Abstract
Glass waste from an industrial wastewater treatment plant (WTP) was studied to recycle the material in ceramic materials. A white kaolinitic clay was used replacing the waste in percentages of 0–30%, producing 115 × 25 × 10 mm specimens pressed with 20 MPa burned in the 850–1050 °C range. Burned specimens were evaluated for plasticity, dry mass density, water absorption, firing shrinkage, flexural strength, optical microscopy and scanning electron microscopy. It was found that the application of the glass waste aided the properties such as water absorption and tensile strength and despite increasing the linear shrinkage, it did not damage this property excessively, except for the application of the waste at a firing temperature of 1050 °C. The properties obtained with the use of glass are attributed to the higher liquid-phase formation promoted by the waste due to the chemical and mineralogical composition of the waste, which presents alkaline compounds. The results proved the viability of recycling the wastewater from WTP glass in ceramic materials, promoting an environmentally correct disposal of the waste. The application of 30% of burnt waste at 850 °C, for example, provides a burn shrinkage of about 2%, 17% water absorption and 7 MPa tensile strength, which enables the values established by technical standards to be met application for both tiles and ceramic bricks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial wastewater treatment plant (WTP) is facilities used to treat urban or industrial waste, before the material is disposed of in the environment [1, 2]. Even if it reduces the levels of contaminants in sewage and effluents, the final disposal of these materials is usually exposing them to the environment, causing numerous contaminations, or having to apply high costs to specialized companies, so that the sludge from the treatment is disposed of in licensed industrial landfills [3, 4].
It is known that the civil construction sector is one of the most consuming natural resources in the world, contributing significantly to the emission of greenhouse gases that are studied during the production of materials such as cement [5, 6]. Thus, the urgency of the application of waste in construction materials is perceived, aiming at achieving the sustainable development of the sector, as reported by several authors [7,8,9,10].
In addition, it is known that the use of waste in clay-based materials, such as ceramic blocks and bricks, for example, promotes the confinement or adsorption of problematic materials present in the waste composition [11]. Because of that, several studies have promoted the application of wastes in ceramic materials, which is also the objective of this work.
Areias et al. [12] studied the incorporation of sludge from the sewage treatment plant in a kaolinitic clay. The authors carried out the physical, chemical, and mineralogical characterization of the two materials and incorporated contents of up to 15% of sludge to replace the studied clay, testing the materials produced by compressive strength and water absorption. The authors verified that although there is a tendency toward a reduction in the compressive strength and an increase in the water absorption of the material, the incorporation of contents up to 2.5% by mass is possible, without excessive loss of properties.
Regarding glass waste, it is worth highlighting the works by Azevedo et al. [13] in which the authors incorporated the waste into adhesive mortars, obtaining satisfactory results; [14], where the authors applied the glass waste to produce a synthetic clay aggregate; the works by Lu et al. [15], Liu et al. [16], and Khan et al. [17], in which the possibility of using glass waste from different sources (bottle glass, household appliances, for example) in cement-based materials such as concrete, pastes, and mortars is addressed. Other important studies were carried out using glass waste in geopolymeric materials. The works by Azevedo et al. [18, 19] who proved the possibility of alkaline activation of these materials.
The work by Gutiérrez et al. [20], where the authors proposed the use of glass waste as a catalyst for the alkali activation reaction of metakaolin-based mortars and obtained excellent results when thermal treatments were performed, since, as highlighted and proven by the authors, the glass waste provides the formation of a vitreous phase during firing, contributing to the increase in the strength of the materials. Raut and Gomez [21] used glass powder to evaluate the efficiency of blocks used for thermal insulation, highlighting that this type of material contributes to improving the thermal efficiency of sealing devices.
Still on the application of glass waste in construction materials, as a possibility of recycling the material, it is worth highlighting the work by Vieira et al. [22] in which the authors applied fluorescent lamp glass waste to red ceramic materials and found that the incorporation of 30% of the waste increased the flexural strength of the materials from 712 to 1361 N, in addition to providing a reduction in water absorption from 24.1 to 15.7%, which was attributed to the formation of a liquid phase and increased vitrification of the studied ceramic artifacts.
