Abstract
Municipal solid waste (MSW) is converted to various materials through treatment processes, which in turn distributes potentially toxic elements (PTEs) to recyclable materials. This study is focused on establishing an inventory of combustible wastes with the objective of identifying specific sources of PTEs (B, Cr, Cu, Zn, As, Sb, Ba, and Pb). The combustible wastes were classified into 26 components by the criterion, which can be conveniently identified by the public. Each component of the combustible wastes was ignited at 450 °C, to reduce organic matters and increase the proportional content of the target PTE, before undergoing inductively coupled plasma analysis. The inventory of PTE contents in the waste components was developed from the ash and the ignition loss of each component. The contribution of waste components to the total amount of PTEs was estimated based on the element content and the proportion in waste. Through this series of processes, specific sources of waste components containing PTEs were presented. This work can contribute to the reduction of toxicity of MSW and incineration residues that are to be recycled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal solid waste (MSW) can be converted into various materials such as compost, solid refuse fuel, and incineration residues (bottom ash and fly ash) during various treatment processes [1,2,3,4], which in turn distribute potentially toxic elements (PTEs) to recyclable materials. Incineration is commonly used for the treatment of MSW, as it has the advantages of not only decreasing the total volume of solid wastes disposed in landfills, but also recovering heat energy. In addition, incineration residues can be utilized for construction materials [5,6,7] and metal resources [8, 9]. The potential toxicity of incineration residues can be reduced by identifying and screening specific wastes as sources of PTEs and removing them before incineration. Therefore, source management is becoming important concerning collection and separation of MSW.
Investigation of element contents in MSW components is necessary to identify specific sources of PTEs. The amount and proportion of MSW category (e.g., food, paper, and textile) have been investigated in many countries and municipalities [10, 11]. The distribution of elements in an incineration process is affected by operation parameters [12]; however, the total amount of elements which behave in the process depends on the input MSW. Combustible waste, which undergoes significant thermochemical reactions, could particularly affect the composition of incineration residues [13, 14]. Hence, an inventory of PTE contents in specific combustible waste components could support to estimate their content in incineration residues by incorporating with a national waste statistics survey for quantity of waste category (hereafter referred to as the waste survey). In future, this survey will contribute to the strategic reduction of toxicity, when materials are recycled for use in the construction industry.
Two approaches have been applied to estimate the PTE composition of input MSW: direct analysis of input MSW and estimation from incineration residues analysis. The estimation from analysis of incineration residues provides the PTE content as the representative value of the whole input waste [15,16,17,18]. However, it is difficult to know the waste source of PTEs, as the element content of incineration residues changes depending on the type of waste.
Therefore, an inventory of waste components for various elements needs to be developed from the direct analysis of different types of wastes. Previous studies have provided information of either various PTEs in a partial waste category or a few types of PTEs from various waste categories, according to the three methods. The first method reported the various PTE contents without classification to estimate the total amount of target elements in MSW [19, 20]. In the second approach, some studies investigated PTE contents in detailed components of one specific category such as plastic [21], food [22], bulky waste [12], or biodegradable MSW [23]. The content of various PTEs was identified, but the entire category of combustible waste was not covered. In the third approach, the entire waste category was investigated; however, only few types of PTEs were targeted. Long et al. reported on the Cu and Zn content in 15 categories of MSWs collected from eight cities in China [24]. However, specific sources of the target PTEs were difficult to identify, as each category comprised various components. Nakamura et al. reported the Cd, Sb, and Pb content in eleven MSW categories; in addition, the Pb and Cd content in 28 kinds of plastics which categorized according to its color were investigated [25]. Another study examined the Cd, Cr, Hg, and Pb content from 60 components in eight categories (as cited in Hasselriis and Licata [26]). However, further investigation is required to estimate the source of various elements introduced to an incineration process.
The analytical method including sample preparation is correspondingly important. Previous studies either did not describe this or only briefly described how the waste was ground or cut [12, 24]. Sufficient amount of the samples should be available for the analysis, as the PTE content varies depending on the fraction used for the analysis. The present study applied the ignition process to reduce organic matter and increase the proportional content of the target PTE in the waste sample, thereby identifying representative PTE content of sufficient amounts of waste in the analytical sample.
The preceding inventory study reported the content of 52 PTEs from six categories and the mass balance result between input MSW and output incineration residues [27]. However, the specific source of PTEs cannot be identified based on the broad-based categories. This study is focused on the detailed analysis of 26 components in seven combustible waste categories: food, paper, textile, wood, plastic, rubber, and particles of 5 mm or less. The ignition of each component is conducted at 450 °C with the objective of preserving the target PTEs from the original combustible components. The inventory of the contents of eight PTEs (B, Cr, Cu, Zn, As, Sb, Ba, and Pb) is developed to identify specific sources of the target elements in MSW.
Materials and methods
Sampling and waste classification
The sampling of MSW was conducted at an incineration facility in Japan following the method for the waste survey [28]. MSW, including household wastes and shredded bulky wastes, had been stored in a pit of incineration facility. More than 200 kg of MSW sample was collected from various parts of the pit using a crane, and approximately 7 kg of representative sample was prepared after applying the conical quarter method. This sample was kept in an air-tight container for transportation to the laboratory.
