0.39 from adult herd immunity effects coming also from a reduction in direct health-care costs in that group. The vaccination approach applied within the model reduces total cost and leads in addition to gains in life years, resulting in a negative cost-effectiveness ratio (−€640 per life year gained and −€567 per QALY), therefore leading to dominance of the vaccination programme. The reduction in indirect costs caused by vaccination adds up to a cost-benefit ratio of 2.29 and to −€5,189 per LYG and −€4,594 per QALY, further increasing the dominance ratio (Table 4).
Results of different sensitivity analyses are presented in Table 5, further confirming that the model’s findings are robust to variations in input parameters. An aging German population and a disproportionately high decline in hospitalisation due to pneumonias will have a favourable effect on the cost-effectiveness of PCV7 from the perspective of the health insurer. The likely underestimation of IPD and also a possible overestimation of CAP within the base case model have only a moderate effect on the cost-effectiveness ratio. As expected, different vaccination schemes, the price of the vaccine and variations of the uptake have an effect on the cost-effectiveness. Using alternative herd immunity figures derived from the Active Bacterial Core Surveillance leads to a similar cost-effectiveness ratio as using the data from the Northern California Kaiser Permanente [55] for the analysis.
Variations of the effect of PCV7 on inpatient utilisation leads to marginally different results compared to the data taken from the clinical studies. The publication of Grijalva et al. [27] has its strength in representing a large secondary data set as well as its close-to-reality illustration of the health service. Every prevented stay in a hospital ward leads to direct cost savings. Furthermore, the results of Grijalva et al. [28] also show that the decrease in inpatient service does not necessarily trigger an intensified use of outpatient services.
The costs of inpatient care for pneumonias have a moderate effect on the cost-effectiveness. This particularly applies to CAP. The average G-DRG lump sum payment not including cases with artificial respiration brings the cost-benefit ratio near the value of 1. However, in this case a problem within the remuneration system is reflected, namely that these specific G-DRG payments underestimate the financial burden of hospitalisation in pneumonia. The break-even of the G-DRG lump sum for pneumonia is 1.42 times higher than the German-wide average cost of the G-DRG in 2005.
Discussion
For the first time, this economic evaluation included cost and benefits of herd immunity in Germany. General vaccination was found to substantially reduce the burden of pneumococcal diseases. The costs of vaccination would be completely refinanced by reductions in direct health-care costs. The savings due to the herd immunity effects and avoidable inpatient treatment that can be expected in the context of pneumococcal pneumonia make a substantial contribution to this result. However, it has to be noted that the individual and collective effectiveness of PCV7 in CAP as well as the incidence of CAP are associated with a higher uncertainty compared to the other investigated pneumococcal diseases. Support and validation of the model results to a certain extent can be found in the research of Grijalva et al. [27]. Hospitalisation rates in pneumonia decrease more strongly than would be expected from clinical trial results in PCV7, since hospitalisation is determined by the number of cases and by the disease severity level.
Uncertainty in health–economic modelling results from different evidence levels applicable to the underlying data and input variables, but also from interim changes in available data. In particular new therapy options trigger the scientific interest and lead to an increased research activity in a wide spectrum of research questions. Hence, the knowledge base constantly expands, as also can be observed in PCV7. To reflect this, different scenarios must be incorporated into a model. In this case, it could be shown in different sensitivity analyses that the cost-effectiveness ratio only slightly varies under different conservative scenarios.
The model reduces the complexity imminent in everyday life, leading to a number of limitations. S. pneumoniae causes a wide range of infections, including sinusitis, endocarditis, arthritis, osteomyelitis, and peritonitis [60]. However, due to a lack of reliable incidence data, the potential impact of vaccination on these infections is not considered. In addition, the model does not consider costs that may arise to the public sector due to special educational needs that children with sequelae to meningitis might have. Other authors have shown previously that avoiding the latter cost factors may lead to an offset of 3.2% of vaccination costs [14].
