Abstract
Ash produced from the combustion of livestock manure contains large amounts of phosphorus (P), which is an important resource as a fertilizer. Some studies have extracted and recovered P from incinerated biomass ash using inorganic acid or alkaline agents, which produce wastewater that requires treatment and is expensive due to the cost of chemicals. Livestock manure ash contains not only P, but also water soluble salts, which could be a negative influence on plant growth and shall be preferably removed from the recovered fertilizer. In this study, we removed salinity from cattle manure incineration ash by simple aqueous leaching, while retaining the P content. The optimal condition was a 20 min leaching time at a liquid/solid (L/S) ratio of 10 mL g-ash−1. Under this condition, over 90 % of Cl and 20 % of Na in the original ash was removed, while over 99 % of the P was retained in the leached residue. The leached residue met the fertilizer standard in Japan in terms of citrate soluble fertilizer components and contained few heavy metals. X-ray analyses of the ash indicated that Cl was mainly present as KCl in the original ash, while P was mainly present as Ca compounds in the ash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Livestock manure is generated worldwide and is one of the most widely produced waste materials in Japan. It is mainly recycled by composting. Composted manure tends to be used near where it is made because of disposal and transportation costs. Therefore, in areas with a high livestock density, excess fertilizer application to agricultural land can cause environmental problems, including groundwater pollution by nitrate-nitrogen [1]. Recently livestock manure incineration has become a concern. Although carbon dioxide (CO2) is generated from its incineration, because livestock manure is a biomass it is considered to be carbon–neutral, and its incineration can recover waste heat, generate electric power, and hygienically reduce the volume of livestock manure [2, 3]. Moreover, manure ash derived from incineration contains large amounts of phosphorus (P) and is more stable and preservable than composted manure. Phosphorus is a very important element for plant growth and in industrial applications, and is a limited resource. Most of the P used in Japan is imported, and the inventory of P in livestock manure is equivalent to that in imported fertilizer [4].
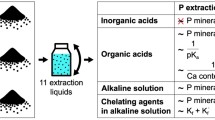
Even though some biomass incineration ash, i.e. incinerated sewage sludge ash (ISSA), contains a high amount of P [5, 6], it cannot be directly applied as a fertilizer without pre-treatment in many cases because it also contains several hazardous elements, such as heavy metals [7], and the chemical form of P in ash is not directly available to plants [8]. Previous studies of P recovery from biomass ash have involved extraction from ISSA, piggery waste ash and chicken manure incineration ash (CMIA) [8–10]. Franz [9] extracted P from ISSA using sulphuric acid and precipitated it with lime water. However, the extracted solution contained heavy metals and had to be purified by removing them with chelating resins. Ottosen et al. [8] extracted P from two types of ISSA using sulphuric acid and indicated that the use of this acid resulted in the formation of a large quantity of gypsum crystals in the remaining ash and the removal of leached heavy metals was required. Kaikake et al. [10] extracted P from CMIA using hydrochloric acid and precipitated it by gradually changing the pH from 3 to 8 using an alkaline solution due to the purification of precipitated P.
Most P recovery processes from biomass incineration ash currently use acid extraction and alkaline precipitation with the contaminant removal stage. Although these processes can successfully recover P in livestock manure ash, as calcium phosphate compounds for use as a fertilizer, they have relatively high costs due to the use of a chemical reagent and generate large amounts of wastewater and extracted residue. A more simplified process to recover P from livestock manure ash is therefore required.
Livestock manure contains sodium (Na), potassium (K) and chlorine (Cl), which are the main components of salinity [11, 12]. Oshita et al. [13] reported that cattle manure ash produced by a fluidized bed incinerator contained not only approximately 4.0 wt% of P but also 1.0 wt% of Na, 4.0 % of K and 10.0 wt% of Cl, and smaller amounts of heavy metals. The Energy Research Centre of the Netherlands (2012) also reported a Na content of 1.7 wt% and Cl content of 3.3 wt% in chicken manure ash in Phyllis2, which is a database providing information on the composition of biomass and waste [14]. While K is a fertiliser component, higher NaCl concentration in soil (>applox. 100 mM) have a harmful effect on plant growth [15]. Although NaCl in soil cannot reach such a high concentration under the normal fertilizing condition, there is some possibility of NaCl harmful effect at local spots in real farm and these components shall preferably not be present in the P-rich materials that are recovered for use as a fertilizer. Fly ash derived from municipal solid waste incineration (MSWI) also contains a large amount of Cl and salts, with most Cl existing as a NaCl, KCl, and CaCl2, which can be easily leached out within a short time (i.e. minutes) by aqueous leaching [16–18]. The Na and Cl in livestock manure ash can also be rapidly removed by aqueous leaching. However, there is little information available regarding livestock manure ash, and the behaviour of salts and P during aqueous leaching will depend on the chemical form of the salts and P in the ash. Even P in livestock manure ash might be removed by aqueous leaching.