The MSW sample was air-dried at 40 °C for 72 h using a drying oven. Large sizes of incombustibles such as aluminum foil, insect screen, and glass were excluded from the sample. Subsequently, the components sized 5 mm or larger were separated from combustibles and categorized into food, paper, textiles, wood, plastics, and rubber. The remaining components, < 5 mm and lumped substances which could not be identified, were classified as the small particles (< 5 mm).
The combustible waste was classified into 26 components (Table 1), and the detailed weight of the sample is shown in Table S1. The classification was attributed to the understanding of the separation of collected waste by the public, as the classification performed according to shape, color, and usage, rather than aspects of the material and manufacturing process. The food category included organic kitchen materials. Paper was classified into eight components according to the purpose of usage. Textiles were sorted according to material color as white or other colors. The wood category included natural branches and leaves, as well as artificially crafted wooden items such as toothpicks, ice cream sticks, and boards. Plastics were classified into film and hard types, with each type sorted into transparent and colored plastics. Afterwards, the plastic components were classified according to the purpose of usage. Sponges and disposable diapers were included in the plastic category because of their synthetic resin content, and rubber bands found in the sample were classified into the rubber category.
Ignition of combustible waste
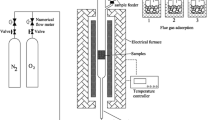
After sorting, each waste component was placed in a ceramic dish, covered with a lid, dried at 105 °C for 4 h using a drying oven, and then cut into pieces < 10 mm. The ignition experiment removed organic material from the waste, improved the detection accuracy of the target PTEs, and ensured that sufficient amounts of the target component were remained in the analytical sample. Ignition methods have been widely applied to estimate the organic content in solid materials [29,30,31]; however, the temperature for the ignition can vary depending on materials. For the ignition of MSW, temperatures between 450 and 500 °C have been recommended to remove organic content, e.g., changes in the structure of paper and pulp were observed after ignition at 500 °C [32], and the organic content of MSW was determined by igniting at 450 °C [33]. In this study, the ignition temperature was set at a lower temperature, so that it would not affect mineral phases. Hence, each dried component of combustible waste was ignited at 450 °C; the ignition was repeated every 1 h until white color ash (hereinafter referred as the ash) remained. The weight of dried waste components and the ash is measured, and ignition loss is calculated by Eq. (1),
where m1 is the weight of dried waste component of sample (g); m2 is the weight of ash (g).
Element content and contribution of combustible waste
The target elements (B, Cr, Cu, Zn, As, Sb, Ba, and Pb) were selected among elements that have been raised environmental concerns in incineration residues [34, 35]. Based on the result of the literature survey as introduced earlier, more detailed sources of Cr, Cu, Zn, Sb, and Pb need to be identified since they could be considerably introduced from combustible waste. Moreover, the detailed components of waste that contain B, As, and Ba have not been identified, thus this requires further examination. Approximately 1 g ash of each component was used for digestion with aqua regia and alkali fusion with lithium metaborate [36, 37]. For components with < 1 g of ignited ash, all the ash were used for the process. The content of the target elements was determined by inductively coupled plasma-optical emission spectrometer (ICP-OES, Agilent 700, Agilent Technologies) and mass spectrometer (ICP-MS, Agilent 7500, Agilent Technologies). The element content of each waste component is calculated from the element content of the ash and the ignition loss based on Eq. (2),
where \(C_{{i,{\text{waste}}}}^{x}\) and \(C_{{i,{\text{ash}}}}^{x}\) are the content of element x in the component i of waste (mg/kg-waste) and ash (mg/kg-ash), respectively. The element content of combustible waste was calculated based on dry weight basis.
The contribution of waste component to the target element was calculated by multiplying the element content of each component and its proportion in the total waste based on Eq. (3).
where Ti is the contribution of waste component (%) and Wi is the proportion of each waste component in the total combustibles.
Results and discussion
Proportion of waste components and ash
The proportion of the ash was changed after the ignition process compared to that of the combustible waste (Table 1). The ignition loss of each component varied from 0.63 to 0.99 depending on the type. Each waste component was converted to the ash except for the colored film-type plastic packaging and the juice cartons. Both metal film and ash were remained after the ignition process; the metal film was identified as aluminum according to x-ray fluorescence analysis. The components of this type of packaging consist of plastic, paper, and aluminum [38] that allows contents to be stored without putrefaction [39].
The plastic (30.1%), textile (26.7%), and paper (25.3%) comprised of the top three categories, and the total proportion of these accounted for 82.1% of the combustible wastes. After the ignition process, the proportion of the ash from paper (38.5%) was the highest due to relatively low ignition loss (0.63–0.94). The ash from plastic was the second highest proportion (24.9%), with an ignition loss in the range of 0.88 to 0.99, followed by the ash from wood (18.6%), textiles (9.0%), small particles (4.0%), and rubber (0.006%). The ignition loss of the small particles was low (0.65), indicating that these may contain incombustibles such as stone, glass, and metal particles.