In this context, the objective of this work is to evaluate the possibility of applying glass waste from an effluent treatment plant of an industry that produces flat glass in ceramic materials, using a white kaolinitic clay with chemical and mineralogical composition usually used in these applications. The main innovations of this article are to carry out the application of waste from a source that has never been studied, from the wastewater treatment plant.
The waste studied in this manuscript was chosen due to its disposal, which is problematic. The materials are disposed of in water resources, using an aqueous solution that contains the glass waste in a concentration of 3–7.5%, according to data from the industry where was extracted. Some data from previous publications with the same waste indicate that the annual generation of the material is approximately 3000 tons per year [18, 19]. This highlights the urgency of recycling the waste, as it contains materials that are harmful to the environment, such as sodium, magnesium, and iron, which will be highlighted in the text below. Therefore, the objective of this manuscript is to recycle the waste extracted from the industry, before it is disposed of in water resources, as this way it is easier to obtain this material.
Methodology
Aiming to study the incorporation of glass waste from WTP in ceramic materials, initially the materials used in the research were collected. The clay used was extracted from Brazil, and is named as white kaolinitic clay. The glass waste was collected from the WTP in Brazil. Figure 1 shows the materials used.
The WTP from which the waste was obtained is an industrial facility and does not serve domestic sewage, which is why its chemical composition is not organic like other sewage obtained from domestic urban waste. WTP only has a primary treatment stage, as the waste generated is predominantly inorganic. This treatment is carried out through grating, desander, and settling tanks. It is at this stage that the residue is extracted, after the decantation process.
The two materials were characterized granulometrically through sieving and sedimentation (ABNT NBR 7181, 2016) [23], mineralogically through X-ray diffraction using a Sheifert brand diffractometer, model URD 65, operating with Cukα and 2θ radiation ranging from 5° to 60° and chemically using X-ray fluorescence spectrometry in Philips PW 2400 equipment.
The glass waste from WTP was thermally analyzed by optical dilatometry using an optical dilatometer brand Misura where a maximum temperature of 1600 °C was used, with a heating rate of 40 °C/min. The glass waste was crushed until it was in powder form, being dried in an oven at 110 °C and later ground in an annular mill. The white kaolinitic clay, on the other hand, was thermally characterized by differential thermal analysis in a thermal analyzer of the TA instruments brand, model SDT2960 with a heating rate of 10 °C/min to speed up the test and a maximum temperature of 1150 °C. The glass waste was also morphologically characterized by scanning electron microscopy (SEM) using a Jeol model JSM 6460 LV microscope with an energy-dispersive spectrometer (EDS). After the characterization of the raw materials, clayey masses were formulated containing 0%, 10%, 20%, and 30% of glass waste in mass to replace white kaolinitic clay. The evaluation of the plasticity parameters of all studied masses was carried out through the Atterberg limits (ABNT NBR 6459, 2016; ABNT NBR 7180, 2016) [24, 25] and specimens were produced through unixial pressing using 8% moisture and applying pressure 20 MPa. The test specimens produced, with dimensions of 115 × 25 × 10 mm, were dried in an oven for 24 h to lose shaping water and later burned using a laboratory furnace type Muffle by Maitec model FL 1300. The firing temperatures were chosen as a function of the thermal analysis performed, using 850 °C, 900 °C, 950 °C, 1000 °C, and 1050 °C. The specimens were tested for dry bulk density, linear burning shrinkage, water absorption, and flexural strength (ABNT NBR 15270-1, 2017; ABNT NBR 15270-2, 2017; ABNT NBR 15310, 2009) [26,27,28]. For further characterization of the material, optical microscopy (OM) was performed using an Olympus model CGA confocal microscope. Finally, the SEM was performed on the fired ceramic bodies to assess the microscopy and rupture surface of the materials.
Results and discussion
Characterization of waste glass and white kaolinitic clay
The chemical analysis of the waste glass from WTP and the white kaolinite clay are shown in Table 1. On the clay used, it can be seen that the material has 49.45% of SiO2 and 31.31% of Al2O3, typical mineral compositions clay. The SiO2/Al2O3 ratio is 1.58, with the theoretical kaolinitic ratio being 1.18 [29]. If the SiO2/Al2O3 ratio was exactly 1.18, this would indicate that the studied clay is entirely kaolinite-based. However, it is observed that the ratio between SiO2/Al2O3 was 1.58, above the theoretical value. Deviation of SiO2 amounts is related to the presence of quartz sand or muscovite mica [30, 31]. The presence of kaolinite in this clay is confirmed by Fig. 2a and by the high loss to fire found for the material, of 14.4% [10, 32].