This study investigated a simple aqueous leaching process for the removal of highly water-soluble salts including Na and Cl from livestock manure ash, while retaining the P content. The concept of this process and its difference from existing P recovery processes is shown in Fig. 1. This process should be located on the side of a livestock manure incineration plant. The leached residue could be dried for simple use as a fertilizer, by using waste heat from the incineration plant. This study specifically focused on the livestock manure ash produced by the incineration of cattle manure. The behaviour of salts and fertilizer elements were investigated during the aqueous leaching of cattle manure ash and the leached residue was evaluated in terms of its quality as a fertilizer. Additionally, the chemical form and changes in the Cl and P content during aqueous leaching experiments are important to determine, to ensure its effective treatment and utilization as a fertilizer. X-ray diffraction (XRD) analysis and X-ray absorption fine structure (XAFS) analysis of Cl and P were conducted to determine the chemical forms of these elements and any changes in their concentration. These data supported the results of the leaching experiment.
Materials and Methods
Sample ash
Two types of cattle manure ash, ash A and ash B, were used in this study. Ash A was produced by the combustion of cattle manure using a pilot-scale rotary kiln incinerator operating at a temperature of 850 °C and an equivalence ratio (ER) of 1.4 [19]. The cattle manure was obtained from the experimental farm of Kochi University in Kochi Prefecture, Japan and dried in sunlight to a water content of about 34 %. After drying, the cattle manure was processed with a 5 mm sieve. Ash B was prepared by the combustion of composted cattle manure using a pilot-scale fluidized bed incinerator at temperatures below 850 °C and an ER above 1.4 [13]. The composted cattle manure (Water content: 39 %) was treated for 3–6 months with turnover, and finally sieved using a 20-mm trommel at a composting facility located at Handa city in Aichi Prefecture, Japan.
Experimental
The ash sample was placed into a beaker and mixed with demineralized water at a liquid/solid ratio (L/S) of 2–30 mL g−1. The mixture was stirred at 250–300 rpm by a magnetic stirrer for 1120 min at room temperature. After leaching, the eluates were separated and filtrated (0.45-μm pore size and 55-mm φ, Whatman, Maidstone, UK) from the leached residue and the pH was measured (D-50, Horiba Ltd., Kyoto, Japan). The weights of leached residues and eluates were measured. Two replications of the leaching experiment were conducted under the same experimental conditions.
To explain the behaviour of each element in the aqueous leaching process, the leaching ratio was used, as defined in Eq. (1):
Analytical methods
Water content and elemental analysis
The water content of leached residues was determined after drying at 110 °C for 24 h. The concentrations of P, sulphur (S), calcium (Ca), magnesium (Mg), K, Na, iron (Fe), aluminium (Al), manganese (Mn), and zinc (Zn) in sample ashes, leached residues, and eluates were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (IRIS Intrepid Duo, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with triplicate measurements. Prior to ICP-AES analysis, original ash samples and leached residues were decomposed by hydrofluoric acid, aqua regia, and perchloric acid on a hot plate at 180 °C [20]. The decomposed solution was then filtrated (0.45-μm pore size and 55-mm φ, Whatman) and analysed by ICP-AES. The concentrations of chromium (Cr), nickel (Ni), arsenic (As), cadmium (Cd), and lead (Pb) in the leached residue were determined using inductively coupled plasma-mass spectrometry (ICP-MS; XSERIES 2, Thermo Fisher Scientific), after pre-treatment by the same method used for ICP-AES samples. Quantitative analysis was performed by the multi-calibration curve method using the yttrium (Y) fixed internal standard method for ICP-AES and ICP-MS. Mercury (Hg) in the leached residue was analysed by a technique based on thermal decomposition, gold amalgamation, and atomic absorption in accordance with US EPA 7473 and ASTM D-6722-01 (MA-2000, Nippon Instruments Corp., Tokyo, Japan).