Inventory of the target PTEs in 26 components of combustible wastes
The PTE content of the ash obtained from the analytical sample and that of the waste component estimated by the ignition loss is shown in Fig. 1. The PTE content is described in integer scale, except for Cu and Zn, where a logarithmic scale was applied due to some specific components exhibiting significantly high element contents. The inventory of the target elements in the 26 waste components and the ash is provided in Table S2 and S3 of the Supplementary Materials.
Boron (B)
Wood and rubber were the major sources of B in the combustible wastes; the highest B content was observed in the wood category. The natural wood (branches and leaves) and wooden items showed 31 mg-B/kg-waste and 16 mg-B/kg-waste, and that of the ash recorded 163 mg-B/kg-ash and 663 mg-B/kg-ash, respectively. Boron is an essential trace element for plants [40], and several studies have investigated B levels in fertilizer and its related toxicity [41, 42]. Food and paper, which are related to biomass like wood, showed relatively high levels of B. Food waste recorded 6.4 mg-B/kg-waste, within the range (not detected–7.0 mg-B/kg) of previous results (as cited in Naghii et al. [22]).
There are diverse industrial uses of B, and it has been used as an additive to improve lubricating properties of rubber products (as cited in Fiume et al. [43]) as well as to improve fire retardant properties of plastics and rubber products [44]. Rubber bands recorded the second highest B content (26 mg-B/kg-waste and 430 mg-B/kg-ash). Moreover, some of the ash from hard type plastics showed relatively high levels of B such as hard type-transparent (HT) food container (250 mg-B/kg-ash), hard type-colored (HC) noodles container (123 mg-B/kg-ash), and HC other plastics (122 mg-B/kg-ash).
Chromium (Cr)
All waste components, excluding the small particles, contained several parts per million (ppm) of Cr. Paper (0.9–8.5 mg-Cr/kg-waste) and wood (0.6–7.3 mg-Cr/kg-waste) showed relatively high Cr content in the combustible waste, followed by plastics (0.2–4.0 mg-Cr/kg-waste), textiles (1.0–2.1 mg-Cr/kg-waste), and food (0.3 mg-Cr/kg-waste). These values may have originated from natural sources, Cr is present at approximately 100 ppm in the Earth’s crust. In addition, Cr is used in various industrial processes such as a textile dye and mordant as well as production of paint, paper, and pulp (as cited in Shahid et al. [45]).
Bode et al. reported the Cr content from several categories of household waste [21]. The Cr content of paper (3.2 mg-Cr/kg), plastics (4.0 mg-Cr/kg), and textiles (5.8 mg-Cr/kg) was comparable with this study, wherein the content was several ppm levels. In contrast, the glass (37 mg-Cr/kg), metals (0.4–23 mg-Cr/kg), organic waste (0.1–38 mg-Cr/kg), and rubber and leather categories (34 mg-Cr/kg) showed relatively high content compared to the categories described above. In this study, small particles showed the highest content of Cr (95 mg-Cr/kg-waste and 272 mg-Cr/kg-ash). In the preceding study, the highest Cr content (47 mg-Cr/kg) was also identified in the < 5 mm component of the waste category which applied the same classification [27]. Based on this comparison, small particles may contain glass and metal particles, supported by the result of low-ignition loss (0.65) as mentioned above.
Copper (Cu)
Colored textiles (156 mg-Cu/kg-waste) and the leaflets of paper (109 mg-Cu/kg-waste) were observed to have remarkably high Cu content. After the ignition, colored textiles also showed the highest content (5370 mg-Cu/kg-ash) among the ash from the combustible wastes. This result was similar to Long et al.’s results, which reported that textile waste contained approximately 100 mg-Cu/kg [24]. Furthermore, Sakanakura et al. observed high Cu content (550 mg-Cu/kg) in paper and cloth [27]. The textile industry is well-known in utilizing several chemicals for fabric dyeing or functional treatments such as odor and wrinkle resistance [46]. Metals are necessary for dyes to impart color [47], in addition, Cu, Sb, and Cr in dyestuffs are used as catalysts in dye intermediates’ synthesis [48]. Based on these results, dyed textile and printed paper can be expected as major sources of Cu in combustible waste.
Zinc (Zn)
Rubber, colored textiles, and HC plastic food containers could have a significant effect on the Zn content of incineration residues. Rubber recorded the highest Zn content (7370 mg-Zn/kg-waste) with the content in the ash approximately 12%. This result corresponded to the aforementioned study that 8360 mg-Zn/kg was detected in the components from the rubber and leather category [27]. These results are reasonably accepted because rubber industry uses significant amounts of zinc oxide as an additive for functional purposes (e.g., vulcanizing activator and improving thermal conductivity) [49]. Small particles recorded the second highest Zn content (710 mg-Zn/kg-waste), followed by the wood and textiles.