The low Fe2O3 contents below 3% indicate that the color of the fired ceramic pieces will not be reddish. The levels obtained for TiO2 above 3%, on the other hand, favor the occurrence of yellow coloration after burning. These two oxides are called dye oxides [2, 33]. The low Na2O and K2O contents, on the other hand, indicate the formation of little liquid phase after burning, as alkaline oxides are melting materials. Furthermore, the low percentage of CaO and MgO is indicative of the absence of carbonates [34]. Still on Table 1, it is possible to verify that the glass waste has a typical sodo-calcium composition. As highlighted by Kielf et al. [35] soda-lime glasses typically have a composition of 74% SiO2, 14% Na2O and 6% CaO, while the glass waste has 71% SiO2, 13.5% Na2O and 10% CaO. Silicon oxide is of great importance in the composition of the glass structure, as they are network-forming oxides. Sodium and calcium oxides are network modifiers, which reduce the viscosity of the glass and allow the formation of a viscous flow that can help to reduce the porosity of the ceramic and thus contribute to improving its mechanical properties. It is worth noting that the high amounts of K2O, which is a fluxing oxide, contributes to the formation of the liquid phase, changing the fluidity and allowing the removal of bubbles and chemical homogenization after burning [34]. It is observed in Table 1 that the waste glass has in its composition 13.57% of Na2O, 2.44% of MgO and 1.25% of Fe2O3. These compounds when diluted in water can cause environmental problems, such as eutrophication of water resources due to algae proliferation, death of aquatic animals and/or contamination of drinking water [36, 37].
Figure 2 shows the mineralogical analysis of white kaolinitic clay and glass waste. It is verified that the clay is predominantly kaolinitic, and this clay mineral adds plasticity to the clayey mass [38]. There is also the presence of quartz, which helps to control post-burn shrinkage, but causes a drop in strength in the material [39] and the presence of gibbsite, muscovite and/or illite mica and microclimate [40]. It is noteworthy that the presence of these minerals helps in the formation of a liquid phase during the sintering of the material, due to the presence of alkaline materials [41].
On the mineralogical analysis of the glass waste (Fig. 2b), it is verified the absence of crystalline phases, containing only bands related to the vitreous phase, characteristic of amorphous materials [42]. This is explained by the rapid cooling process characteristic of glass production. The peaks detected in the figure between 20 and 30º are the result of the presence of semi-crystalline silica [43]. The presence of amorphous material is desirable for application in ceramic materials, since this crystal structure favors the occurrence of a liquid phase and vitrification of the material, in addition to helping to reduce post-firing porosity [44].
Figure 3 shows the thermal analysis of clay. Some events can be identified and are described below: an exothermic peak occurs between 65 °C and 70 °C attributed to the loss of kneading water that causes a mass loss of approximately 0.96%; another exothermic peak occurs between 265 °C and 270 °C, with a mass loss of 2.15%, related to the transformation of gypsum; another exothermic peak is identified at approximately 490 °C, with a mass loss of almost 9%, attributed to the transformation of kaolinite into metakaolinite, proving that the clay is kaolinitic in nature, and finally an endothermic peak is identified at approximately 980 °C, attributed the sintering of the material and transformation of metakaolinite to form resistant phases [29]. This helps to justify the chosen burning temperatures, three below 980 °C and two above this value.
Figure 4 shows the thermal analysis of the glass waste using optical dilatometry. Two important events in the application of waste glass are noticeable in the figure: around 850 °C, the softening of the glass occurs, where the material starts to behave as a superheated and low-viscosity fluid, which can help the vitrification and densification of ceramic materials. Another event that can be described is the melting of glass, which occurs just before 1100 °C. At this stage, the glass turns into a liquid and is easier to flow in the ceramic matrix, causing the occurrence of defects in the material, such as segregation [45]. Therefore, the chosen firing temperatures were limited to the range of 850 °C–1100 °C, since below this range, the glass tends to have no effect on the material, and above this range, the incorporation of glass is not beneficial.