The Cl concentration in sample ashes and leached residues was determined according to test method: JCI-SC4 [21]. Samples were preliminary treated by nitric acid, with a 2 mol L−1 solution of nitric acid added to 1 g of sample, stirred until the pH of the mixture was below 3, and then boiled for 5 min. This mixture was then neutralized by approximately 3 g of CaCO3 and repeatedly boiled for 2 min. After filtration (5C, JIS P 3801, 55-mm φ, Advantec, Dublin, CA, USA), the Cl concentration in the filtrate was determined by ion chromatography (HIC-20A Super, Shimadzu Corp., Kyoto, Japan). The Cl concentrations in eluates following aqueous leaching were determined by ion chromatography without pre-treatment.
Fertilizer content analysis
The plant-availability of leached residue as a fertilizer was estimated by an extraction using citric acid solution. The extractable P, K, and Mg in citric acid solution were determined by a testing method for fertilizers [22]. One gram of leached residue was mixed with 150 mL of citric acid solution (20 g L−1) and shaken at 30–40 rpm and 30 °C for 1 h. After extraction, the cooled mixture was filtered (0.45-μm pore size and 55-mm φ, Whatman). The P, K, and Mg concentrations in the eluates were determined by ICP-AES.
Particle size distribution
Because cattle manure ashes can contain large amounts of salts, which might be water soluble, the particle size distribution would be influenced and changed by aqueous leaching. Therefore, the particle size distribution was determined by the dry and wet laser diffraction method using a particle size analyser (SALD-2300 and SALD-2200, Shimadzu Corp.). While the particle size distribution of the original ash was analysed by the dry method using a cyclone type analytical unit (SALD-DS5, Shimadzu Corp.), the change in the particle size distribution due to aqueous leaching was evaluated by a flow type cell using deionized water (SALD-BS2, Shimadzu Corp.).
XRD analysis
The main crystalline compounds in the original ash samples and leached residues were identified using a powder X-ray diffractometer (model RINT-Ultima PC, Rigaku Co., Ltd., Tokyo, Japan) with the characteristic Cu radiation and a solid state 1-D detector (D/teX Ultra, Rigaku Co., Ltd.).
Ash A and B, and leached residue A and B were used for XRD analysis. Each leached residue was generated under the conditions of L/S: 2 and 1 min or 2 h aqueous leaching of ash. These samples were dried at 110 °C for 24 h and homogenized to a powder mounted on a glass plate. The measurement was made using a continuous scan in the 5°–90° region in 2θ, with a scan speed of 10° min−1. The obtained XRD patterns were analysed using XRD analysis software (PDXL2, Rigaku Co., Ltd.).
XAFS analysis
When a sample of thickness t (m) is irradiated by X-rays with an energy of I 0 (eV), the X-ray intensity transmitted through the sample is given by Beer’s Law, as follows:
where μ is the absorption coefficient of the X-rays (m−1).
If I 0 changes around the absorption edge energy of an element in the sample, μ fluctuates greatly and this produces the XAFS spectrum. The local structure and electronic states of a target element can be determined from the XAFS spectrum. The comparison of the XAFS spectrum of an unknown sample with spectra of standard compounds can be used to evaluate the chemical states of the element in the unknown sample. Unlike XRD analysis, high crystallinity and a vacuum are not required for XAFS analysis [23].
To determine the chemical forms of Cl and P in the ashes and the leached residues, the Cl and P K-edge XAFS spectra of samples were measured at the BL-10 beam line of Ritsumeikan University SR Center (Shiga, Japan) and the BL-11B beam line of the Photon Factory (Ibaragi, Japan), respectively. A monochromator with Ge (111) and Si (111) crystals was used to access the energy Cl K-edge at 2822.4 keV and P K-edge at 2145.5 keV, respectively. Energy calibration for the Cl and P K edge was performed by the first peak position of the spectrum of KCl and AlPO4.