For the ash content, colored textiles (4800 mg-Zn/kg-ash) recorded the second highest content, followed by HC plastic food container (2280 mg-Zn/kg-ash). HC food containers included plastic cups and bowls commonly sold in supermarkets and convenience store; use of these single-use plastics should be reduced to control Zn levels in incineration residues. In addition, high Zn content was detected in the ash from various plastic components such as HC other plastics (1210 mg-Zn/kg-ash), HC noodles containers (1160 mg-Zn/kg-ash), and film type-transparent (FT) packaging and bag (1060 mg-Zn/kg-ash).
Arsenic (As)
Arsenic content of less than one ppm was detected from the target waste, other than from the small particles (2.9 mg-As/kg-waste) and branches and leaves (1.3 mg-As/kg-waste). Arsenic content in the branches and leaves was within the ranges of that in natural biomass such as straw and wood (0.15–3.5 mg-As/kg) which was reported in previous studies [50, 51]. A variety of chemical forms of As is presented in water and sediment in nature [52], and plants uptake various metal(loid)s including As, regardless of whether it is essential for plant growth or not [53, 54]. Hence, certain metals can be detected in natural wood and wooden items. For the ash, the highest As content was recorded for wooden items and small particles (8.3 mg-As/kg-ash), followed by the white textiles (7.4 mg-As/kg-ash). The As content recorded in the present study corresponds to the preceding study which found 0.8–1.3 mg-As/kg from components > 5 mm of combustibles and 16.4 mg-As/kg from small particles (< 5 mm) [27]. In another study, relatively high levels of As (1.2–3.7 mg-As/kg) were observed in glass and ferrous metals [21]. Consequently, the small particles may contain glass and metal particles.
Antimony (Sb)
Textiles and HT plastic food containers were clearly indicated as sources of Sb in combustible wastes. Textiles presented the highest Sb content (60–95 mg-Sb/kg-waste). Other components recorded < 10 mg-Sb/kg-waste, except some of plastics such as HT food containers (13 mg-Sb/kg-waste), film type-colored (FC) bags and labels (12 mg-Sb/kg-waste), and HC food containers (11 mg-Sb/kg-waste). For the ash, HT food container (e.g., plastic bottles and packaging materials), recorded 4590 mg-Sb/kg-ash, it was followed by the textiles which presented 2070–3040 mg-Sb/kg-ash. Previous studies reported similar Sb content in the plastics: 12.8 mg-Sb/kg [27] and 12.9 mg-Sb/kg [25]. Moreover, 25.9 mg-Sb/kg was recorded for textiles, and specific components of textiles showed remarkable Sb content: 156 mg-Sb/kg in bedding clothes and 2100 mg-Sb/kg in curtains [25]. Antimony is widely used as a catalyst to make polyethylene terephthalate [55] as well as synergist of flame retardants in textiles [56]. These results clearly indicated that textiles and HT food containers need to be better managed to control Sb levels in MSW incineration residues.
Barium (Ba)
FC plastics and white textiles were sources of Ba in combustible wastes with 99–179 and 27–100 mg-Ba/kg-waste, respectively. Additionally, wood (16–61 mg-Ba/kg-waste) and paper (11–49 mg-Ba/ka-waste) also contained several tens of ppm of Ba content. In contrast, hard type plastics, sponges, disposable diapers, and rubber showed less than 5 mg-Ba/kg-waste. In terms of Ba content in the ash, FC bags and labels (3310 mg-Ba/kg-ash) and white textiles (3190 mg-Ba/kg-ash) presented significant result, followed by FC plastic packaging (1370 mg-Ba/kg-ash) and colored textiles (920 mg-Ba/kg-ash). Barium can be detected from various components, because it is present as approximately 400 ppm in Earth’s crust and is relatively abundant in the environment [57]. In addition, barium sulfate is widely utilized in various industries for its chemical inertness, acid and alkali resistance, and high-whitening properties [59].
Lead (Pb)
Recycled paper and colored plastic bags and labels were significant regarding Pb content. Recycled paper showed relatively high Pb content, particularly toilet paper (16 mg-Pb/kg-waste) and newspaper (9.9 mg-Pb/kg-waste). Although paper recycling has been promoted, concerns related to the potential harmful chemical substances have been raised [59]. A lot of toilet paper is manufactured from recycled pulp [60]. Paper quality may decrease during the recycling loop, even though the Pb content in newspaper was reduced by the replacement of pigments from inks [61].
FC plastic bags and labels and FC plastic packaging are similar in that these are the film type-colored plastic; however, different Pb contents were detected. The FC bags and labels, which included single-use color plastic bags and labels from plastic bottles, recorded 16 mg-Pb/kg-waste of Pb content. This result supported Nakamura et al.’s findings that Pb content of white and yellow plastic bags were 16 mg-Pb/kg and 54 mg-Pb/kg, respectively [25]. In contrast, FC packaging, which included packaging materials for consumer products such as biscuits and tea bags, recorded 0.8 mg-Pb/kg-waste. Pb compounds have been used for coloring agents and stabilizers for plastics [25, 62]. However, PTE content in the FC plastic packaging is better managed due to regulations of hazardous additives for packaging materials [63].
Pb content in the ash was the highest for the FC bags and labels (292 mg-Pb/kg-ash), followed by recycled toilet paper (276 mg-Pb/kg-ash) and the wooden items (157 mg-Pb/kg-ash). These components could influence the Pb content of MSW incineration residues depending on their proportion.