Like in all other published health–economic evaluations of PCV7, the present model uses a deterministic instead of a dynamic approach, so that transmission of S. pneumoniae from a human to another human as well as nasopharyngeal carriage is not modelled. Also, we did not include any impact on antibiotic resistance that might result from reduced cases and nasopharyngeal carriage. The vaccine PCV7 might undoubtedly have a favourable influence on the future development of antibiotic resistance, since with implementation of PCV7 there would be fewer people with carriage, and the circulation of S. pneumoniae would be reduced. As a result, the lack of specific data for these issues leads to an underestimation of the cost-effectiveness within the model. As the model resulted in a dominance of the vaccination programme, the inclusion could only widen the cost-saving ratio. On the other hand, a possible serotype replacement discussed in the literature could lead to an over-estimation of the cost-effectiveness. Analysis of US data sets about effectiveness of PCV7 in everyday life point out that this effect is present, but absolute numbers that could be incorporated in the model are inconclusive. In future re-evaluations serotype replacement should be an important aspect that should not be neglected.
This model only considers average infection rates across the overall annual birth cohort. However, risk factors are not equally distributed. In the literature risk factors for pneumococcal diseases are present in approximately 81,000 of a birth cohort per year [35]. The cost-effectiveness of giving a PCV7 vaccination only to those at risk has been estimated to be €38,222 per life year gained [44]. Hence, looking at the results of this study, considering herd immunity may enhance the cost-effectiveness of universal vaccination.
Occurrence of IPD, especially in children, is well documented, but data on the incidence of CAP and otitis media are not available. Therefore, incidence rates had to be extrapolated from secondary data sources. The model did not adjust for reduction in vaccine efficacy over time. This effect is unlikely to be substantial, as children are at their highest risk in the years immediately following the vaccination, and herd immunity will continue to support individual immunisation. Serotype replacement has been reported in AOM [52] and therefore has already been considered in the model. Replacement data of IPD are limited. In the model, serotype replacement in IPD is accounted for among non-vaccinated individuals (herd immunity), but not among the vaccine’s target age groups. Cost of adverse events has also been excluded as only limited information is available [8].
A number of previous economic evaluations have been conducted in 12 different countries: Australia [13], Belgium [6], Germany [14, 44], Finland [62], Italy [45], Canada [16, 24, 40, 49], the Netherlands [10], Norway [77], Spain [1, 50], Switzerland [21], the UK [46–48], and the USA [30, 43, 55, 73]. All studies comprise a decision-analytic model. Five studies use a 2 + 1 vaccination schedule while incorporating a vaccine effectiveness that is comparable to the 3 + 1 vaccination schedule. In this context it needs to be stated that there is no evidence of equivalent reduction in pneumococcal carriage or herd immunity.
All hypothetical target cohorts differ from the study populations of the two primary studies, and only a few authors had access to primary epidemiological data sources about incidences of pneumococcal-related diseases, case fatality rates, rates of sequelae, and serotype structure. Although the incorporated efficacy data in all publications were derived from the two primary studies, results varied substantially. In papers published before mid-2003, herd immunity could not be considered due to lack of evidence. In some publications incidences are mixed with utilisation data. Furthermore, the variation of utilisation data were not subject to any sensitivity analyses. Ten evaluations chose a cost-utility-analysis approach. QALY or disability-adjusted life years (DALY) values are literature-based. In one study the authors conducted their own elicitation of preference-based health-related quality-of-life utility values [54]. All relevant direct medical costs, except treatment costs for sequelae, were considered. Cost estimates were given either through an expert panel and/or an analysis of secondary data (e.g. billing, health insurance, and hospital data). All authors combined data on prices and costs per unit with assumptions or estimates. Indirect costs were considered in 11 publications covering loss of working time by parents by using the friction cost approach. Six articles measure indirect costs via the human capital approach reflecting productivity losses due to premature deaths. Adverse vaccination effects are included in only a few publications. Discounting of future costs (with rates of 3–6%) and outcomes (with rates of 0–6%) were chosen by nearly all authors.