Only ash A and leached residue A were used for XAFS analysis. Leached residue A was generated under the conditions of L/S: 2 and 1 min aqueous leaching of ash A. These samples were dried at 110 °C for 24 h and homogenized to a powder. Powder samples were mounted on carbon tape, and the spectral data of Cl and P were collected under vacuum pressure using the total electron yield and fluorescence yield techniques, respectively.
The spectrum data were analysed using XAFS analysis software (REX-2000 Ver2.5, Rigaku Co., Ltd.).
Results and discussion
Elemental composition of the samples
Table 1 compares the elemental compositions of ashes A and B with those of other daily cattle manure ash and ISSA [24–26].
Although cattle manure ash contained large amounts of P, Ca, Mg, and K, indicating its suitability for use as a fertilizer, the Na and Cl concentrations exceeded 1.1 and 1.9 wt%, respectively. While the Na, Fe, Mn, and Zn concentrations in ash A were markedly higher than in ash B, the Cl concentration in ash A was lower than in ash B. Comparing ashes A and B with other cattle manure, the P and K concentrations of ashes A and B were slightly higher and lower respectively, those in other daily cattle manure. These differences probably depended on the kind of feed or bedding materials used for the cattle or the moisture control materials used during compost processing.
The concentrations of P in ashes A and B were almost identical to that in ISSA, and cattle manure ash can be recycled as a P resource. While the levels of K and Mg were higher, and those of Fe, Al, and Zn were lower, the Na and Cl concentration in the cattle manure ash was more than 2.9 and 6.5 times higher than in ISSA, respectively.
Particle size distribution
Figure 2 shows the particle size distribution of original ashes with d values of each sample measured by the dry and wet methods of laser diffraction. The particle size of cattle manure ash ranged from 0.1 to 200 μm and the median particle sizes (d 50) of original ash were 28.2 μm for ash A and 62.8 μm for ash B, respectively. The particle size distribution of ash B was shifted towards a larger size than that of ash A. These ranges of particle size were almost the same as those reported for MSWI fly ash [18] and fly ash generated by the combustion of coal, wood bark, and sewage sludge using a circulating fluidized bed incinerator [27].
The particle size distributions determined by the wet method of laser diffraction were smaller than those determined by the dry method of laser diffraction, although the particle size of ash A was shifted to a greater size (over 50 μm). The medians in this case were 23.6 μm for ash A and 44.2 μm for ash B, respectively, and were 15–30 % lower than for the original ashes. This was because the water soluble content, i.e. chloride and salt, was dissolved by the wet method and the particle size was therefore decreased.
Leaching characteristics
Figure 3a, b showed the relationship between L/S and leaching ratios of each element in ashes A and B, respectively. Figure 4a, b showed the relationship between leaching time and leaching ratios of each element in ashes A and B, respectively. The behaviour of each element in ashes A and B had a similar tendency. The pH ranged from 10.1 to 10.5 in ash A and from 9.8 to 10.2 in ash B.
From Fig. 3a, b, the leaching ratios of Cl, Na and K were increased along with the increasing of L/S up to 10. The 90 % of Cl in the both ashes were leached at L/S:10 within 1 min. About 60–80 % of the Cl content in fly ash from MSWI was readily leached by aqueous leaching at L/S: 2 and 5 min leaching [16] or at L/S: 2 and 60 min leaching [28]. However, only about 10–30 % of the Cl content in bottom ash from a MSWI plant was leached by aqueous leaching at L/S: 20 and 5 min leaching [29]. As with the MSWI fly ash, the Cl content in cattle manure ash was also rapidly leached by aqueous leaching. Only 20 % of the Na in both ashes was leached at L/S:10 within 1 min. Approximately 55 % of the Na in fly ash from MSWI was leached at L/S: 2 and 60 min leaching [28]. Therefore the proportion of water soluble Na in cattle manure ash is likely lower than that of MSW fly ash. The remaining Na could exist as water insoluble compounds, such as aluminosilicates [6], as in the leached residues and could have some harmful effects on plant growth. The highest leaching ratios of K were approximately 50 and 35 % for ashes A and B, respectively. The difference in the leaching ratios of K between ashes A and B depended on not only the concentration of each element in the original ash, but also the ratio of the water soluble K. In this process, 35–50 % of the K in the manure ashes was lost to the leachate. K is an important fertilizer element and should be recovered when possible. Although we did not attempt to recover K from the leachate, potassium struvite (KMgPO4) could be precipitated by controlling the P and Mg concentrations in the leachate [30].