Contribution of seven waste categories for target PTEs
Attention is particularly required where the contribution to the amount of specific PTE was higher than the proportion of the ash by weight. The proportion of the ash indicates the more accessible mass of sample to evaluate the contribution to the target elements because organic matter was removed. The element content in the ash is also a more accessible value to estimate potential toxicity in incineration residues. The contribution of seven categories to the total amount of target PTEs is shown in Fig. 2 based on the proportion of waste and ash (Table 1) and its representative PTE content (Table 2). The representative PTE content of each category can be utilized to estimate element flow by applying the result of waste proportion from the waste survey. Additionally, Table S4 reports the contribution of 26 waste components to the total amount of target PTEs.
The food contribution to the amount of B was 6.1%, slightly higher than the proportion of the ash (5.0%). However, contribution of food to the total amount of PTEs, except for the B, was lower than its proportion of the ash.
The paper, which accounted to the highest proportion (38.5%) in the ash, recorded the highest contribution to Pb (36.2%) and Cr (32.1%) and the second highest contribution to As (31.2%) under the proportion of combustible waste. Recycled paper (toilet paper and newspaper) was the major sources of Pb contributing 27.0% to the total amount in combustible wastes (Table S4). Recycling loop may influence the presence of PTEs in recycled paper as discussed earlier. Moreover, the toilet paper found in this study appeared to have wiped off various contaminants in addition to their use in toilets. This kind of usage of toilet paper as a wiping material could lead it to contain PTEs.
Textiles recorded the highest contribution to Sb (89.17%), Cu (70.2%), and Zn (45.5%) among the seven categories. In addition, the contribution of Ba (30.1%) was approximately 3.3 times higher than the proportion of textiles (9.0%), meaning that the separation of textiles from MSW could reduce the content of Sb, Cu, Zn, and Ba in incineration residues.
For wood, the contribution to B (55.4%) and As (33.9%) was the highest among the seven categories; Pb (22.9%), Zn (19.0%), and Cr (17.2%) levels were highly influenced by wood. Wood components could absorb various elements form nature, therefore a certain level of PTEs in incineration residues would be unavoidable. Moreover, the increase in the amount of branches and leaves by seasonal events (e.g., weeding and fallen leaf) could result in a high content of these elements in incineration residues. Additionally, wood treated with preservatives could increase the toxicity of incineration residues. Wood treated by chromated copper arsenate (CCA) had been used for increasing durability against insects and water [64]. Those treatments were regulated due to possible releases of toxic elements, but CCA-treated wood still remained in various products [65,66,67]. Wood treated with preservatives was not found in this study when considering that they were reported to contain several thousand ppm levels of Cr, Cu, and As [68, 69].
Plastic recorded the highest contribution to the amount of Ba (32.5%), and the contribution to that of Pb (22.3%) was higher than the proportion of the ash derived from the plastic. More specifically, FC plastic bags and labels accounted to 23.4% of Ba and 19.6% of Pb of the total amount, while recording considerable contents in the ash: 3310 mg-Ba/kg-ash and 292 mg-Pb/kg-ash. Film type plastics are used as raw materials of solid refuse fuel [70], and plastics were reported to contribute approximately half of Pb content in solid refuse fuel [71]. These results indicated that FC plastic bags and labels could be the sources of Pb in solid refuse fuel. Since PTEs firmly bound in carbon-based plastics could be actively released after thermal degradation [55], FC plastic bags and labels could result in significantly high levels of PTEs in incineration residues.
The contribution of rubber to the amount of Zn was approximately 1.0%, a significantly high value compared to the proportion of the ash (0.006%). This indicated that the contribution to Zn could be drastically increased under the presence of high proportion of rubber in waste.
The small particles recorded the higher contributions than the proportion of the ash other than B and Sb. Incombustibles, such as glass and metal particles, would be included in the small particles as stated earlier, and this should be separated from the waste collection stage. Additionally, metal particles can be separated from incineration residues, particularly bottom ash [72, 73]. The further separation of metal particles can lead to the reduction of potential toxicity in incineration residues.
Conclusions
This study investigated 26 components of combustible wastes with objective of identifying the sources of eight PTEs (B, Cr, Cu, Zn, As, Sb, Ba, and Pb). The ignition process was applied for reducing organic matter in the waste components, and the content of PTEs and the proportion in the ash indicated more accessible value for estimating the contribution of waste components to the total amount of PTEs in incineration residues. Specific sources of PTEs were identified by examining their contents in waste components and the ash as well as the contribution to the total amount of elements in the collected combustible wastes. Recycled paper (toilet paper and newspaper) and FC plastic bags and labels were the major sources of Pb. Plastics were identified as sources of various PTEs, i.e., FC plastic (3310–1370 mg-Ba/kg-ash and 68–276 mg-Pb/kg-ash), HC plastic food containers (2280 mg-Zn/kg-ash), and HT plastic food containers (4590 mg-Sb/kg-ash). Colored textiles and leaflets contained high levels of Cu, from dyeing and ink in industrial processes. Branches and leaves were the major sources of B and As. Rubber was a notable source of Zn. The specific sources of waste containing PTEs need to be further separated in the stage of source management, thereby the toxicity of incineration residues can be reduced to recycle as construction materials. The scope of the inventory should be extended to incorporate more components and elements, and further incineration experiments are needed to estimate PTE composition in individual incineration residues (e.g., bottom ash and fly ash) depending on the proportion of MSW.