Figure 5 shows the particle size of the materials used in this research. It is verified that the glass waste presents a very fine granulometry, being relatively similar to the clay granulometry used in the research, which is a positive characteristic to substitute the clay for the waste under study. This fact can be proven by analyzing the equivalent diameters D20 and D85, where it is clear that there is an overlapping of the materials curve. The fineness of the waste is proven through scanning electron microscopy, illustrated in Fig. 6, where it is noticed that the waste particles have diameters in micrometric orders, ranging from about 2 to 20 µm.
Characterization of ceramic masses
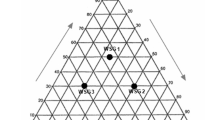
Figure 7 is defined through the parameters plasticity limit and plasticity index, which is obtained through the numerical difference between the liquidity limit and the plasticity limit. It is verified that the 0% mass is within the acceptable extrusion range, since the clay used is very plastic. Using 10% waste glass shifts the plasticity of the material to the optimal extrusion zone, while using 20% and 30% excessively reduces the plasticity of the material. This fact makes the masses containing 20% and 30% of the waste present plasticity slightly lower than the recommended range for extrusion of ceramic materials. Thus, following the plasticity criterion, the mass containing 10% of waste is the most suitable. The 20% and 30% masses were not discarded, as the technological properties obtained by these materials will be evaluated.
Figure 8 shows the dry bulk density of the studied masses. It is verified that the use of WTP glass waste made the ceramic mass lighter. This fact is directly related to the amount of clay used in the material. It is observed that the masses containing greater amounts of clay have higher dry density, because, as they are more plastic, they keep more water bound to clay mineral particles, especially kaolinite particles, the main compound responsible for plasticity [38, 46]. Thus, the results obtained by Fig. 8 confirm and prove the results obtained by Fig. 7.
Figure 9 shows the post-firing properties obtained by the ceramic masses. It can be seen from Fig. 9a that there was an increase in post-burning retraction as the burning temperature increased and as the percentage of waste used increased. This fact can be attributed to the greater amount of liquid-phase formation promoted by the waste [47], which causes an effect of reducing the size of the material. Although the Brazilian standard does not present maximum retraction values, [48] recommend that shrinkage be limited to a maximum of 7% for ceramic materials that require high-dimensional accuracy. Thus, of the masses burned at a temperature of 1050 °C, only the percentage with 0% meets the recommended value. At other temperatures, all studied masses present shrinkage values less than 7%. This discards the use of waste at a burning temperature of 1050 °C.
Regarding water absorption, as seen in Fig. 9b, there are two patterns of values obtained: at temperatures of 950 °C, 1000 °C, and 1050 °C, there was a reduction in water absorption as higher waste contents were incorporated, due to liquid-phase formation and vitrification promoted by the waste [49]. At lower temperatures, this reduction did not occur, which shows that the formation of a liquid phase by the waste at lower temperatures was not as intense. If the waste does not act to form a liquid phase, the presence of this material in the ceramic matrix only causes defects, increasing porosity and water absorption. Brazilian standards for the application of ceramic tiles limit the absorption of water to 22% [28], while limiting the absorption to 25% for the application of ceramic blocks [26]. As a result, it is verified that all the studied masses at all fired temperatures can be applied both for tiles and for ceramic blocks. This fact is beneficial with regard to recycling WTP glass waste into ceramic materials.
Regarding the flexural strength, illustrated in Fig. 9c, it appears that there is no defined standard for the values obtained, which is attributed to the large number of variables involved in the analysis, such as: application of the glass waste, which forms different liquid-phase proportions depending on the temperature and proportion of waste applied, chemical and mineralogical composition of the white kaolinitic clay, which provides different amounts of flux minerals, minerals that act as fillers and minerals that act in a plastic way, different calcination temperatures, which provide different effects to the materials studied [50, 51]. In general, it appears that the most resistant compound formed was calcined at 1050 °C containing 10% waste, while the least resistant was calcined at 950 °C containing 30% waste. Based on these results, it appears that there is an optimal WTP glass waste content to be used in ceramic materials; since the more glass waste is applied, the more liquid phase is formed after firing. However, the formation in excess of the liquid phase is as harmful to mechanical strength as the non-formation of this phase [47]. On the minimum values stipulated by Brazilian recommendations, above 6.5 MPa is adopted for ceramic tiles [2, 51]. This value is obtained using 10% of waste burned at 1000 °C or 1050 °C, and using 20% burned at 950 °C or 1050 °C. With the use of 30% acceptable values are obtained for all temperatures with the exception of 950 °C. It is interesting to note that for the reference mass, only at 1050 °C, it is possible to obtain flexural strength values greater than 6.5 MPa. This fact proves the beneficial nature of the application of the waste on ceramic materials. For ceramic blocks, the minimum value is 2.5 MPa [2, 51]. It is observed that all evaluated compositions meet this value. Thus, although only some compositions can be used in the manufacture of ceramic tiles, a material whose commercial value is greater, and all compositions can be applied to the manufacture of bricks.