The leaching ratios of P, Mg and Ca were very low and these elements were hardly leached from ash A or ash B. The aqueous leaching process leaves over 99 % of these elements as a fertilizer component in the leached residue.
From Fig. 4a, b, the leaching ratios of Cl, Na and K with the increasing of the leaching time were more stable than these with the increasing of L/S in Fig. 3a, b. Therefore this salt leaching system would be almost controlled by the solubility of salts to water. The leaching ratio of Cl was initially over 75 %, which is relatively high. The leaching ratio of Cl from ash A increased to 95 % after a 20 min leaching time, and then gradually decreased to 80 %. This tendency was also observed to some extent for ash B. There are two possible reasons for this. The first is that the leached Cl− from ash reacted with tricalcium aluminate (3CaO·Al2O3) to form 3CaO·Al2O3·CaCl2·10H2O, which is Friedel’s salt and is poorly water soluble [31]; the other is that the solubility of salts containing Cl were relatively decreased with the progress of the dissolution of other salts.
From the all results of aqueous leaching experiments, the optimum condition to remove Na and Cl from ashes and to retain P in ashes was considered to be L/S:10 and 20 min of the leaching time. Under this condition, over 90 % of Cl and 20 % of Na could be removed from the cattle manure ashes.
Figure S1 in the supplemental information shows the elemental balances for Cl, P, Ca, Mg, Na and K between the input ash (ash A and B) and the aqueous leaching product under the conditions of Fig. 4a, b. The results shown in Fig. S1 indicate that the sum of the amount in leached residue and eluate was approximately 100 %, although in the case of K it was slightly higher than 100 %. The validity of the elemental balance and the results in the leaching experiments were thus demonstrated.
Fertilizer contents
Table 2 shows the fertilizer components and harmful heavy metals in leached residue and the Japanese standards for a fused sewage sludge ash compound fertilizer. Leached residues A and B were refined by the aqueous leaching of ashes A and B, respectively, under the conditions of L/S: 2 and 1 min leaching. The P2O5 content in both leached residues was over 15 %, and the citrate soluble P2O5 content was over 12 %, which is the Japanese standard. The K2O content was over 6.80 % in both leached residues, and met the Japanese standard (over 1 %). Most of the MgO was present as citrate soluble MgO in both of the leached residues.
The Cr, Ni, As, Cd, Pb, and Hg concentrations in both leached residues were below the Japanese standards. This is because the levels of harmful heavy metals in Japanese cattle manure are generally very low [12]. The phosphate rock used as a raw material for fertilizer often contains hazardous elements such as Cd and uranium and the average Cd concentration in phosphate rock is 20.6 mg kg−1, which is 68.7 times the average content in shale (0.3 mg kg−1) [32]. The Cd concentrations in leached residues A and B were 1.35 and 2.16 mg kg−1, respectively. The mean Cd concentration in composted cattle manure in Japan is 0.26 mg kg−1 (range 0.11–0.44 mg kg−1) [33]. These values are lower than that of phosphate rock. Therefore, the recycling of cattle manure ash as a fertilizer is also important in terms of the management of hazardous elements.
XRD analysis
Figure 5a, b shows the XRD pattern of the original sample and leached residue of ashes A and B, respectively. The Cl in crystalline compounds of both ashes mainly existed as KCl and their peak intensities decreased with aqueous leaching. These results, together with the Cl behaviour in Figs. 3 and 4, suggest that the Cl removal mechanism could depend on the dissolution of KCl in the original ash by aqueous leaching. The P in crystalline compounds of ashes mainly exists as Ca compounds: Ca9MgK(PO4)7, NaCa9Fe(PO4)7 or Ca3(PO4)2. This is consistent with the fact that calcium phosphate compounds were sparingly soluble in water and displayed citrate solubility [34], with the results shown in Figs. 3, 4 and Table 2. In ash B, part of the K and Na in crystalline compounds was present as K3Na(SO4)2 (glaserite), which was not detected in ash A and the leached residues A and B, because the sulphur concentration in ash B was higher than in ash A, and could be removed by aqueous leaching.