All publications confirm that with the introduction of PCV7, morbidity will decrease. The incremental cost-effectiveness ratios were close to or exceeded common societal willingness-to-pay thresholds. With consideration of herd immunity effects, the cost-effectiveness ratio moves closely forward to cost neutrality. The immune protection of individuals leads to an average refinancing of vaccination costs by 25.2%. Including herd immunity this refinancing effect increases by an additional 47.3% (Table 6).
Conclusion
Direct cost savings resulting from an implementation of an universal vaccination programme would be sufficient to offset total direct health-care costs of the vaccination programme. Additionally, herd immunity effects resulting from a vaccination of children can provide protection of the elderly. Sensitivity analyses showed that the result is based on conservative estimates and is robust with respect to expected changes. At first sight, a reduction in the number of vaccine shots may result in a positive effect on the cost-effectiveness ratio of PCV7. However, before changes are considered, it will be critical to analyse effectiveness data and to assess specific differences in countries applying the 2 + 1 scheme (e.g. public support for vaccine uptake and maintenance of vaccination rates). Otherwise, there will be a risk of thwarting the current German PCV7 programme.
References
Asensi, F., De Jose, M., Lorente, M., Moraga, F., Ciuryla, V., Arikian, S., Casciano, R., Vento, M.: A pharmacoeconomic evaluation of seven-valent pneumococcal conjugate vaccine in Spain. Value. Health. 7, 36–51 (2004)
Balk, R.A.: Severe sepsis and septic shock. Crit. Care Clin. 16, 179–192 (2000)
Barker, L., Luman, E., Zhao, Z., Smith, P., Linkins, R., Santoli, J., Rodewald, L., McCauley, M.: National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2001. Morb. Mortal. Wkly. Rep. 51, 664–666 (2002)
Baxter, R., Black, S., Shinefield, H., Fireman, B.: Impact of seven-valent pneumococcal conjugate vaccine (PCV) on antibiotic resistance within Northern California Kaiser Permanente (NCKP) (abstract). In: 41st Annual Meeting of IDSA, San Diego, 9–12 October 2003
Bernard, G.R., Vincent, J.L., Laterre, P.F., LaRosa, S.P., Dhainaut, J.F., Lopez-Rodriguez, A., Steingrub, J.S., Garber, G.E., Helterbrand, J.D., Ely, E.W., Fisher, C.J. Jr.: Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 (2001)
Beutels, P., Van Damme, P., Oosterhuis-Kafeja, F.: Effects and costs of pneumococcal conjugate vaccination of Belgian children. Health. Technol. Assess. (HTA). Brussels (2006)
Black, S.: Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. J. Pediatr. 143, 688–689 (2003)
Black, S., Shinefield, H., Fireman, B., Lewis, E., Ray, P., Hansen, J.R., Elvin, L., Ensor, K.M., Hackell, J., Siber, G., Malinoski, F., Madore, D., Chang, I., Kohberger, R., Watson, W., Austrian, R., Northern California Kaiser Permanente Vaccine Study Center Group: efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19, 187–195 (2000)
Black, S.B., Shinefield, H.R., Ling, S., Hansen, J., Fireman, B., Spring, D., Noyes, J., Lewis, E., Ray, P., Lee, J., Hackell, J.: Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21, 810–815 (2002)
Bos, J.M., Rumke, H., Welte, R., Postma, M.J.: Epidemiologic impact and cost-effectiveness of universal infant vaccination with a 7-valent conjugated pneumococcal vaccine in the Netherlands. Clin. Ther. 25, 2614–2630 (2003)