Figure 10 shows optical microscopy (OM) for the studied masses burned at 1050 °C. It is verified that there was an intensification of the vitreous phase formed in the materials [47, 49], which has a whiter appearance in the OM. It is also possible to notice the greater presence of glass crystals in the masses that have a higher percentage of waste, and a smaller amount of pores in materials containing a higher percentage of waste. The image that has the reference composition, for example, has voids in dark form that are easy to detect in the image. These images help to confirm the results obtained in the parameters evaluated and discussed in the previous paragraphs.
Figure 11 shows the scanning electron microscopy (SEM) for the masses containing 30% of waste burned at the temperatures of 850 °C, 950 °C, and 1000 °C. The images demonstrate how the waste provided pore reduction in the ceramic matrix, especially in the 1000 °C image, where even with a 100 × magnification in the material, the pores are not easily detected. It is verified that the WTP glass waste was satisfactorily homogenized in the ceramic matrix, since even evaluating the mass with higher waste contents, it is not possible to easily detect the presence of glass particles. This proves that the waste acts as a flux and forms a liquid phase, being incorporated into the matrix after burning, not just occupying empty spaces. In other words, the waste actually acts as a liquid-phase former, and not only as a material filling effect, as is the case with materials such as quartz sand [52].
Conclusion
The objective of this work was to evaluate the recycling of waste glass extracted from WTP into ceramic materials. The main conclusions obtained were:
-
The characterization of clay and glass residue proved that both materials are suitable for use in ceramic materials. The presence of alkaline materials in the glass residue is a beneficial factor, because it contributes to the formation of a liquid phase during firing, improving ceramic properties.
-
The plasticity analysis showed that the residue is not plastic, affecting the prognosis of the extrusion. With 10% waste, however, the ceramic mass was in the ideal extrusion zone.
-
An increase in shrinkage was observed with the increase in the burning temperature and the percentage of waste used, which proves the formation of a greater liquid phase promoted by the glass waste. It was found that burning at 1050 °C causes excessive retraction for the masses containing residues, which discards the application of the material at that temperature.
-
The values obtained for water absorption are compatible for application on ceramic bricks (maximum 25%) and ceramic coverings (maximum 22%).
-
The flexural strength values obtained were greater than 2.5 MPa recommended for ceramic bricks in all compositions. Only the composites with 20% burning at 950 °C or 1050 °C and the compositions with 30% burning at any temperature, except 950 °C, met the minimum value for application on 6.5 MPa tiles.
As a result, the feasibility of recycling glass waste into ceramic materials in different compositions is proven. Some examples of viable compositions are 10% waste burned at 1000 °C and/or 20% waste burned at 950 °C for ceramic tiles; and 30% of waste burned at 850 °C for ceramic bricks. Therefore, the objective of the article was achieved.