XAFS analysis
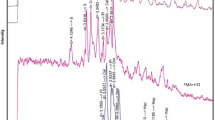
The Cl K-edge and P-K edge X-ray absorption near edge structure (XANES) spectra of standard substances (Cl: AlCl3, CaCl2, MgCl2, NaCl, KCl, and polyvinyl chloride (PVC), P: AlPO4, FePO4, triphenyl phosphate, PX-200™, Na3PO4·12H2O, sodium polyphosphate, adenosine 5′-triphosphate (disodium salt) (ATP), Ca3(PO4)2, and CaHPO4·12H2O), in ash A and leached residue A, are shown in Fig. 6a, b, respectively. In this study, the XANES spectra of the ash samples were compared with those of standard substances. From Fig. 6a, the Cl K-edge XANES spectra of standard substances were very different within the range 2820–2850 eV. The XANES spectrum of ash A had a maximum positive peak (the white line with approximately 2826 eV of peak top energy) and secondary positive peak with 2830 eV of peak top energy, and was not like the spectrum for NaCl but similar to the spectrum for KCl. In addition, the XANES spectra of ash A and leached residue A were almost identical, although the spectra of leached residue A exhibited greater fluctuations, with small noise peaks due to the low Cl concentration in the leached residue. Therefore, the Cl in ash A was present as KCl and was leached by aqueous leaching, which is consistent with the results of the XRD analysis. Although KCl is highly water soluble, the small amount of Cl remaining in leached residue A was still present as KCl, and its chemical form was not changed by aqueous leaching. If most of the Cl in ash A exists as KCl, approximately 35 % of the K in ash A was determined to exist as KCl, as shown in Table 1. This value is slightly lower than the leaching ratio of K in Figs. 3 and 4. Therefore leached K is not derived only the dissolution of KCl. Although the Cl K-edges XANES spectrum of ash B was not analysed in this study, the Cl in ash B could also exist as KCl based on the results of the XRD analysis (Fig. 5b). However, as shown in Table 1, there was more K in ash B than Cl. Therefore, Cl in ash B existed as KCl and other soluble salts, such as NaCl and CaCl2, as in MSWI fly ash [17, 35].
a Cl K-edge X-ray absorption near edge structure (XANES) spectra of Cl compounds, ash A, and leached residue A and b P K-edge XANES spectra of P compounds, ash A, and leached residue A. Leached residue A was generated under conditions of L/S:2 and 1 min aqueous leaching of ash A. PVC, polyvinyl chloride; ATP, adenosine 5′-triphosphate (disodium salt); PX-200TM, resorcinol bis (dixylenyl phosphate)
Although from Fig. 6b the P K-edge XANES spectra of standard substances were less different than the Cl K-edge XANES spectra, they differed within the photon energy range of 2149–2165 eV. The XANES spectrum of ash A had a positive peak (white line with approximately 2152 eV peak top energy), and appeared to be similar to the spectra for CaHPO4·12H2O and Ca3(PO4)2 in terms of the peak top energy, with a shoulder range from 2153 to 2157 eV, and a small and broad peak from 2159 to 2163 eV. From the XRD results (Fig. 5a), the P in crystalline compounds in ash A could be present as a form of Ca3(PO4)2. Although the XANES spectrum of Ca9MgK(PO4)7 and NaCa9Fe(PO4)7 was not analysed, the P K-edge XANES spectrum of calcium phosphate minerals involving apatite group minerals displays a shoulder on the main peak [36]. The main chemical form of P in ash A could be Ca compounds at least. This can also be seen for ash B, from the XRD results in Fig. 5b. In addition, the XANES spectra of ash A and leached residue A were almost identical.
Therefore, P in the original sample ash was present mainly in the chemical form of Ca compounds, and could not be leached or changed to other chemical forms by aqueous leaching. The fact that calcium phosphate compounds were sparingly soluble in water and displayed citrate solubility [34] explains the results in Figs. 3, 4 and Table 2.
Conclusions
This study investigated salinities removal from two types of cattle manure ash by simple aqueous leaching for final use as a fertilizer.