References
Sakanakura H, Yui K, Kuramochi H, et al (2016) Concentration and distribution of 56 elements in residues from stoker-type municipal solid waste incineration. 9th International Conference on Combustion, Incineration/Pyrolysis, Emission and Climate Change (9th i-CIPEC), Kyoto, Japan
Wei Y, Li J, Shi D et al (2017) Environmental challenges impeding the composting of biodegradable municipal solid waste: a critical review. Resour Conserv Recycl 122:51–65. https://doi.org/10.1016/j.resconrec.2017.01.024
Iacovidou E, Hahladakis J, Deans I et al (2018) Technical properties of biomass and solid recovered fuel (SRF) co-fired with coal: impact on multi-dimensional resource recovery value. Waste Manag 73:535–545. https://doi.org/10.1016/j.wasman.2017.07.001
Yi S, Jang Y-C (2018) Life cycle assessment of solid refuse fuel production from MSW in Korea. J Mater Cycles Waste Manag 20:19–42
Keulen A, van Zomeren A, Harpe P et al (2016) High performance of treated and washed MSWI bottom ash granulates as natural aggregate replacement within earth-moist concrete. Waste Manag 49:83–95
Joseph MA, Snellings R, Van den Heede P et al (2018) The use of municipal solid waste incineration Ash in various building materials: a Belgian point of view. Mater. 11:141
Sakita S, Nishimura K (2016) Physical containment of municipal solid waste incineration bottom ash by accelerated carbonation. J Mater Cycles Waste Manag 18:687–694. https://doi.org/10.1007/s10163-015-0369-8
Holm O, Wollik E, Johanna Bley T (2018) Recovery of copper from small grain size fractions of municipal solid waste incineration bottom ash by means of density separation. Int J Sustain Eng 11:250–260
Syc M, Simon F-G, Biganzoli L et al (2018) Resource recovery from incineration bottom ash: Basics, concepts, principles. In: Holm O, Thome-Kozmiensky E (eds) Removal, treatment and utilisation of waste incineration bottom ash. Thomé-Kozmiensky Verlag GmbH, Neuruppin, pp 1–10
Yang N, Damgaard A, Scheutz C et al (2018) A comparison of chemical MSW compositional data between China and Denmark. J Environ Sci 74:1–10
Areeprasert C, Kaharn J, Inseemeesak B et al (2018) A comparative study on characteristic of locally source-separated and mixed MSW in Bangkok with possibility of material recycling. J Mater Cycles Waste Manag 20:302–313. https://doi.org/10.1007/s10163-017-0583-7
Jung CH, Matsuto T, Tanaka N, Okada T (2004) Metal distribution in incineration residues of municipal solid waste (MSW) in Japan. Waste Manag 24:381–391. https://doi.org/10.1016/S0956-053X(03)00137-5
Helsen L, Bosmans A (2010) Waste-to-Energy through thermochemical processes: matching waste with process. In: Proceedings of the 1st International Academic Symposium on Enhanced Landfill Mining. Haletra; Houthalen-Helchteren, pp 133–180
Ménard Y, Asthana A, Patisson F et al (2006) Thermodynamic study of heavy metals behaviour during municipal waste incineration. Process Saf Environ Prot 84:290–296
Riber C, Fredriksen GS, Christensen TH (2005) Heavy metal content of combustible municipal solid waste in Denmark. Waste Manag Res 23:126–132. https://doi.org/10.1177/0734242X05051195
Morf LS, Gloor R, Haag O et al (2013) Precious metals and rare earth elements in municipal solid waste—sources and fate in a Swiss incineration plant. Waste Manag 33:634–644. https://doi.org/10.1016/j.wasman.2012.09.010
Belevi H, Langmeier M (2000) Factors determining the element behavior in municipal solid waste incinerators. 2. Laboratory experiments. Environ Sci Technol 34:2507–2512
Astrup T, Riber C, Pedersen AJ (2011) Incinerator performance: effects of changes in waste input and furnace operation on air emissions and residues. Waste Manag Res 29:S57–S68. https://doi.org/10.1177/0734242X11419893
Law SL, Gordon GE (1979) Sources of metals in municipal incinerator emissions. Environ Sci Technol 13:432–438
Zhang H, He P-J, Shao L-M (2008) Flow analysis of heavy metals in MSW incinerators for investigating contamination of hazardous components. Environ Sci Technol 42:6211–6217. https://doi.org/10.1021/es800548w
Bode P, De Bruin M, Aalbers TG, Meyer PJ (1990) Plastics from household waste as a source of heavy metal pollution. In: Zeisler R, Guinn VP (eds) Nuclear analytical methods in the life sciences. Humana Press, Totowa, pp 377–383
Naghii MR, Wall PM, Samman S (1996) The boron content of selected foods and the estimation of its daily intake among free-living subjects. J Am Coll Nutr 15:614–619. https://doi.org/10.1080/07315724.1996.10718638