References
Smiri M, Elarbaoui S, Missaoui T, Ben Dekhil A (2015) Micropollutants in sewage sludge: elemental composition and heavy metals uptake by phaseolus vulgaris and vicia faba seedlings. Arab J Sci Eng 40:1837–1847. https://doi.org/10.1007/s13369-015-1639-4
Girondi Delaqua GC, Marvila MT, Souza D, Sanchez Rodriguez RJ, Colorado HA, Fontes Vieira CM (2020) Evaluation of the application of macrophyte biomass salvinia auriculata aublet in red ceramics. J Env Manage 275:111253. https://doi.org/10.1016/j.jenvman.2020.111253
Zhou Z, Tang Y, Dong J, Chi Y, Ni M, Li N, Zhang Y (2018) Environmental performance evolution of municipal solid waste management by life cycle assessment in Hangzhou China. J Env Manage 227:23–33. https://doi.org/10.1016/j.jenvman.2018.08.083
de Azevedo ARG, Alexandre J, de Xavier GC, Pedroti LG (2018) Recycling paper industry effluent sludge for use in mortars: a sustainability perspective. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.05.011
Marvila MT, Azevedo ARG, Alexandre J, Colorado H, Pereira Antunes ML, Vieira CMF (2020) Circular economy in cementitious ceramics: replacement of hydrated lime with a stoichiometric balanced combination of clay and marble waste. Int J Appl Ceram Technol. https://doi.org/10.1111/ijac.13634
Marvila MT, Alexandre J, de Azevedo ARG, Zanelato EB (2019) Evaluation of the use of marble waste in hydrated lime cement mortar based. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-019-00878-6
Marvila MT, Azevedo ARG, Barroso LS, Barbosa MZ, de Brito J (2020) Gypsum plaster using rock waste: a proposal to repair the renderings of historical buildings in Brazil. Constr Build Mater 250:118786. https://doi.org/10.1016/j.conbuildmat.2020.118786
de Azevedo ARG, Marvila MT, Barroso LS, Zanelato EB, Alexandre J, Xavier GC, Monteiro SN (2019) Effect of granite residue incorporation on the behavior of mortars. Mater (Basel). https://doi.org/10.3390/ma12091449
França BR, Azevedo ARG, Monteiro SN, Da Costa F, Filho G, Marvila MT, Alexandre J, Zanelato EB (2018) Durability of soil-Cement blocks with the incorporation of limestone residues from the processing of marble. Mater Res 21:1–6. https://doi.org/10.1590/1980-5373-MR-2017-1118
Girondi GD, Marvila MM, de Azevedo ARG, de Souza CC, Souza D, de Brito J, Vieira CMF (2020) Recycling potential of powdered cigarette waste in the development of ceramic materials. J Mater Cycles Waste Manag 22:1672–1681. https://doi.org/10.1007/s10163-020-01058-7
Mohajerani A, Kadir AA, Larobina L (2016) A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. https://doi.org/10.1016/j.wasman.2016.03.012
Areias IOR, Vieira CMF, Intorne AC (2017) Incorporação de lodo da estação de tratamento de esgoto (ETE) em cerâmica vermelha (incorporation of sludge of the sewage treatment station). Cerâmica 63:343–349
de Azevedo ARG, Alexandre J, Zanelato EB, Marvila MT (2017) Influence of incorporation of glass waste on the rheological properties of adhesive mortar. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2017.04.208
de Oliveira HA, dos Santos CP, Oliveira RMPB, de Jesus E, Macedo ZS (2019) Produção de agregado sintético de argila com reaproveitamento de resíduo de vidro. Matéria (Rio Janeiro). https://doi.org/10.1590/s1517-707620190001.0653
Lu J-X, Zheng H, Yang S, He P, Poon CS (2019) Co-utilization of waste glass cullet and glass powder in precast concrete products. Constr Build Mater 223:210–220. https://doi.org/10.1016/j.conbuildmat.2019.06.231
Liu G, Florea MVA, Brouwers HJH (2019) Characterization and performance of high volume recycled waste glass and ground granulated blast furnace slag or fly ash blended mortars. J Clean Prod 235:461–472. https://doi.org/10.1016/j.jclepro.2019.06.334
Khan MNN, Saha AK, Sarker PK (2020) Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: a review. J Build Eng 28:101052. https://doi.org/10.1016/j.jobe.2019.101052