Ash produced from the combustion of cattle manure contains not only P, but also Na and Cl, which shall preferably be removed. The particle size of the ashes ranged from 0.1 to 200 μm and became smaller following aqueous leaching due to the dissolution of salts. The optimized condition for aqueous leaching was 20 min leaching time at an L/S of 10. Under these conditions, over 90 % of Cl and 20 % of Na in the original ash was removed and over 99 % of the P remained in leached residue. Aqueous leached residue met the fertilizer standard in Japan in terms of citrate soluble fertilizer components, and did not contain heavy metals. XRD and XAFS analysis of cattle manure ash and the leached residue indicated that Cl mainly exists as KCl in cattle manure ash and was removed by aqueous leaching, while P was mainly present as Ca compounds in the cattle manure ash and was retained in the leached residue.
References
Goldberg VM (1989) Groundwater pollution by nitrates from livestock wastes. Environ Health Perspect 83:25–29
Kelleher BP, Leahy JJ, Henihan AM, O’Dwyer TF, Sutton D, Leahy MJ (2002) Advances in poultry litter disposal technology—a review. Bioresour Technol 83:27–36
Reijnders L, Huijbregts MAJ (2005) Life cycle emissions of greenhouse gases associated with burning animal wastes in countries of the European Union. J Clean Prod 13:51–56
Matsubae-Yokoyama K, Kubo H, Nakajima K, Nagasaka T (2009) A material flow analysis of phosphorus in Japan, the iron and steel industry as a major phosphorus source. J Ind Ecol 13(5):687–705
Baeyens J, Van Puyvelde F (1994) Fluidized bed incineration of sewage sludge: a strategy for the design of the incinerator and the future for incinerator ash utilization. J Hazard Mater 37(1):179–190
Vassilev SV, Baxter D, Vassileva CG (2013) An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 112:391–449
Adam C, Peplinski B, Michaelis M, Kley G, Simon F-G (2009) Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag 29(3):1122–1128
Ottosen LM, Kirkelund GM, Jensen PE (2013) Extracting phosphorous from incinerated sewage sludge ash rich in iron or aluminum. Chemosphere 91(7):963–969
Franz M (2008) Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag 28(10):1809–1818
Kaikake K, Sekito T, Dote Y (2009) Phosphate recovery from phosphorus-rich solution obtained from chicken manure incineration ash. Waste Manag 29(3):1084–1088
Font-Palma C (2012) Characterisation, kinetics and modelling of gasification of poultry manure and litter: an overview. Energy Convers Manag 53(1):92–98
Oshita K, Sun X, Takaoka M, Matsukawa K, Fujiwara T (2011) Investigation of livestock manure as a resource for phosphorus and fuel in Japan. In: 8th IWA International Symposium on Waste Management Problems in Agro-Industries, Izmir, Turkey, 22–24 June
Oshita K, Kawaguchi K, Takaoka M, Matsukawa K, Fujimori T, Fujiwara T (2015) Emission and control of nitrous oxide and composition of ash derived from cattle manure combustion using a pilot-scale fluidized bed incinerator. Environ Technol. doi:10.1080/09593330.2015.1077190
Energy research Centre of the Netherlands, Phyllis2 (2012) Ash from chicken manure (#3499). Available at https://www.ecn.nl/phyllis2/Browse/Standard/ECN-Phyllis#3499. Accessed 30 Dec 2015
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250
Zhu F, Takaoka M, Oshita K, Takeda N (2009) Comparison of two types of municipal solid waste incinerator fly ashes with different alkaline reagents in washing experiments. Waste Manag 29(1):259–264
Zhu F, Takaoka M, Shiota K, Oshita K, Kitajima Y (2008) Chloride chemical form in various types of fly ash. Environ Sci Technol 42(11):3932–3937
Colangelo F, Cioffi R, Montagnaro F, Santoro L (2012) Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag 32(6):1179–1185
Oshita K, Sun X, Taniguchi M, Takaoka M, Matsukawa K, Fujiwara T (2012) Emission of greenhouse gases from controlled incineration of cattle manure. Environ Technol 33(13):1539–1544