Garcı́a AJ, Esteban MB, Márquez MC, Ramos P (2005) Biodegradable municipal solid waste: characterization and potential use as animal feedstuffs. Waste Manag 25:780–787. https://doi.org/10.1016/j.wasman.2005.01.006
Long Y-Y, Shen D-S, Wang H-T et al (2011) Heavy metal source analysis in municipal solid waste (MSW): case study on Cu and Zn. J Hazard Mater 186:1082–1087. https://doi.org/10.1016/j.jhazmat.2010.11.106
Nakamura K, Kinoshita S, Takatsuki H (1996) The origin and behavior of lead, cadmium and antimony in MSW incinerator. Waste Manag 16:509–517
Hasselriis F, Licata A (1996) Analysis of heavy metal emission data from municipal waste combustion. J Hazard Mater 47:77–102. https://doi.org/10.1016/0304-3894(95)00107-7
Sakanakura H, Back S, Naruoka T (2019) Contribution of Each Combustible Waste to the Element Content of MSW Incineration Residue. IOP Conf Ser Earth Environ Sci 265:12003. https://doi.org/10.1088/1755-1315/265/1/012003
MOEJ (2018) Instruction on guidance for general waste disposal (in Japanese)
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Matthiessen MK, Larney FJ, Brent Selinger L, Olson AF (2005) Influence of loss-on-ignition temperature and heating time on ash content of compost and manure. Commun Soil Sci Plant Anal 36:2561–2573
Donkin MJ (1991) Loss-on-ignition as an estimator of soil organic carbon in a-horizon forestry soils. Commun Soil Sci Plant Anal 22:233–241. https://doi.org/10.1080/00103629109368411
Sun CL, Koziński JA (2000) Ignition behaviour of pulp and paper combustible wastes. Fuel 79:1587–1593
Yesiller N, Hanson JL, Cox JT, Noce DE (2014) Determination of specific gravity of municipal solid waste. Waste Manag 34:848–858
Huber F, Blasenbauer D, Aschenbrenner P, Fellner J (2019) Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Manag 95:593–603
Quina MJ, Bordado JC, Quinta-Ferreira RM (2008) Treatment and use of air pollution control residues from MSW incineration: an overview. Waste Manag 28:2097–2121
Oguchi M, Sakanakura H, Terazono A, Takigami H (2012) Fate of metals contained in waste electrical and electronic equipment in a municipal waste treatment process. Waste Manag 32:96–103. https://doi.org/10.1016/J.WASMAN.2011.09.012
Kida A, Miyazaki T, Kuramochi H (2011) Analytical methods for elements including precious metals and rare metals in electric and electronic products. 22:19–27. https://doi.org/10.3985/mcwmr.22.19
Korkmaz A, Yanik J, Brebu M, Vasile C (2009) Pyrolysis of the tetra pak. Waste Manag 29:2836–2841. https://doi.org/10.1016/j.wasman.2009.07.008
Czajczyńska D, Anguilano L, Ghazal H et al (2017) Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog 3:171–197
Blevins DG, Lukaszewski KM (1994) Proposed physiologic functions of boron in plants pertinent to animal and human metabolism. Environ Health Perspect 102:31–33
Martens DC, Westermann DT (1991) Fertilizer application for correcting micronutrient deficiencies. Micronutr Agric. 4:549–592
Abat M, Degryse F, Baird R, McLaughlin MJ (2015) Boron phosphates (BPO4) as a seedling-safe boron fertilizer source. Plant Soil 391:153–160. https://doi.org/10.1007/s11104-015-2424-6
Fiume MM, Bergfeld WF, Belsito DV et al (2015) Safety assessment of Boron Nitride as used in cosmetics. Int J Toxicol 34:53S–60S. https://doi.org/10.1177/1091581815617793
Wilkie CA, Morgan AB (2009) Fire Retardancy of Polymeric Materials, 2nd edn. CRC Press, Boca Raton
Shahid M, Shamshad S, Rafiq M et al (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Allam OG (2011) Improving functional characteristics of wool and some synthetic fibres. Open J Org Polym Mater 3:8–19
Madhav S, Ahamad A, Singh P, Mishra PK (2018) A review of textile industry: wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manag 27:31–41
Sungur Ş, Gülmez F (2015) Determination of metal contents of various fibers used in textile industry by MP-AES. J Spectrosc 2015:1–5. https://doi.org/10.1155/2015/640271
Moezzi A, McDonagh AM, Cortie MB (2012) Zinc oxide particles: synthesis, properties and applications. Chem Eng J 185:1–22
Nzihou A, Stanmore B (2013) The fate of heavy metals during combustion and gasification of contaminated biomass—a brief review. J Hazard Mater 256:56–66
Wang T, Yang Q, Wang Y et al (2020) Arsenic release and transformation in co-combustion of biomass and coal: effect of mineral elements and volatile matter in biomass. Bioresour Technol 297:122388. https://doi.org/10.1016/j.biortech.2019.122388