de Azevedo ARG, Teixeira Marvila M, de Barbosa OL, Macario Ferreira W, Colorado H, Rainho Teixeira S, Mauricio Fontes VC (2021) Circular economy and durability in geopolymers ceramics pieces obtained from glass polishing waste. Int J Appl Ceram Technol. https://doi.org/10.1111/ijac.13780
Azevedo ARG, Marvila MT, Rocha HA, Cruz LR, Vieira CMF (2020) Use of glass polishing waste in the development of ecological ceramic roof tiles by the geopolymerization process. Int J Appl Ceram Technol. https://doi.org/10.1111/ijac.13585
de Mejía Gutiérrez R, Villaquirán-Caicedo MA, Guzmán-Aponte LA (2020) Alkali-activated metakaolin mortars using glass waste as fine aggregate: mechanical and photocatalytic properties. Constr Build Mater 235:117510. https://doi.org/10.1016/j.conbuildmat.2019.117510
Raut AN, Gomez CP (2020) Utilization of glass powder and oil palm fibers to develop thermally efficient blocks. Arab J Sci Eng 45:3959–3972. https://doi.org/10.1007/s13369-019-04316-5
Vieira CMF, Morais ASC, Monteiro SN, Delaqua GCG (2016) Teste industrial de cerâmica vermelha incorporada com resíduo de vidro de lâmpada fluorescente. Cerâmica 62:376–385. https://doi.org/10.1590/0366-69132016623642035
ABNT NBR 7181 (2016) - Soil - Grain size analysis. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
ABNT NBR 6459 (2016) - Soil — Determination of the liquidity limit. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
ABNT NBR 7180 (2016) - Soil — Determination of plasticity limit. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
ABNT NBR 15270-1 (2005) - Ceramic components. Part 1: Ceramic Blocks for Sealing Masonry - Terminology and Requirements. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
ABNT NBR 15270-2 (2017) - Ceramic components ― blocks and bricks for masonry, Part 2: Test methods. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
ABNT NBR 15310 (2005) - Ceramic components — Tiles — Terminology, requirements and test methods. associate Brazil Technical Norms, Rio de Janeiro (In Portuguese).
Vieira CMF, Pinheiro RM (2011) Avaliação de argilas cauliníticas de campos dos goytacazes utilizadas para fabricação de cerâmica vermelha. Ceramica 57:319–323. https://doi.org/10.1590/s0366-69132011000300010
Monteiro SN, Silva FAN, Vieira CMF (2006) Microstructural evaluation of a clay ceramic incorporated with petroleum waste. Appl Clay Sci 33:171–180. https://doi.org/10.1016/j.clay.2006.04.005
Chin CL, Ahmad ZA (2020) Optimization of ceramic tile properties from three malaysian clays via statistical mixture design. Arab J Sci Eng 45:275–290. https://doi.org/10.1007/s13369-019-04150-9
Amaral LF, De Carvalho JPRG, Da Silva BM, Delaqua GCG, Monteiro SN, Vieira CMF (2019) Development of ceramic paver with ornamental rock waste. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2018.05.009
Vigneron TQG, Vieira CMF, Delaqua GCG, Vernilli Júnior F, Cristante NÂ (2019) Incorporation of mold flux waste in red ceramic. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2019.09.038
Eliche-Quesada D, Corpas-Iglesias FA, Pérez-Villarejo L, Iglesias-Godino FJ (2012) Recycling of sawdust, spent earth from oil filtration, compost and marble residues for brick manufacturing. Constr Build Mater 34:275–284. https://doi.org/10.1016/j.conbuildmat.2012.02.079
Kiefer P, Balzer R, Deubener J, Behrens H, Waurischk T, Reinsch S, Müller R (2019) Density, elastic constants and indentation hardness of hydrous soda-lime-silica glasses. J Non Cryst Solids 521:119480. https://doi.org/10.1016/j.jnoncrysol.2019.119480
Zang N, Zhu J, Wang X, Liao Y, Cao G, Li C, Liu Q, Yang Z (2022) Eutrophication risk assessment considering joint effects of water quality and water quantity for a receiving reservoir in the South-to-North water transfer project China. J Clean Prod 331:129966. https://doi.org/10.1016/j.jclepro.2021.129966
Cui J, Jin Z, Wang Y, Gao S, Fu Z, Yang Y, Wang Y (2021) Mechanism of eutrophication process during algal decomposition at the water/sediment interface. J Clean Prod 309:127175. https://doi.org/10.1016/j.jclepro.2021.127175