Breiman, R.F., Spika, J.S., Navarro, V.J., Darden, P.M., Darby, C.P.: Pneumococcal bacteremia in Charleston county, South Carolina. A decade later. Arch. Intern. Med. 150, 1401–1405 (1990)
Bundesverband der Pharmazeutischen Industrie e.V. (BPI) (Hrsg.): Pharma-Daten 2006, 36. überarbeitete Auflage. Berlin (2006)
Butler, J.R., McIntyre, P., MacIntyre, C.R., Gilmour, R., Howarth, A.L., Sander, B.: The cost-effectiveness of pneumococcal conjugate vaccination in Australia. Vaccine 22, 1138–1149 (2004)
Claes, C., Schulenburg, J.M. Graf von der.: Cost effectiveness of pneumococcal vaccination for infants and children with the conjugate vaccine PnC-7 in Germany. Pharmacoeconomics 21, 587–600 (2003)
Committee for Proprietary Medicinal Products: European Public Assessment Report (EPAR). Prevenar. URL: http://www.emea.europa.eu/humandocs/Humans/EPAR/prevenar/prevenar.htm. Accessed 10 Jan 2008)
De Wals, P., Petit, G., Erickson, L.J., Guay, M., Tam, T., Law, B., Framarin, A.: Benefits and costs of immunization of children with pneumococcal conjugate vaccine in Canada. Vaccine 21, 3757–3764 (2003)
Deutsche Rentenversicherung Bund (Hrsg.): Rentenversicherung in Zahlen 2007. Berlin (2007)
Eisenmenger, M.: Sterbetafel 2001/2003. Wirtschaft und Statistik. 5, 463–478 (2005)
Eisenmenger, M., Poetzsch O., Sommer B.: 11. Koordinierte Bevölkerungs-Vorausberechnung. Annahmen und Ergebnisse, Wiesbaden (2006)
Eskola, J., Kilpi, T., Palmu, A., Jokinen, J., Haapakoski, J., Herva, E., Takala, A., Käyhty, H., Karma, P., Kohberger, R., Siber, G., Mäkelä, P.H.: Finnish Otitis Media Study Group: efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344, 403–409 (2001)
Ess, S.M., Schaad, U.B., Gervaix, A., Pinosch, S., Szucs, T.D.: Cost-effectiveness of a pneumococcal conjugate immunisation program for infants in Switzerland. Vaccine 21, 3273–3281 (2003)
Fedson, D.S., Musher, D.M.: Pneumococcal vaccine. In: Plotkin, S.A., Mortimer, E Jr. (eds.) Vaccine, 2nd edn pp. 517–564. Saunders, Philadelphia (1994)
Fireman, B., Black, S.B., Shinefield, H.R., Lee, J., Lewis, E., Ray, P.: Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr. Infect. Dis. J. 22, 10–16 (2003)
Ford, M.W., Grace, E., Wand, E.C.: The clinical and economic impact of pneumococcal conjugate vaccine associated herd immunity in Canada. J. Med. Econ. 7, 85–92 (2004)
Gemeinsamer Bundesausschuss (Hrsg.): Beschluss des Gemeinsamen Bundesausschusses über eine Richtlinie über Schutzimpfungen nach § 20 d Abs. 1. SGB V (Schutzimpfungs-Richtlinie / SiR): Regelung des Anspruches der Versicherten auf Leistungen für Schutzimpfungen gemäß § 29 Abs. 1 Satz 2 Nr. 15 SGB V vom 21. Juni 2007. URL: http://www.g-ba.de/cms/front_content.php?idcat=56 (Accessed 10 Jan 2008)
Gesetz zur Stärkung des Wettbewerbs in der gesetzlichen Krankenversicherung (GKV-Wettbewerbsstärkungsgesetz — GKV-WSG) vom 26. März 2007. Bundesgesetzblatt Teil 1 Nr. 11, 378–473 (2007)
Grijalva, C.G., Nuorti, J.P., Arbogast, P.G., Martin, S.W., Edwards, K.M., Griffin, M.R.: Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369, 1179–1186 (2007)
Grijalva, C.G., Poehling, K.A., Nuorti, J.P., Zhu, Y., Martin, S.W., Edwards, K.M., Griffin, M.R.: National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 118, 865–873 (2006)