Hasegawa H, Rahman MA, Matsuda T et al (2009) Effect of eutrophication on the distribution of arsenic species in eutrophic and mesotrophic lakes. Sci Total Environ 407:1418–1425
Tangahu BV, Abdullah S, Rozaimah S, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011:1–31. https://doi.org/10.1155/2011/939161
Abbas G, Murtaza B, Bibi I et al (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Van Oyen A, Kühn S, van Franeker JA, Ortlieb M, Egelkraut-Holtus M (2016) Plastic and restricted heavy metals. In: MICRO Conference fate and impact of microplastics in marine ecosystems, Lanzarote, Spain
Rovira J, Nadal M, Schuhmacher M, Domingo JL (2015) Human exposure to trace elements through the skin by direct contact with clothing: risk assessment. Environ Res 140:308–316
Oskarsson A, Reeves AL (2007) CHAPTER 20 - Barium. In: Third E (ed) Nordberg GF, Fowler BA, Nordberg M, Friberg LTBT-H on the T of M. Academic Press, Burlington, pp 407–414
Kresse R, Baudis U, Jäger P, Riechers HH, Wagner H, Winkler J, Wolf HU (2000) Barium and barium compounds. Wiley, VCH Verlag GmbH & Co. KGaA, Weinheim
Pivnenko K, Eriksson E, Astrup TF (2015) Waste paper for recycling: overview and identification of potentially critical substances. Waste Manag 45:134–142
Masternak-Janus A, Rybaczewska-Błażejowska M (2015) Life cycle analysis of tissue paper manufacturing from virgin pulp or recycled waste paper. Manag Prod Eng Rev 6:47–54
Tucker P, Douglas P, Durrant A, Hursthouse AS (2000) Heavy metal content of newspapers: longitudinal trends. Environ Manag Heal 11:47–66
Weisel C, Demak M, Marcus S, Goldstein BD (1991) Soft plastic bread packaging: lead content and reuse by families. Am J Public Health 81:756–758
European Union (1994) European Parliament and Council Directive 94/62/EC of 20 December 1994 on packaging and packaging waste
Helsen L, Van den Bulck E (2005) Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste, with detailed analyses of thermochemical conversion processes. Environ Pollut 134:301–314. https://doi.org/10.1016/j.envpol.2004.07.025
Augustsson A, Sörme L, Karlsson A, Amneklev J (2017) Persistent Hazardous Waste and the Quest Toward a Circular Economy: the Example of Arsenic in Chromated Copper Arsenate-Treated Wood. J Ind Ecol 21:689–699
Hata T, Bronsveld P, Kakitani T, Meier D, Imamura Y (2004) Environmental impact of CCA-treated wood in Japan. In: Environmental impacts of preservative-treated wood, Orlando, USA
Kartal SN, Imamura Y (2005) Removal of copper, chromium, and arsenic from CCA-treated wood onto chitin and chitosan. Bioresour Technol 96:389–392. https://doi.org/10.1016/j.biortech.2004.03.004
Clausen C (2004) Improving the two-step remediation process for CCA-treated wood: Part I. Evaluating oxalic acid extraction. Waste Manag 24:401–405. https://doi.org/10.1016/j.wasman.2003.11.008
Nico PS, Fendorf SE, Lowney YW et al (2004) Chemical structure of arsenic and chromium in CCA-treated wood: implications of environmental weathering. Environ Sci Technol 38:5253–5260
Nasrullah M, Vainikka P, Hannula J et al (2015) Mass, energy and material balances of SRF production process. Part 3: solid recovered fuel produced from municipal solid waste. Waste Manag Res 33:146–156. https://doi.org/10.1177/0734242X14563375
Rotter VS, Kost T, Winkler J, Bilitewski B (2004) Material flow analysis of RDF-production processes. Waste Manag 24:1005–1021. https://doi.org/10.1016/j.wasman.2004.07.015
International Solid Waste Association (2015) Bottom ash from WTE plants: metal recovery and utilization. ISWA Report
Šyc M, Simon FG, Hykš J et al (2020) Metal recovery from incineration bottom ash: state-of-the-art and recent developments. J Hazard Mater 393:122433. https://doi.org/10.1016/j.jhazmat.2020.122433
Acknowledgements
This study is supported by the Environmental Research and Technology Development Fund (3-1804) of the Environmental Restoration and Conservation Agency and Japan Waste Research Foundation.
Author information
Authors and Affiliations
Contributions
Seungki Back: Writing original draft; Methodology; Data curation and validation; Visualization; Review and editing of the manuscript; Supervision. Hirofumi Sakanakura: Conceptualization; Data validation; Resources; Review and editing of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Back, S., Sakanakura, H. Inventory of twenty-six combustible wastes as sources of potentially toxic elements: B, Cr, Cu, Zn, As, Sb, Ba, and Pb. J Mater Cycles Waste Manag 23, 664–675 (2021). https://doi.org/10.1007/s10163-020-01155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01155-7