Marvila MT, Alexandre J, Azevedo ARG, Zanelato EB, Xavier GC, Monteiro SN (2019) Study on the replacement of the hydrated lime by kaolinitic clay in mortars. Adv Appl Ceram. https://doi.org/10.1080/17436753.2019.1595266
Chen Y, Zhang Y, Chen T, Zhao Y, Bao S (2011) Preparation of eco-friendly construction bricks from hematite tailings. Constr Build Mater 25:2107–2111. https://doi.org/10.1016/j.conbuildmat.2010.11.025
Mahmoudi S, Srasra E, Zargouni F (2014) composition and ceramic properties of carbonate-bearing: illitic clays from North-Eastern Tunisia. Arab J Sci Eng 39:5729–5737. https://doi.org/10.1007/s13369-014-1145-0
Menezes RR, Ferreira HS, Neves GA, de Lira HL, Ferreira HC (2005) Use of granite sawing wastes in the production of ceramic bricks and tiles. J Eur Ceram Soc 25:1149–1158. https://doi.org/10.1016/j.jeurceramsoc.2004.04.020
Li L, Wang F, Liao Q, Wang Y, Zhu H, Zhu Y (2020) Synthesis of phosphate based glass-ceramic waste forms by a melt-quenching process: the formation process. J Nucl Mater 528:151854. https://doi.org/10.1016/j.jnucmat.2019.151854
Mehta A, Ashish DK (2020) Silica fume and waste glass in cement concrete production: a review. J Build Eng 29:100888. https://doi.org/10.1016/j.jobe.2019.100888
Chen X, Zheng W, Zhang J, Liu C, Han J, Zhang L, Liu C (2020) Enhanced thermal properties of silica-based ceramic cores prepared by coating alumina/mullite on the surface of fused silica powders. Ceram Int 46:11819–11827. https://doi.org/10.1016/j.ceramint.2020.01.216
Karamanov A, Dzhantov B, Paganelli M, Sighinolfi D (2013) Glass transition temperature and activation energy of sintering by optical dilatometry. Thermochim Acta 553:1–7. https://doi.org/10.1016/j.tca.2012.10.006
Marvila MT, Azevedo ARG, Alexandre J, Zanelato EB, Azeredo NG, Simonassi NT, Monteiro SN (2019) Correlation between the properties of structural clay blocks obtained by destructive tests and ultrasonic pulse tests. J Build Eng. https://doi.org/10.1016/j.jobe.2019.100869
Kaczmarczyk K, Partyka J (2019) Effect of ZrSiO4 addition on sintering and selected physicochemical parameters of glass-ceramic materials from the SiO2-Al2O3-Na2O-K2O-CaO-MgO system in the presence of barium oxide. Ceram Int 45:22813–22820. https://doi.org/10.1016/j.ceramint.2019.07.323
Dondi M, Raimondo M, Zanelli C (2014) Clays and bodies for ceramic tiles: reappraisal and technological classification. Appl Clay Sci 96:91–109. https://doi.org/10.1016/j.clay.2014.01.013
Enríquez E, Fuertes V, Cabrera MJ, Seores J, Muñoz D, Fernández JF (2019) Absence of surface flaking in hierarchical glass-ceramic coating: high impact resistant ceramic tiles. J Eur Ceram Soc 39:4450–4456. https://doi.org/10.1016/j.jeurceramsoc.2019.05.047
Muñoz Velasco P, Morales Ortíz MP, Mendívil Giró MA, Muñoz Velasco L (2014) Fired clay bricks manufactured by adding wastes as sustainable construction material–a review. Constr Build Mater 63:97–107. https://doi.org/10.1016/j.conbuildmat.2014.03.045
Girondi GD, Marvila MM, de Azevedo ARG, de Souza CC, Souza D, de Brito J, Vieira CMF (2020) Recycling potential of powdered cigarette waste in the development of ceramic materials. J Mater Cycle Waste Manag. https://doi.org/10.1007/s10163-020-01058-7
Vieira CMF, Pinheiro RM, Rodriguez RJS, Candido VS, Monteiro SN (2016) Clay bricks added with effluent sludge from paper industry: technical, economical and environmental benefits. Appl Clay Sci 132–133:753–759. https://doi.org/10.1016/j.clay.2016.07.001
Funding
CNPq, 301634/2018.1, Carlos Maurício F. Vieira, FAPERJ, E-26/202.773/2017, Carlos Maurício F. Vieira.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Faria Busch, P., Marvila, M.T., Girondi Delaqua, G.C. et al. Recycling of waste glass extracted from a WTP into ceramic materials. J Mater Cycles Waste Manag 24, 763–774 (2022). https://doi.org/10.1007/s10163-022-01358-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01358-0