Hallauer, J.F.: Aktueller Stand de Pneumokokkenerkrankungen in Deutschland und der Nutzung der konjugierten Pneumokokkenvakzine. Gesundheitsökonomie und Qualitätsmanagement 11, 43–55 (2006)
Hueston, W.J., Mainous, A.G. III, Brauer, N.: Predicting cost-benefits before programs are started: looking at conjugate vaccine for invasive pneumococcal infections. J. Community. Health. 25, 23–33 (2000)
Hülße, C., Ley, S., Stück, B.: Ärztemerkblatt Pneumokokken. Verlag im Kilian, Marburg (1999)
IMS Health Deutschland: Verschreibungsindex für Pharmazeutika (VIP). URL: http://www.imshealth.de (Accessed 10 Jan 2008)
Imran, M.N., Leng, P.H., Yang, S., Kurup, A., Eng, P.: Early predictors of mortality in pneumococcal bacteraemia. Ann. Acad. Med. Singapore. 34, 426–431 (2005)
Institut für das Entgeltsystem im Krankenhaus gGmbH (InEK): G-DRG-System 2007, Reportbrowser 2005/2007. URL: http://www.g-drg.de (Accessed 10 Jan 2008)
Kalies, K., Hermann, M., Schmitt, H.J., Kries, R.V.: Prävention invasiver Pneumokokken Infektionen im Kindersalter. Welche Impfstrategie ist zu empfehlen? Kinderärztliche Praxis. 72, 90–98 (2001)
Kalin, M., Ortqvist, A., Almela, M., Aufwerber, E., Dwyer, R., Henriques, B., Jorup, C., Julander, I., Marrie, T.J., Mufson, M.A., Riquelme, R., Thalme, A., Torres, A., Woodhead, M.A.: Prospective study of prognostic factors in community-acquired bacteremic pneumococcal disease in 5 countries. J. Infect. Dis. 182, 840–847 (2000)
Kaplan, V., Angus, D.C., Griffin, M.F., Clermont, G., Scott, W.R., Linde-Zwirble, W.T.: Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am.J. Respir. Crit. Care Med. 165, 766–772 (2002)
von Kries, R., Siedler, A., Schmitt, H.J., Reinert, R.R.: Proportion of invasive pneumococcal infections in German children preventable by pneumococcal conjugate vaccines. Clin. Infect. Dis. 31, 482–487 (2000)
von Kries, R., Toschke, A.M., Siedler, A., Reinert, R.R.: Population-based nationwide study on invasive pneumococcal infections among children in Germany (1997–2003). (2005)
Lebel, M.H., Kellner, J.D., Ford-Jones, E.L., Hvidsten, K., Wang, E.C., Ciuryla, V., Arikian, S., Casciano, R.: A pharmacoeconomic evaluation of 7-valent pneumococcal conjugate vaccine in Canada. Clin. Infect. Dis. 36, 259–268 (2003)
Lexau, C.A., Lynfield, R., Danila, R., Pilishvili, T., Facklam, R., Farley, M.M., Harrison, L.H., Schaffner, W., Reingold, A., Bennett, N.M., Hadler, J., Cieslak, P.R., Whitney, C.G.: Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. J. Am. Med. Assoc. 294, 2043–2051 (2005)
Ley, S., Stück, B.: Pneumokokken-Infektionen: Ältere Menschen. Immunologie & Impfen. 2, 128–133 (1999)
Lieu, T.A., Ray, G.T., Black, S.B., Butler, J.C., Klein, J.O., Breiman, R.F., Miller, M.A., Shinefield, H.R.: Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. J. Am. Med. Assoc. 283, 1460–1468 (2000)
Lloyd, A., Patel, N., Scott, D.A., Runge, C., Claes, C., Rose, M.: Cost-effectiveness of heptavalent conjugate pneumococcal vaccine (Prevenar) in Germany: considering a high-risk population and herd immunity effects. Eur. J. Health. Econ. [Epub ahead of print] (2007)
Marchetti, M., Colombo, G.L.: Cost-effectiveness of universal pneumococcal vaccination for infants in Italy. Vaccine 23, 4565–4576 (2005)
McIntosh, E.D., Conway, P., Willingham, J., Hollingsworth, R., Lloyd, A.: Pneumococcal pneumonia in the UK—how herd immunity affects the cost-effectiveness of 7-valent pneumococcal conjugate vaccine (PCV). Vaccine 23, 1739–1745 (2005)