Nakazato N (2012) Basic knowledge of pretreatment for sample analysis- Pretreatment for determination of heavy metals in soil. Bunseki 7:352–357 (in Japanese)
JCI-SC4 (2004) Method for determination of chloride quantity in the hardening concrete. Japan Concrete Institute, Tokyo (in Japanese)
Incorporated Administrative Agency, Food and Agricultural Materials Inspection Center (2013) Testing Methods for Fertilizers. Available at https://www.famic.go.jp/ffis/fert/obj/TestingMethodsForFertilizers2013.pdf. Accessed 30 Sep 2015
Calvin S (2013) XAFS for Everyone. CRC Press, Boca Raton
Hioki M, Sakakibara M (2013) Effects of daily manure compost ash as fertilizer. Res Bull Aichi Prefect Agric Res Cent 45:135–141 (in Japanese)
Mattenberger H, Fraissler G, Brunner T, Herk P, Hermann L, Obernberger I (2008) Sewage sludge ash to phosphorus fertiliser: variables influencing heavy metal removal during thermochemical treatment. Waste Manag 28(12):2709–2722
Mattenberger H, Fraissler G, Jöller M, Brunner T, Obernberger I, Herk P, Hermann L (2010) Sewage sludge ash to phosphorus fertiliser (II): influences of ash and granulate type on heavy metal removal. Waste Manag 30(8–9):1622–1633
Van de Velden M, Baeyens J, Dougan B, McMurdo A (2007) Investigation of operational parameters for an industrial CFB combustor of coal, biomass and sludge. China Particuol 5(4):247–254
Wang K-S, Chiang K-Y, Lin K-L, Sun C-J (2001) Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 62(2):73–81
Boghetich G, Liberti L, Notarnicola M, Palma M, Petruzzelli D (2005) Chloride extraction for quality improvement of municipal solid waste incinerator ash for the concrete industry. Waste Manag Res 23:57–61
Wilsenach JA, Schuurbiers CAH, Van Loosdrecht MCM (2007) Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res 41(2):458–466
Birnin-Yauri UA, Glasser FP (1998) Friedel’s salt, Ca2Al(OH)6(Cl, OH)·2H2O: its solid solutions and their role in chloride binding. Cem Concr Res 28(12):1713–1723
Dissanayake CB, Chandrajith R (2009) Phosphate mineral fertilizers, trace metals and human health. J Natl Sci Found Sri Lanka 37(3):153–165
Hioki M, Kitamura H, Kuno C, Kato T (2001) The manure elements of the livestock excrement compost produced in Aichi Prefecture. Res Bull Aichi Prefect Agric Res Cent 33:237–244 (in Japanese)
Somani LL (1989) Dictionary of Soils and Fertilizers, vol IV. Part Four, Mittal Publications, New Delhi, p 1067
Kitamura H, Sawada T, Shimaoka T, Takahashi F (2015) Geochemically structural characteristics of municipal solid waste incineration fly ash particles and mineralogical surface conversions by chelate treatment. Environ Sci Pollut Res. doi:10.1007/s11356-015-5229-5
Ingall ED, Brandes JA, Diaz JM, de Jonge MD, Paterson D, McNulty I, Elliotte WC, Northrup P (2011) Phosphorus K-edge XANES spectroscopy of mineral standards. J Synchrotron Rad 18:189–197
Acknowledgments
Part of this study was supported financially by the Japan Science and Technology Agency (JST/CREST; T. Fujiwara). The XAFS spectra of P and Cl were measured at BL-11B in the Photon Factory, with the approval of the Photon Factory Program Advisory Committee (Proposal No. 2009G044 and 2011G174) supported by Dr. Yoshinori Kitajima, and at BL-10 of Ritsumeikan University SR Center, (Proposal No. Rits S23-18) supported by Prof. Toshiaki Ota and Dr. Koji Nakanishi. We would like to thank Dr. Hitoshi Shinjo, associate professor in Graduate school of Global environmental studies, Kyoto University, Japan for his advice of NaCl harmful effects on the plant growth.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oshita, K., Sun, X., Kawaguchi, K. et al. Aqueous leaching of cattle manure incineration ash to produce a phosphate enriched fertilizer. J Mater Cycles Waste Manag 18, 608–617 (2016). https://doi.org/10.1007/s10163-016-0528-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0528-6