McIntosh, E.D., Conway, P., Willingham, J., Lloyd, A.: The cost-burden of paediatric pneumococcal disease in the UK and the potential cost-effectiveness of prevention using 7-valent pneumococcal conjugate vaccine. Vaccine 21, 2564–2572 (2003)
Melegaro, A., Edmunds, W.J.: Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine 22, 4203–4214 (2004)
Moore, D., Bigham, M., Patrick, D.: Modelling the costs and effects of a universal infant immunization program using conjugated pneumococcal vaccine in British Columbia. Can. Commun. Dis. Rep. 29, 97–104 (2003)
Navas, E., Salleras, L., Gisbert, R., Dominguez, A., Timoner, E., Ibanez, D., Prat, A.: Cost-benefit and cost-effectiveness of the incorporation of the pneumococcal 7-valent conjugated vaccine in the routine vaccination schedule of Catalonia (Spain). Vaccine 23, 2342–2348 (2005)
Overturf, G.D., American Academy of Pediatrics, Committee on Infectious Diseases: technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics 106, 367–376 (2000)
Pelton, S.I.: Acute otitis media in the era of effective pneumococcal conjugate vaccine: will new pathogens emerge? Vaccine 19, S96–S99 (2000)
Poethko-Müller, C., Kuhnert, R., Schlaud, M.: Durchimpfung und Determinanten des Impfstatus in Deutschlang. Ergebnisse des Kinder-und Jugendgesundheitssurveys (KiGGS). Bundesgesundheitsblatt — Gesundheitsforschung — Gesundheitsschutz 50, 851–862 (2007)
Prosser, L.A., Ray, G.T., O’Brien, M., Kleinman, K., Santoli, J., Lieu, T.A.: Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics. 113, 283–290 (2004)
Ray, G.T., Whitney, C.G., Fireman, B.H., Ciuryla, V., Black, S.B.: Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr. Infect. Dis. J. 25, 494–501 (2006)
Reinert, R.R., Haupts, S., van der Linden, M., Heeg, C., Cil, M.Y., Al-Lahham, A., Fedson, D.S.: Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin. Microbiol. Infect. 11, 985–991 (2005)
Robert Koch Institut (RKI): Zur Impfung gegen Pneumokokken-Infektionen. Epidemiol. Bull. 12, 97–99 (2000)
Rote Liste® Service GmbH (Hrsg.): Rote Liste. Arzneimittelinformationen für Deutschland (einschließlich EG-Zulassungen und bestimmter Medizinprodukte). Frankfurt (2005)
Rote Liste® Service GmbH (Hrsg.): Rote Liste online. Arzneimittelinformationen für Deutschland (einschließlich EG-Zulassungen und bestimmter Medizinprodukte). URL: http://www.rote-liste.de (Accessed 10 Jan 2008)
Ryan, K.J., Ray, C.G., Sherris, J.C.: (eds.): Sherris Medical Microbilogoy: an introduction to infectious diseases. McGraw-Hill, Cincinnati (2004)
Rüggeberg, J.U., Ketteler, K., MacKenzie, C.R., von Kries, R., Reinert, R.R., Schroten, H.: Blood culture sampling rates at a German pediatric university hospital and incidence of invasive pneumococcal disease. Infection 32, 78–81 (2004)
Salo, H., Sintonen, H., Pekka Nuorti, J., Linna, M., Nohynek, H., Verho, J., Kilpi, T.: Economic evaluation of pneumococcal conjugate vaccination in Finland. Scand. J. Infect. Dis. 37, 821–832 (2005)
Schnoor, M., Hedicke, J., Dalhoff, K., Raspe, H., Schafer, T.: Approaches to estimate the population-based incidence of community acquired pneumonia. J. Infect. 55, 233–239 (2007)
Scholz, H.: Akute Otitis media — Krankheitsbild und Behandlung im Kindesalter. In: Stück B., von Voss H. (eds.) Pneumokokken-Erkrankungen bei Säuglingen und Kleinkindern, pp. 15–22. Verlag im Kilian, Marburg (2001)
Schulenburg, J.M. Graf von der, Greiner, W., Jost, F., Klusen, N., Kubin, M., Leidl, R., Mittendorf, T., Rebscher, H., Schöffski, O., Vauth, C., Volmer, T., Wahler, S., Wasem, J., Weber, C., und die Mitglieder des Hannoveraner Konsens. Deutsche Empfehlungen zur gesundheitsökonomischen Evaluation — dritte und aktualisierte Fassung des Hannoveraner Konsens. Gesundheitsökonomie und Qualitätsmanagement 12, 285–290 (2007)
Schulze-Gattermann, H.: Kosten-Nutzen-Analyse der Cochlea-Implantation bei Kindern. Dissertation, Medizinische Hochschule Hannover (2000)
Schulze-Gattermann, H., Illg, A., Schoenermark, M., Lenarz, T., Lesinski-Schiedat, A.: Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol. Neurotol. 23, 674–681 (2002)
Statistisches Bundesamt (Hrsg.): Bevölkerung nach Altersgruppen, Familienstand und Religionszugehörigkeit. URL: http://www.destatis.de (Accessed 10 Jan 2008)
Statistisches Bundesamt (Hrsg.): Diagnosedaten der Krankenhäuser nach Behandlungsort ab 2000 (Fälle/Sterbefälle, Pflegetage, durchschnittliche Verweildauer. Gliederungsmerkmale: Jahre, Behandlungsort, Alter, Geschlecht, Verweildauer, ICD10. URL: http://www.gbe-bund.de (Accessed 10 Jan 2008)
Statistisches Bundesamt (Hrsg.): Leben und Arbeiten in Deutschland. Sonderheft 2: Vereinbarkeit von Familie und Beruf. Ergebnisse des Mikrozensus 2005. Wiesbaden (2006)
Statistisches Bundesamt (Hrsg.): Statistisches Jahrbuch 2006. Für die Bundesrepublik Deutschland. Wiesbaden (2006)
Ständige Impfkommission am Robert Koch-Institut (STIKO): Empfehlung der Ständigen Impfkommission (STIKO) am Robert Koch Institut, Stand: Juli 2006. Epidemiol. Bull. 235–254 (2006)
Weycker, D., Richardson, E., Oster, G.: Childhood vaccination against pneumococcal otitis media and pneumonia: an analysis of benefits and costs. Am. J. Manage. Care. 6, S526–S535 (2000)
Whitney, C.G.: Effect of pneumococcal conjugate vaccine on invasive disease in the U.S. (abstract). 4th international symposium on pneumococci and pneumococcal diseases, Helsinki 9–13 May 2004
Whitney, C.G.: Impact of conjugate pneumococcal vaccines. Pediatr. Infect. Dis. J. 24, 729–730 (2005)
Whitney, C.G., Farley, M.M., Hadler, J., Harrison, L.H., Bennett, N.M., Lynfield, R., Reingold, A., Cieslak, P.R., Pilishvili, T., Jackson, D., Facklam, R.R., Jorgensen, J.H., Schuchat, A.: Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348, 1737–1746 (2003)
Wisloff, T., Abrahamsen, T.G., Bergsaker, M.A., Lovoll, O., Moller, P., Pedersen, M.K., Kristiansen, I.S.: Cost effectiveness of adding 7-valent pneumococcal conjugate (PCV-7) vaccine to the Norwegian childhood vaccination program. Vaccine 24, 5690–5699 (2006)
Zentralinstitut (ZI): Abrechnungsdatentraeger-Panel (ADT-Panel). URL: http://www.zi-berlin.de (Accessed 10 Jan 2008)
Acknowledgments
The authors would like to thank the members of the German Advisory Board Prevenar® PCV7 for their valuable comments.
Competing interest
This study was supported by an unrestricted educational grant from Wyeth Pharma, Münster, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Claes, C., Reinert, R.R. & von der Schulenburg, JM.G. Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur J Health Econ 10, 25–38 (2009). https://doi.org/10.1007/s10198-008-0098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-008-0098-1